Abstract
Objective
Long-term abstinence can be undermined by cessation fatigue-an exhaustion of coping resources due to quitting smoking/staying quit. The current study examines the predictive validity of a Cessation Fatigue Scale (CFS; three subscales). Among current smokers, we hypothesized higher fatigue would predict longer latency to both quit initiation and achieving 7-day point prevalence abstinence (7-day PPA). Among recent quitters, we expected higher cessation fatigue would confer greater lapse/relapse risk. Lower rates of abstinence at 2-month follow-up were expected for those with higher fatigue.
Method
Current smokers motivated to quit in the next month (n=301) and recent quitters (n=242) were assessed biweekly over the course of two months. Retention rates were high (>85%). Cox and logistic regression analyses tested hypotheses.
Results
Among smokers, greater emotional exhaustion predicted longer delay to achieving 7-day PPA (HR=.53, 95% CI=.40–.68, p<.001) and lower likelihood of 7-day PPA at 2-month follow-up (OR=.27, 95% CI=.16–.46, p<.001), even after controlling for nicotine dependence and motivation to quit. Among recent quitters, emotional exhaustion progressively increased over the first six weeks since quit initiation. Elevated exhaustion was associated with greater lapse (HR=1.65, 95% CI=1.06–2.56, p<.05) and relapse (HR=2.33, 95% CI=1.37–3.97, p<.01) risk, and lower likelihood of 7-day PPA at 2-month follow-up (OR=.39, 95% CI=.16–.94, p<.05), even after controlling for nicotine withdrawal and motivation to quit.
Conclusions
Cessation fatigue, as measured by the CFS’s emotional exhaustion subscale, prospectively predicted important cessation milestones. Findings suggest cessation fatigue is a novel process that undermines smoking cessation and a viable target for intervention.
Keywords: quitting, relapse, smoking cessation, nicotine dependence, cessation fatigue
Despite advances in smoking cessation treatments and tobacco control efforts, 45% of smokers do not make a quit attempt in a given year (Lavinghouze, Malarcher et al. 2015). Among those who make a quit attempt, relapse is the most likely outcome even when the most effective smoking cessation treatments are used (Cahill, Stevens et al. 2013). Clinicians and researchers need a better understanding of the processes that undermine cessation milestones at each stage of the quit attempt (Shiffman, Scharf et al. 2006): initial attempts to quit, lapse (i.e., initial smoking after quitting), and relapse (i.e., progression to regular smoking). Identifying factors that have predictive validity across all phases of the quitting process will offer novel therapeutic targets to optimize treatment development (Baker, Piper et al. 2016).
Cessation fatigue, or tiredness of trying to quit smoking, is one promising individual difference characteristic that has received limited empirical attention to date (Piper 2015). Cessation fatigue was first conceptualized over 15 years ago as a latent construct comprising loss of motivation to quit, loss of hope in cessation success, reduced coping skills utilization, decreased self-efficacy, and exhaustion of self-control resources (Piasecki, Fiore et al. 2002). Drawing from the large body of self-control literature (Muraven and Baumeister 2000), cessation fatigue was posited to increase over the course of a quit attempt as the cumulative toll of remaining abstinent depletes an individual’s coping resources. It was theorized to increase vulnerability to relapse, particularly in the weeks or months after a quit attempt, once acute withdrawal symptoms had abated.
To date, two studies have prospectively examined cessation fatigue among smokers undergoing a quit attempt. One study examined a single-item measure of cessation fatigue, “I am tired of trying to quit smoking,” within a large clinical trial (Liu, Li et al. 2013). Cessation fatigue increased over the first two weeks of a quit attempt, was positively associated with craving and negative affect, was reduced by pharmacotherapy intervention, and importantly, was negatively associated with abstinence at 6-month follow-up. Using this same single-item measure of cessation fatigue, a second investigation found that smokers who had a history of anxiety disorders, relative to those who did not, had greater increases in cessation fatigue during the week leading up to a quit attempt and the week after the quit day (Piper, Cook et al. 2011). The authors concluded that anxiety might limit coping resources and resilience in the face of acute nicotine withdrawal, leading to greater fatigue and relapse vulnerability. This interpretation is consistent with a workload-capacity model, as presented below. Though these initial findings suggest that cessation fatigue may be strongly associated with important treatment outcomes, their significance is limited by reliance on a single-item measure. A multi-item cessation fatigue scale would be less susceptible to random measurement error, allow for computation of internal consistency reliability, and take into account other cognitive and emotional factors that may comprise cessation fatigue.
Building on the foundational work of others, Mathew and colleagues (2017) developed a 17-item Cessation Fatigue Scale (CFS), which showed strong psychometric properties among 484 smokers who had relapsed within the past 30 days. Exploratory factor analysis identified three CFS factors: 1) emotional exhaustion (i.e., negative emotional impact due to quitting/burnout), 2) pessimism (i.e., disbelief in skills/capacity to quit), and 3) devaluation (i.e., the notion that quitting is not important). Higher CFS scores were associated with greater nicotine withdrawal severity and difficulty quitting, and lower self-efficacy, abstinence-related motivational engagement, and quit intentions. Modest associations between cessation fatigue and motivation to quit suggested these constructs were overlapping, but distinct. Findings were limited by their cross-sectional nature but suggest cessation fatigue is relevant to smokers not in the midst of a quit attempt, and may uniquely predict future quitting. An important next step is to test the predictive validity of the CFS in both current smokers and recent quitters. Presumably, cessation fatigue will reduce the likelihood of both initiating and maintaining quit attempt, analogous to the role physical fatigue plays with initiating and maintaining exercise engagement.
The purpose of the current study was to examine cessation fatigue as a prospective predictor of engaging in a quit attempt (any duration), achieving 7-day point prevalence abstinence (7-day PPA), lapse, and relapse. To assess fatigue in relation to each of these milestones our study comprised two groups: current smokers with immediate interest in quitting, and recent quitters (1–5 weeks abstinent). Both samples were assessed longitudinally over the course of two months. Among current smokers, we hypothesized that higher cessation fatigue would predict longer latency to quit initiation and achieving 7-day PPA. Among recent quitters, we expected that higher cessation fatigue would confer greater lapse/relapse risk. Across both groups, lower rates of abstinence at the final follow-up were expected for those with higher fatigue.
Secondarily, we assessed construct validity through examination of psychosocial variables that may have exacerbated or attenuated cessation fatigue. Variables examined were informed by a workload-capacity model for patients with chronic health conditions, such as tobacco use disorder (Heckman, Mathew, & Carpenter, 2015). Workload is comprised of demands associated with quitting and general life demands, while capacity refers to coping resources. Fatigue levels are expected to intensify as workload exceeds capacity, and consequentially treatment outcomes become compromised. We hypothesized that cessation fatigue would be positively associated with smoking-specific (e.g., withdrawal severity) and general (e.g., stress, depression, anxiety) workload factors, and negatively associated with capacity factors (e.g., resilience, distress tolerance). That is, those with higher coping demands or lower coping resources are expected to be more susceptible to cessation fatigue.
