Abstract
BACKGROUND
Myocarditis is an uncommon, but potentially fatal, toxicity of immune checkpoint inhibitors (ICI). Myocarditis after ICI has not been well characterized.
OBJECTIVES
The authors sought to understand the presentation and clinical course of ICI-associated myocarditis.
METHODS
After observation of sporadic ICI-associated myocarditis cases, the authors created a multicenter registry with 8 sites. From November 2013 to July 2017, there were 35 patients with ICI-associated myocarditis, who were compared to a random sample of 105 ICI-treated patients without myocarditis. Covariates of interest were extracted from medical records including the occurrence of major adverse cardiac events (MACE), defined as the composite of cardiovascular death, cardiogenic shock, cardiac arrest, and hemodynamically significant complete heart block.
RESULTS
The prevalence of myocarditis was 1.14% with a median time of onset of 34 days after starting ICI (inter-quartile range: 21 to 75 days). Cases were 65 ± 13 years of age, 29% were female, and 54% had no other immune-related side effects. Relative to controls, combination ICI (34% vs. 2%; p < 0.001) and diabetes (34% vs. 13%; p = 0.01) were more common in cases. Over 102 days (interquartile range: 62 to 214 days) of median follow-up, 16 (46%) developed MACE; 38% of MACE occurred with normal ejection fraction. There was a 4-fold increased risk of MACE with troponin T of ≥1.5 ng/ml (hazard ratio: 4.0; 95% confidence interval: 1.5 to 10.9; p = 0.003). Steroids were administered in 89%, and lower steroids doses were associated with higher residual troponin and higher MACE rates.
CONCLUSIONS
Myocarditis after ICI therapy may be more common than appreciated, occurs early after starting treatment, has a malignant course, and responds to higher steroid doses.
Keywords: cardio-oncology, checkpoint inhibitor, ipilimumab, myocarditis, nivolumab, pembrolizumab
Immune checkpoint inhibitors (ICI) represent a novel category of drugs that help direct the immune system to recognize and target cancer cells (1,2). The suppression of immune regulation is associated with immune-mediated adverse events. During initial regulatory approval in 2014, immune-mediated adverse events involving the neurological, endocrine, pulmonary, gastrointestinal, and renal systems were reported (3,4). Recently, in small case series, myocarditis was identified as a side effect of immune checkpoint inhibitors (5), but data on presentation, risk factors and outcomes are limited (6–9). At present, in the United States, there are almost 600,000 patients who may be eligible for ICI therapy(10), and the use of ICI is expected to increase significantly in the coming years (11). Hence, there is a need to better characterize ICI-associated myocarditis. After the observation of sporadic cases of ICI-associated myocarditis, we created a retrospective and prospective multicenter registry to provide data on 35 patients with myocarditis following ICI and compared them with contemporaneous ICI-treated patients who did not develop myocarditis; we detail the cardiovascular outcomes, as well as the presentation, treatment, and testing variables associated with those outcomes.
METHODS
PATIENTS.
Cases were derived from an 8-center institutional registry (Online Table 1). The registry was formed in 2016 specifically designed for collating suspected cases of myocarditis related to ICI. The registry included both retrospective and prospective cases; the first case in the registry was from November 2013, and cases were included until July 2017. Controls were randomly derived from a single-center registry (Massachusetts General Hospital, Boston, Massachusetts) of all 964 patients started on ICI in the same time interval who did not develop clinical myocarditis. The number of patients treated with ICI therapy at Massachusetts General Hospital during the study period was confirmed by 2 independent researchers. Controls (3:1 ratio) were not pre-selected to match cases on any variables. To compare different types of steroids, the dose was converted to equivalent methylprednisolone units using standard conversion factors (12). The study was approved by each center’s institutional review board, and the requirement for written informed consent was waived.
COVARIATES.
Data on covariates of interest were extracted retrospectively from electronic medical records including standard demographics, cardiovascular risk factors, medications, electrocardiograms (ECGs), and echocardiographic variables. Admission troponin was defined as first measured serum troponin; peak troponin was maximum measured troponin; and discharge/final troponin was defined as troponin measured at discharge from index hospitalization or the troponin before an event if that event occurred on the index admission. Cancer-specific covariates included the type, ICI treatments, prior cardiotoxic chemotherapy, and prior radiation. Covariates specific to myocarditis also included clinical presentation, physical examination, coronary angiography (invasive or computed tomography) or stress testing, admission, peak and discharge cardiac biomarkers, and if available, cardiac magnetic resonance data, endomyocardial biopsy, and autopsy results, as well as treatments for myocarditis.
DEFINITIONS AND OUTCOME OF INTEREST.
Myocarditis was diagnosed in 2 ways: 1) standard histological features present on endomyocardial biopsy or autopsy; and 2) a guideline-recommended scoring system for clinically suspected myocarditis that incorporates several variables including the clinical, biomarker and imaging features (13). Adverse event grading was performed using Common Toxicity Criteria for Adverse Events (version 4.0) (14). The outcome of interest, major adverse cardiac events (MACE), was a composite of cardiovascular death, cardiac arrest, cardiogenic shock, and hemodynamically significant complete heart block (CHB). In cases where cardiac arrest, cardiogenic shock, or CHB led to a death, that case was counted as a cardiac death. Standard definitions were used for cardiovascular death (15), cardiac arrest (16), and cardiogenic shock (17). Hemodynamically significant CHB was defined as a complete absence of atrial-to-ventricular conduction requiring a temporary pacemaker (18).
STATISTICAL ANALYSIS.
