Abstract
Angiopoietin-1 (Ang-1) is a well-known endothelial growth factor, but its effects on neurons have yet to be elucidated. We show that Ang-1 is rapidly downregulated in the injured brain after controlled cortical impact (CCI), a mouse experimental traumatic brain injury (TBI) model and in etoposide-induced neuronal apoptosis in vitro. Ang-1 treatment inhibits etoposide-induced upregulation of proapoptotic B-cell lymphoma 2 (Bcl-2) family members Noxa, p53 upregulated modulator of apoptosis (Puma), Bcl-2 interacting mediator of cell death (Bim), and Bcl-2-associated X protein (Bax); reduces markers of caspase-dependent (cytochrome c release/caspase activation) and caspase-independent (apoptosis-inducing factor release) pathways; and limits neuronal cell death. Ang-1 treatment phosphorylates receptors Tunica interna endothelial cell kinase 2 (Tie2), and β1-integrin and limits the etoposide-induced decrease in protein kinase B (Akt) activity. Blocking Tie2 and β1-integrin signaling reduces Ang-1 neuroprotective effects. After both TBI and etoposide treatment microRNA (miR)-711 are upregulated, consistent with its putative role as a negative regulator of Ang-1. We show that miR-711 directly targets the Ang-1 messenger RNA (mRNA), decreasing Ang-1 expression. Increased levels of miR-711 and Ang-1 mRNA are found in the RNA-induced silencing complex complex site of miR-mediated degradation of target mRNAs after etoposide treatment and the miR-711mimic downregulates Ang-1. Administration of miR-711 inhibitor elevates Ang-1 after TBI whereas Ang-1 administration increases Akt activation; reduces Puma, Noxa, Bim, and Bax levels; and attenuates caspase-dependent and -independent neuronal apoptosis 24 h after TBI. Ang-1 also attenuates neuronal degeneration, increases gene expression of molecules that maintain blood–brain barrier integrity, and reduces post-traumatic lesion volume/edema 24 h after TBI. Although we only observed short-term neuroprotective effects after Ang-1 administration, miR-711-dependent downregulation of Ang-1, followed by Akt pathway inhibition, may play a role in neuronal cell death after neuronal injury in vitro and after experimental TBI.
Keywords: : angiopoietin-1 (Ang-1), cell death, microRNA (miRNA), traumatic brain injury
Introduction
Angiopoietin 1 (Ang-1) is a secreted oligomeric glycoprotein and a member of the angiopoietin family of growth factors. Ang-1 can bind and signal through the receptor tyrosine kinase, Tunica interna endothelial cell kinase 2 (Tie2),1,2 and integrins.3,4 Ang-1 inhibits apoptosis in endothelial cells,5,6 and its anti-apoptotic effects involve activation of protein kinase B (Akt) through the phosphatidylinositol 3-kinase (PI3K) pathway.7,8 Neurons express Ang-1, Tie2,9 and various integrin subunits.10 Ang-1 can attenuate neuronal cell death induced by serum deprivation through activation of the PI3K/Akt pathway9 and protects neurons against zinc toxicity.10
Ang-1 signaling through the Tie2 receptor promotes endothelial cell survival, stabilizes blood vessels and attenuates leakiness, and reduces leukocyte infiltration, resulting in protection after spinal cord injury.11 Ang-1–mediated angiogenesis has also been shown to reduce infarct volume and neurological deficits in a model of brain ischemia,12 and its neurotrophic effects can attenuate neuropathy in a sciatic nerve injury model.13 However, the role played by Ang-1 in models of neuronal cell death or in neuronal loss after traumatic brain injury (TBI) remains unclear.
MicroRNAs (miRs) are short (20–23 nucleotide) noncoding RNAs that negatively regulate gene expression at the post-transcriptional level by binding to the 3′-untranslated region (UTR) of target messenger RNA (mRNAs), leading to their degradation and/or translational inhibition.14 They have been implicated in the pathophysiology of brain seizures, ischemia, and trauma15,16 and are powerful modulators of neuronal cell death pathways.17,18 The ability of individual miRs to target multiple genes across various connected pathways enables concerted action on cellular signaling mechanisms and makes miRs promising candidates for therapeutic intervention.19
Recently, we showed that miR-711 is rapidly up-regulated after controlled cortical impact (CCI) and in etoposide-induced neuronal cell death.
Our previous data suggested that miR-711 contribute to neuronal cell death by directly targeting Akt for downregulation.20 Ang-1 is another predicted target for miR-711, and the present study explores the significance of this link in neuronal apoptosis.