Finally, time-varying effects models (TVEMs) were used to assess the time course and the naturalistic trajectory of cessation fatigue during a quit attempt. This flexible approach can be used to identify how relapse risk factors change over the course of quit attempt (i.e., early vs. later in quit attempt) because they take into account temporal dynamics of the risk factor and covariation with other variables in flux. TVEMs have proved useful for understanding craving (Lanza, Vasilenko et al. 2014) and smoking (Vasilenko, Piper et al. 2014). The current study uses TVEMs to examine how cessation fatigue and its association with nicotine withdrawal unfold over 12-weeks of a quit attempt. We hypothesized fatigue would increase over time, as expected by Piasecki’s (2002) account. This discovery is especially important, as most relapse risk factors dissipate during this time frame (e.g., withdrawal). Given that withdrawal symptoms last approximately 2–4 weeks (Hughes 2007), we expected the influence of withdrawal on cessation fatigue would diminish over the initial 12-weeks of a quit attempt.
Method
Participants
Participants were recruited nationwide via ads posted on Craigslist and ResearchMatch, with embedded links to an online survey. Participants were eligible for the study if they were between the ages 18–65, fluent in English, and met criteria for one of two groups: current smokers or recent quitters. Current smokers (n=301) smoked at least 25 days out of the last 30, at least 10 cigarettes per day, and reported a 7 or higher on a 0–10 scale of intent to quit smoking within the next 30 days. Recent quitters (n=242) smoked at least 25 days per month and at least 10 cigarettes per day prior to their quit date. We stratified by time since quit date, with at least 50 participants who had quit for 1–2, 2–3, 3–4, and 4–5 weeks, to create a balanced accelerated longitudinal cohort study design (Galbraith, Bowden et al. 2014). The consistent overlap between successive cohorts allowed us to model recent quitters’ cessation fatigue over 12 weeks even though assessments were completed over eight weeks.
Procedures
Following initial assessment, eligible participants were re-contacted by email every two weeks over an 8-week follow-up period. Retention was high overall, with completion rates ranging from 86–94% across assessment time points. Study data were collected and managed using REDCap electronic data capture tools (Harris, Taylor et al. 2009). Participants were compensated up to $50 in electronic gift cards for assessment completion. Upon study completion, participants were debriefed and provided with contact information for a toll-free, national smoking cessation quitline. The Institutional Review Board at the Medical University of South Carolina approved all procedures.
Measures
The following self-report measures were completed at the baseline study assessment. Cessation milestones were assessed at each follow-up time point (i.e., weeks 2, 4, 6, and 8). Cronbach’s coefficient alpha values from the current study are presented for both current smokers and recent quitters (current/quitters).
Participant Characteristics
Demographic and smoking history information was collected, including electronic cigarette use in the past seven days. Nicotine dependence was assessed with the Heaviness of Smoking Index (Heatherton, Kozlowski et al. 1989; HSI).
Cessation Fatigue Scale (CFS)
The wording of the original CFS was specific to current smokers (Mathew, Heckman et al. 2017). To improve relevance to both current and recent quitters the original 17-item CFS was slightly modified so that all items referred to quitting smoking or staying quit (see Supplemental Table 1). Item responses ranged from 1 (Strongly Disagree) to 5 (Strongly Agree), some of which were reverse coded prior to computing subscale scores. Subscale scores are averaged such that higher scores indicate greater fatigue.
Confirmatory factor analyses (CFA) were conducted on the CFS using the maximum likelihood estimation method via AMOS. Data were fit to 1-factor and 3-factor models. Better fit for the 1-factor model would suggest the CFS was unidimensional, whereas better fit for the 3-factor model would support our prior work that the CFS was multidimensional. Models were evaluated based on three fit indices (Jackson, Gillaspy et al. 2009): the comparative fit index (CFI), the root mean square error of approximation (RMSEA), and the standardized root mean square residual (SRMR). Good model fit is indicated by values of CFI >.90, RMSEA <.08, and SRMR <.08. Fit indices were best for the 3-factor model (CFI=.84; RMSEA=.079; SRMR=.074). We also examined standardized factor loadings, with those <.40 indicative of poor item fit and candidates for trimming. Fit indices for the 3-factor model were improved (CFI=.87; RMSEA=.077; SRMR=.071) after trimming two items (8 and 16) that had low loadings (see Supplemental Table 1).
Internal consistency was computed for each subscale and adequate reliability was observed for both the emotional exhaustion (α=.74/.86) and devaluation (α=.80/.72) subscales. Due to low internal consistency within the pessimism scale (α=.63/.59), it was omitted from further analyses. Pessimism as a part of cessation fatigue should perhaps be considered exploratory for future research. Emotional exhaustion was positively correlated with devaluation in both the current smoker (r=.16, p<.01) and recent quitter (r=.27, p<.001) samples.
Construct Validity
We assessed several constructs hypothesized to contribute to cessation fatigue because they may impose additional demands on coping resources. Current craving was assessed with the 4-item Questionnaire on Smoking Urges (Carter and Tiffany 2001; α = .90/.95). Current negative affect was assessed via the Mood Form (Diener, Larsen et al. 1984; α = .85/.92). Past-24 hour nicotine withdrawal was assessed with the 9-item version of the Minnesota Nicotine Withdrawal Scale (Hughes and Hatsukami 1986; α = .85/.95). Depressive symptoms over the past two weeks were assessed with the 8-item Patient Health Questionnaire (Kroenke, Strine et al. 2009; α = .88/.96). Social anxiety symptoms over the past two weeks was assessed with the 3-item Social Phobia Inventory (Connor, Kobak et al. 2001; α = .81/.94).
The following measures represent constructs that may enhance coping resources, and therefore buffer against cessation fatigue. Current positive affect was assessed via the Mood Form (Diener, Larsen et al. 1984; α = .78/.84). Distress tolerance was assessed with the 15-item Distress Tolerance Scale (Simons and Gaher 2005; α = .89/.91). Resilience was assessed with the 6-item Brief Resilience Scale (Smith, Dalen et al. 2008; α = .77/.77).
Lastly, the following measures represent cessation-related processes that may be associated with cessation fatigue. The 5-item Abstinence-Related Motivational Engagement short-form scale (ARME) assessed current efforts towards abstinence (Simmons, Heckman et al. 2010; α = .64/.76). Confidence (i.e., self-efficacy) and intentions to quit or stay quit over the next 30 days were assessed with a single item (0–10) Contemplation Ladder (Biener and Abrams 1991).
Cessation Milestones
At each follow-up assessment participants indicated whether they had smoked any cigarettes (even a puff) since last completing the survey (date of last survey provided). This single-item approach has shown strong concordance with timeline follow-back methods (Bernstein, Rosner et al. 2016). Those who reported any smoking were asked how many times they tried to quit smoking (i.e., quit attempt) since the last survey, number of days smoked over the past two weeks, and when they last smoked. For current smokers, the primary dependent variables were latency to initiating a quit attempt and latency to 7-day PPA, defined as self-reported abstinence for the preceding seven days at each follow-up (Hughes, Keely et al. 2003). For recent quitters, the primary dependent variables were latency to lapse (first instance of smoking/puff) and latency to relapse (seven consecutive days of smoking) (Ossip-Klein, Bigelow et al. 1986). We also examined 7-day PPA at the 2-month follow-up for both groups.
Analytic Strategy
Construct Validity
Pearson’s correlations and t-tests were used to examine bivariate relationships among baseline CFS subscales and relevant baseline measures. Correlations of .1, .3, and .5 represent small, medium, and large effect sizes, respectively (Cohen 1988).