Continuous variables are presented as mean ± SD or median (interquartile range), as appropriate, and categorical variables are presented as percentages. Continuous data were compared with the use of unpaired Student’s t-tests or Wilcoxon rank sum tests. Categorical data were compared using the Fisher exact test. To determine factors associated with MACE, covariates were compared between patients with myocarditis with and without this composite outcome. To calculate the optimal combination of sensitivity and specificity for troponin T in the prediction of MACE, a receiver-operating characteristic curve was constructed. Hazards ratios for MACE with 95% confidence intervals were calculated using Cox regression analysis for the optimal troponin T values. All statistical tests were 2 sided, and 5% was set as the level of significance. Statistical analyses were performed using IBM SPSS Statistics version 24 (IBM, Armonk, New York).
RESULTS
PATIENT CHARACTERISTICS.
The mean age of patients (n = 35) who developed ICI-associated myocarditis was 65 ± 13 years with 29% being female (Table 1). Before myocarditis, the pre-ICI left ventricular ejection fraction (LVEF) was ≥50% in all those with a baseline measurement (Table 1). In comparison with controls, myocarditis cases had a higher prevalence of diabetes mellitus and sleep apnea, and a higher body mass index (Table 1).
TABLE 1.
Description of Cases and Controls
| Myocarditis (n = 35) | Controls (n = 105) | Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|---|
| Age at start of ICI, yrs | 65 ± 13 | 65 ± 13 | – | – | 0.85 |
| Female | 10 (29.0) | 33 (31.0) | 0.87 | 0.38–2.02 | 0.83 |
| CV risk factors | |||||
| Current or prior smoking | 15 (43.0) | 65 (62.0) | 0.51 | 0.23–1.13 | 0.075 |
| Hypertension | 25 (71.0) | 65 (62.0) | 1.54 | 0.67–3.53 | 0.31 |
| Diabetes mellitus | 12 (34.0) | 14 (13.0) | 3.36 | 1.37–8.20 | 0.01 |
| No CV risk factors | 1 (2.9) | 4 (3.8) | 0.74 | 0.08–6.90 | – |
| Coronary artery disease | 7 (20.0) | 17 (16.0) | 1.29 | 0.49–3.44 | 0.61 |
| Prior myocardial infarction | 3 (8.6) | 6 (5.7) | 1.55 | 0.37–6.54 | 0.69 |
| Prior coronary stenting | 2 (5.7) | 2 (1.9) | 3.13 | 0.42–23.26 | – |
| Coronary artery bypass graft | 3 (8.6) | 7 (6.7) | 1.31 | 0.32–5.38 | 1.00 |
| Stroke | 2 (5.7) | 11 (10.5) | 0.52 | 0.11–2.46 | 0.52 |
| Heart failure | 1 (2.9) | 9 (8.6) | 0.31 | 0.04–2.57 | 0.45 |
| Chronic obstructive pulmonary disease | 4 (11.0) | 14 (13.0) | 0.84 | 0.26–2.74 | 1.00 |
| Obstructive sleep apnea | 5 (14.0) | 4 (3.8) | 4.2 | 1.06–16.67 | 0.04 |
| Chronic kidney disease* | 2 (5.9) | 19 (18.0) | 0.28 | 0.06–1.28 | 0.10 |
| Body mass index, kg/m2 | 29.0 ± 8.4 | 26.0 ± 6.0 | – | – | 0.02 |
| Primary cancer type | |||||
| Head and neck | 2 (5.7) | 10 (9.5) | 0.58 | 0.12–2.76 | 0.73 |
| Breast | 1 (2.9) | 0 (0.0) | – | – | – |
| Hodgkin’s lymphoma | 1 (2.9) | 2 (1.9) | 1.52 | 0.13–17.24 | – |
| Melanoma | 16 (46.0) | 50 (48.0) | 0.19 | 0.02–1.51 | 1.00 |
| Non-small cell lung cancer | 4 (11.0) | 26 (25.0) | 0.39 | 0.13–1.22 | 0.15 |
| Small cell lung cancer | 0 (0.0) | 4 (3.8) | – | – | 0.57 |
| Pancreatic | 1 (2.9) | 0 (0.0) | – | – | – |
| Renal cell carcinoma | 2 (5.7) | 1 (1.0) | 6.29 | 0.55–71.43 | – |
| Glioblastoma | 1 (2.9) | 0 (0.0) | – | – | – |
| Other | 7 (20.0) | 12 (11.0) | 1.94 | 0.70–5.41 | 0.25 |
| Prior chemotherapy or radiation | |||||
| Radiation | 14 (40.0) | 61 (58.0) | 0.48 | 0.22–1.05 | 0.078 |
| Anthracyclines | 2 (5.7) | 3 (2.9) | 2.06 | 0.33–12.82 | 0.60 |
| Vascular endothelial growth factor inhibitors | 1 (2.9) | 2 (1.9) | 1.52 | 0.13–17.24 | – |
| Pre-ICI treatment echocardiogram† | |||||
| LVEF, % | 60 ± 7 | 62 ± 10 | – | – | 0.28 |
| Left ventricular internal dimension in diastole, mm | 43 ± 6 | 45 ± 6 | – | – | 0.38 |
| Pre-ICI treatment ECG | |||||
| Sinus rhythm | 27 (96.0) | 83 (91.0) | 0.50 | 0.18–1.37 | 0.81 |
| ST-segment or T-wave changes | 6 (21.0) | 32 (35.0) | 2.60 | 0.31–21.74 | 0.199 |
| Heart rate, beats/min | 81 ± 15 | 82 ± 24 | – | – | 0.82 |
| QRS interval, ms | 96 ± 17 | 94 ± 23 | – | – | 0.71 |
| QTc interval, ms | 443 ± 26 | 440 ± 31 | – | – | 0.63 |
| Pre-ICI home CV medications | |||||
| Statin | 11 (31.0) | 26 (25.0) | 1.39 | 0.60–3.23 | 0.51 |
| Aspirin | 12 (34.0) | 23 (22.0) | 1.86 | 0.81–4.29 | 0.18 |
| Beta-blockers | 10 (29.0) | 34 (32.0) | 0.84 | 0.36–1.93 | 0.83 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 15 (43.0) | 20 (19.0) | 3.18 | 1.39–7.30 | 0.007 |
| Calcium-channel blocker | 4 (11.4) | 20 (19.0) | 0.55 | 0.17–1.73 | 0.44 |
Values are mean ± SD or n (%), unless otherwise indicated.
