Abstract
Exposure to bisphenols (BPA and BPS) during pregnancy can significantly affect fetal development and increase risk of adverse health consequences, however the underlying mechanisms are not fully elucidated. In human placenta, the efflux transporter P-glycoprotein (P-gp), encoded by the ABCB1 gene, extrudes its substrates from the trophoblasts back into the maternal circulation. Alterations in levels of placental P-gp could therefore significantly affect fetal exposure to xenobiotics that are P-gp substrates. The ABCB1 promoter contains many single nucleotide polymorphisms (SNPs). In the genome, SNPs are not arrayed as independent variants but as combinations forming defined haplotypes. Recently, we determined the haplotype sequences encompassing the ABCB1 promoter SNPs and found that promoter haplotypes differentially affect ABCB1 promoter activity. Here we investigate the effect of BPA and BPS on ABCB1 promoter activity by testing the hypothesis that BPA and BPS exposure affect ABCB1 promoter activity in a haplotype-dependent manner. Our data indicate that acute exposure to 50nM BPA induced a significant haplotype-dependent increase in ABCB1 promoter activity (P<0.05). However, acute exposure to 0.5nM BPS induced a significant decrease (P<0.05) in promoter activity that was haplotype-dependent. Chronic exposure to BPA and BPS individually (5nM and 0.3nM, respectively) or as a mixture (5nM BPA:1.5nM BPS) induced significant haplotype-dependent increases (P<0.01) in ABCB1 promoter activity. Our data indicate that BPA and BPS significantly alter ABCB1 promoter activity in a haplotype- and exposure type- dependent manners. Such alteration could significantly impact placental P-gp levels and alter fetal exposure to many therapeutic and environmental xenobiotics.
Keywords: BPA/BPS, ABCB1, polymorphisms, haplotypes, P-glycoprotein, placenta
Introduction
Bisphenol A (BPA, 4,4’-Isopropylidenediphenol) is produced in mass quantities for use in polycarbonate plastics and many daily-use consumer products including water bottles, canned food inner-linings, thermal receipt paper, and many more (Biedermann et al., 2010; Chen et al., 2016; Liao and Kannan, 2013). BPA is a xenoestrogen that is thought to mimic natural estrogens’ action through binding to estrogen receptors and regulating expression of target genes (Bonefeld-Jørgensen et al., 2007; Richter et al., 2007). Evidence for the estrogenic effects of BPA was first identified in 1936 and subsequently health risks associated with chronic low-dose exposure have been reported (Dodds and Lawson, 1936; Krishnan et al., 1993; Rochester, 2013). BPA exposure-associated health effects have led to the emergence of many other bisphenol derivatives as BPA replacements that are now appearing at measurable quantities in many of the same goods (Liao et al., 2012; Liao and Kannan, 2013). One of the common substitutes is bisphenol S (BPS, 4,4’-sulfonyldiphenol), with measurable amounts observed in surface waters, sewage effluent (Xuan et al., 2014; Yamazaki et al., 2015) and indoor dust at concentrations as high as 25% of that of BPA (0.34μg/g; 1.33μg/g) (Liao and Kannan, 2013). Chronic exposure to both of these chemicals has led to detectable, quantifiable amounts of BPA and BPS in human tissues and fluids, raising concerns for potential health hazards resulting from exposure to these chemicals individually or as a mixture (Ikezuki et al., 2002).
During pregnancy, bisphenol exposure was reported to significantly affect fetal development and increase the risk of adverse health consequences. Associations between BPA exposure and reproductive dysfunction (Naderi et al., 2014; Sharpe and Skakkebaek, 1993), obesity (Rubin and Soto, 2010; Vafeiadi et al., 2016; Valvi et al., 2013), developmental behavioral problems (Braun et al., 2011; Kundakovic et al., 2013; Miodovnik et al., 2011; Wolstenholme et al., 2012, 2011b, 2011a) and cancer (Boyerinas et al., 2012; Doherty et al., 2010; Prins et al., 2008; Sengupta et al., 2013) have been reported. Animal studies have also shown that BPA can diffuse across the placenta to the fetal circulation and into fetal tissues (Takahashi and Oishi, 2000). In humans, BPA has been detected at early gestation in maternal serum at ng/ml levels and in significantly higher concentrations (up to 5-fold and greater) in amniotic fluid, suggesting accumulation during fetal development (Ikezuki et al., 2002; Schönfelder et al., 2002).
During pregnancy, the human placenta serves as an interface for regulating xenobiotic distribution between the fetal and the maternal circulation. In the placenta, large, multinuclear syncytiotrophoblasts form a physiological barrier on the apical membrane, where they contact maternal blood (Joshi et al., 2016). This brush-border membrane is the site of nutrient and waste exchange, and is also where regulation of drug and chemical transport between maternal and fetal circulations occurs through a group of transporter proteins (St-Pierre et al., 2000; Walker et al., 2017). One of the major placental transporters is P-glycoprotein (P-gp), which is encoded by the gene ABCB1. P-gp, is expressed throughout gestation, but its expression varies in each trimester (Mathias et al., 2005). P-gp interacts with a wide variety of structurally diverse compounds (Ceckova-Novotna et al., 2006) and extrudes its substrates out from the trophoblasts back into the maternal circulation, thereby limiting their entry into the fetal circulation (Nakamura et al., 1997). The loss or pharmacological inhibition of P-gp has been demonstrated to dramatically influence fetal exposure to xenobiotics that are P-gp substrates (Smit et al., 1999). Alterations in the level and/or activity of placental P-gp can therefore significantly impact fetal exposure to many xenobiotics during pregnancy.
Steroid hormones such as progesterone, although not transported by P-gp, were found to influence P-gp transport activity depending on concentration where both low inhibitory and stimulatory effects were observed (Orlowski et al., 1996; Shapiro et al., 1999). Due to their structural similarity to natural steroid hormones, it is conceivable that environmental estrogens such as BPA and BPS may also alter P-gp transport activity, which would consequently affect fetal exposure to many xenobiotics that are P-gp substrates. Consistent with this notion, using BeWo cells as a placental model, Jin and Audus (2005) reported that acute exposure to BPA stimulated P-gp transport activity.
