Abstract
Medical and surgical interventions for elevated breast cancer risk (e.g., BRCA1/2 mutation, family history) focus on reducing estrogen exposure. Women at elevated risk may be interested in less aggressive approaches to risk reduction. For example, exercise might reduce estrogen, yet has fewer serious side effects and less negative impact than surgery or hormonal medications. Randomized controlled trial. Increased risk defined by risk prediction models or BRCA mutation status. Eligibility: Age 18–50, eumenorrheic, non-smokers, and body mass index (BMI) between 21 and 50 kg/m2. 139 were randomized. Treadmill exercise: 150 or 300 min/week, five menstrual cycles. Control group maintained exercise<75 min/week. Primary outcome: Area under curve (AUC) for urinary estrogen. Secondary measures: urinary progesterone, quantitative digitized breast dynamic contrast-enhanced magnetic resonance imaging background parenchymal enhancement. Mean age 34 years, mean BMI 26.8 kg/m2. A linear dose-response relationship was observed such that every 100 min of exercise is associated with 3.6 % lower follicular phase estrogen AUC (linear trend test, p = 0.03). No changes in luteal phase estrogen or progesterone levels. There was also a dose–response effect noted: for every 100 min of exercise, there was a 9.7 % decrease in background parenchymal enhancement as measured by imaging (linear trend test, p = 0.009). Linear dose–response effect observed to reduce follicular phase estrogen exposure measured via urine and hormone sensitive breast tissue as measured by imaging. Future research should explore maintenance of effects and extent to which findings are repeatable in lower risk women. Given the high benefit to risk ratio, clinicians can inform young women at increased risk that exercise may blunt estrogen exposure while considering whether to try other preventive therapies.
Keywords: Breast cancer, Exercise, Estrogens, Clinical trial, Breast MRI
The strongest basis for breast cancer prevention among high risk women is through hormonal intervention. Prophylactic oophorectomy can reduce risk by 50–70 % [1–3]. Selective Estrogen Receptor Modulators (SERMS, e.g., tamoxifen and raloxifene) are shown effective at reducing risk by approximately 50 % [4]. However, surgical and drug interventions have serious negative long-term consequences, including loss of fertility, increased risk of heart disease, and poor bone health outcomes [2, 5]. BRCA1/2 carriers often do not choose prophylactic mastectomy, even though reduction of risk is substantial [1, 2]. Women at elevated risk for breast cancer are motivated to reduce their risk [6].
An exercise intervention might reduce sex hormones, and have a positive health impact. In observational studies, adolescent physical activity decreased breast cancer risk or delayed diagnosis among BRCA1/2 carriers [7]. Weight loss in early adult life reduced breast cancer risk among BRCA1/2 carriers by 34 % [8]. The hypothesized mechanisms for these effects included reduced steroid hormone exposure [9].
Progesterone also has long been thought to be anti-proliferative in the premenopausal breast. Its effects are opposite to estrogen-induced epithelial proliferation in the endometrium in the luteal phase of the menstrual cycle [10]. That said, there are animal data that implicate progesterone as contributing to mammary endothelial proliferation [11], while epidemiologic data supporting the protective effect of progesterone [12, 13]. Prior studies have shown that exercise reduces [14] or does not change progesterone [15].
Evidence supports a positive relationship of breast cancer risk with the amount of fibroglandular tissue and specifically with background parenchymal enhancement (BPE) as evaluated by DCE-MRI [16–18], which can differentiate the non-enhancing fibrous tissues from the hormonally responsive enhancing glandular tissue [19–21]. To our knowledge, there have been no prior studies that have examined the effects of exercise on BPE.
The Women In Steady Exercise Research (WISER) Sister study was a randomized controlled trial with a primary objective to test dose–response effects of 150 and 300 min/week of aerobic exercise training over five menstrual cycles among healthy premenopausal women at elevated risk for breast cancer. Our primary hypothesis was that exercise training would decrease endogenous estrogen exposure in a dose–response manner. Herein we also report on two secondary outcomes, urinary progesterone and BPE. Our hypotheses for these secondary outcomes were similar: that exercise training would result in dose–response decreases.
Methods
Study subjects
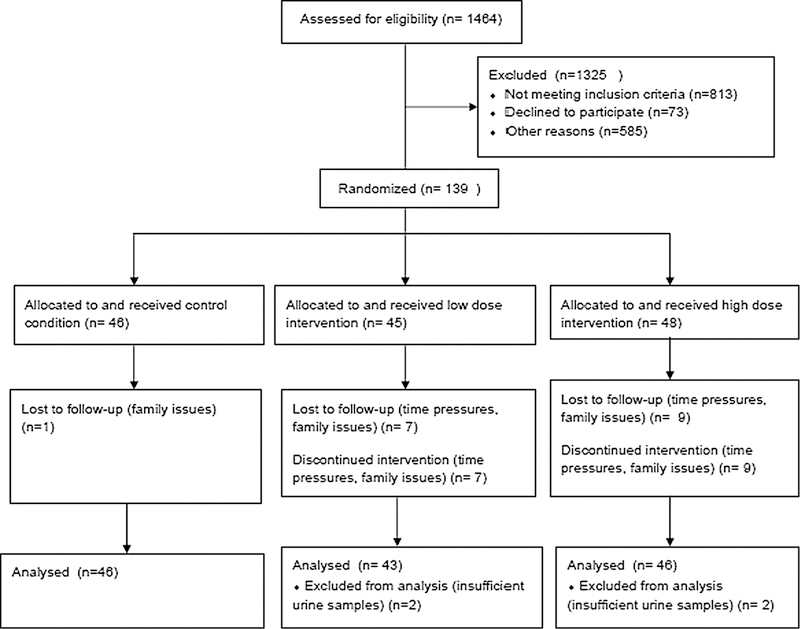
Recruitment focused on organizations that worked with women at elevated risk, such as Facing Our Risk of Cancer Empowered (FORCE) (www.facingourrisk.org) and the Cancer Genetics Network [22]. Eligibility requirements included: female sex, no personal history of breast cancer, lifetime breast cancer risks of ≥ 18 % evidenced by documented BRCA 1 or 2 mutation for participant or first degree relative, and/or Claus model risk of [18 %, [23] and/or Gail model risk of ˃ 18 % [24]. The cut-off of ˃ 18 % was based on feasibility and to ensure the sample were truly at elevated risk. The background lifetime risk level is 12.4 % [25]. Additional eligibility factors were body mass index (BMI) between ≥ 21 and ≤50 kg/m2, age 18–50, eumenorrheic (menstrual cycles 23–35 days in length), gynecologic age ≥ 4 years, intact ovaries and uterus, no hormonal contraceptive use (past 3 months for oral and vaginal methods, past 12 months for medroxyprogesterone), prior tubal ligation or willingness to use non-hormonal birth control during study, no history of cancer, no eating disorders (screened using Eating Disorder Diagnostic Scale [26]), not currently in a weight loss program, not pregnant in past 6 months, not currently breastfeeding, not planning to become pregnant, no more than 7 alcoholic beverages per week, self-reported aerobic exercise of < 75 min/week over past 6 months, not planning to move during study; and no medical conditions that would preclude safe participation. Figure 1 presents the Consort flow diagram and final distribution of 139 randomized participants. Further details of recruitment are provided elsewhere [27].