Chronic kidney disease = glomerular filtration rate <60 ml/min/1.73 m2.
Twenty-three of the 35 cases had a pre-treatment echocardiogram, and 50 of the 105 controls had a pre-treatment echocardiogram.
CV = cardiovascular; ECG = electrocardiogram; ICI = immune checkpoint inhibitors; QTc = QT interval corrected for heart rate.
CANCER AND TREATMENT CHARACTERISTICS.
The most common indications for ICI were melanoma (Table 2) and non-small cell lung cancer. All cases with ICI-associated myocarditis had ICIs permanently discontinued. Compared with controls, myocarditis cases were more likely to have received combination ICI at any stage in treatment and were more likely to be treated with combination ICI therapy at the time of statistical analysis, that is, a median 281 days follow-up for controls, and 209 days for myocarditis cases (Table 2). Of the combination therapies at the time of presentation, anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA4) with anti-programmed cell death protein 1 (anti-PD1) was the most frequent in cases. However, overall, myocarditis was more commonly seen with concurrent single ICI therapy (66%). A complete description of the comparisons of ICI therapies between cases and controls separated by those on combination therapy at presentation, on single therapy at presentation, and by previous therapies administered before the current regimen is presented in Table 2. None of the cases with melanoma were on trametinib. A description of cancer types when restricted to those on single ICI therapy alone is also provided (Online Table 2). Approximately one-half (54%) of the myocarditis cases had not experienced another ICI-related side effect. There was generally no difference in the overall prevalence of other ICI-related side effects between cases and controls; however, myocarditis cases who did have an additional previous immune-related side effect had higher rates of hepatitis.
TABLE 2.
Baseline Cancer Demographics
| Myocarditis (n = 35) | Control (n = 105) | p Value | |
|---|---|---|---|
| Single agent vs. combined | |||
| Combination (ever received) | 12 (34.3) | 10 (9.5) | <0.001 |
| Combination (current regiment) | 12 (34.3) | 2 (1.9) | <0.001 |
| Monotherapy (current regiment) | 23 (65.7) | 103 (96.0) | |
| Combined ICI | |||
| Ipilimumab (anti-CTLA4) + nivolumab (anti-PD1) | 9 (26.0) | 9 (8.6) | 0.02 |
| Ipilimumab (anti-CTLA4) + pembrolizumab (anti-PD1) | 1 (2.9) | 0 (0.0) | 0.25 |
| Tremelimumab (anti-CTLA4) + avelumab (anti-PDL1) | 1 (2.9) | 0 (0.0) | 0.25 |
| Tremelimumab (anti-CTLA4) + durvalumab (anti-PDL1) | 1 (2.9) | 1 (1.0) | 0.44 |
| Current monotherapy ICI* | |||
| Pembrolizumab (anti-PD1) | 11 (31.0) | 41 (39.0) | 0.54 |
| Nivolumab (anti-PD1) | 7 (20.0) | 53 (51.0) | 0.002 |
| Ipilimumab (anti-CTLA4) | 2 (5.7) | 9 (8.6) | 0.73 |
| Tremelimumab (anti-CTLA4) | 1 (2.9) | 0 (0.0) | 0.25 |
| Atezolizumab (anti-PDL1) | 2 (5.7) | 0 (0.0) | 0.06 |
| Avelumab (anti-PDL1) | 0 (0.0) | 0 (0.0) | – |
| Durvalumab (anti-PDL1) | 0 (0.0) | 0 (0.0) | – |
| Overall types of ICI | |||
| Any anti-PD1 | 28 (80.0) | 102 (97.0) | 0.002 |
| Any anti-CTLA4 | 18 (51.0) | 29 (27.0) | 0.01 |
| Any anti-PDL1 | 4 (11.0) | 2 (2.0) | 0.03 |
| Pembrolizumab | 16 (46.0) | 49 (47.0) | 1.00 |
| Nivolumab | 16 (46.0) | 60 (57.0) | 0.25 |
| Ipilimumab | 15 (43.0) | 28 (27.0) | 0.09 |
| Atezolizumab | 2 (5.7) | 1 (1.0) | 0.15 |
| Avelumab | 1 (2.9) | 0 (0.0) | 0.25 |
| Durvalumab | 1 (2.9) | 1 (1.0) | 0.44 |
| Tremelimumab | 3 (8.6) | 1 (1.0) | 0.048 |
| Days of follow-up | 209 ±300 | 281 ± 286 | 0.69 |
| Other immune side effects during treatment | |||
| No other immune side effects | 19 (54.0) | 61 (58.0) | 0.70 |
| Hypophysitis/pituitary/adrenal | 3 (8.6) | 6 (5.7) | 0.69 |
| Pneumonitis | 3 (8.6) | 11 (10.5) | 1.00 |
| Hepatitis | 7 (20.0) | 5 (4.8) | 0.01 |
| Colitis | 4 (11.0) | 15 (14.0) | 0.78 |
| Dermatitis | 4 (11.0) | 3 (2.9) | 0.065 |
| Neurological | 4 (11.0) | 3 (2.9) | 0.065 |
| Gastritis | 0 (0.0) | 4 (3.8) | 0.57 |
Values are n (%) or mean ± SD. All cases with ICI-associated myocarditis had ICI permanently discontinued.
If most recent ICI therapy was monotherapy.
anti-CTLA4 = anti-cytotoxic T-lymphocyte-associated protein 4; anti-PD1 = anti-programmed cell death protein 1; anti-PDL1 = anti-programmed death-ligand 1; ICI = immune checkpoint inhibitors.