Trajectories
Recent quitters’ cessation fatigue trajectories were modeled over the first three months since quit initiation, with week as the unit of time. Because we stratified recruitment of recent quitters by time since quit initiation (i.e., 1–2, 2–3, 3–4, and 4–5 weeks), these data reflect a balanced accelerated longitudinal cohort study design (Galbraith, Bowden et al. 2014). That is, recent quitters completed assessments over eight weeks, with consistent overlap between successive cohorts. To aid in comparability across studies, we used a similar analytic approach (time-varying effects models; TVEMs) and covariates (withdrawal and occurrence of a lapse since last assessment) as reported in prior study of post-quit cessation fatigue trajectories (Liu, Li et al. 2013). TVEMs provide a powerful tool to examine time-varying trends (i.e., early vs. later in quit attempt) and how relations with covariates change over time (Shiyko, Lanza et al. 2012). TVEMs have been used to examine the temporal dynamics of craving (Lanza, Vasilenko et al. 2014) and smoking (Vasilenko, Piper et al. 2014). Analyses were conducted via SAS 9.4 based on the macro (%TVEM version 3.1.0; p-spline method) available at http://www.methodology.psu.edu.
Predictive Validity
Cox regression survival analyses were used to test baseline CFS subscales as predictors of cessation milestones, with week as the unit of time and missing observations due to attrition treated as censored (Hughes, Keely et al. 2003). Outcomes for current smokers included making a quit attempt and 7-day PPA. Outcomes for recent quitters included lapse and relapse. Hazard ratios are presented to indicate the risk of making a quit attempt, reaching 7-day PPA, lapsing, and relapsing for every additional point on the CFS. Logistic regression was used to examine 7-day PPA at the 2-month follow-up for both current and recent quitters. Intent-to-to treat and per-protocol results were similar; the latter is reported below.
Across all outcomes, unadjusted and adjusted models were tested. Given that lack of multicollinearity (along with proportional hazards) is an assumption for properly conducting cox models, we elected to include as many variables as possible until collinearity diagnostics indicated otherwise. Variance inflation factors (VIFs) greater than 2.5 (or tolerances less than .40) were considered problematic (Allison 2012), and covariates were included if this threshold was maintained. We started with the most common predictors (e.g., dependence/withdrawal, motivation to quit) that have been found to be consistently associated with cessation outcomes, as identified by a systematic review of population-based studies (Vangeli, Stapleton et al. 2011). Confidence to quit or stay quit (i.e., self-efficacy) was explored initially, but we removed it from the final models because VIFs exceeded 3 when it was included. As would be expected, there were strong correlations among many of the other covariates. For example, withdrawal symptoms are inclusive of craving (r=.72) and negative affect (r=.83), and withdrawal severity was strongly associated with depression (r=.89) and anxiety severity (r=.71). Including all variables in our analyses led to multicollinearity and overfitting issues. To avoid redundancy, we elected to only include withdrawal in our final models because withdrawal encompassed both craving and negative affect. Adjusted models for current smokers included nicotine dependence and motivation to quit, as measured by the single-item intention scale and ARME. Adjusted models for recent quitters included time since quit initiation, nicotine withdrawal, and motivation to stay quit measures. We also tested adjusted models that included demographics (e.g., age, sex, ethnicity) and negative emotional states (e.g., depression, anxiety), but the more parsimonious models are presented because the pattern of results were similar. Analyses were conducted via SPSS v24, with traditional significance levels set at p<.05 (two-tailed).
Results
Participant Characteristics
Table 1 displays the characteristics of the current smoker (n=301) and recent quitter (n=242) samples, and their bivariate associations with CFS emotional exhaustion and devaluation subscale scores.
Table 1.
Participant Characteristics and Bivariate Associations with Cessation Fatigue Subscales
| Variable | Current Smokers (n=301) | Recent Quitters (n =242) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Emotional Exhaustion | Devaluation | Emotional Exhaustion | Devaluation | |||
|
| ||||||
| M (SD) | r | r | M (SD) | r | r | |
| Age | 35.1 (9.5) | −.17** | −.11 | 33.5 (7.5) | −.16* | −.14* |
| CPD | 18.6 (5.8) | .35*** | .06 | 20.5 (7.6) | .57** | .19** |
| Dependence | 2.9 (1.1) | .13* | −.28*** | 3.5 (.9) | .02 | −.06 |
|
|
||||||
| n (%) | M (SD) | M (SD) | n (%) | M (SD) | M (SD) | |
|
|
||||||
| Sex | ns | ns | *** | *** | ||
| Male | 207 (68.8) | 3.0 (.6) | 2.1 (.7) | 185 (76.4) | 3.2 (.7) | 2.2 (.6) |
| Female | 94 (31.2) | 2.8 (.7) | 2.1 (.9) | 57 (23.6) | 2.5 (.8) | 1.7 (.7) |
| Race | ** | ns | *** | ns | ||
| Non-White | 85 (28.2) | 2.8 (.7) | 2.2 (.9) | 45 (18.6) | 2.6 (.8) | 2.0 (.8) |
| White | 216 (71.8) | 3.0 (.6) | 2.1 (.7) | 197 (81.4) | 3.1 (.8) | 2.1 (.6) |
| Rx Status | ** | ns | *** | ** | ||
| Single | 97 (32.2) | 2.8 (.7) | 2.1 (.9) | 85 (35.1) | 2.5 (.7) | 1.9 (.7) |
| In Rx | 204 (67.8) | 3.0 (.6) | 2.1 (.7) | 157 (64.9) | 3.3 (.7) | 2.1 (.6) |
| Education | ns | *** | ns | ns | ||
| ≤HS | 68 (22.6) | 3.0 (.5) | 2.5 (.8) | 21 (8.7) | 2.8 (.8) | 2.3 (.8) |
| >HS | 233 (77.4) | 2.9 (.7) | 2.0 (.7) | 221 (91.3) | 3.0 (.8) | 2.0 (.6) |
| Employed | ns | ** | * | * | ||
| Unemployed | 41 (13.6) | 2.8 (.8) | 1.8 (.8) | 9 (3.7) | 2.5 (.7) | 1.5 (.8) |
| ≥part-time | 260 (86.4) | 3.0 (.6) | 2.2 (.7) | 233 (96.3) | 3.0 (.8) | 2.1 (.6) |
| Income | *** | *** | *** | * | ||
| <$40,000 | 142 (47.2) | 2.7 (.6) | 1.8 (.6) | 65 (26.9) | 2.5 (.7) | 1.9 (.6) |
| ≥$40,000 | 157 (52.2) | 3.1 (.6) | 2.4 (.7) | 176 (72.7) | 3.2 (.7) | 2.1 (.7) |
| DK | 2 (.7) | - | - | 1 (.4) | - | - |
| QA in past year | ns | *** | *** | ** | ||
| No | 230 (76.4) | 2.9 (.6) | 2.2 (.7) | 141 (58.3) | 3.3 (.7) | 2.1 (.6) |
| Yes | 70 (23.3) | 3.0 (.8) | 1.7 (.7) | 81 (41.7) | 2.6 (.8) | 1.9 (.7) |
| EC use in past 7 days | *** | ns | *** | ns | ||
| No | 226 (75.1) | 2.8 (.6) | 2.1 (.7) | 191 (78.9) | 2.8 (.8) | 2.0 (.7) |
| Yes | 75 (24.9) | 3.2 (.6) | 2.2 (.8) | 51 (21.1) | 3.6 (.6) | 2.0 (.5) |
Note. ns=nonsignificant; CPD=cigarettes per day; Rx=relationship; HS=high school; DK=don’t know; QA=quit attempt; EC=electronic cigarette; Emotional exhaustion and devaluation scores can range from 1–5.