We demonstrated that: Ang-1 is a target for miR-711 in neuronal injury; Ang-1 attenuates neuronal apoptosis pathways, including the induction of proapoptotic Bcl-2 interacting mediator of cell death (Bcl2) family molecules; and Ang-1 is neuroprotective and reduces acute neuronal cell death after TBI.
Methods
Animals
Studies were performed using young-adult (3 months of age, 22–26 g) male C57Bl/6 mice. All surgical procedures complied with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH; DHEW publication NIH 85-23-2985). Males and females have differences in response to TBI.21 In this study, we used only male mice to avoid influence of reproductive cycle and hormone fluctuation.
Controlled cortical impact
CCI is an experimental model of TBI.22 Our custom-designed CCI device23 consists of a microprocessor-controlled pneumatic impactor with a 3.5-mm diameter tip. Mice were anesthetized with isoflurane evaporated in a gas mixture containing 70% N2O and 30% O2 and administered through a nose mask (induction at 3% and maintenance at 1.5%). The head was mounted in a stereotaxic frame, a 10-mm midline incision was made over the skull, and the skin and fascia were reflected. A 5-mm craniotomy was made on the central aspect of the left parietal bone. Moderate-level injury was induced by using an impactor velocity of 6 m/s and deformation depth of 2 mm as previously described.24 After injury and intracerebroventricular (i.c.v.) injection, the incision was closed (the craniotomy was not closed to avoid additional injury) with 9-mm EZ clips (Steeling), anesthesia was terminated, and the animal placed into a heated cage to maintain normal core temperature for 45 min post-injury. In this study, we only used male C57Bl/6 mice to avoid the disrupting influence of the reproductive cycle and hormone fluctuation in female mice on cell death mechanisms. References to TBI in this work refer only to this CCI model.
In vivo drug treatments
Fifteen minutes post-injury, mice received a single i.c.v. injection of 0.3125 or 1.25 μg of recombinant Ang-1 or artificial cerebrospinal fluid (ACSF; vehicle). Drugs were administered in ACSF and injected into the left ventricle (coordinates from bregma = A: −0.5; L: −1.0; and V: −2.0) using a 30-gauge needle attached to a Hamilton syringe at a rate of 0.5 mL/min, with a final volume of 5 μL of 0.1 mM of miR hairpin inhibitor or 5 μL of 0.25 μg of Ang-1.
In vitro cell culture
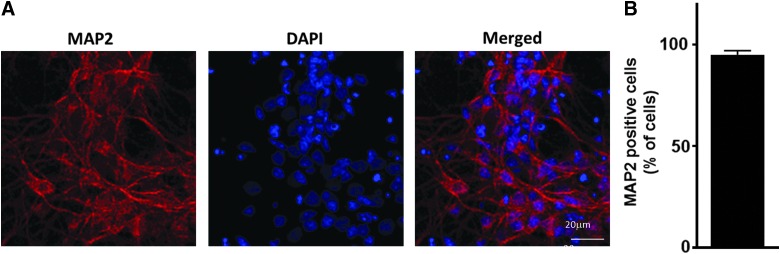
Rat cortical neurons (RCNs) were derived from rat embryonic cortices as previously described.25 Neurons were maintained in serum-free conditions using the B27 supplement. Purity of the neuronal culture was confirmed by MAP-226 staining (a marker for viable neurons) showing the cultures were >95% neurons (Fig. 1). Glial fibrillary acidic protein (GFAP; astrocyte marker) staining was used to determinate the presence of astrocytes. We found that 2.3% of cells in culture were GFAP positive (data not shown). Primary cortical neurons grown on glass cover-slips were fixed and immunostained using standard immunocytochemistry techniques. Sections were incubated with Alexa Fluor goat antimouse and -rabbit antibodies; 4′,6-diamidino-2-phenylindole (DAPI) was used as a nuclear counter stain. Imaging was performed using a Leica TCS SP5 II Tunable Spectral Confocal microscope (Leica Microsystems Inc., Bannockburn, IL). We examined nine independent fields of control primary neurons identifying live cells based on lack of chromatin condensation (DAPI; the number of total counted cells/field [DAPI] ranged from 211 to 359. The number of neurons in these populations were identified as microtubule-associated protein 2 (MAP2) positive whereas astrocytes were identified as GFAP positive.
FIG. 1.