We (Hemauer et al., 2010) and others (Hitzl et al., 2004; Tanabe et al., 2001) have previously demonstrated wide interindividual variability in placental P-gp expression and activity. Such variability was largely attributed to single nucleotide polymorphisms (SNPs) of the ABCB1 gene which could affect P-gp expression and function (Hitzl et al., 2004, 2001; Hoffmeyer et al., 2000; Kim et al., 2001; Mölsä et al., 2005; Salama et al., 2006; Tanabe et al., 2001). There are many SNPs in the ABCB1 promoter that could affect P-gp expression by affecting ABCB1 transcription. While a few studies evaluated the effect of some of these SNPs on ABCB1 expression, discrepant results were reported where the same SNPs were associated with increased and decreased expression and also with no effect (Ito et al., 2001; Lourenço et al., 2008; Sai et al., 2010, 2006; Takane et al., 2004; Tanabe et al., 2001). The lack of reproducibility between studies is not surprising because SNPs do not occur individually in the genome, but rather form defined combinations or haplotypes due to varying degrees of linkage disequilibrium between them. In recent studies from our laboratory, we found that the phenotypic effect of a SNP is not always consistent but varies depending on its presence in given haplotypes (Speidel et al., 2018; Xu et al., 2016, 2014). Recently, we determined the haplotype sequences of the ABCB1 promoter and found that the haplotypes differentially regulate ABCB1 promoter activity (Speidel et al., 2018). Here we investigate the interaction between BPA/BPS exposures with the different ABCB1 haplotypes. Our working hypothesis is that BPA and BPS exposure affects ABCB1 promoter activity in a haplotype-dependent manner.
Materials and Methods
Chemicals
4,4’-Isopropylidenediphenol (Bisphenol A; 97% purity) was purchased from Acros Organics (Geel, Belgium). 4,4’-sulfonyldiphenol (Bisphenol S; 99% purity) was purchased from SigmaAldrich (St. Louis, MO). All other chemicals and supplies were purchased from Fisher Scientific unless otherwise stated.
Bisphenol A and bisphenol S were dissolved in 50% ethanol (PHARMCO-AAPER, Toronto) and 50% Minimal Essential Medium with Earle’s Salts and L-Glutamine (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Flowery Branch, GA). Serial dilutions were then performed which allowed for a 10μM BPA or BPS working solution in a final ethanol concentration of 0.01%.
ABCB1 promoter haplotype luciferase reporter generation
Previously, we generated luciferase reporters representing different ABCB1 promoter haplotypes using the NanoLuc™ Luciferase vector system (Promega, Madison, WI) to determine the effects of the different haplotypes on promoter activity (Speidel et al., 2018). Based on our results, for the current study we selected 4 haplotypes (Table 1), namely the ancestral haplotype 1, representing the reference promoter activity (100%), haplotypes 4 and 29 representing high basal promoter activity (3.9- and 3.5-fold higher activity than haplotype 1, respectively) and haplotype 30 representing low basal promoter activity (6% activity compared to haplotype 1). For the acute exposure experiments, vectors containing the different haplotypes were generated using the NanoLuc Luciferase pNL1.1 vector (Promega, Madison, Wisconsin, USA). For the evaluation of chronic exposures to BPA and BPS, luciferase reporters for the designated haplotypes were generated using the NanoLuc™ pNL1.2 vector (Promega, Madison, WI). This vector (no PEST domain) was used for the chronic experiments because the response would be measured after several days rather than a few hours. In brief, reporter constructs representing the different haplotypes were generated by inserting the appropriate promoter sequences into the vector after double digestion with KpnI-HF and NheI-HF (New England Biolabs, Ipswich, MA). Subsequently, E. coli DH5α (New England Biolabs, Ipswitch, MA) were transformed with the generated reporters and plated on 100 μg/ml ampicillin LB agar plates. Individual colonies were selected and grown in LB media containing 100 μg/ml ampicillin for 18–24 hours at 37ºC. Plasmids were isolated using the endotoxin free ZR Plasmid Miniprep™ - Classic kit (Zymo Research Corp, Irvine, CA) and quantified at 260nm using a DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE). The isolated plasmids were sequenced to verify the proper promoter haplotype was present in the reporter plasmid and to ensure no additional mutations were introduced during the preparation. Isolated plasmids were stored at 20ºC to maintain plasmid integrity until transfection.
Table 1.
Select ABCB1 promoter haplotypes evaluated in this study
| Haplotype† | ABCB1 Promoter Haplotype‡ | Promoter Activity (a.u.) |
|---|---|---|
| 1 | −1572aA/−1517aT/−1459aG/−1157aG/−1017aT/−684aA/−274aG/−41aA/−240G/−129T/−43A/133C | 1.00 ± 0.08 |
| 4 | −1572aA/−1517aT/−1459aG/−1157aG/−1017aT/−684aA/−274aG/−41aA/−240A/−129T/−43A/133C | 3.90 ± 1.81 |
| 29 | −1572aA/−1517aT/−1459aA/−1157aG/−1017aT/−684aA/−274aG/−41aA/−240G/−129C /−43A/133C | 3.47 ± 1.43 |
| 30 | −1572aA/−1517aT/−1459aG/−1157aA/−1017aC/−684aA/−274aG/−41aA/−240G/−129T/−43A/133C | 0.06 ± 0.02 |
Haplotype 1 (ancestral haplotype) is used as a reference for promoter activity comparisons. Haplotypes 4 and 29 have significantly higher basal promoter activity and haplotype 30 has a significantly lower basal promoter activity compared to haplotype 1 (Speidel et al., 2018).
Small ‘a’ nomenclature represents nucleotide before the nontranscribed exon 1. The (−) without the ‘a’ are within or after exon 1 before the transcription start site. Bold denotes variant(s) present in the haplotype (see Speidel et al., 2018 for additional details). Variants in the different haplotypes are G-240A (rs35265821); G-1459aA (rs12720464); T129C(rs3213619); G-1157aA (rs28381797) and T-1017aC (rs28746504).