p<.05;
p<.01;
p<.001
Current Smokers
Bivariate analyses indicated greater emotional exhaustion was associated with younger age and with greater CPD and nicotine dependence. Emotional exhaustion scores were higher among those who reported being White, were in a relationship, had higher income, and had used an electronic cigarette within the past seven days. When participant characteristics were entered simultaneously into a multi-predictor analysis (ANOVA) exhaustion was predicted by age (F(1, 287)=8.86, p=.003), CPD (F(1, 287)=18.60, p<.001), relationship status (F(1, 287)=5.64, p=.02), income (F(2, 287)=2.66, p<.001), and electronic cigarette use (F(1, 287)=8.89, p=.003). Bivariate analyses found greater devaluation was associated with lower nicotine dependence. Devaluation scores were higher among those who reported lower education, were currently employed, had higher income, and had not made a quit attempt in the past year. Age (F(1, 287)=9.73, p=.002), CPD (F(1, 287)=15.77, p<.001), nicotine dependence (F(1, 287)=27.41, p<.001), sex (F(1, 287)=4.36, p=.04), education (F(1, 287)=24.49, p<.001), income (F(2, 287)=11.43, p<.001), and quit attempt in the past year (F(1, 287)=15.76, p<.001) predicted devaluation in multi-predictor analyses.
Recent Quitters
Bivariate analyses showed greater emotional exhaustion was associated with younger age and with greater pre-quit date CPD. Emotional exhaustion scores were higher among those who were male, reported being white, were in a relationship, were employed, had higher income, had not made a previous quit attempt in the past year, and had used an electronic cigarette within the past seven days. When participant characteristics were entered simultaneously into a multi-predictor analysis (ANOVA) exhaustion was predicted by age (F(1, 229)=7.99, p=.005), CPD (F(1, 229)=33.25, p<.001), nicotine dependence (F(1, 229)=11.38, p=.001), income (F(2, 229)=3.31, p=.04), and electronic cigarette use (F(1, 229)=16.15, p<.001). The same pattern of bivariate results was observed for devaluation, with the exception of non-significant relationships for race and electronic cigarette use. However, only sex (F(1, 229)=17.09, p<.001) and education (F(1, 229)=8.69, p=.001) were significant predictors of devaluation in multi-predictor analyses.
Construct Validity
Table 2 reveals bivariate associations between CFS subscale scores and relevant psychosocial variables. Patterns of association differed by subscale, but were largely consistent across samples.
Table 2.
Bivariate Correlations between Cessation Fatigue Subscales and Putatively Related Constructs
| Variable | Current Smokers (n=301) | Recent Quitters (n=242) | ||
|---|---|---|---|---|
|
| ||||
| Emotional Exhaustion | Devaluation | Emotional Exhaustion | Devaluation | |
| Craving | .37*** | .11 | .71*** | .12 |
| Negative Affect | .52*** | .14* | .67*** | .06 |
| Nicotine Withdrawal | .52*** | −.11 | .66*** | −.07 |
| Depression | .57*** | .12* | .67*** | .02 |
| Social Anxiety | .36*** | .09 | .70*** | .26*** |
| Positive Affect | −.03 | .13* | −.11 | −.13* |
| Distress Tolerance | −.44*** | .19** | −.65*** | −.18** |
| Resilience | −.53*** | −.28*** | −.47*** | −.27*** |
| Confidence | −.45*** | .20*** | −.73*** | −.08 |
| Intention | −.08 | .02 | −.13* | .08 |
| ARME | −.09 | −.13* | −.23*** | −.50*** |
Note. ARME=Abstinence-Related Motivational Engagement.
p<.05;
p<.01;
p<.001
Current Smokers
Greater emotional exhaustion was associated with higher craving, negative affect, withdrawal, and symptoms of depression and social anxiety, all with medium to large effect sizes. Large negative associations were observed for emotional exhaustion and distress tolerance, resilience, and confidence to quit. In contrast, devaluation was positively associated with negative affect, depression, positive affect, distress tolerance, and confidence to quit, all with small effect sizes. Small negative associations were observed between devaluation and both resilience and abstinence-related motivational engagement.
Recent Quitters
Greater emotional exhaustion was associated with higher craving, negative affect, withdrawal, depression, and social anxiety, all with large effect sizes. Large negative associations were observed for emotional exhaustion and distress tolerance, resilience, and confidence to quit. Emotional exhaustion had small positive associations with abstinence intentions and abstinence-related motivational engagement. In contrast, devaluation was positively associated with social anxiety only, and this relationship was small in magnitude. Small negative associations were observed between devaluation and positive affect, distress tolerance, and resilience. Devaluation had a large negative association with abstinence related-motivational engagement.
Trajectories
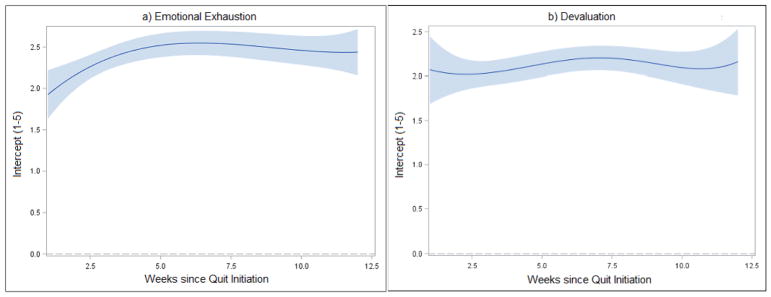
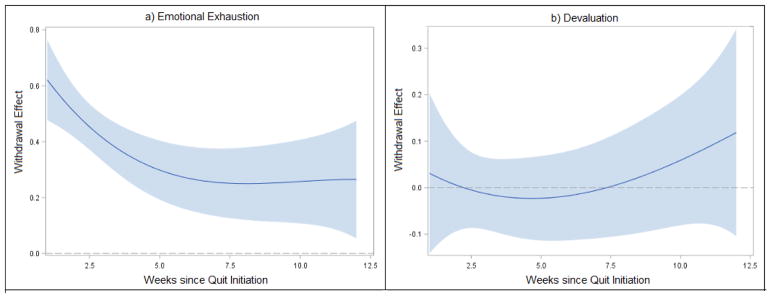
Nonparametric curves that characterize growth of recent quitters’ emotional exhaustion and devaluation over the first three months of a quit attempt are presented in Figure 1 (intercept function). Emotional exhaustion increased over the first six weeks and then remained stable through week 12 (panel a). In contrast, negligible change over time was observed for devaluation (panel b). Figure 2 depicts the dynamic associations between withdrawal and cessation fatigue (coefficient function); confidence bands that do not cross zero indicate significant effects of withdrawal on fatigue. The estimated effect of withdrawal on emotional exhaustion decreased rapidly over the first six weeks (panel a; confidence bands do not cross zero), and remained significant through 12 weeks. Lapse predicted greater emotional exhaustion (estimate=.39; p<.001). In contrast, neither withdrawal (panel b; confidence bands overlap with zero) nor lapse (estimate=−.14; p=.89) were significantly related to devaluation.