Fig. 1.
Consort diagram
Women were placed into three exercise groups (control, low dose, and high dose) through a blocked randomization process, and using 1:1:1 ratio. We stratified to balance important potential confounders: gynecological age (<10 vs. ≥10), obesity (BMI <30 vs. ≥30 kg/m2). The study was approved by the University of Pennsylvania IRB. Women provided written informed consent and written clearance from a physician prior to participation.
Intervention
Exercise took place over five menstrual cycles, on in-home treadmills provided to each intervention group participant (Smooth Fitness, model 5.65, King of Prussia, PA). Women wore a downloadable heart rate (HR) monitor during each exercise session (model RS400 Polar Electro Inc., Lake Success, NY). Exercise logs included date, time, average HR, and workout duration. Study staff reviewed logs and objectively monitored HR data weekly. Exercise intensity was set at 65–70 % age predicted max HR for weeks 1–4 (max HR = 220-age), 70–80 % of max HR for remainder of study. The low-dose exercise group completed 150 min/week. The high-dose exercise group started with 150 min/week and increased to 300 min/week by week 11, which was then sustained to study completion. Control group participants were asked not to engage in new activities during study participation, and were provided the in-home treadmill at study completion. Further details of the intervention are provided elsewhere [27].
Measurements
Baseline and follow-up measurements were completed by trained staff blinded to treatment allocation, using standardized methods. Demographic characteristics were self-reported at baseline. Total treadmill time from the Bruce protocol [28] was used to assess aerobic fitness. Anthropometry measures included weight, height (baseline only). Body composition was measured by dual energy X-ray absorptiometry (DXA) (QDR 4500 Discovery A, Hologic, Bedford MA). Three day food records were entered into the Nutrition Data System for Research software (Nutrition Coordinating Center, University of Minnesota, (2009 version)). Physical activity was assessed via a modified version of the Modifiable Activity Questionnaire [29].
Urinary hormones
All women provided first morning urine samples daily during cycles 1 and 2 (pre-intervention) as well as 6 and 7 (last 2 months of intervention, called ‘post-intervention’ going forward)). All urine samples were corrected for specific gravity using a hand refractometer (NSG Precision Cells, Inc., Farmingdale, NY) to account for hydration status. Pre- and post-intervention urine samples were grouped to run within the same reagent batch.
Microtiter plate competitive enzyme immunoassays (EIA) were used to measure the urinary metabolites of estrogen and progesterone, i.e., Estrone-3-Glucuronide (E1G) and Pregnanediol-3-Glucuronide (PdG), respectively. The secretion of these metabolites in the urine parallels serum concentrations of the parent hormones [30]. The intra-assay coefficients of variation for high and low internal controls for the E1G assay were 1.8 and 4.9 %. The intra-assay coefficients of variation for high and low internal controls for the PdG assay were 5.2 and 11.0 %.
Urinary luteinizing hormone (LH) was determined by double antibody radioimmunoassay (Diagnostic Products Corp., Los Angeles, CA, USA). The sensitivity of the LH assay is 0.6 mIU/L. The intra-assay and inter-assay coefficients of variation were 1.6 and 7.1 %, respectively. Day of the ovulation was identified by the day of the urinary LH peak and by the association of urinary LH peak with the mid-cycle peak of E1G (occurring 1–2 days prior to the urinary LH peak) [31]. The total menstrual cycle length was defined, by self-report logs, as the number of days from day 1 of menses to the day before the next menses. Follicular phase length was defined as the number of days from day 1 of menses up to and including the day of ovulation (determined by LH surge, described above). Luteal phase length was defined as the number of days beginning from the day after ovulation to the day before next menses. The area under the curve (AUC) for E1G and PdG for follicular phase, luteal phase, and full cycle were computed using trapezoidal rule [32].
MR image acquisition and analysis
Due to budget cuts MR imaging was completed for the first 68 women (22, 22, and 24 in the control, low-, and high-dose groups, respectively). Besides fewer minority women than white women (p = 0.001), there were no other observed differences between women receiving MR imaging versus not. Bilateral dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) examinations were performed between days 6 and 10 of the menstrual cycle at month one and seven using a 1.5-T Siemens scanner according to published methods [33]. At follow-up, images were obtained using the precise field of view (FOV) and slab dimensions used in the initial examinations to ensure consistency when comparing over time. We used our previously validated fully automated computerized methods [34] to quantitatively measure BPE [35–38].