Cell Culture
The human trophoblastic 3A placental cell line (CRL-1584) was purchased from American Type Culture Collection (ATCC, Manassas, VA) and used as the host cell for all bisphenol exposures and haplotype construct transfections. The 3A cells were chosen as the experimental cell type because they represent the human placenta and are well characterized. They have normal trophoblast traits (e.g. they synthesize human chorionic gonadotropin and alkaline phosphatase) and, in culture, they are heterogeneous in nature, containing both cytotrophoblasts (small precursor cells) and syncytiotrophoblasts (large, mature, multinucleated cells) (You et al., 2002). Cells were maintained in 75cm2 flasks with Minimal Essential Medium (MEM) with Earle’s Salts and L-Glutamine (Gibco Cat. 11095–080, Grand Island, NY) supplemented with 10% FBS in 5% CO2 at 37°C. Cells were passaged at 85% confluency (2–3 days) and sub-cultured at a 3:1 ratio. A solution of 0.25% (w/v) Trypsin – 0.53mM EDTA was used to detach the cells for subculture or transfer to 6-well plates for transfection.
MTT assay for cytotoxicity and cell viability determination
The tetrazolium MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Acros Organics, Belgium) was dissolved in phosphate buffered saline (PBS, Sigma-Aldrich, St. Louis, MO) at a concentration of 5mg/mL. The MTT solution was then filter-sterilized using a 0.22micron syringe filter (Merck Millipore, Billerica, MA) and placed in a light-protected bottle. To each well of a 96-well plate containing human 3A placenta cells, 20μL of the MTT solution was added and the cells were incubated at 37°C for 3.5 hours. The media was removed and 150μL MTT solvent [4mM hydrochloric acid (Sigma-Aldrich, St. Louis, MO), 0.1% NP-40 substitute (US Biological, Salem, MA) in isopropanol (EMD Millipore, Billerica, MA)] was added. The plate was then covered with aluminum foil and incubated on a shaker at room temperature for 15 minutes. Absorbance at 590nm was measured in triplicate using a Tecan GenIOS Pro plate reader (Tecan, Durham, NC)
Acute Bisphenol Exposures and Transfection
Untreated placental 3A cells were trypsin digested and transferred into 6-well plates at low confluency (≤40%). After allowing the cells to adhere to the plate (14–18 hours), cells were transfected using a mixture of 600ng haplotype promoter plasmid DNA, 66ng firefly luciferase control plasmid pGL4.53 PGK (Promega, Madison, WI) and 2μL Lipofectamine 3000 transfection reagent (Invitrogen, Carlsbad, CA). After transfection, cells were allowed to recover for 36–48h and were then exposed to BPA, BPS or a mixture of both bisphenols dissolved in 3 × 10−5 % ethanol. For the initial acute exposures, the experiment was carried out for 90 minutes with samples taken at t=0, 15, 30, 45, 60 and 90 minutes. At each time point, cells were lysed with 500μL Passive Lysis Buffer (PLB, Promega, Madison WI) and the effects of acute exposure were measured using the NanoGlo Dual-Luciferase® Assay (Promega, Madison, WI). The maximal signal was observed for both bisphenols at 15 minutes. Therefore, for all subsequent acute exposures, samples were collected after 15 minutes.
Chronic Bisphenol Exposures and Transfection
Human 3A placental cells were split into six T-10 flasks at low confluency ≤20% and chronically exposed to BPA or BPS individually or as a mixture of both bisphenols. Complete MEM with BPA and/or BPS added was replaced every day for 12 days (~4 passages).
After 12 days of bisphenol exposure, cells were trypsin digested, and transferred to 6-well plates. Bisphenols were then added to their respective wells, and after allowing 12–16 hours for cells to adhere, transfections were performed in the 6-well plates between passages 9–10 with cells at low confluency (≤ 40%). For each transfection, cells were treated with a mixture of 600ng haplotype plasmid DNA, 66ng PGL4.53K vector and 2μL Lipofectamine 3000 transfection reagent. Six hours after transfection, the media was replaced and bisphenols were added. Treatment continued with another media change/exposure occurring 24 hours after transfection. Cells were harvested 36–48 hours after transfection using 500μL PLB and effect of chronic exposure was measured using the NanoGlo Dual-Luciferase Assay.
NanoGlo Dual-Luciferase Assay to determine the effect of bisphenols on the activity of different ABCB1 promoter haplotypes
The Nano-Glo Dual-Luciferase Reporter Assay was performed according to the manufacturer’s instructions. Briefly, 3A cells were harvested using 500μL PLB and the lysates were then used immediately or stored at −80°C for later analysis. Luciferase activity was measured according to the manufacturer’s recommendations, and luminescence was measured in triplicate using a Tecan GenIOS Pro plate reader (Tecan, Durham, NC). Luminescence was measured as relative light units after normalization against the co-transfected Firefly luciferase. Each experiment was repeated at least three times.
Statistical Analysis
The Shapiro-Wilk normality test was used to determine the distribution of the data for all subsequent statistical analyses. One-Way ANOVA was used for the MTT assay to compare the effects of chronic BPA and/or BPS exposure on cell-viability. Posthoc analysis using the multiple comparison Holm-Šídák method was used to compare the exposure groups to nontreated and ethanol controls. The nonparametric Kruskal-Wallis One-Way ANOVA was used to compare luminescence values corresponding to the different haplotypes to determine their effect on ABCB1 promoter activity. Posthoc analysis using the rank-based Dunn’s Method was used to compare the luminescence values for the individual haplotypes against the ancestral haplotype. One-Way ANOVA was then used to compare luminescence values within each haplotype. Posthoc analysis using Dunnett’s method was used to compare the luminescence values for the individual treatments against the EtOH control. One-Way ANOVA was used to compare the effects of chronic BPA and BPS exposure on ABCB1 promoter activity. Posthoc analysis using the Holm-Šídák method was used to compare the exposed groups with their respective haplotype ethanol control. P-values <0.05 were considered significant.