MYOCARDITIS PRESENTATION.
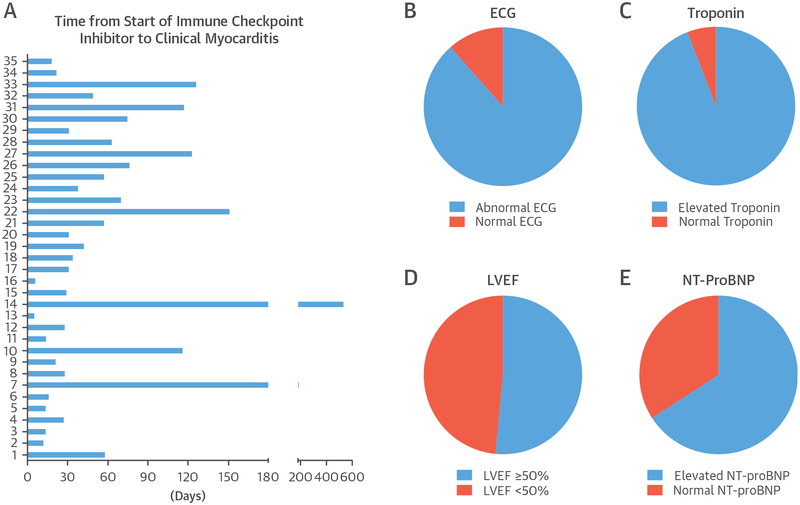
Median time to onset of myocarditis from first ICI was 34 days (interquartile range 21 to 75 days) (Figure 1) with 81% presenting within 3 months of starting therapy. Nearly all myocarditis cases had a troponin elevation (94%) (Figure 1) and an abnormal ECG (89%) (Figure 1). The LVEF was normal in 51% of cases of myocarditis (Figure 1). Overall, 11 patients had a cardiac biopsy or autopsy, and 31 had a cardiac magnetic resonance imaging (MRI) study. The consistent finding noted on histology was a patchy to gross, T-cell–predominant lymphocytic infiltrate within the myocardium, which was similar in findings to those seen in cardiac transplant rejection; no granulomas or giant cells were noted. In another 20 cases, the diagnosis was made with the combination of an elevated troponin and the presence of late gadolinium enhancement (Table 3) on a cardiac magnetic resonance study in a pattern typical for myocarditis(19) and were without evidence of coronary ischemia on standard testing. In the remaining 4 cases, the patients did not have a biopsy or late gadolinium enhancement on a cardiac MRI but presented with typical cardiovascular symptoms, congestive heart failure, a reduced LVEF, an elevated troponin, and were without evidence of coronary ischemia using coronary angiography. In these 4 cases, the discharge diagnosis was myocarditis both by the primary team and using the standardized criteria.
FIGURE 1. Clinical Presentation of Patients With ICI-Associated Myocarditis.
Time from ICI to onset of clinical myocarditis in each of the 35 cases of myocarditis (A). The ICI was administered on day 0. A description of the results for the ECG (B), troponin (C), LVEF (D), and natriuretic peptides (E), standard tests performed at the time of presentation with myocarditis, in patients with myocarditis related to ICI. ECG = electrocardiography; ICI = immune checkpoint inhibitors; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
TABLE 3.
Comparison of Myocarditis Cases With and Without MACE
| No MACE (n = 19) |
MACE (n = 16) | p Value | |
|---|---|---|---|
| Age at start of ICI, yrs | 66.0 ± 13.2 | 63.0 ± 13.6 | 0.44 |
| Female | 6 (32.0) | 4 (25.0) | 0.72 |
| ICI to onset of myocarditis | 57 (6–235) | 31 (4–151) | 0.135 |
| Number of ICI doses | 5.2 ± 8 | 3 ± 3 | 0.40 |
| CV risk factors | |||
| Current or prior smoking | 7 (37.0) | 8 (50.0) | 0.51 |
| Hypertension | 14 (74.0) | 11 (69.0) | 1.00 |
| Diabetes mellitus | 7 (37.0) | 5 (31.0) | 1.00 |
| Single agent vs. combined | |||
| Combination (current regiment) | 9 (47.0) | 3 (19.0) | 0.08 |
| Monotherapy (current regiment) | 10 (53.0) | 13 (81.0) | |
| Type of combined ICI | |||
| Ipilimumab + Nivolumab | 6 (32.0) | 3 (19.0) | 0.46 |
| Ipilimumab + Pembrolizumab | 1 (5.3) | 0 (0.0) | 1.00 |
| Tremelimumab + Avelumab | 1 (5.3) | 0 (0.0) | 1.00 |
| Tremelimumab + Durvalumab | 1 (5.3) | 0 (0.0) | 1.00 |
| Type of monotherapy ICI* | |||
| Pembrolizumab | 9 (47.0) | 2 (13.0) | 0.035 |
| Nivolumab | 0 (0.0) | 7 (44.0) | 0.002 |
| Ipilimumab | 1 (5.3) | 1 (6.3) | 1.00 |
| Tremelimumab | 0 (0.0) | 1 (6.3) | 0.46 |
| Atezolizumab | 0 (0.0) | 2 (13.0) | 0.20 |
| Avelumab | 0 (0.0) | 0 (0.0) | – |
| Durvalumab | 0 (0.0) | 0 (0.0) | – |
| Overall types of ICI | |||
| Any anti-PD1 | 16 (84.0) | 12 (75.0) | 0.68 |
| Any anti-CTLA4 | 12 (63.0) | 6 (38.0) | 0.19 |
| Any anti-PDL1 | 2 (11.0) | 2 (13.0) | 1.00 |
| Pembrolizumab | 11 (58.0) | 5 (31.0) | 0.18 |
| Nivolumab | 6 (32.0) | 10 (63.0) | 0.07 |
| Ipilimumab | 10 (53.0) | 5 (31.0) | 0.31 |
| Tremelimumab | 2 (10.5) | 1 (6.3) | 1.00 |
| Atezolizumab | 0 (0.0) | 2 (13.0) | 0.20 |
| Durvalumab | 1 (5.3) | 0 (0.0) | 1.00 |
| Avelumab | 1 (5.3) | 0 (0.0) | 1.00 |
| Myocarditis presentation | |||
| Chest pain | 7 (37.0) | 5 (31.0) | 1.00 |
| Shortness of breath | 11 (58.0) | 14 (88.0) | 0.07 |