Statistical analysis
Descriptive statistics were computed for study variables. All data are presented as mean ± SD for continuous variable and N (%) for categorical variables. The primary outcomes for this study were the AUC for urinary estrogen (hereafter E1G-AUC) for the follicular phase and luteal phase. Based on prior research, we expected a mean baseline value of 550 ng/mL in E1G-AUC, a standard deviation of 240 units and a correlation over time of approximately 0.77. Given an anticipated final sample size of approximately 42 women per group, we determined the power to detect a 10 and 20 % change in E1G-AUC in the low- and high-dose groups, respectively, to be 87 % for a two-sided, 0.05-level test. This corresponded to an effect size [39] of approximately 0.47.
One-way ANOVA was used for baseline comparisons of aerobic fitness, anthropometry, diet, and physical activity variables. Normality of the outcome variables was tested by Shapiro–Wilk test. A log10 transformation for estrogen AUC, BPE, progesterone AUC, and physical activity level were taken to improve distribution normality. Geometric means were reported for the transformed variable when appropriate. To analyze the primary aim of treatment effects on estrogen AUC, a series of linear mixed effects models was fitted to the transformed outcome measures. Under the intention to treat framework, the models included a random effect for individual to account for up to four repeated measures per individual and fixed effects for group (low and high dose), indicator of post-intervention period, and the interaction between treatment group and post period. A missing at random mechanism was assumed and each model was adjusted for predictors associated with drop out: cycle length, age, BMI, marital status, race (except for BPE), and education [27]. Comparison between groups was done with a Bonferroni adjustment for Type I error (α = 0.025). Percent change in outcomes from pre to post and post/pre ratio in the mean values was estimated from the adjusted models. To evaluate the presence of dose–response relationships, a test of linear trend was conducted by testing linear and quadratic contrasts. A non-significant p value for the quadratic contrast along with a significant linear contrast provides evidence that a linear trend in dose holds. Secondary outcomes were analyzed in the same fashion. Statistical analysis was performed using SAS 9.3 (Cary, NC) or STATA 13.0 (College Station, TX).
Results
Table 1 shows the 139 randomized participants, including 17 women (12.2 %) lost to follow-up. Participants were 18–49 years of age at baseline, 15 % self-reported minority race/ethnicity. The majority were college educated, married, employed full time, and had children. Of the 69 participants aged ≥35, the average lifetime risk level as assessed by the Gail model [24] ranged from 9.6 to 51.4 %. Of the 134 participants for whom it was possible to calculate the Claus model risk prediction [23], the average lifetime risk level ranged from 8.3 to 46.0 %. All participants met the eligibility criteria for 18 % lifetime risk using at least one method of prediction. Objective HR monitor data indicated that women in the low-dose and high-dose exercise groups completed 85 and 81 % of prescribed exercise minutes, respectively. Further detail on exercise adherence is provided elsewhere [27].
Table 1.
Demographic characteristics of women at elevated risk for breast cancera, recruited from across the United States (N = 139) mean (SD) or N (%)
| Control | Low dose | High dose | All | |
|---|---|---|---|---|
| N | 46 | 45 | 48 | 139 |
| Age | 34.6 (7.47) | 35.2 (6.38) | 33.4 (6.76) | 34.35 (6.75) |
| Body mass index (kg/m2) | 26.8 (6.2) | 26.8 (6.0) | 26.7 (6.5) | 26.8 (6.2) |
| Race | ||||
| White | 39 (85 %) | 40 (89 %) | 39 (81 %) | 118 (85 %) |
| Black | 5 (11 %) | 4 (9 %) | 5 (11 %) | 14 (10 %) |
| Other | 2 (4 %) | 1 (2 %) | 4 (9 %) | 7 (5 %) |
| Ethnicity | ||||
| Hispanic | 3 (7 %) | 3 (7 %) | 4 (8 %) | 10 (7 %) |
| Education Level | ||||
| ≤HS | 3 (7 %) | 1 (2 %) | 0 (0 %) | 4 (3 %) |
| Some college | 12 (26 %) | 13 (29 %) | 15 (31 %) | 40 (29 %) |
| ≥College | 31 (67 %) | 31 (69 %) | 33 (69 %) | 95 (68 %) |
| Employed full time (% yes) | 24 (52 %) | 28 (62 %) | 28 (58 %) | 80 (58 %) |
| Marital status | ||||
| Single/divorced/separated | 23 (50 %) | 11 (24 %) | 21 (44 %) | 55 (40 %) |
| Married/partnered | 23 (50 %) | 34 (76 %) | 27 (56 %) | 84 (60 %) |
| Parity | ||||
| Nulliparous | 22 (48 %) | 12 (27 %) | 23 (48 %) | 51 (41 %) |
| Primaparous | 5 (11 %) | 5 (11 %) | 7 (15 %) | 17 (12 %) |
| Multiparous | 19 (41 %) | 28 (62 %) | 18 (38 %) | 65 (47 %) |
| Children (% yes) | 24 (52 %) | 34 (76 %) | 25 (52 %) | 83 (60 %) |
| BRCA gene mutation status | ||||
| Positive | 14 (32 %) | 18 (56 %) | 17 (37 %) | 49 (35 %)b |
| Negative | 7 (15 %) | 2 (4 %) | 3 (6 %) | 12 (9 %)c |
| Not tested | 25 (54 %) | 25 (56 %) | 28 (59 %) | 78c (56 %)d |
| Lifetime risk % for all (tested and non-tested) | ||||
| n = 27 | n = 24 | n = 18 | n = 69e | |
| Gail | 25.02 (10.25) | 21.45 (8.06) | 20.67 (5.89) | 22.65 (8.64) |
| n = 45 | n = 45 | n = 45 | n = 135f | |
| Claus | 24.09 (10.13) | 24.27 (10.05) | 25.13 (11.02) | 24.62 (10.34) |