Results
Effect of acute exposure to BPA and BPS on ABCB1 promoter activity
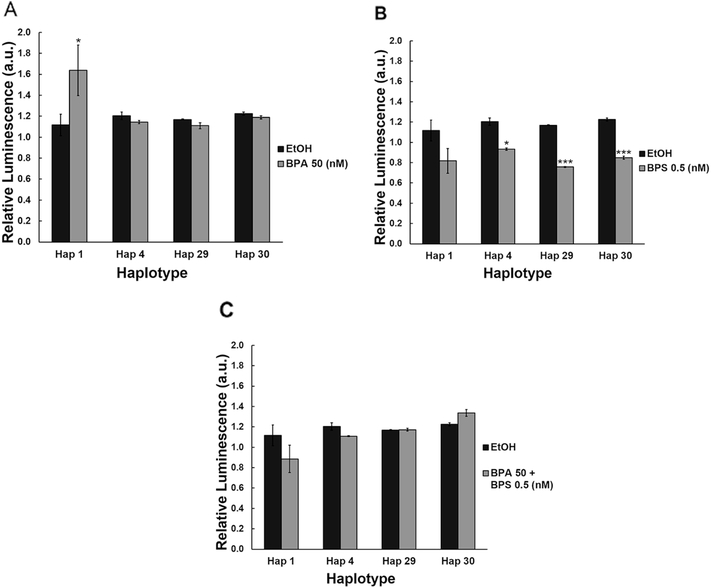
The concentration of BPA and BPS used in our investigation was determined from an initial dose-response study conducted with BPA and BPS concentrations ranging from 0.5nM to 500nM. The maximal signal was observed for both bisphenols at 15 minutes. Cells transfected with the Nanoluciferase reporter plasmid containing the ancestral ABCB1 haplotype 1 were exposed for 15 minutes to BPA or BPS and the effect of exposures on ABCB1 promoter activity was determined. There was no effect on cell viability within this concentration range as determined by the MTT assay (Levitz and Diamond, 1985; Mosmann, 1983) (data not shown). There was a significant increase (p<0.001) in luminescence (indicative of increased ABCB1 promoter activity) when cells were exposed to 50nM BPA. In contrast, no statistically significant change in promoter activity was observed with any of the BPS concentrations tested. Based on these initial results, the concentration of 50nM for BPA and the lowest concentration tested of BPS (0.5nM) were used in the subsequent acute exposure experiments.
To test for potential haplotype-exposure interaction on ABCB1 promoter activity, we exposed the four different ABCB1 promoter haplotypes to 50nM BPA alone or to 0.5nM BPS alone. Apart from the significant increase observed with haplotype 1 (46.7% increase, P<0.05; Figure 1A), exposure to 50nM BPA alone had no significant effect on ABCB1 promoter activity with the other haplotypes tested. In contrast, exposure to 0.5nM BPS alone induced a significant reduction in promoter activity that was haplotype dependent. As shown in Figure 1B, while exposure of haplotype 1 to BPS had no significant effect on promoter activity, haplotypes 4, 29 and 30 all displayed significant decreases in ABCB1 promoter activity (22.4%, P<0.05; 35.3%, P<0.001 and 30.8%, P<0.001, respectively).
Figure 1. Haplotype specific response to acute BPA and BPS exposures.
Luficerase reporters containing ABCB1 promoter haplotypes were acutely exposed to (A) 50nM BPA, (B) 0.5nM BPS, or (C) a mixture of 50nM BPA and 0.5nM BPS. Data are reported as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001.
Because human exposure to these xenoestrogens occurs more commonly as a mixture, we also evaluated the effect of combined acute exposure to both BPA and BPS on ABCB1 promoter activity. Our data indicate that co-exposure to a mixture of BPA and BPS at the concentrations of 50 and 0.5nM, respectively, had no effect on promoter activity regardless of the haplotype evaluated (Figure 1C).
Chronic BPA and PBS Exposure and ABCB1 Promoter Activity
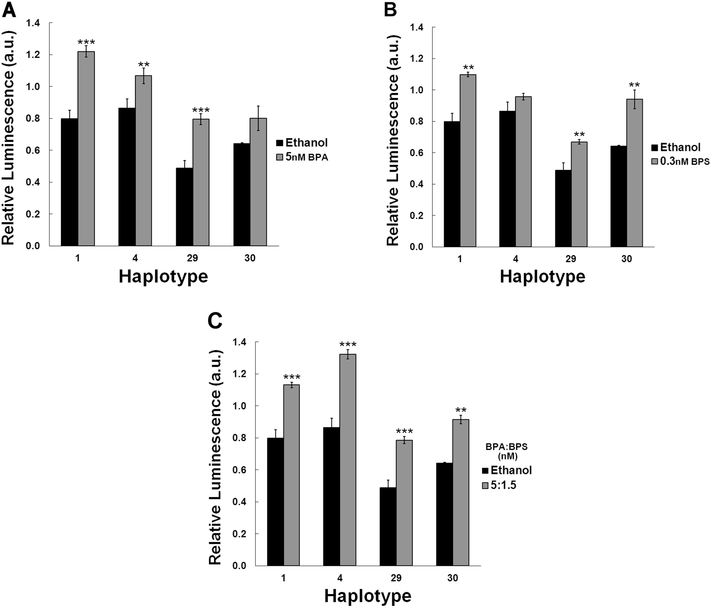
Since pregnant women could be chronically exposed to BPA and BPS individually or as a mixture, we also tested the effect of chronic exposure to BPA alone (5nM; Figure 2A), BPS alone (0.3nM; Figure 2B), and both together as a mixture (5nM BPA:1.5nM BPS; Figure 2C). The ratio for the mixture was chosen based on a recent report indicating that BPS concentrations levels observed in humans were between 12% and 38% that of BPA in human urine (Thayer et al., 2016). Our data indicate a haplotype dependent response to these exposures on promoter activity. While chronic exposure to BPA alone induced a highly significant increase in promoter activity of haplotypes 1 (52.8%; P<0.001), 4 (23.3%; P<0.01) and 29 (62.7%; P<0.001), it had no statistically significant effect on the promoter activity of haplotype 30 (Figure 2A). Similarly, this haplotype-exposure interaction was observed with BPS. While chronic exposure to 0.3nM BPS significantly induced the promoter activity of haplotypes 1, 29 and 30 by 37.6%, 37% and 46.3%, respectively (p<0.01), it did not affect the activity of haplotype 4 (Figure 2B). Exposure to a mixture of BPA and BPS, however, induced a highly significant increase in ABCB1 promoter activity for all haplotypes tested where haplotypes 1, 4, 29 and 30 activities were increased by 41.6.8% (P<0.001), 52.8% (P<0.001), 60.7% (P<0.001) and 42.4% (P<0.01), respectively (Figure 2C).