Figure 1.
Cessation fatigue trajectories with 95% confidence bands. The intercept functions represent the time-varying mean fatigue scores during first 12 weeks of quit attempt.
Figure 2.
Time-varying effect of withdrawal on cessation fatigue with 95% confidence bands. Confidence bands that do not overlap with zero represent significant correlations between withdrawal and fatigue, whereas overlap with zero indicates non-significant effects.
Predictive Validity: Cessation Milestones
Cox and logistic regression results for current and recent quitters are shown in Tables 3 and 4, respectively.
Table 3.
Current Smoker Cessation Milestones (n=301)
| Predictor | Time to Quit Attempt | Time to 7-day PPA | 7-day PPA at 2-month Follow-Up | |||
|---|---|---|---|---|---|---|
|
| ||||||
|
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
|
| Emotional Exhaustion | .80 (.67–.96)* | .84 (.70–1.01) | .51 (.40–.65)*** | .53 (.40–.68)*** | .21 (.13–.36)*** | .27 (.16–.46)*** |
| Devaluation | 1.13 (.96–1.33) | 1.07 (.90–1.27) | 2.72 (2.15–3.43)*** | 2.60 (2.02–3.34)*** | 4.50 (2.80–7.25)*** | 3.79 (2.24–6.40)*** |
| Nicotine Dependence | .88 (.78–.99)* | .72 (.62–.83)*** | .49 (.35–.67)*** | |||
| Abstinence Intentions | 1.09 (.94–1.25) | 1.25 (1.04–1.51)* | 1.39 (.97–2.00) | |||
| ARME | 1.03 (.87–.1.22) | .98 (.77–1.25) | 1.07 (.68–1.67) | |||
Note. HR=hazard ratio; OR; odds ratio; PPA=Point Prevalence Abstinence; ARME=Abstinence-Related Motivational Engagement.
p<.05;
p<.001
Table 4.
Recent Quitter Cessation Milestones (n=242)
| Predictor | Time to Lapse (initial smoking/puff) | Time to Relapse (seven consecutive days smoking) | 7-day PPA at 2-month Follow-Up | |||
|---|---|---|---|---|---|---|
|
| ||||||
|
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
|
| Emotional Exhaustion | 3.89 (2.75–5.51)*** | 1.65 (1.06–2.56)* | 5.63 (3.64–8.70)*** | 2.33 (1.37–3.97)** | .07 (.03–.14)*** | .39 (.16–.94)* |
| Devaluation | .60 (.41–.87)** | .71 (.45–1.14) | .80 (.52–1.23) | .92 (.54–1.54) | 2.48 (1.35–4.57)** | 1.63 (.64–4.12) |
| Nicotine Withdrawal | 2.85 (1.98–4.08)*** | 3.72 (2.38–5.81)*** | .11 (.05–.24)*** | |||
| Abstinence Intentions | 1.03 (.90–1.18) | 1.22 (1.01–1.49)* | .73 (.53–1.01) | |||
| ARME | .79 (.57–1.10) | .64 (.43–.95)* | 2.60 (1.27–5.34)** | |||
| Time since Quit Initiation | 1.09 (.92–1.29) | 1.13 (.93–1.36) | .70 (.47–1.05) | |||
Note. HR=hazard ratio; OR; odds ratio; ARME=Abstinence-Related Motivational Engagement.
p<.05;
p<.01;
p<.001
Current Smokers
By expectation and by design, nearly all of the current smokers made a quit attempt (91.7%) over the course of the 8-week follow-up period. The majority (72.4%) of quit attempts were made within the initial two weeks of follow-up, with another 9.6% by four weeks, 7.0% by six weeks, and 2.7% by eight weeks. Emotional exhaustion significantly predicted time to initial quit attempt in the unadjusted model, such that smokers with greater exhaustion had longer latency to making a quit attempt. However, this relationship was no longer statistically significant (p=.06) when nicotine dependence and motivation to quit measures were added to the model. Devaluation was not associated with time to making a quit attempt, in either unadjusted or adjusted models.
Seven-day PPA was achieved by 56.5% of the current smokers over the course of the 8-week follow-up period. Most smokers (30.2%) achieved 7-day PPA within the initial two weeks of follow-up, with an additional 5.3% by four weeks, 7.3% by six weeks, and 13.6% by eight weeks. Both emotional exhaustion and devaluation predicted time to 7-day PPA in the unadjusted and adjusted models. Greater exhaustion was associated with longer latency to 7-day PPA, such that likelihood of abstinence was reduced 47% for every 1-point increase in exhaustion, after controlling for nicotine dependence and motivation to quit measures. In contrast, those with greater devaluation had faster time to 7-day PPA, such that likelihood nearly tripled for every 1-point increase in devaluation.
Over half (per protocol: 58.2%; intent-to-treat: 56.5%) of the smokers reported 7-day PPA at the 2-month follow-up. Per-protocol results are reported here, and the same pattern was observed for intent-to-treat analyses. Emotional exhaustion and devaluation significantly predicted abstinence in the unadjusted and adjusted models. Greater exhaustion was associated with lower likelihood of abstinence, whereas elevated devaluation predicted higher likelihood of abstinence. Smokers were 73% less likely to be abstinent for each 1-point increase in exhaustion, but nearly four times more likely to report abstinence for each 1-point increase in devaluation. Nicotine dependence also predicted abstinence, whereas motivation to quit measures did not.
Recent Quitters
Lapse (i.e., at least a puff) was observed for 40.1% of the recent quitters over the course of the 8-week follow-up period. Most (36.4%) first lapses occurred within the initial two weeks of follow-up, with an additional 2.1% by four weeks, 0.4% by six weeks, and 1.2% by eight weeks. Emotional exhaustion and devaluation significantly predicted time to initial lapse in the unadjusted model, but only exhaustion remained significant in the adjusted model. Greater exhaustion was associated with greater risk for lapse. Controlling for nicotine withdrawal and motivation to remain quit measures, each 1-point increase in exhaustion was associated with a 67% increase in the risk of lapse risk.
Relapse (i.e., seven consecutive days smoking) was observed for 32.6% of the recent quitters over the course of the 8-week follow-up period. Most (28.1%) relapse was observed within the initial two weeks of follow-up, with an additional 2.1% by four weeks, 1.2% by six weeks, and 1.2% by eight weeks. Emotional exhaustion significantly predicted time to relapse in the unadjusted and adjusted models (ps<.01). Greater exhaustion was associated with greater relapse risk. For each 1-point increase in exhaustion, the risk of relapsing increased by 129%, after controlling for nicotine withdrawal and motivation to remain quit measures. Devaluation was not associated with relapse risk.
Over half (per protocol: 59.9%; intent-to-treat: 58.7%) of the recent quitters reported 7-day PPA at the 2-month follow-up. Per-protocol results are reported here, and the same pattern was observed for intent-to-treat analyses. Emotional exhaustion and devaluation significantly predicted abstinence in the unadjusted model, but only exhaustion remained significant in the adjusted model. Abstinence was also predicted by nicotine withdrawal and abstinence-related motivation engagement. Greater exhaustion was associated with a lower likelihood of abstinence. For every 1-point increase in exhaustion recent quitters were 61% less likely to be abstinent at the final follow-up.