Study eligibility required that all women not be taking any hormonal contraception or any other hormonal medications for specific time periods. For example: no oral contraceptives for 3 months, no depo-provera for 12 months, and no vaginal estrogen ring for 6 months. Women who stopped these medications to enter the study were required to show they had at least 3 menstrual cycles that fit our criteria of normal’ (25–32 days) before being admitted to the trial
49 participants tested positive for the BRCA1 or BRCA2 gene mutation. Those who tested positive were immediately deemed the appropriate risk level for the study
10 participants tested negative for both the BRCA1 and BRCA2 gene mutations. These participants were eligible for the study due to their lifetime risk, as calculated via the Gail and/or Claus models
78 participants were not tested for the BRCA1 and BRCA2 gene mutations. These participants were eligible for the study due to their lifetime risk, as calculated via the Gail and/or Claus models
A Gail score is not calculated for women below the age of 35, which is why the Gail N does not equal the total N randomized
A Claus score is not calculated for women who lack female first and/or second degree relatives with breast cancer, which is why the Claus N does not equal the total N randomized
Table 2 shows baseline and follow-up data for fitness, anthropometry, diet, and physical activity. We observed a −2.1, +13.4, and +17.7 % change in aerobic fitness in the control-, low-, and high-dose groups, respectively. Menstrual cycle length and anthropometric measurements did not differ between groups at baseline or follow-up. Self-reported physical activity improved in the two exercise groups. No between-group differences were noted in energy intake at baseline or follow-up. There were no unexpected or serious adverse events related to the intervention.
Table 2.
Levels of aerobic fitness, anthropometry, diet, and physical activity among 139 premenopausal women at elevated breast cancer risk, before and after a 5-month exercise intervention
| Variables | Baseline | Follow-up | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Low dose | High dose | p valuea | Control | Low dose | High dose | p valuea | |||||||
| N | Mean (SD) | N | Mean(SD) | N | Mean(SD) | N | Mean(SD) | N | Mean(SD) | N | Mean (SD) | |||
| Aerobic fitness | ||||||||||||||
| Treadmill test duration (s) | 46 | 483 (98.5) | 45 | 476 (93.3) | 48 | 498 (114) | 0.58 | 45 | 473 (109) | 38 | 540 (95.2) | 39 | 586 (110) | <0.001 |
| Menstrual cycle lengthd | ||||||||||||||
| Total | 46 | 29.1 (4.4) | 43 | 29.1 (5.6) | 48 | 30.1 (4.4) | 0.54 | 45 | 28.4 (3.1) | 37 | 28.4 (5.6) | 38 | 29.5 (4.3) | 0.46 |
| Follicular | 46 | 18.8 (5.1) | 39 | 18.0 (6.3) | 48 | 18.8 (4.9) | 0.73 | 40 | 17.5 (4.2) | 32 | 17.2 (4.2) | 35 | 17.9 (3.7) | 0.79 |
| Luteal | 46 | 10.2 (2.3) | 39 | 10.6 (2.1) | 48 | 10.8 (1.8) | 0.38 | 40 | 10.5 (2.9) | 32 | 11.1 (2.6) | 35 | 11.5 (3.0) | 0.30 |
| Anthropometry | ||||||||||||||
| Weight (kg) | 46 | 73.8 (16.2) | 45 | 74.0 (17.3) | 48 | 73.0 (18.0) | 0.96 | 45 | 74.1 (17.1) | 38 | 71.7 (17.7) | 39 | 68.3 (15.7) | 0.30 |
| Body mass index (kg/m2) | 46 | 26.8 (6.2) | 45 | 26.9 (6.0) | 48 | 26.7 (6.5) | 0.99 | 45 | 26.9 (6.5) | 38 | 26.1 (6.2) | 39 | 25.1 (5.9) | 0.38 |
| % body fat | 46 | 38.9 (7.0) | 45 | 39.8 (6.1) | 48 | 38.2 (7.1) | 0.52 | 45 | 39.1 (7.1) | 37 | 38.0 (6.7) | 39 | 35.7 (7.8) | 0.10 |
| Fat mass (kg) | 46 | 28.9 (11.3) | 45 | 29.6 (11.1) | 48 | 28.2 (11.9) | 0.83 | 45 | 29.5 (12.1) | 38 | 27.7 (11.7) | 39 | 24.7 (11.0) | 0.18 |
| Lean mass (kg) | 46 | 45.3 (5.8) | 45 | 44.9 (7.0) | 48 | 45.2 (7.2) | 0.95 | 45 | 45.7 (6.4) | 38 | 44.5 (6.8) | 39 | 44.1 (6.2) | 0.50 |
| Diet and physical activity | ||||||||||||||
| Energy intake (Kcals/day) | 44 | 1840 (554) | 42 | 1804 (556) | 46 | 1867 (500) | 0.86 | 44 | 1821 (445) | 38 | 1800 (5426) | 39 | 1829 (429) | 0.93 |
| Alcohol intake (drinks/day)b | 44 | 43 | 46 | 44 | 38 | 37 | ||||||||
| 0 | 16 (36) | 15 (35) | 17 (37) | 11 (25) | 11 (29) | 13 (35) | ||||||||
| 1 | 24 (55) | 22 (51) | 24 (52) | 0.9755 | 30 (68) | 21 (55) | 21 (57) | 0.5543 | ||||||
| >1 | 4 (9) | 6 (14) | 5 (11) | 3 (7) | 6 (16) | 3 (8) | ||||||||
| Physical activity (MET-h/week)c | 46 | 5.57 (3.33) | 45 | 4.82 (4.14) | 48 | 4.44 (3.08) | 0.65 | 45 | 1.10 (0.98) | 38 | 7.82 (1.88) | 39 | 7.98 (3.00) | <0.001 |
p value for test of group differences separately at baseline and follow-up using analysis of variance (df = 2) for all variables except alcohol intake which is the Fisher’s exact p value
Values reported as N (%)
Values reported are geometric means at baseline and follow-up are for log transformed physical activity levels to improve normality of distribution. Standard deviations for the untransformed version were approximated from SD of the log transformed version using the delta method
Sample sizes for menstrual cycle length vary because it was not possible to calculate follicular and luteal phase lengths if the cycle was anovulatory
Table 3 shows urinary hormone data and the BPE data from MR imaging. Hormonal data included all 135 women who provided adequate baseline urinary data. The four women for whom we had insufficient hormonal data were in the low-dose (2 women) and high-dose (2 women) groups. There were no between group differences in any hormonal or MRI-BPE measurements at baseline.