Figure 2. Haplotype specific response to chronic BPA and BPS exposures.
Luficerase reporters containing ABCB1 promoter haplotypes were chronically exposed to (A) 5nM BPA, (B) 0.3nM BPS, or (C) a mixture of 5:1.5nM BPA:BPS. Data are reported as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001.
Discussion
P-gp, encoded by the polymorphic ABCB1 gene, is one of the major efflux transporters highly expressed in the apical membrane of the placental syncytiotrophoblast. P-gp interacts with a wide variety of structurally diverse compounds and, in the placenta, it exports its substrates out from the trophoblasts back into the maternal circulation, thus limiting their entry into the fetal circulation (Nakamura et al., 1997). Alteration in expression and/or activity of placental P-gp can therefore have serious health consequences since it could significantly impact maternal and fetal exposure to medications and environmental toxins that are P-gp substrates. Alteration of Pgp transport activity was reported to be influenced by steroid hormones (Orlowski et al., 1996; Shapiro et al., 1999). Because of their structural similarity to natural steroid hormones, it is conceivable that environmental estrogens such as BPA and BPS may also alter P-gp transport activity. Recently, we reported that single nucleotide polymorphisms of the ABCB1 promoter, segregated in specific combinations forming defined haplotypes that differentially affect ABCB1 promoter activity (Speidel et al., 2018). In the current investigation, we evaluated the interaction between ABCB1 promoter haplotypes and acute and chronic exposure to BPA and BPS.
Data reported on serum levels of BPA in humans varied considerably between different studies, probably because the materials used for the analysis could have potentially contained trace amounts of BPA, increasing contamination possibility (Calafat et al., 2013; Twaddle et al., 2010; Ye et al., 2013). In non-pregnant subjects, the observed serum BPA levels varied from 0.13nM to 10.95nM (Vandenberg et al., 2012, 2010). In maternal and fetal cord blood, the concentrations were much higher, ranging from 2.0 to 39.6nM (Vandenberg et al., 2012, 2010). In term human placenta, concentrations were found to be even higher, with concentrations ranging from 4.4nM to 459.5nM (Schönfelder et al., 2002; Vandenberg et al., 2012, 2010). A recent study evaluating both BPA and BPS concentrations in urine of cashiers found that BPS levels were between 12% and 38% of urine concentrations of BPA (Thayer et al., 2016). Because of the structural and chemical property similarities of the two molecules, the ratio of BPS to BPA in the serum should be similar to the ratio in urine levels. In studies investigating serum BPS, concentrations were found much lower than BPA at concentrations between 0.04nM and 0.45nM (Thayer et al., 2016). In our studies, we used a BPA concentration of 50nM for acute exposure and 5nM for chronic exposure (10% of the chronic dose). For BPS, 0.5nM was used for acute exposure and the 0.3nM concentration for chronic BPS exposures. For mixed BPA/BPS exposures, we used the ratio of 5nM:1.5nM (BPA:BPS) in keeping with the ratio observed in exposed humans reported by Thayer et al., 2016
The effect of acute exposures on ABCB1 promoter activity was measured using the Nanoluciferase-PEST (NlucP) plasmid. This plasmid produces a luciferase protein with an attached c-terminal peptide sequence of Proline-Glutamic Acid-Serine-Threonine (PEST) which has been demonstrated to enhance proteasomal degradation (Li et al., 1998). The half-life of the NlucP protein varies between cell lines (~25–30 min), but this short half-life and strong signal result in the protein as a good candidate for acute exposure studies (Hall et al., 2012). The effect of BPA/BPS exposure on the activity of different ABCB1 promoter haplotypes has not yet been evaluated. However, several other xenoestrogens have been evaluated for their effect on ABCB1 mRNA expression and the resulting P-gp expression. Both the synthetic estrogen ethynyl estradiol and the phytoestrogen genistein were reported to alter the expression of ABCB1 mRNA as well as the P-gp protein expression (Arias et al., 2014).
In our study, when placental 3A cells were acutely exposed to BPA alone we observed a significant increase in ABCB1 promoter activity with haplotype 1 only. However, when placental 3A cells were acutely exposed to BPS alone, there were significant decreases in promoter activity of 3 of the 4 ABCB1 haplotypes tested (haplotype 4, 29, and 30). These data indicate that structurally similar xenoestrogens do not exert the same effects on ABCB1 promoter activity. These data also indicate that the same concentrations of a bisphenol can induce a different response on ABCB1 promoter activity depending on the haplotype, strongly suggesting a possible haplotype-exposure interaction.
When cells were exposed to a mixture of the two tested bisphenols at the same concentrations evaluated individually, no significant difference in ABCB1 promoter activity was observed depending on the haplotype. Although exposure to BPS alone had a significant effect on promoter activity, the difference in response could be due to the presence of BPA in the mixture. At a concentration 5 times higher than that of BPS, it is possible that BPA could be saturating potential target sites thus masking the effect of BPS. BPA has been shown to be a substrate for P-gp in the intestine in vitro (Yoshikawa et al., 2002) and to stimulate the drug efflux mechanism in human BeWo choriocarcinoma cell lines, indicating the possibility of regulation through P-gp (Jin and Audus, 2005).
For chronic BPA and BPS exposures, in contrast to acute BPA exposure, our data indicate that chronic exposure to 5nM BPA, a concentration 10 times lower than the acute concentration tested, induced a significant increase (p<0.05) in ABCB1 promoter activity for 3 of the 4 haplotypes evaluated (haplotypes 1, 4 and 29). Chronic exposure to BPS also induced a significant increase in promoter activity that was also haplotype-dependent. An increase in activity was observed with haplotypes 1, 29 and 30 but not haplotype 4. The same significant increase in promoter activity (p<0.01) was observed with chronic exposure to the BPA and BPS mixture for all haplotypes evaluated. These data with chronic exposure contrasts those observed with acute exposure to the same compounds, suggesting that promoter activity in response to bisphenols is not only haplotype-dependent, but is also exposure-type dependent (acute vs. chronic).