| Orthopnea | 3 (16.0) | 7 (44.0) | 0.13 |
| Paroxysmal nocturnal dyspnea | 3 (16.0) | 3 (19.0) | 1.00 |
| Fatigue | 4 (21.0) | 6 (38.0) | 0.45 |
| Admission examination | |||
| Jugular venous distention | 7 (37.0) | 6 (38.0) | 1.00 |
| Crackles on lung examination | 10 (53.0) | 8 (50.0) | 1.00 |
| Admission vitals | |||
| Heart rate, beats/min | 87 ± 20 | 98 ± 27 | 0.21 |
| Systolic blood pressure, mm Hg | 130 ± 22 | 117 ± 25 | 0.14 |
| Diastolic blood pressure, mm Hg | 75 ± 10 | 66 ± 11 | 0.007 |
| Respiratory rate, rate/min | 23 ± 8 | 21 ± 5.6 | 0.69 |
| Oxygen requirement and delivery | |||
| Room air | 15 (79.0) | 6 (38.0) | 0.037 |
| Nasal cannula | 4 (21.0) | 4 (25.0) | |
| Intubated | 0 (0.0) | 4 (25.0) | |
| ECG, myocarditis admission | |||
| Sinus rhythm | 17 (90.0) | 10 (63.0) | 0.10 |
| QRS interval, ms | 102 ± 19 | 103 ± 20 | 0.83 |
| QTc interval, ms | 457 ± 28 | 457 ± 44 | 0.98 |
| Echocardiography, myocarditis admission | |||
| New LVEF, % | 49 ± 17 | 41 ± 18 | 0.25 |
| Change in LVEF from baseline | 16 ± 16 | 19 ± 11 | 0.66 |
| Left ventricular internal dimensions in diastole, mm | 49 ± 6 | 47 ± 14 | 0.41 |
| Pericardial effusion | 5 (26.0) | 1 (7.7) | 0.19 |
| Late gadolinium enhancement on a cardiac magnetic resonance study† | |||
| None | 6 (33.0) | 2 (15.0) | 0.41 |
| Subepicardial | 3 (16.0) | 3 (23.0) | 1.00 |
| Mid-myocardial | 4 (21.0) | 8 (62.0) | 0.06 |
| Diffuse | 5 (26.0) | 4 (31.0) | 1.00 |
| Elevated troponin | 17 (90.0) | 16 (100.0) | 0.48 |
| Troponin T, ng/ml‡ | |||
| Admission | 0.54 (0.01–1.55) | 1.18 (0.19–5.90) | 0.01 |
| Peak | 1.33 (0.01–3.5) | 2.68 (0.24–7.63) | 0.01 |
| Final/discharge | 0.14 (0.01–1.55) | 1.45 (0.03–6.41) | 0.002 |
| BNP or NT-proBNP | 12 (63.0) | 11 (69.0) | 1.00 |
| Serum sodium (admission), mEq/l | 137.0 ± 3.9 | 135.0 ± 4.1 | 0.144 |
| Serum creatinine (admission), mg/dl | 1.1 (0.5–2.6) | 1.1 (0.5–3.9) | 0.84 |
| White cell count (admission), cells/ml3 | 8.4 (4.4–14.5) | 11.6 (3.1–35.7) | 0.133 |
| Hemoglobin (admission), g/dl | 12.0 ± 1.9 | 12.8 ± 2.9 | 0.31 |
| Number of patient on steroids before myocarditis | 4 (21.0) | 3 (19.0) | 1.00 |
| Initial steroid dose, mg | 160.0 (0.0–1,000.0) | 72.5 (0.0–1,000.0) | 0.055 |
| Initial steroid dose/body weight, (mg/kg) | 2.06 (0.00–20.20) | 0.84 (0.00–14.40) | 0.041 |
| Time from admission to steroid administration, h | 18.3 ± 12.8 | 27.2 ± 17.5 | 0.12 |
Values are mean ± SD, n (%), or median (interquartile range).
If most recent ICI therapy was monotherapy.
Patients could have more than 1 pattern of late gadolinium enhancement; 18 of the patients without MACE and 13 of the patients with MACE had a cardiac magnetic resonance study.
Admission troponin was the first measured serum troponin; peak troponin was the maximum measured troponin, and discharge/final troponin was defined as the troponin measured at discharge from index hospitalization or the troponin before an event if that event occurred on the index admission.
BNP = B-type natriuretic peptide; MACE = major adverse cardiac events; NT-proBNP = N-terminal pro–B-type natriuretic peptide; other abbreviations as in Table 1.
MAJOR ADVERSE CARDIOVASCULAR EVENTS.
The median follow-up of myocarditis cases was 102 days (interquartile range 62 to 214 days). Nearly one-half of all myocarditis cases experienced a MACE: cardiovascular death (n = 6), cardiogenic shock (n = 3), cardiac arrest (4), or CHB (n = 3) (16 of 35 myocarditis cases, 46%). Causes of death included 2 sudden deaths, 1 witnessed and 1 unwitnessed, 2 documented ventricular arrhythmias, and 2 of progressive cardiogenic shock. Of the 16 MACE, 6 (38%) occurred in patients with a normal LVEF. Among cases with myocarditis, those with and without MACE were compared (Table 3). On admission, cases who experienced a subsequent MACE were more likely to be oxygen-dependent and have a lower diastolic blood pressure.