Table 3.
Pre and post intervention levels of estrogen and progesterone (urinary conjugate, ng/mL) and hormonally sensitive breast tissue (MR imaging) among premenopausal women at elevated risk for breast cancer (N = 135 for hormonal data, N = 68 for imaging data)
| N | Pre level [95 % CI] | Post level [95 % CI] | |||||
|---|---|---|---|---|---|---|---|
| Control | Low dose | High dose | Control | Low dose | High dose | ||
| Estrogen (ng/mL) | |||||||
| Follicular | 135 | 790 [687, 908] |
785 [674, 915] |
802 [694, 927] |
882 [767, 1014] |
769 [659, 898] |
804 [693, 932] |
| Luteal | 135 | 499 [418, 599] |
487 [400, 592] |
541 [449, 651] |
519 [434, 621] |
511 [419, 624] |
579 [479, 702] |
| Progesterone (ng/mL) | |||||||
| Follicular | 135 | 18.8 [15.5, 22.8] |
19.5 [15.8, 24.0] |
19.2 [15.7, 23.4] |
20.0 [16.5 24.2] |
20.2 [16.3, 25.0] |
18.1 [14.7, 22.2] |
| Luteal | 135 | 54.1 [44.7, 65.6] |
62.2 [50.4, 76.7] |
61.3 [50.2, 74.8] |
51.2 [42.2, 62.1] |
57.7 [46.5, 71.5] |
66.6 [54.2, 81.9] |
| Imaging outcome (cm2) | |||||||
| Background parenchymal enhancement (cm2) |
68a | 263 [213, 326] |
268 [214, 337] |
265 [215, 327] |
317 [256, 392] |
289 [229, 364] |
234 [188, 292] |
Measured in a subset of participants, image acquisition during follicular phase at months 1 and 7
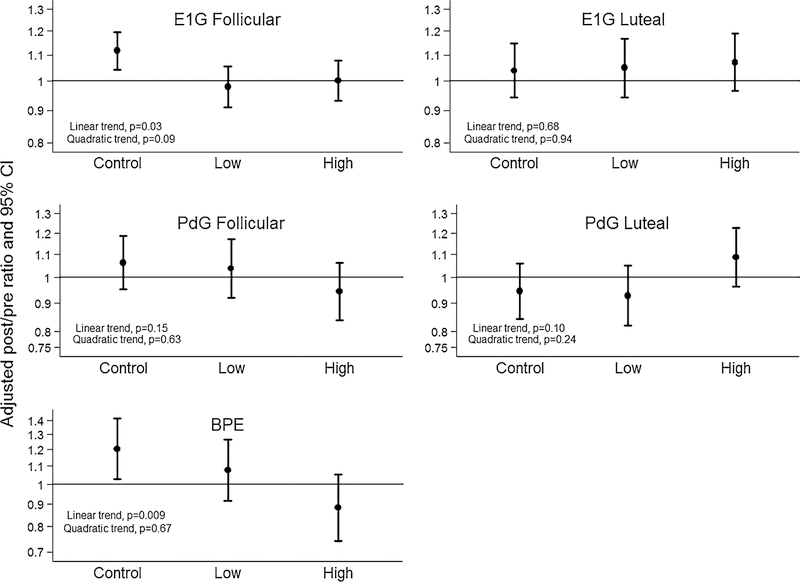
Figures 2 and 3 shows the adjusted ratio of post: pre values for urinary estrogen, urinary progesterone, and MRI-BPE outcomes, during the follicular phase (all variables), and luteal phase (hormonal outcomes only). The primary analysis was to evaluate for a linear dose–response effect. The hypothesis of linear dose–response was supported by a non-significant quadratic trend (p = 0.09) and a significant linear trend (p = 0.03). For every 100 min per week of exercise, a 3.6 % [95 CI 0.3, 6.8] reduction in follicular phase estrogen AUC was estimated. At follow-up, the control group exhibited an 11.6 % increase in follicular phase estrogen AUC, compared to a −2.1 and a 0.2 % change in the low- and high-dose groups, respectively. No linear trend or between group differences were noted for luteal phase urinary estrogen AUC. No linear trend or between group differences were noted for follicular or luteal phase progesterone. Sensitivity analyses based on a pattern-mixture model were performed to determine what would have been different if the MAR assumption was violated. Even if the missing E1G follicular values were on average as much as 25 % higher OR lower than observed, the pattern of findings remained the same.
Fig. 2.