Our data suggest there may be a difference in the mechanisms by which acute and chronic exposures alter the promoter activity of ABCB1 haplotypes. For example, it is possible that chronic exposure to BPA or BPS, alone or in mixture, differentially alter transcription factor (TF) binding profile of ABCB1 promoter. We have recently identified several TF binding sites in the ABCB1 promoter that differed depending on the haplotype (Speidel et al., 2018), and chronic exposure could induce the translation of select alternate/additional TFs that could alter ABCB1 expression depending on the haplotype. Other potential mechanisms include potential epigenetic modifications, such as histone modification or alteration of methylation patterns on the ABCB1 promoter. Within the ABCB1 promoter, there are several CpG islands (Li et al., 2015; Takai and Jones, 2003), which are often targets for DNA methylation which results in decreased activity of the promoter.
Alternatively, the effect observed with acute exposures could be due to different mechanisms. For example, acute exposures could exert their observed effects by initiating nongenomic signaling events that activate modifying enzymes (kinase, acetylase, methyltransferase) that would in turn inactivate certain TFs that normally bind to ABCB1 promoter to induce (or repress) expression or activate alternative transcription factors that could activate (or repress) expression in a haplotype-dependent manner. This non-genomic signaling has been demonstrated with BPA and BPS exposures in pituitary cells when evaluating c-JunN-terminal kinases as well as extracellular signal-regulated kinases (Viñas and Watson, 2013a, 2013b).
In summary, we showed the ability of both BPA and BPS to alter the activity of ABCB1 promoter in human placental 3A cells. Such alteration could significantly impact placental P-gp levels. We also demonstrated that the effects of BPA and BPS on ABCB1 promoter activity depend on the ABCB1 promoter haplotype. The results of our study have important health implications for pregnant women and their fetuses. The significant decrease in ABCB1 promoter activity resulting from acute BPS exposure observed with three of four haplotypes tested could indicate reduced placental P-gp levels with these haplotypes in mothers exposed to this compound. Such decreases could increase fetal exposure to environmental and pharmaceutical xenobiotics that are P-gp substrates. In contrast, ABCB1 increased expression observed with acute exposure to BPA and chronic exposure to BPA and BPS may lead to placental P-gp overexpression with certain haplotypes, ultimately altering the distribution of endogenous or exogenous P-gp substrates in the maternal and fetal circulation. The importance of P-gp in the placenta warrants further studies to affirm our results in vivo, and to understand the mechanisms by which exposure to bisphenols alter ABCB1 expression in different haplotypes.
Highlights.
BPA and BPS alter ABCB1 promoter activity in a haplotype-dependent manner.
Acute BPA exposure elicits haplotype-dependent increases in ABCB1 promoter activity
Acute BPS exposure elicits haplotype-dependent decreases in ABCB1 promoter activity
Chronic exposure to BPA/BPS elicits haplotype-dependent increases in ABCB1 activity
Alteration of ABCB1 promoter activity could significantly affect P-gp levels.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health [T32-ES007254 to J.S.] and the John Sealy Memorial Endowment fund for Biomedical Research (to S.A.R.). Additional partial support was provided by the NIEHS Center in Environmental Toxicology at the University of Texas Medical Branch (UTMB) funded through P30 ES006676, the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award [8UL1TR000071] from the National Center for Research Resources, now the National Center for Advancing Translational Sciences and the Obstetric-Fetal Pharmacology Research Center at UTMB funded through 2 U54 HD047891.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arias A, Rigalli JP, Villanueva SSM, Ruiz ML, Luquita MG, Perdomo VG, et al. (2014). Regulation of expression and activity of multidrug resistance proteins MRP2 and MDR1 by estrogenic compounds in Caco-2 cells. Role in prevention of xenobiotic-induced cytotoxicity. Toxicology 320, 46–55. 10.1016/j.tox.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Biedermann S, Tschudin P, Grob K (2010). Transfer of bisphenol A from thermal printer paper to the skin. Anal. Bioanal. Chem 398, 571–576. 10.1007/s00216-010-3936-9 [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Long M, Hofmeister M,V, Vinggaard AM (2007). Endocrinedisrupting potential of Bisphenol A, Bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-noctylphenol in vitro: New data and a brief review. Environ. Health Perspect 115, 69–76. 10.1289/ehp.9368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyerinas B, Park SM, Murmann AE, Gwin K, Montag AG, Zillhardt M, et al. (2012). Let-7 modulates acquired resistance of ovarian cancer to Taxanes via IMP-1-mediated stabilization of multidrug resistance 1. Int. J. Cancer 130, 1787–1797. 10.1002/ijc.26190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. (2011). Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 128, 873–82. 10.1542/peds.2011-1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, et al. (2013). Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res. 15, 403 10.1186/bcr3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceckova-Novotna M, Pavek P, Staud F (2006). P-glycoprotein in the placenta: Expression, localization, regulation and function. Reprod. Toxicol 22, 400–410. 10.1016/j.reprotox.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Chen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, et al. (2016). Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity - A Review. Environ. Sci. Technol 50, 5438–5453. 10.1021/acs.est.5b05387 [DOI] [PubMed] [Google Scholar]
- Dodds EC, Lawson W (1936). Synthetic estrogenic agents without the phenanthrene nucleus. Nature 996. [Google Scholar]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS (2010). In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: An epigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer 1, 146–155. 10.1007/s12672-010-0015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MP, Unch J, Binkowski BF, Valley MP, Butler BL, Wood MG, et al. (2012). Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol 7, 1848–1857. 10.1021/cb3002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, Patrikeeva SL, Hankins GDV, Ahmed MS(2010). Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem. Pharmacol 79, 921–925. 10.1016/j.bcp.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzl M, Drescher S, van der Kuip H, Schäffeler E, Fischer J, Schwab M, et al. (2001). The C3435T mutation in the human MDR1 gene is associated with altered efflux of the Pglycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics 11, 293–298. 10.1097/00008571-200106000-00003 [DOI] [PubMed] [Google Scholar]