Purity of primary rat cortical neuron (RCN) cultures. (A) RCN cultures were fixed and stained with MAP2 (neuronal marker, red) and 4′,6-diamidino-2-phenylindole (DAPI; nuclear marker, blue) at 7 days in vitro. (B) Percent of MAP2-positive cells to total number of cells (DAPI; N = 9). MAP2, microtubule-associated protein 2. Color image is available online at www.liebertpub.com/neu
Transfection of RCNs was performed at 6 days in vitro. RCNs were transfected with miR mimics and hairpin inhibitors using the LipofectamineRNAiMAX Transfection Reagent (Invitrogen, Life Technologies, Carlsbad, CA), according to the manufacturer's protocol. Based on preliminary titration experiments, we chose a final concentration of 50 nM for the miR mimics, hairpin inhibitors, and their negative controls. This concentration resulted in optimal transfection efficiency (∼50%), was devoid of nonspecific changes in nontargeted miRs, and had no neurotoxic effects.18 Four hours after transfection, media were replaced with normal condition media and cells treated with etoposide or β-amyloid. The following miR mimics and hairpin inhibitors were used: miRIDIAN microRNA Mimic Negative Control (−ve con mimic; CN-001000-01-05); miRIDIAN Mimic, Rat rno-miR-711 (C-320669-00-0005); miRIDIAN microRNA Hairpin Inhibitor Negative Control (−ve con inhibit; IN-001005-01-05); and miRIDIAN microRNA Rat rno-miR-711 Hairpin Inhibitor (IH-HMR-XX-0005) (Dharmacon Inc.). Sequences of both miRIDIAN microRNA Mimic Negative Control and miRIDIAN microRNA Hairpin Inhibitor Negative Control are based on Caenorhabditis elegans miRs and have minimal sequence identity in human, mouse, and rat.
Rat cortical neuron treatment
DNA damage, including DNA breaks produced by oxidative injury and other mechanisms, is a key inducer of neuronal cell death after TBI.27 Etoposide is an anticancer drug that produces DNA breaks in neurons by inhibiting DNA-topoisomerase-II, resulting in caspase-dependent and -independent apoptosis.28,29
Etoposide treatment
Cells were treated with 12.5 μM of etoposide diluted in normal condition media for 3 h. After 3 h, etoposide media were replaced with normal condition media.
β-amyloid treatment
Cells were treated with 50 uM of β-amyloid for 24 h. Carrier Free Recombinant Human Angiopoietin-1 Protein (Ang-1; cat# 923-AN-025/CF; R&D Systems, Minneapolis, MN) that shares 97% amino acid sequence identity with mouse and rat Ang-1 has been used in this study. RCNs were treated with Ang-1 at a final concentration 200 ng/mL.
Small interfering RNA gene silencing
RCNs were transfected with ON-TARGET plus SMART pool Angpt1 siRNA (#L-089028-02) and ON-TARGET plus Non-targeting Pool (#D-001810-10; GE Healthcare Dharmacon Inc., Lafayette, CO) using the Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, Life Technologies), according to the manufacturer's protocol. SMART pool small interfering RNA (siRNA) is a pool of four chemically modified siRNAs. Using pool of siRNAs lowers the effective concentration of each individual siRNA. Chemical modifications of the siRNA itself impede sense-strand entry into RISC and other potential off-targets. Dharmacon siRNA design considers miRNA-like seed regions as one of the filters for siRNA screening. These three approaches help to eliminate siRNA-induced nonspecific gene modulation by siRNAs.
Function-blocking experiments
The following reagents were used in signaling-blocking experiments: antibodies against β1-integrin (5 μg/mL; Clone JB1A; Chemicon, Temecula, CA); antibodies against Tie2 (50 μg/mL; #AF762; R&D Systems); focal adhesion kinase (FAK) inhibitor PF573228 (100 nM; TocrisBioscience, Bristol, UK)30; PLC inhibitor U73122 (1 μM; Sigma-Aldrich, St. Louis, MO); and U-73344 (1 μM; Sigma-Aldrich) as control/inactive analog of PLC inhibitor10; Akt inhibitor (1L6-hydroxymethyl-chiro-inositol-2-(R)-2-O-methyl-3-O-octadecyl-sn-glycerocarbonate; Calbiochem, Burlington, MA) selectively inhibits Akt (PKB; half maximal inhibitory concentration [IC50] of 5.0 μM) and moderately inhibits PI3-K activity (IC50 = 83.0 μM).
Cell death, cell viability, and in-plate fluorometric caspase-3 assays
Cell death, cell viability, and in-plate fluorometric caspase-3 activity were measured using the LDH, Calcein AM, and DEVD-AMC assays, respectively, as previously described.18 Each individual treatment/time point reflects six replicates for all assays performed in RCNs cultured in 96-well plates.