Discussion
The current study is the first to test whether a multi-dimensional measure of cessation fatigue can prospectively predict important smoking-cessation milestones. Our study provides a significant contribution by showing the importance of this construct for both current smokers and recent quitters, based on its relevance to a range of milestones, including outcomes that have yet to be explored empirically (e.g., time-to-event analyses). We also demonstrate the utility of balanced accelerated longitudinal cohort study design for smoking cessation research, with time since quit date as the unit of time. The consistent overlap between successive cohorts, based on time since quit date, allowed us to model trajectories over 12 weeks from eight weeks of assessments. Together with our analytical approach (TVEM, survival analyses), the current study design represents a strong and efficient methodological approach to delineate how the temporal dynamics of smoking cessation fatigue unfolds.
Of the three CFS subscales, emotional exhaustion was the strongest predictor of cessation outcomes. Consistent with the initial CFS study (Mathew, Heckman et al. 2017), emotional exhaustion demonstrated good internal consistency and strong construct validity. The current study extended these findings to establish emotional exhaustion as a robust prospective predictor of cessation outcomes among both current and recent quitters. Moreover, while fatigue is conceptually similar to existing constructs (e.g., motivation, withdrawal), the current study is the first to show it predicts behavioral outcomes above and beyond these constructs. Consistent with laboratory-based evidence (Heckman, MacQueen et al. 2017), our findings underscore the role of depleted self-control resources in precipitating smoking lapse. Higher exhaustion was associated with longer delay to initial cessation (7-day PPA) among current smokers, and higher lapse/relapse risk among recent quitters. Across both samples, those with greater exhaustion were less likely to report 7-day PPA at the 2-month follow-up. All results were observed beyond the effects of nicotine dependence/withdrawal and motivation to quit. Unadjusted analyses suggested emotional exhaustion predicted time to quit attempt as well, but this effect no longer met traditional levels of significance in adjusted analyses. This pattern of findings is unique, as psychosocial measures do not typically predict both cessation and relapse (Vangeli, Stapleton et al. 2011).
Additionally, our study provides novel data to delineate the temporal dynamics of cessation fatigue among recent quitters. Consistent with Piasecki’s (2002) prognostication, emotional exhaustion increased over time since quit initiation. Building upon evidence that fatigue increases over the first two weeks of a quit attempt (Liu, Li et al. 2013), we observed peak values at around six weeks at which point they plateaued and remained stable through 12 weeks. Studying fatigue over a longer timeframe is an important next step. We suspect fatigue may decrease as living smoke-free becomes more routine over time, given that relapse curves show drastic reductions in relapse risk after 100 days of abstinence (Hughes, Keely et al. 2004, Kirshenbaum, Olsen et al. 2009). TVEM results suggest that withdrawal effects on cessation fatigue dissipated over the first six weeks of a quit attempt at which point they plateaued and remained stable through 12 weeks. Thus, the initial demands of coping with withdrawal may have lasting effects on self-control depletion and cessation fatigue, consistent with theoretical accounts (Muraven, Tice et al. 1998, Muraven and Baumeister 2000, Piasecki, Fiore et al. 2002).
While the main goal of the current study was to examine the CFS’s predictive validity, our findings also shed light on the critical question of which smokers are most likely to be ‘fatigue-prone.’ We found several sociodemographic variables that predicted elevated emotional exhaustion across both current smokers and recent quitters, including: being younger, smoking more CPD, having higher income, and recent electronic cigarette use (past seven days). These findings are, in part, consistent with Mathew and colleagues (2017), who found similar results for age and CPD, but not income. To our knowledge, the current study is the first to examine the relationship between electronic cigarette use and cessation fatigue. The nature of this relationship should be explored further given the high rates of electronic cigarette use for cessation (NASEM 2018). We corroborate that the characteristics of anxiety, negative affect, and craving are positively associated with fatigue (Piper, Cook et al. 2011, Liu, Li et al. 2013), and found this pattern extends to other known relapse risk factors such as depression. However, it is unclear if fatigue drives these negative affective states, or whether a general tendency to experience negative emotions in turn increases perceived fatigue. Interestingly, emotional exhaustion was more strongly linked to negative affect, depression, and social anxiety among recent quitters versus current smokers, underscoring the importance of considering smoking status and other contextual factors. Further, another important factor to consider is the role of an individual’s response to cessation fatigue. It may be that fatigue is a much more potent predictor of relapse risk in the context of high versus low coping resources. The current study is the first to demonstrate that resilience and distress tolerance may serve as protective factors against emotional exhaustion. Taken together, we believe that work refining the construct of cessation fatigue remains at a nascent stage, and further efforts to delineate this dynamic construct, and its potential interactions with sociodemographic and contextual factors, are greatly needed.
The compelling emotional exhaustion findings were not replicated across the CFS’s other two subscales. In contrast to the initial CFS validation study (Mathew, Heckman et al. 2017), the pessimism scale did not have adequate internal consistency so it was not examined further. The devaluation subscale showed good reliability, but findings with regard to construct and predictive validity were counterintuitive. Although we did not have an a priori hypothesis regarding nicotine dependence, its negative association with devaluation is surprising. We found positive associations with positive distress tolerance, and confidence to quit, which was contrary to our hypotheses. Moreover, outcomes significantly predicted by devaluation were opposite the expected direction. Greater devaluation was associated with shorter time to 7-day PPA and higher abstinence rates at 2-month follow-up. We suspect devaluation and pessimism subscales exhibited measurement issues because they were comprised entirely of reverse-scored items, which increases the likelihood of misresponse (Swain, Weathers et al. 2008). That lower education predicted elevated devaluation across both current smokers and recent quitters may suggest the need to improve item content or instruction set. While we believe that devaluation and pessimism are conceptually relevant to the cessation fatigue construct, empirically this was not the case. Our results suggest further work is needed to extend assessment of these domains and validate their role as meaningful constructs in relation to fatigue and cessation outcomes. Future studies should include other relevant assessments to help disentangle the nature of this construct. While we examined several constructs specific to smoking cessation (e.g., withdrawal, motivation), there is a need to explore other constructs related to behavior change and maintenance more generally (e.g., satisfaction, perceptions of the costs of behavior change, delay discounting).
Several limitations are noteworthy. Online recruitment allowed for enhanced generalizability, but limited outcomes to self-report alone. Although this conforms with recommendations for observational studies (SRNT 2002), future studies could incorporate remote bioverification of smoking status to overcome this limitation (McClure and Gray 2013). Recruitment methods may have underrepresented smokers unconnected to the internet. However, we also view the online nature of the study as a strength because this approach is more convenient than traditional laboratory based methods and may reach a larger portion of the smoking population (e.g., those unwilling/unable to visit a laboratory). We did not design our study to test for individual difference factors in cessation fatigue, but report them to help guide future research. Population-based studies that are well-powered and have more representative samples will provide the evidence necessary to reach stronger conclusions about subpopulations more susceptible to cessation fatigue, and the degree to which the validity of the CFS varies by subpopulations. While several theoretically-relevant constructs were correlated with fatigue and fatigue predicted cessation milestones, formal mediation analysis of the protective/risk factors would better explain mechanisms through which fatigue influences capacity to quit successfully. Future research should also examine other conceptually relevant constructs that may moderate susceptibility to cessation fatigue, such as personality (e.g., negative emotionality; Leventhal, Japuntich et al. 2012) and transdiagnostic psychopathology (e.g., anhedonia; Leventhal and Zvolensky 2015). Finally, cessation fatigue and outcomes were assessed every two weeks. Given that motivation and quitting can change rapidly (Hughes, Solomon et al. 2014), future research should capture the temporal dynamics of fatigue with greater precision by including more frequent assessments (e.g., ecological momentary assessment).