- Hitzl M, Schaeffeler E, Hocher B, Slowinski T, Halle H, Eichelbaum M, et al. (2004). Variable expression of P-glycoprotein in the human placenta and its association with mutations of the multidrug resistance 1 gene (MDR1, ABCB1). Pharmacogenetics 14, 309–318. 10.1097/01.fpc.0000114729.08559.09 [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, et al. (2000). Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. U. S. A 97, 3473–8. 10.1073/pnas.050585397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y (2002). Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod 17, 2839–2841. 10.1093/humrep/17.11.2839 [DOI] [PubMed] [Google Scholar]
- Ito S, Ieiri I, Tanabe M, Suzuki A, Higuchi S, Otsubo K (2001). Polymorphism of the ABC transporter genes, MDR1, MRP1 and MRP2/cMOAT, in healthy Japanese subjects. Pharmacogenetics 11, 175–84. [DOI] [PubMed] [Google Scholar]
- Jin H, Audus KL (2005). Effect of bisphenol A on drug efflux in BeWo, a human trophoblast-like cell line. Placenta 26 Suppl A, S96–S103. 10.1016/j.placenta.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Joshi AA, Vaidya SS, St-Pierre MV, Mikheev AM, Desino KE, Nyandege AN, et al. (2016). Placental ABC Transporters: Biological Impact and Pharmaceutical Significance. Pharm. Res 10.1007/s11095-016-2028-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, et al. (2001). Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin. Pharmacol. Ther 70, 189–199. 10.1067/mcp.2001.117412 [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D (1993). Bisphenol-A: An Estrogenic Substance Is Released from Polycarbonate Flasks during Autoclaving. Endocrinology 132, 2279–2286. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, et al. (2013). Sexspecific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. U. S. A 110, 9956–61. 10.1073/pnas.1214056110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz SM, Diamond RD (1985). A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J. Infect. Dis 152, 938–945. 10.1093/infdis/152.5.938 [DOI] [PubMed] [Google Scholar]
- Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, et al. (2015). The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. 43, W580–W584. 10.1093/nar/gkv279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, et al. (1998). Generation of destabilized green fluorescent protein as a transcription reporter. J. Biol. Chem 273, 34970–34975. 10.1074/jbc.273.52.34970 [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K (2013). Concentrations and profiles of bisphenol a and other bisphenol analogues in foodstuffs from the united states and their implications for human exposure. J. Agric. Food Chem 61, 4655–4662. 10.1021/jf400445n [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Kannan K (2012). Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ. Sci. Technol 46, 6515–22. 10.1021/es300876n [DOI] [PubMed] [Google Scholar]
- Lourenço JJ, Maia RC, Scheiner MAM, Vasconcelos FC, Moreira MAM (2008). Genomic variation at the MDR1 promoter and P-glycoprotein expression and activity in AML patients. Leuk. Res 32, 976–979. 10.1016/j.leukres.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Mathias AA, Hitti J, Unadkat JD (2005). P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am. J. Physiol. Regul. Integr. Comp. Physiol 289, R963–9. 10.1152/ajpregu.00173.2005 [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. (2011). Endocrine disruptors and childhood social impairment. Neurotoxicology 32, 261–7. 10.1016/j.neuro.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölsä M, Heikkinen T, Hakkola J, Hakala K, Wallerman O, Wadelius M, et al. (2005). Functional role of P-glycoprotein in the human blood-placental barrier. Clin. Pharmacol. Ther 78, 123–131. 10.1016/j.clpt.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Mosmann T (1983). Rapid Colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J. Immunol. Methods 65, 55–63. 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- Naderi M, Wong MYL, Gholami F (2014). Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol 148, 195–203. 10.1016/j.aquatox.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ikeda Sichi, Furukawa T, Sumizawa T, Tani A, Akiyama S ichi, et al. (1997). Function of P-glycoprotein expressed in placenta and mole. Biochem. Biophys. Res. Commun 235, 849–853. 10.1006/bbrc.1997.6855 [DOI] [PubMed] [Google Scholar]
- Orlowski S, Mir LM, Belehradek J, Garrigos M (1996). Effects of steroids and verapamil on Pglycoprotein ATPase activity: progesterone, desoxycorticosterone, corticosterone and verapamil are mutually non-exclusive modulators. Biochem. J 317, 515–522. 10.1042/bj3170515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Tang WY, Belmonte J, Ho SM (2008). Developmental exposure to bisphenol A increases prostate cancer susceptibility in adult rats: epigenetic mode of action is implicated. Fertil. Steril 89, e41 10.1016/j.fertnstert.2007.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Taylor JA, Ruhlen RL, Welshons WV, vom Saal FS (2007). Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ. Health Perspect 115, 902–908. 10.1289/ehp.9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR (2013). Bisphenol A and human health: A review of the literature. Reprod. Toxicol 42, 132–155. 10.1016/j.reprotox.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Rubin BS, Soto AM (2010). Bisphenol A: Perinatal exposure and body weight. Mol. Cell. Endocrinol 304, 1–17. 10.1016/j.mce.2009.02.023.Bisphenol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai K, Itoda M, Saito Y, Kurose K, Katori N, Kaniwa N, et al. (2006). Genetic variations and haplotype structures of the ABCB1 gene in a Japanese population: an expanded haplotype block covering the distal promoter region, and associated ethnic differences. Ann. Hum. Genet 70, 605–22. 10.1111/j.1469-1809.2006.00260.x [DOI] [PubMed] [Google Scholar]
- Sai K, Saito Y, Maekawa K, Kim S-R, Kaniwa N, Nishimaki-Mogami T, et al. (2010). Additive effects of drug transporter genetic polymorphisms on irinotecan pharmacokinetics/pharmacodynamics in Japanese cancer patients. Cancer Chemother. Pharmacol 66, 95–105. 10.1007/s00280-009-1138-y [DOI] [PubMed] [Google Scholar]
- Salama NN, Yang Z, Bui T, Ho RJY (2006). MDR1 haplotypes significantly minimize intracellular uptake and transcellular P-gp substrate transport in recombinant LLC-PK1 cells. J. Pharm. Sci 95, 2293–2308. 10.1002/jps.20717 [DOI] [PubMed] [Google Scholar]