Dose–response effects of aerobic exercise training on estrogen and progesterone (urinary conjugate, ng/mL) and hormonally sensitive breast tissue (MR imaging) among premenopausal women at elevated risk for breast cancer (N = 135 for hormonal data, N = 68 for MRI data)
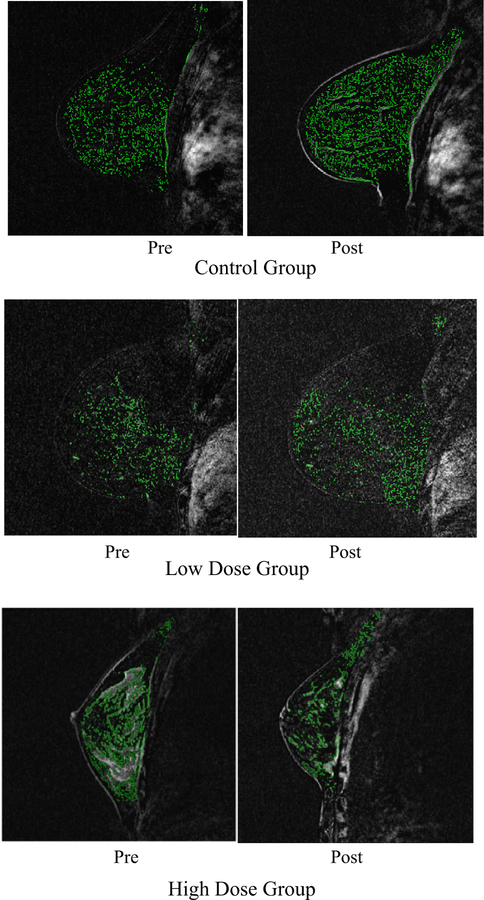
Fig. 3.
An example magnetic resonance image from three women among the 68 women at elevated breast cancer risk who underwent the imaging study. This image that shows examples of background parenchymal enhancement for 1 patient in each group: control-, low-, and high-dose groups, from baseline (month 1) and follow-up (month 7)
Within the subset of 68 women who underwent MR imaging, there were no baseline between group differences in quantitative BPE (Table 3). The hypothesis of a dose– response linear trend was supported by a non-significant quadratic trend (p = 0.67) and a significant linear trend (p = 0.009). For every 100 min per week of exercise, a 9.7 % [95 % CI 0.02, 16.4] reduction in BPE was estimated. At follow-up, the control group exhibited a 20.4 % increase, compared to −7.7 % and −11.6 % decreases in the low- and high-dose groups, respectively. Adding body fat to the models that evaluated MR imaging did not alter these findings.
Comment
A 5-month aerobic exercise intervention among pre-menopausal women at elevated breast cancer risk resulted in significant linear dose–response reductions in follicular phase estrogen and hormonally sensitive breast tissue, measured by imaging. High-risk women may ask clinicians whether there is anything beyond drugs or surgery that may reduce breast cancer risk. The results herein are relevant to that discussion.
The pattern of results differed across the estrogen and imaging outcomes. Estrogen and hormonally sensitive breast tissue increased in the control group. However, estrogen stayed the same in the intervention groups, while hormonally sensitive breast tissue decreased in a linear fashion in the low- and high-dose exercise groups. To our knowledge this trial is the first to document such increases over time in estrogens and hormonally sensitive breast tissue in high risk women. Future research is needed to confirm and explain this pattern. One testable hypothesis is that the exercise intervention had a more direct effect on breast tissue (as assessed by imaging) than on systemic estrogens.
Ours is the first dose–response exercise intervention trial to assess effects on endogenous sex hormone exposure among premenopausal women. In contrast to our results, a prior study of 150 min/week of exercise over four menstrual cycles did not result in reductions of estrone or estradiol, as measured from three 24 h urine collections in the follicular phase [40]. Our findings on estrogen exposure concur with prior observational studies in premenopausal women [41]. A key innovation of our trial was use of daily hormonal data collection to assess AUC for hormonal exposure [32, 42]. Data from the Nurses Health Study indicate 11 % higher urinary estrogens among premenopausal women who developed breast cancer than women who did not develop breast cancer [43]. This is similar to the difference in percent change in urinary estrogens when comparing the low (13.1 %)- and high (11.4 %)-dose exercise groups, respectively, to the control group.
BPE is strongly predictive of breast cancer risk [16, 18] and is more sensitive than mammographic density. Another innovation of our study is the fully automated BPE measurements, which alleviate the subjectivity of visual assessment [16, 20, 44]. Prior studies have used a qualitative approach to analyzing BPE when characterizing the relationship with breast cancer risk. This challenges direct comparison of our results to those prior studies. However, it is plausible that the difference in the percent change of 12.7 and 32 % from pre-to-post intervention in BPE, we observed between the control group and the low- and high-dose groups, respectively, represent at least a quintile category shift of BPE data [16, 20], which has been associated with breast cancer risk odds ratios ranging from 3.3 up to 10.1 [16].
Strengths of WISER Sister include the high exercise adherence, high proportion of subjects with full follow-up, daily hormone measurements, study duration, and national recruitment. Limitations include that MR imaging was completed in a subset of participants. Further, there were four women whose baseline urine samples were insufficient for analysis.
In conclusion, we observed a dose–response effect of aerobic exercise training on estrogen and breast tissue, assessed by imaging. Future research should explore maintenance of effects and extent to which findings are repeatable in lower risk women. The high quality measurements of endogenous hormone exposure used in WISER Sister need to be applied to a lower risk population to discern whether effects of exercise on estrogens might partly explain the well-documented epidemiologic association of exercise and reduced breast cancer risk [45]. While the relationship between exercise and pre-menopausal breast cancer is not as clear as for exercise and post-menopausal cancer, it is biologically plausible that hormonal exposures prior to menopause are relevant to post-menopausal breast cancer risk. Clinicians have conversations with young women every day that include providing options for interventions to reduce elevated breast cancer risk. Women at elevated risk should be guided to include at least 150 and up to 300 min per week of aerobic exercise to reduce estrogen exposure and hormonally sensitive breast tissue.