BIOMARKERS AND MACE.
In 86% of cases, a fourth-generation troponin T assay was the troponin assay used. As compared with non-MACE myocarditis cases, those who experienced a MACE had a higher admission, peak, and discharge/final troponin T value (Table 3). The diagnostic accuracy for troponin T and MACE was highest for discharge/final troponin T (area under the curve [AUC]: 0.81; p = 0.004) and fair for admission and peak troponin T (AUC: 0.76; p = 0.010; and AUC: 0.76; p = 0.010, respectively). A final/discharge troponin T of ≥1.5 ng/ml was associated with a 4-fold increased risk of MACE (hazard ratio 4.0; 95% confidence interval 1.5 to 10.9; p = 0.003) with a specificity of 95% and a sensitivity of 63% for MACE. Among the group not meeting criteria for MACE, there was also a high rate of cardiac events (Online Table 3). For example, 26% had atrial arrhythmias requiring intervention, and 42% developed heart failure requiring intravenous diuretics.
TREATMENT.
Steroids were initial treatment of myocarditis in 31 (89%) cases. The mean time from admission to steroid initiation was 21.4 ± 16 h (range 1 to 60 h). There was no difference in time from admission to steroid administration in the group with MACE as compared with no MACE. The median equivalent dose of methylprednisolone was 120 mg (range 0 to 1,000 mg). A higher initial steroid dose was associated with lower rate of MACE (Table 3). However, MACE still occurred in 2 patients who received an initial dose of methylprednisolone of 1,000 mg. The final/discharge serum troponin was also lower among those who were treated with higher initial doses of steroids (median troponin [inter-quartile range]: 0.165 [0.01 to 6.41] ng/l vs. 1.55 [0.01 to 3.19] ng/l; p = 0.007). In the follow-up period, there was no case of recurrent myocarditis after the initial myocarditis event. Other immunosuppression therapies were also administered; intravenous immunoglobulin was used in 2 patients, mycophenolate was used in 2 patients, antithymocyte globulin was used in 1 case, and infliximab in 3 cases. In these cases, successfully treated myocarditis was achieved in 1 case each with intravenous immunoglobulin, mycophenolate, infliximab, and antithymocyte globulin.
PREVALENCE OF MYOCARDITIS.
We do not have a denominator for all patients started on ICI at each of the 8 centers. However, of the 964 patients at Massachusetts General Hospital who received an ICI between November 2013 and July 2017, 1.14% (11 patients) developed myocarditis and 0.52% developed a MACE. Myocarditis was noted in 0.5% of patients on anti-PD1 alone, 2.4% of anti-programmed death-ligand 1 (anti-PDL1) alone, 3.3% with all anti-CTLA4 alone, 2.4% with combined anti-PD1/anti-CTLA4 antibodies, and 1% with combined anti-CTLA4/anti-PDL1 antibodies.
DISCUSSION
ICIs have improved treatment of several cancers. Immune-mediated side effects have been reported in up to 90% of patients and are generally manageable, and fatal or fulminant events were previously felt to be rare (20). We found that myocarditis with ICI occurred early during treatment and was more common with combination ICI, although 66% of cases occurred with single therapy. Pre-ICI treatment LVEF and ECG were normal in the majority, and myocarditis was the first presentation of an adverse immune-related side effect in approximately 50% of patients. Serious cardiovascular sequelae occurred in 46% of patients who developed myocarditis after immune therapy, and importantly, in comparison to non-immune therapy–related myocarditis (21), a depressed LVEF was not a prerequisite for serious adverse cardiovascular events. By contrast, serum troponin was abnormal in 94% of cases, and the degree of troponin elevation was also a reasonable predictor of adverse events. Most patients were treated with steroids, and higher doses of steroids were associated with lower adverse cardiac events and a lower serum troponin level.
The median time from starting ICI to clinical myocarditis was 34 days. This early onset is similar in timing to reported side effects with either combination or single therapy. For example, with combination therapy, immune therapy–mediated side effects affecting the renal, hepatic, endocrine, pulmonary, gastrointestinal, and dermatologic organ systems are reported at 3.75, 2.62, 2.16, 1.93, 1.63, and 0.71 months, respectively (22). Screening is considered when a cardiac toxicity is identified from cancer therapies (23). Our data suggest that neither a screening ECG nor measurement of the LVEF would provide any utility. Specifically, all patients who had a baseline echocardiogram had a normal LVEF before ICI, and most had a normal ECG. Surveillance is also considered when a cardiac toxicity is identified from cancer therapies (23). In 94% of patients, troponin was elevated at clinical presentation, the LVEF was normal in 51%, natriuretic peptides were abnormal in 66%, and an ECG was abnormal in 89%. An ECG is an inexpensive test and widely available, and conduction abnormalities have been reported in the setting of ICI-associated myocarditis (5); however, it lacks sensitivity and specificity for the diagnosis of myocarditis (24). Serum troponin is also inexpensive and widely available, and an elevation in troponin generally suggests myocyte death. Therefore, because the median time to myocarditis was 34 days, that is, after the second dose of ICI (2-week or 3-week infusions), checking troponin levels at baseline and at each cycle may be of value. An elevated troponin should warrant consideration of myocarditis and immediate referral to cardiology/cardio-oncology for further evaluation.