- Schönfelder G, Wittfoht W, Hopp H, Talsness CECE, Paul M, Chahoud I (2002). Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect 110, A 703–A 707. 10.1289/ehp.021100703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Obiorah I, Maximov PY, Curpan R, Jordan VC (2013). Molecular mechanism of action of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br. J. Pharmacol 169, 167–78. 10.1111/bph.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AB, Fox K, Lam P, Ling V (1999). Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone: Evidence for a third drug-binding site. Eur. J. Biochem 259, 841–850. 10.1046/j.1432-1327.1999.00098.x [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE (1993). Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341, 1392–1396. 10.1016/0140-6736(93)90953-E [DOI] [PubMed] [Google Scholar]
- Smit JW, Huisman MT, Van Tellingen O, Wiltshire HR, Schinkel AH (1999). Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure. J. Clin. Invest 104, 1441–1447. 10.1172/JCI7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidel JT, Xu M, Abdel-Rahman SZ (2018). Differential effect of ABCB1 haplotypes on promoter activity. Pharmacogenet. Genomics 28, 69–77. 10.1097/FPC.0000000000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, Lauper U, et al. (2000). Expression of members of the multidrug resistance protein family in human term placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol 279, R1495–R1503. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Oishi S (2000). Disposition of orally administered 2,2-bis(4hydroxyphenyl)propane (bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ. Health Perspect. 108, 931–935. 10.1289/ehp.00108931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai D, Jones PA (2003). The CpG island searcher: a new WWW resource. In Silico Biol. 3, 235–40. [PubMed] [Google Scholar]
- Takane H, Kobayashi D, Hirota T (2004). Haplotype-oriented genetic analysis and functional assessment of promoter variants in the MDR1 (ABCB1) gene. J. Pharmacol. Exp. Ther 311, 1179–1187. 10.1124/jpet.104.069724.ymous [DOI] [PubMed] [Google Scholar]
- Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, et al. (2001). Expression of Pglycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J. Pharmacol. Exp. Ther 297, 1137–43. [PubMed] [Google Scholar]
- Thayer KA, Taylor KW, Garantziotis S, Schurman SH, Kissling GE, Hunt D, et al. (2016). Bisphenol A, Bisphenol S, and 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) in Urine and Blood of Cashiers. Environ. Health Perspect 124, 437–444. 10.1289/ehp.1409427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaddle NC, Churchwell MI, Vanlandingham M, Deorge DR (2010). Quantification of deuterated bisphenol A in serum, tissues, and excreta from adult Sprague-Dawley rats using liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom 24, 3011–3020. 10.1002/rcm.4733 [DOI] [PubMed] [Google Scholar]
- Vafeiadi M, Roumeliotaki T, Myridakis A, Chalkiadaki G, Fthenou E, Dermitzaki E, et al. (2016). Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ. Res 146, 379–387. 10.1016/j.envres.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Valvi D, Casas M, Mendez M a, Ballesteros-Gómez A, Luque N, Rubio S, et al. (2013). Prenatal bisphenol a urine concentrations and early rapid growth and overweight risk in the offspring. Epidemiology 24, 791–9. 10.1097/EDE.0b013e3182a67822 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V (2012). Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Cien. Saude Colet. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G (2010). Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 10.1289/ehp.0901716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñas R, Watson CS (2013a). Mixtures of xenoestrogens disrupt estradiol-induced nongenomic signaling and downstream functions in pituitary cells. Environ. Heal. 12, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñas R, Watson CS (2013b). Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: Effects on cell functions. Environ. Health Perspect 121, 352–358. 10.1289/ehp.1205826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N, Filis P, Soffientini U, Bellingham M, O’Shaughnessy PJ, Fowler PA (2017). Placental transporter localization and expression in the human: The importance of species, sex, and gestational age difference. Biol. Reprod 96, 733–742. 10.1093/biolre/iox012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SRJ, Gatewood JD, Taylor JA, Rissman EF, et al. (2012). Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Neuroendocrinology 153, 1–11. 10.1210/en.2012-1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ (2011a). The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav 59, 296–305. 10.1016/j.yhbeh.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Taylor JA, Shetty SRJ, Edwards M, Connelly JJ, Rissman EF (2011b). Gestational Exposure to Low Dose Bisphenol A Alters Social Behavior in Juvenile Mice. PLoS One 6, e25448 10.1371/journal.pone.0025448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Cross CE, Speidel JT, Abdel-Rahman SZ (2016). MGMT DNA repair gene promoter/enhancer haplotypes alter transcription factor binding and gene expression. Cell. Oncol 39, 435–447. 10.1007/s13402-016-0286-4 [DOI] [PubMed] [Google Scholar]
- Xu M, Nekhayeva I, Cross CE, Rondelli CM, Wickliffe JK, Abdel-Rahman SZ (2014). Influence of promoter/enhancer region haplotypes on MGMT transcriptional regulation: A potential biomarker for human sensitivity to alkylating agents. Carcinogenesis 35, 564–571. 10.1093/carcin/bgt355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan M, Li H, Fu R, Yang Y, Zhang D, Zhang X, et al. (2014). Association of ABCB1 gene polymorphisms and haplotypes with therapeutic efficacy of glucocorticoids in Chinese patients with immune thrombocytopenia. Hum. Immunol 75, 317–321. 10.1016/j.humimm.2014.01.013 [DOI] [PubMed] [Google Scholar]
- Yamazaki E, Yamashita N, Taniyasu S, Lam J, Lam PKS, Moon HB, et al. (2015). Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf 122, 565–572. 10.1016/j.ecoenv.2015.09.029 [DOI] [PubMed] [Google Scholar]
- Ye X, Zhou X, Hennings R, Kramer J, Calafat AM (2013). Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environ. Health Perspect 121, 283–286. 10.1289/ehp.1206093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y, Hayashi A, Inai M, Matsushita A, Shibata N, Takada K (2002). Permeability characteristics of endocrine-disrupting chemicals using an in vitro cell culture model, Caco-2 cells. Curr. Drug Metab 3, 551–7. [DOI] [PubMed] [Google Scholar]
- You H, Liu Y, Carey MJ, Lowery CL, Hermonat PL (2002). Defective 3A trophoblastendometrial cell adhesion and altered 3A growth and survival by human papillomavirus type 16 oncogenes. Mol. Cancer Res 1, 25–31. [PubMed] [Google Scholar]