Acknowledgments
The authors wish to acknowledge the advocacy organizations that assisted with recruitment for this study, principally FORCE (Facing Our Risk of Cancer Empowered), the study participants, and the National Institutes of Health/National Cancer Institute for Grant R01-CA131333. The work of Kelsey Pears and Ellen Bingham is gratefully acknowledged. This work was also supported by discounts for treadmills from Smooth Fitness, Inc., King of Prussia, PA. The project described was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003. Several study investigators were supported by the Basser Center at the University of Pennsylvania’s Abramson Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors have no financial relationship with the organization that sponsored the research.
Compliance with ethical standards
Registered through ClinicalTrials.Gov (http://clinicaltrials.gov/ct2/home) Registration number NCT00892515.
Footnotes
Conflict of interest The authors declare they have no conflict of interest.
References
- 1.Metcalfe KA, Goel V, Lickley L, Semple J, Narod SA (2002) Prophylactic bilateral mastectomy: patterns of practice. Cancer 95(2):236–242 [DOI] [PubMed] [Google Scholar]
- 2.Wainberg S, Husted J (2004) Utilization of screening and preventive surgery among unaffected carriers of a BRCA1 or BRCA2 gene mutation. Cancer Epidemiol Biomarkers Prev 13(12):1989– 1995 [PubMed] [Google Scholar]
- 3.Uyei A, Peterson SK, Erlichman J, Broglio K, Yekell S, Schmeler K, Lu K, Meric-Bernstam F, Amos C, Strong L et al. (2006) Association between clinical characteristics and risk-reduction interventions in women who underwent BRCA1 and BRCA2 testing: a single-institution study. Cancer 107(12):2745–2751 [DOI] [PubMed] [Google Scholar]
- 4.Narod SA (2006) Modifiers of risk of hereditary breast cancer. Oncogene 25(43):5832–5836 [DOI] [PubMed] [Google Scholar]
- 5.Eccles DM (2004) Hereditary cancer: guidelines in clinical practice. Breast and ovarian cancer genetics. Ann Oncol 15(Suppl 4:iv):133–138 [DOI] [PubMed] [Google Scholar]
- 6.Cooper BT, Murphy JO, Sacchini V, Formenti SC (2013) Local approaches to hereditary breast cancer. Ann Oncol 24(Suppl 8):viii54–viii60 [DOI] [PubMed] [Google Scholar]
- 7.King MC, Marks JH, Mandell JB (2003) New York Breast Cancer Study G: Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302(5645):643–646 [DOI] [PubMed] [Google Scholar]
- 8.Kotsopoulos J, Olopado OI, Ghadirian P, Lubinski J, Lynch HT, Isaacs C, Weber B, Kim-Sing C, Ainsworth P, Foulkes WD et al. (2005) Changes in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res 7(5): R833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colilla S, Kantoff PW, Neuhausen SL, Godwin AK, Daly MB, Narod SA, Garber JE, Lynch HT, Brown M, Weber BL et al. (2006) The joint effect of smoking and AIB1 on breast cancer risk in BRCA1 mutation carriers. Carcinogenesis 27(3):599–605 [DOI] [PubMed] [Google Scholar]
- 10.Genuth SM (1993) The endocrine system In: Berne RM, Levy MN (eds) Physiology, 3rd edn. Mosby Year Book, Philadelphia, pp 813–1024 [Google Scholar]
- 11.Ismail PM, Amato P, Soyal SM, DeMayo FJ, Conneely OM, O’Malley BW, Lydon JP (2003) Progesterone involvement in breast development and tumorigenesis—as revealed by progesterone receptor “knockout” and “knockin” mouse models. Steroids 68(10–13):779–787 [DOI] [PubMed] [Google Scholar]
- 12.Micheli A, Muti P, Secreto G, Krogh V, Meneghini E, Venturelli E, Sieri S, Pala V, Berrino F (2004) Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer 112(2):312–318 [DOI] [PubMed] [Google Scholar]
- 13.Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H et al. (2005) Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 97(10):755–765 [DOI] [PubMed] [Google Scholar]
- 14.Kossman DA, Williams NI, Domchek SM, Kurzer MS, Stopfer JE, Schmitz KH (2011) Exercise lowers estrogen and progesterone levels in premenopausal women at high risk of breast cancer. J Appl Physiol 111(6):1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AJ, Phipps WR, Arikawa AY, O’Dougherty M, Kaufman B, Thomas W, Schmitz KH, Kurzer MS (2011) Effects of aerobic exercise on premenopausal sex hormone levels: results of the WISER study, a randomized clinical trial in healthy, sedentary, eumenorrheic women. Cancer Epidemiol Biomarkers Prev 20(6):1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA (2011) Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 260(1):50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price ER, Brooks JD, Watson EJ, Brennan SB, Comen EA, Morris EA (2014) The impact of bilateral salpingo-oophorectomy on breast MRI background parenchymal enhancement and fibroglandular tissue. Eur Radiol 24(1):162–168 [DOI] [PubMed] [Google Scholar]
- 18.Pike MC, Pearce CL (2013) Mammographic density, MRI background parenchymal enhancement and breast cancer risk. Ann Oncol 24(Suppl 8):viii37–viii41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA (2012) Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol 22(12):2641–2647 [DOI] [PubMed] [Google Scholar]
- 20.DeLeo MDS, Kontos D, Conant E, Chen J, Weinstein S (2015) Breast MRI fibroglandular volume and parenchymal enhancement in BRCA1 and BRCA2 mutation carriers before and immediately after risk-reducing salpingooophorectomy. Am J Roentgenol 3:669–673 [DOI] [PubMed] [Google Scholar]