The rate of serious adverse cardiovascular events was high among patients who developed ICI-associated myocarditis. In our registry, incidence of grade 4 or 5 cardiovascular adverse events was noted in nearly one-half of cases (14). This contrasts with the low incidence of severe pneumonitis with anti-PD1 or anti-PDL1 (4% grade 4 or 5) (25), severe hepatitis with anti-PD1 and anti-CTLA4 as single agents or in combination (0% to 18% grade 4, no grade 5) (26–28), and severe diarrhea or colitis with anti-PD1 (0% grade 4 or 5) (28). The major cardiac event rate of almost 50% was markedly increased compared with cases of myocarditis in broad populations. For example, among 670 patients admitted to hospital with myocarditis unrelated to ICI, a fulminant course was noted in 15% of patients (29,30). An additional difference is that, in our cohort, 38% of patients who had a fulminant course had a normal LVEF. This finding contrasts with studies in broad populations of patients admitted with fulminant myocarditis in which all have a markedly depressed LVEF (29). The implication of this finding is that clinicians should not rely on ejection fraction as a discriminator of severity in ICI-associated myocarditis. By contrast, we did find that the degree of troponin elevation was useful in determining adverse cardiac outcomes. Specifically, we found that troponin T >1.5 ng/dl had a 95% specificity for the development of MACE. However, there were still 4 (11%) major cardiac events among subjects who had a troponin below that cutoff.
There is a variation in the reported rates of myocarditis with ICI-based therapies. However, the number of cases at 1 of the registry institutions was compared to the number of individual patients who received ICI in the same time interval. Overall, just >1% developed myocarditis after ICI therapy and approximately 0.5% had a MACE. Anti-PD1 therapy accounted for nearly all myocarditis cases—1.3% with pembrolizumab alone, and 0.6% with nivolumab alone—a rate higher than the 0.4% in a study of 496 patients receiving anti-PD1 therapy (9). The prevalence of myocarditis was even higher (2.4%) with anti-PD1/anti-CTLA4 combined therapy. The trend for more adverse events with combination therapy has been reported for noncardiac side effects; for example, such as in a pooled analysis of 448 patients in phase I to III trials of combination anti-PD1/anti-CTLA4 (nivolumab with ipilimumab), which did not report any incidence of myocarditis (22). Our reported prevalence of myocarditis is higher than the 0.09% reported rate of myocarditis in the Bristol-Myers Squibb safety database of nivolumab and ipilimumab given in combination or as single therapy (5), and in a pharmacovigilance registry of 388 patients using single-agent ICI followed for 18 months, where no cases of heart failure or CHB were noted (31). The reason for the difference in the reported prevalence of myocarditis is not clear but may relate to patient selection, pre-specified adjudication, a well-recognized difficulty with the diagnosis of myocarditis, or a reporting bias by investigators not suspecting this as a potential toxicity.
STUDY LIMITATIONS.
Some weaknesses to this data merit mention. This was a retrospective case-control study where cases were derived from multiple institutions and cases were derived from a single institution. However, we also compared baseline characteristics between cases and controls at the same institution and had similar findings. As this was a retrospective study, institutional standards were employed, and the MRI protocol was not prespecified. However, each cardiac MRI protocol included balanced cine steady-state free precession imaging for cardiac function and mass, contrast administration, and magnitude and phase-sensitive inversion recovery images for late gadolinium enhancement at 10 to 15 min. The control cohort did not undergo testing to exclude myocarditis; this may have led to an underestimation of the occurrence of myocarditis. Additionally, it is possible that patients prescribed ICI presented to other facilities or other providers with myocarditis, also leading to an additional underestimation of the incidence rate.
There is a clear need for an improved risk evaluation and mitigation strategy for ICI-associated myocarditis, as has been done for other ICI related adverse events (32). Steroids are standard initial therapy for most serious ICI-related immune events and steroids were prescribed in 89% of our cases. In our study, serum troponin was lower and there was a lower MACE rate with higher starting doses of immunosuppression. This trend is supported by the limited prior published reports. Specifically, Johnson et al. (5) reported 2 cases of ICI leading to myocarditis and cardiac death. In both cases, patients were treated with 1 mg per kg of methylprednisolone per day. Thus, our practice has changed and, despite limited data, we administer 1,000 mg of methylprednisolone daily as a standard starting dose for treatment of ICI-related myocarditis. However, 2 patients (12.5%) who were initially treated with 1,000 mg/day still developed serious adverse cardiac outcomes, suggesting more insight is needed. Some of the additional relevant questions include how the timing of the steroid prescription, total steroid administered, and the steroid taper influences time to resolution of ICI-associated myocarditis, and importantly, an enhanced understanding of the molecular mechanisms involved to determine targeted alternatives to steroids. The successful resolution of myocarditis is important because these patients face limited options for the management of advanced heart failure. For example, active malignancy is an absolute contraindication for cardiac transplantation(33), and although active malignancy does not preclude temporary mechanical circulatory support for cardiogenic shock, it does influence candidacy for subsequent durable mechanical circulatory support.
CONCLUSIONS
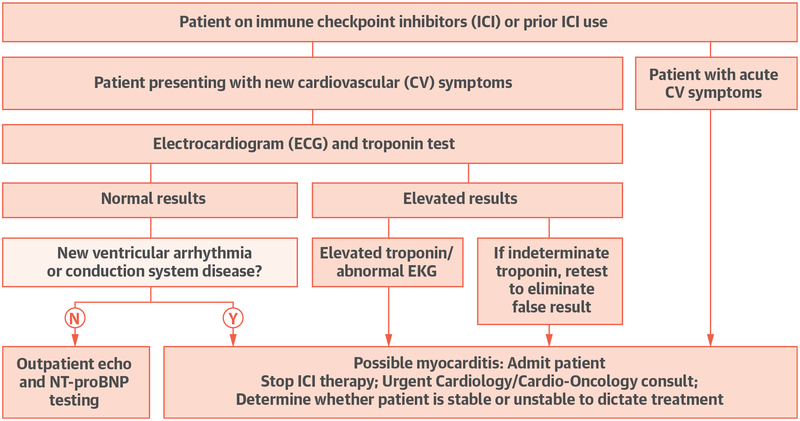
Myocarditis is an uncommon but often fulminant and severe side effect of ICI. Our study provides new insights into the occurrence, timing, associations and prognosis of patients who develop ICI-associated myocarditis, and emphasizes the pressing need for vigilance and further studies (Central Illustration).