Clinical Implications
That cessation fatigue may impede quitting and disrupt abstinence has novel clinical implications. With further refinement, we envision the 7-item emotional exhaustion subscale to be used as a triage tool to identify relapse-vulnerable individuals. Elevated fatigue may signal limited self-control resources that if not addressed will undermine quit attempts. Future research should determine the degree to which evidence-based treatments impact fatigue and need for interventions specific to cessation fatigue.
As proposed by a workload-capacity model of health behaviors (Heckman, Mathew et al. 2015), there are several pathways to buffer against cessation fatigue. Reducing the workload associated with general life demands and the process of quitting can attenuate cessation fatigue. Effective stress management is paramount (Baker, Piper et al. 2004) as stressors reliably increase motivation to smoke (Heckman, Kovacs et al. 2013, Heckman, Carpenter et al. 2015). Behavioral intervention technologies offer unique opportunities to transform continuous care delivery (Mohr, Schueller et al. 2014). Mobile health (mHealth) interventions can increase accessibility, facilitate treatment engagement, and reduce patient efforts. Telehealth approaches can reduce stress associated with treatment adherence (e.g., transportation costs, time, childcare). The next generation of mHealth is working towards automatic yet personalized treatment delivery by leveraging smartphone-embedded sensors (e.g., global positioning system [GPS]). Context sensing interventions can reduce the amount of effort typically needed to monitor and cope with high-risk situations and have shown potential for relapse prevention (Gustafson, McTavish et al. 2014).
Additionally, capacity can be increased by alleviating the negative consequences associated with cessation and improving general coping resources. Self-control resources are greatly compromised during a quit attempt when smokers must cope with nicotine withdrawal (Baker, Japuntich et al. 2006). Pharmacotherapies decrease both withdrawal and cessation fatigue (Liu, Li et al. 2013). Behavioral interventions designed to increase withdrawal coping skills appear promising (Hendricks, Hall et al. 2016, McCarthy, Bold et al. 2016), and may improve cessation outcomes by enhancing self-control capacity. These approaches are similar to self-control strength training interventions (Muraven 2010). Many promising approaches focused on increasing resilience are on the horizon, including: positive psychotherapy (Kahler, Spillane et al. 2015), distress tolerance (Brown, Reed et al. 2013), and acceptance and commitment therapy (Bricker, Wyszynski et al. 2013).
Finally, the robust emotional exhaustion results reported here demonstrate the relevance of cessation fatigue to the science of behavior change (Davis, Campbell et al. 2015). Future research should evaluate the concept of fatigue in relation to other types of substance use disorders and other chronic conditions (e.g., obesity). Chronic disease management among people living with diabetes and human immunodeficiency virus (HIV) has been associated with treatment fatigue (Fritschi and Quinn 2010, Claborn, Meier et al. 2014), which in turn can reduce treatment adherence. It is unclear if fatigue will manifest differently when attempting to modify behaviors that are hard to reduce (e.g., smoking), hard to sustain (e.g., exercise, medication adherence), or both (e.g., diet) (Borland 2014).
Conclusions
Our results, and those from three prior studies, indicate cessation fatigue is experienced by both current and recent quitters. The cumulative toll associated with efforts to maintain abstinence can be indexed by the CFS’s 7-item emotional exhaustion subscale to determine the sustainability of behavioral change. That fatigue impeded both quitting and maintaining abstinence suggests the need for ongoing efforts to monitor and reduce fatigue. Innovations in technology-based interventions (e.g., mHealth) are well positioned to meet these needs.
Supplementary Material
Public Health Significance.
This study suggests cessation fatigue is an obstacle to quitting smoking and precipitates smoking relapse. Assessment of cessation fatigue over the course of a quit attempt is recommended to identify individuals at elevated relapse risk. Interventions targeting this novel risk factor may help improve smoking-cessation and reduce relapse rates.
Acknowledgments
Role of Funding Source: This research was supported by the Chairman’s Research Development Fund Pilot Grant Program, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina. BWH was supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (grant numbers K12 DA031794 and K23 DA041616). JD was supported by T32 DA007288. Data collection via REDCap was supported by the National Center for Advancing Translational Sciences (UL1 TR001450). The research presented in this paper is that of the authors and does not reflect the official policy of the funders. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors are grateful to Amy Boatright, Amy Wahlquist, Hannah Shoemaker, Caitlyn Hood, and Alexander Hirsch for their valuable assistance.
Footnotes
Contributors: BWH had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to study design and manuscript preparation.
Conflicts of Interest: none
References
- Allison PD. Logistic Regression Using SAS. SAS Institute; 2012. [Google Scholar]
- Baker TB, Japuntich SJ, Hogle JM, McCarthy DE, Curtin JJ. Pharmacologic and Behavioral Withdrawal From Addictive Drugs. Current Directions in Psychological Science. 2006;15(5):232–236. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction Motivation Reformulated: An Affective Processing Model of Negative Reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, Fiore MC. Effects of Nicotine Patch vs Varenicline vs Combination Nicotine Replacement Therapy on Smoking Cessation at 26 Weeks: A Randomized Clinical Trial. JAMA. 2016;315(4):371–379. doi: 10.1001/jama.2015.19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SL, Rosner J, Toll B. Concordance between timeline follow-back and single-question assessment of self-reported smoking in a clinical trial. Subst Abus. 2016;37(3):398–401. doi: 10.1080/08897077.2016.1154494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10(5):360. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Borland R. Understanding hard to maintain behaviour change : a dual process approach. Hoboken, New Jersey: John Wiley & Sons Inc; 2014. [Google Scholar]
- Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine Tob Res. 2013;15(10):1756–1764. doi: 10.1093/ntr/ntt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Gifford EV, Hayes SC. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine Tob Res. 2013;15(12):2005–2015. doi: 10.1093/ntr/ntt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:Cd009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9(2):183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Claborn KR, Meier E, Miller MB, Leffingwell TR. A systematic review of treatment fatigue among HIV-infected patients prescribed antiretroviral therapy. Psychol Health Med. 2014:1–11. doi: 10.1080/13548506.2014.945601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, editor. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Connor KM, Kobak KA, Churchill LE, Katzelnick D, Davidson JRT. Mini-SPIN: A brief screening assessment for generalized social anxiety disorder. Depression and Anxiety. 2001;14(2):137–140. doi: 10.1002/da.1055. [DOI] [PubMed] [Google Scholar]
- Davis R, Campbell R, Hildon Z, Hobbs L, Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychol Rev. 2015;9(3):323–344. doi: 10.1080/17437199.2014.941722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Larsen RJ, Emmons RA. Person × Situation interactions: Choice of situations and congruence response models. Journal of Personality and Social Psychology. 1984;47(3):580–592. doi: 10.1037//0022-3514.47.3.580. [DOI] [PubMed] [Google Scholar]