- 21.Weinstein S, Rosen M (2010) Breast MR imaging: current indications and advanced imaging techniques. Radiol Clin North Am 48(5):1013–1042 [DOI] [PubMed] [Google Scholar]
- 22.Anton-Culver H, Ziogas A, Bowen D, Finkelstein D, Griffin C, Hanson J, Isaacs C, Kasten-Sportes C, Mineau G, Nadkarni P et al. (2003) The cancer genetics network: recruitment results and pilot studies. Community Genet 6(3):171–177 [DOI] [PubMed] [Google Scholar]
- 23.Claus EB, Risch N, Thompson WD (1994) Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 73(3):643–651 [DOI] [PubMed] [Google Scholar]
- 24.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81(24):1879–1886 [DOI] [PubMed] [Google Scholar]
- 25.Howlader N, Noone NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (2014) SEER cancer statistics review, 1975–2011 National Cancer Institute, Bethesda [Google Scholar]
- 26.Stice E, Telch CF, Rizvi SL (2000) Development and validation of the Eating Disorder Diagnostic Scale: a brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess 12(2):123–131 [DOI] [PubMed] [Google Scholar]
- 27.Schmitz KH, Williams NI, Kontos D, Kurzer MS, Schnall M, Domchek S, Stopfer J, Galantino ML, Hwang WT, Morales K et al. (2015) Women In Steady Exercise Research (WISER) Sister: study design and methods. Contemp Clin Trials 41C:17–30 [DOI] [PubMed] [Google Scholar]
- 28.Bruce RA, Kusumi F, Hosmer D (1973) Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 85(4):546–562 [DOI] [PubMed] [Google Scholar]
- 29.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH (1990) Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 13(4):401–411 [DOI] [PubMed] [Google Scholar]
- 30.Munro C, Stbenfeldt G, Cragun J, Addiego L, Overstreet J, Lasley B (1991) Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their majority urinary metabolites as measured by enzyme immunoassay and radioassay. Clin Chem 37:838–844 [PubMed] [Google Scholar]
- 31.Beitins IZ, McArthur JW, Turnbull BA, Skrinar GS, Bullen BA (1991) Exercise induces two types of human luteal dysfunction: confirmation by urinary free progesterone. J Clin Endocrinol Metab 72(6):1350–1358 [DOI] [PubMed] [Google Scholar]
- 32.Williams NI, Reed JL, Leidy HJ, Legro RS, De Souza MJ (2010) Estrogen and progesterone exposure is reduced in response to energy deficiency in women aged 25–40 years. Hum Reprod 25(9):2328–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boston RC, Schnall MD, Englander SA, Landis JR, Moate PJ (2005) Estimation of the content of fat and parenchyma in breast tissue using MRI T1 histograms and phantoms. Magn Reson Imaging 23(4):591–599 [DOI] [PubMed] [Google Scholar]
- 34.Schmitz KH, Williams NI, Kontos D, Kurzer M, Schnall M, Domchek S, Stopfer J, Galantino ML, Hwang W, Morales K et al. (2015) Women In Steady Exercise Research (WISER) Sister: study design and methods. Contemp Clin Trials 41:17–30 [DOI] [PubMed] [Google Scholar]
- 35.Wu S, Weinstein SP, Conant EF, Schnall MD, Kontos D (2013) Automated chest wall line detection for whole-breast segmentation in sagittal breast MR images. Med Phys 40(4):042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, Weinstein SP, Conant EF, Kontos D (2013) Fully-auto-mated fibroglandular tissue segmentation and volumetric density estimation in breast MRI by integrating a continuous max-flow model and a likelihood atlas. In: Novak C, Aylward S (eds) SPIE medical imaging: computer aided diagnosis, vol. 8670 Orlando, FL: 86701C [Google Scholar]
- 37.Wu S, Weinstein S, Kontos D (2012) Atlas-based probabilistic fibroglandular tissue segmentation in breast MRI. Med Image Comput Comput Assist Interv 15(Pt 2):437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S, Weinstein SP, Conant EF, Kontos D (2013) Quantitative background parenchynal enhancement estimation on breast DCE-MRI by measuring relative voxel-wise enhancement. In: International Society for Magnetic Resonance in Medicine Annual Meeting. Salt Lake City, UT [Google Scholar]
- 39.Cohen J (1992) Statistical power analysis in the behavioral sciences, 2nd edn. Lawrence Erlbaum, Hillsdale [Google Scholar]
- 40.Smith AJ, Phipps WR, Thomas W, Schmitz KH, Kurzer MS (2013) The effects of aerobic exercise on estrogen metabolism in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev 22(5):756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews CE, Fortner RT, Xu X, Hankinson SE, Eliassen AH, Ziegler RG (2012) Association between physical activity and urinary estrogens and estrogen metabolites in premenopausal women. J Clin Endocrinol Metabol 97(10):3724–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL (2001) Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab 86(11):5184–5193 [DOI] [PubMed] [Google Scholar]
- 43.Eliassen AH, Spiegelman D, Xu X, Keefer LK, Veenstra TD, Barbieri RL, Willett WC, Hankinson SE, Ziegler RG (2012) Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res 72(3):696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melsaether A, McDermott M, Gupta D, Pysarenko K, Shaylor SD, Moy L (2014) Inter- and intrareader agreement for categorization of background parenchymal enhancement at baseline and after training. AJR Am J Roentgenol 203(1):209–215 [DOI] [PubMed] [Google Scholar]
- 45.Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, van Leeuwen FE (2007) Physical activity and breast cancer: a systematic review. Epidemiology 18(1):137–157 [DOI] [PubMed] [Google Scholar]