CENTRAL ILLUSTRATION. Algorithm for Work-Up and Management of Immune-Mediated Myocarditis.
Algorithm based on study findings, and institutional experience with 35 cases of ICI-associated myocarditis. CVD = cardiovascular; ECG = electrocardiogram; ICI = immune checkpoint inhibitors.
Supplementary Material
PERSPECTIVES.
COMPETENCIES IN PATIENT CARE AND PROCEDURAL SKILLS: In patients receiving ICI therapy for cancer, myocarditis develops at a median of 34 days. Elevation of serum troponin levels may signal the need to consider myocarditis. Checking troponin levels at baseline and at each 21-day infusion cycle or at alternate cycles for those receiving 14-day infusion therapy may be useful.
TRANSLATIONAL OUTLOOK: Future studies should determine the response of ICI-related myocarditis to escalating doses of corticosteroids and other immunotherapies such as infliximab, intravenous immunoglobulin, mycophenolate, and antithymocyte globulin.
ACKNOWLEDGMENTS
The authors acknowledge the help of Michael Griffin, PhD, and Ms. Grace Griffin for their efforts in language editing and proofreading.
Dr. Mahmood has been supported by the Sarnoff Cardiovascular Research Foundation. Drs. Chen and Gupta are supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support grant P30 CA008748. Dr. Thavendiranathan is supported by Canadian Institutes of Health Research New Investigator Award (FRN 147814). Dr. Neilan was supported in part through the Kohlberg Foundation, American Heart Association Fellow to Faculty Award 12FTF12060588, NIH/National Heart, Lung, and Blood Institute grants 1R01HL130539–01A1, 1R01HL137562–01A1, and K24HL113128–06, and NIH/Harvard Center for AIDS Research grant P30 AI060354. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ABBREVIATIONS AND ACRONYMS
- anti-CTLA4
anti-cytotoxic T-lymphocyte-associated protein 4
- anti-PD1
anti-programmed cell death protein 1
- anti-PDL1
anti-programmed death-ligand 1
- AUC
area under the curve
- CHB
complete heart block
- ECG
electrocardiogram
- ICI
immune checkpoint inhibitor
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiac events
- MRI
magnetic resonance imaging
Footnotes
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;2010: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364: 2517–26. [DOI] [PubMed] [Google Scholar]
- 3.Approved Drugs - Nivolumab (Dec 22, 2014). Silver Spring, MD: U.S. Food and Drug Administration, 2014. [Google Scholar]
- 4.Approved Drugs - Pembrolizumab (Sep 4, 2014). Silver Spring, MD: U.S. Food and Drug Administration, 2014. [Google Scholar]
- 5.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375: 1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadokoro T, Keshino E, Makiyama A, et al. Acute lymphocytic myocarditis with anti-PD-1 antibody nivolumab. Circ Heart Fail 2016;9:e003514. [DOI] [PubMed] [Google Scholar]
- 7.Laubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer 2015;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210–25. [DOI] [PubMed] [Google Scholar]
- 10.Andrews A Treating with checkpoint inhibitors—figure $1 million per patient. Am Health Drug Benefits 2015;8 Spec Issue:9. [PMC free article] [PubMed] [Google Scholar]
- 11.Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov 2014;13:883–4. [DOI] [PubMed] [Google Scholar]
- 12.Lexicomp. Drug Information Handbook: A Clinically Relevant Resource for All Healthcare Professionals. 26 edition Indianapolis, IN: Wolters Kluwer, 2017. [Google Scholar]
- 13.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34: 2636–48. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version4.0. Bethesda, MD: U.S. Department of Health and Human Services, 2009. [Google Scholar]
- 15.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–69. Erratum in: J Am Coll Cardiol 2015;66:982. [DOI] [PubMed] [Google Scholar]
- 16.Neilan TG, Farhad H, Mayrhofer T, et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. J Am Coll Cardiol Img 2015; 8:414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008;117:686–97. [DOI] [PubMed] [Google Scholar]
- 18.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013;61: e6–75. [DOI] [PubMed] [Google Scholar]
- 19.Neilan TG, Shah RV, Abbasi SA, et al. The incidence, pattern, and prognostic value of left ventricular myocardial scar by late gadolinium enhancement in patients with atrial fibrillation. J Am Coll Cardiol 2013;62:2205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation 2008;118:639–48. [DOI] [PubMed] [Google Scholar]
- 22.Sznol M, Ferrucci PF, Hogg D, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017;35(34): 3815–22. [DOI] [PubMed] [Google Scholar]
- 23.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;35:893–911. [DOI] [PubMed] [Google Scholar]
- 24.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009;53:1475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti–programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2016;35:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernardo SG, Moskalenko M, Pan M, et al. Elevated rates of transaminitis during ipilimumab therapy for metastatic melanoma. Melanoma Res 2013;23:47–54. [DOI] [PubMed] [Google Scholar]
- 27.Huffman BM, Kottschade LA, Kamath PS, Markovic SN. Hepatotoxicity after immune checkpoint inhibitor therapy in melanoma: natural progression and management. Am J Clinical Oncol 2017. July 26 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 29.Ammirati E, Cipriani M, Lilliu M, et al. Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation 2017;136:529–45. [DOI] [PubMed] [Google Scholar]
- 30.Gräni C, Eichhorn C, Bière L, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017;70: 1964–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ederhy S, Voisin AL, Champiat S. Myocarditis with immune checkpoint blockade. N Engl J Med 2017;376:290–1. [DOI] [PubMed] [Google Scholar]
- 32.Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28: iv119–42. [DOI] [PubMed] [Google Scholar]
- 33.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016; 35:1–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.