- Fritschi C, Quinn L. Fatigue in patients with diabetes: a review. J Psychosom Res. 2010;69(1):33–41. doi: 10.1016/j.jpsychores.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith S, Bowden J, Mander A. Accelerated longitudinal designs: An overview of modelling, power, costs and handling missing data. Statistical Methods in Medical Research. 2014;26(1):374–398. doi: 10.1177/0962280214547150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DH, McTavish FM, Chih MY, Atwood AK, Johnson RA, Boyle MG, Levy MS, Driscoll H, Chisholm SM, Dillenburg L, Isham A, Shah D. A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA Psychiatry. 2014;71(5):566–572. doi: 10.1001/jamapsychiatry.2013.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Heckman BW, Carpenter MJ, Correa JB, Wray JM, Saladin ME, Froeliger B, Drobes DJ, Brandon TH. Effects of experimental negative affect manipulations on ad libitum smoking: a meta-analysis. Addiction. 2015;110(5):751–760. doi: 10.1111/add.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman BW, Kovacs MA, Marquinez NS, Meltzer LR, Tsambarlis ME, Drobes DJ, Brandon TH. Influence of affective manipulations on cigarette craving: a meta-analysis. Addiction. 2013;108(12):2068–2078. doi: 10.1111/add.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman BW, MacQueen DA, Marquinez NS, MacKillop J, Bickel WK, Brandon TH. Self-control depletion and nicotine deprivation as precipitants of smoking cessation failure: A human laboratory model. J Consult Clin Psychol. 2017;85(4):381–396. doi: 10.1037/ccp0000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman BW, Mathew AR, Carpenter MJ. Treatment burden and treatment fatigue as barriers to health. Current Opinion in Psychology. 2015;5:31–36. doi: 10.1016/j.copsyc.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Hall SM, Tyus LR, Thorne CB, Lappan SN, McMurray MV, Bailey WC, Cropsey KL, Baker TB. Withdrawal exposure with withdrawal regulation training for smoking cessation: a randomized controlled pilot trial. Drug Alcohol Depend. 2016;164:28–37. doi: 10.1016/j.drugalcdep.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of general psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5(1):13–25. [PubMed] [Google Scholar]
- Hughes JR, Solomon LJ, Naud S, Fingar JR, Helzer JE, Callas PW. Natural History of Attempts to Stop Smoking. Nicotine & Tobacco Research. 2014;16(9):1190–1198. doi: 10.1093/ntr/ntu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DL, Gillaspy JA, Purc-Stephenson R. Reporting practices in confirmatory factor analysis: an overview and some recommendations. Psychol Methods. 2009;14(1):6–23. doi: 10.1037/a0014694. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Day AM, Cioe PA, Parks A, Leventhal AM, Brown RA. Positive Psychotherapy for Smoking Cessation: A Pilot Randomized Controlled Trial. Nicotine Tob Res. 2015;17(11):1385–1392. doi: 10.1093/ntr/ntv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Olsen DM, Bickel WK. A quantitative review of the ubiquitous relapse curve. J Subst Abuse Treat. 2009;36(1):8–17. doi: 10.1016/j.jsat.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders. 2009;114(1):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Vasilenko SA, Liu X, Li R, Piper ME. Advancing the Understanding of Craving During Smoking Cessation Attempts: A Demonstration of the Time-Varying Effect Model. Nicotine & Tobacco Research. 2014;16(Suppl_2):S127–S134. doi: 10.1093/ntr/ntt128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavinghouze SR, Malarcher A, Jama A, Neff L, Debrot K, Whalen L. Trends in Quit Attempts Among Adult Cigarette Smokers—United States, 2001–2013. MMWR Morbidity and mortality weekly report. 2015;64(40):1129. doi: 10.15585/mmwr.mm6440a1. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Japuntich SJ, Piper ME, Jorenby DE, Schlam TR, Baker TB. Isolating the role of psychological dysfunction in smoking cessation: relations of personality and psychopathology to attaining cessation milestones. Psychol Addict Behav. 2012;26(4):838–849. doi: 10.1037/a0028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychol Bull. 2015;141(1):176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li R, Lanza ST, Vasilenko SA, Piper M. Understanding the role of cessation fatigue in the smoking cessation process. Drug Alcohol Depend. 2013;133(2):548–555. doi: 10.1016/j.drugalcdep.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew AR, Heckman BW, Meier E, Carpenter MJ. Development and initial validation of a cessation fatigue scale. Drug and Alcohol Dependence. 2017;176:102–108. doi: 10.1016/j.drugalcdep.2017.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Bold KW, Minami H, Yeh VM. A randomized clinical trial of a tailored behavioral smoking cessation preparation program. Behav Res Ther. 2016;78:19–29. doi: 10.1016/j.brat.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Gray KM. The Remote Monitoring of Smoking in Adolescents. Adolesc Psychiatry (Hilversum) 2013;3(2):156–162. doi: 10.2174/2210676611303020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Schueller SM, Montague E, Burns MN, Rashidi P. The behavioral intervention technology model: an integrated conceptual and technological framework for eHealth and mHealth interventions. J Med Internet Res. 2014;16(6):e146. doi: 10.2196/jmir.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M. Practicing self-control lowers the risk of smoking lapse. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2010;24(3):446–452. doi: 10.1037/a0018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychol Bull. 2000;126(2):247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Muraven M, Tice DM, Baumeister RF. Self-control as limited resource: Regulatory depletion patterns. Journal of Personality and Social Psychology. 1998;74:774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- NASEM. Public health consequences of e-cigarettes. Washington, DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- Ossip-Klein DJ, Bigelow G, Parker SR, Curry S, Hall S, Kirkland S. Task Force 1: Classification and assessment of smoking behavior. Health Psychology. 1986;5(Suppl):3–11. [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97(9):1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Piper ME. Withdrawal: Expanding a Key Addiction Construct. Nicotine Tob Res. 2015;17(12):1405–1415. doi: 10.1093/ntr/ntv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106(2):418–427. doi: 10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. Journal of consulting and clinical psychology. 2006;74(2):276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Shiyko MP, Lanza ST, Tan X, Li R, Shiffman S. Using the Time-Varying Effect Model (TVEM) to Examine Dynamic Associations between Negative Affect and Self Confidence on Smoking Urges: Differences between Successful Quitters and Relapsers. Prevention Science. 2012;13(3):288–299. doi: 10.1007/s11121-011-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons VN, Heckman BW, Ditre JW, Brandon TH. A measure of smoking abstinence-related motivational engagement: Development and initial validation. Nicotine & tobacco research. 2010:ntq020. doi: 10.1093/ntr/ntq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Gaher RM. The Distress Tolerance Scale: Development and Validation of a Self-Report Measure. Motivation and Emotion. 2005;29(2):83–102. [Google Scholar]
- Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: Assessing the ability to bounce back. International Journal of Behavioral Medicine. 2008;15(3):194–200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- SRNT. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Swain SD, Weathers D, Niedrich RW. Assessing Three Sources of Misresponse to Reversed Likert Items. Journal of Marketing Research. 2008;45(1):116–131. [Google Scholar]
- Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction. 2011;106(12):2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- Vasilenko SA, Piper ME, Lanza ST, Liu X, Yang J, Li R. Time-Varying Processes Involved in Smoking Lapse in a Randomized Trial of Smoking Cessation Therapies. Nicotine & Tobacco Research. 2014;16(Suppl_2):S135–S143. doi: 10.1093/ntr/ntt185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.