RNA-interacting protein immunoprecipitation using argonaute 2–specific antibodies
One strand of the mature miR binds to argonaute (Ago) proteins to form the RNA-induced silencing complex (RISC), and miR acts as a template for RISC to recognize and cleave complementary mRNA. miRNA-mRNA target pairs can be purified by immunoprecipitation of the RISC components to confirm mRNA targets. Ago2 immunoprecipitation was performed as previously described.31 Briefly, RCNs were suspended in 500 μL of lysis buffer (150 mM of KCl, 25 mM of Tris-HCl [pH 7.4], 5 mM of ethylenediaminetetraacetic acid [EDTA], 0.5% NP-40, 5 mM of dithiothreitol [DTT]) and protease inhibitor and phosphatase inhibitor (2, 3) cocktails (Sigma-Aldrich) at 4°C for 20 min., and cell lysates were separated by centrifugation at 12,000g for 20 min at 4°C. A volume of 50 μL of protein A/G UltraLink Resin (Thermo Fisher Scientific, Waltham, MA) and 20 μL of argonaute 2 (Ago2) antibody (Cell Signaling Technology, Danvers, MA) were added to 400 μL of cell lysate (in a final 1-mL mixture filled with lysis buffer), and the mixture was rotated for 4 h at 4°C. Beads were washed three times with 1 mL of lysis buffer to remove nonspecific binding. RNAs bound on the beads were extracted by the miRNeasy Kit (Qiagen, Hilden, Germany). miR and gene expression was analyzed by quantitative real-time polymerase chain reaction (qPCR; as described below).
Construction of reporter plasmids and luciferase assays
An RNA hybrid miR target prediction tool32 predicted two sites for rno-miR-711 in 3′UTR of rat Ang1 (at positions 1872 and 1820; NCBI Reference Sequence: NM_053546.1); six sites for has-miR-711 were predicted in 3′-UTR of human Ang-1 (NCBI Reference Sequence: NM_001146.4); and four sites for mmu-miR-711 were predicted in 3″UTR of mouse Ang-1 (NCBI Reference Sequence: NM_009640.4). To produce reporter plasmids containing 3′-UTRs of rat Ang-1, sequences were PCR amplified, digested, gel purified, ligated, and cloned into pmirGLO vector (Promega, Madison, WI) digested with XbaI and SalI restriction enzymes. The following primers were used to amplify 3′-UTR of rat Ang-1; 3′-UTR forward primer 5′-tgTCTAGACTGAAGGCGCTATGCC TAGTA-3′, reverse primer 5′- tgGTCGACTGGAACGGAGACAATGCTGG-3′. XbaI site was added on the 5′ end of forward primers, and SalI site was added to the 5′ end of reverse primers for cloning into pmirGLO vector XbaI, SalI-digested vector (XbaI and SalI sites are marked bold). RCNs were cultured in 96-well plates and transfected as described above. All assays were performed at 24 h after transfection with the dual luciferase assay (Promega) on a BioTek Synergy HT Microplate Reader (BioTek Instruments, Inc., Winooski, VT). Firefly luciferase activity was normalized to Renilla luciferase activity. Experiments were performed in triplicate.
RNA isolation
Total RNA was isolated using miRNeasy Kit (Qiagen). During the process of isolation, samples were treated with RNase-free DNase (Qiagen) to digest DNA contamination of the samples according to the manufacturer's protocol.
Quantitative real-time polymerase chain reaction
The Verso cDNA Kit (Thermo Scientific) was used to synthesize complementary DNA (cDNA) from purified total RNA as described previously.18 qPCR amplification was performed by using cDNA TaqMan Universal Master Mix II (Applied Biosystems, Foster City, CA). TaqMan Gene Expression assays for following mouse (Mm) and rat (Rt) genes were performed: glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_g1; Rn01775763_g1); p53 upregulated modulator of apoptosis (PUMA; Mm00519268_m1); Noxa (Mm00451763_m1); Bcl-2 interacting mediator of cell death (Bim; Mm00437796_m1); Bcl-2-associated X protein (Bax; Mm00432051_m1); Ang-1 (Mm00456503_m1; Rn01504818_m1); neurogranin (Nrgn; Mm01178296_g1); neurofilament medium polypeptide (Nefm; Mm00456201_m1); and synapsin I (Syn-1; Mm00449772_m1; Applied Biosystems). Primers for tight junction protein 1 (TJP1) and occludin (Ocln) were described previously.33 Reactions were amplified and quantified using a 7900HT Fast Real-Time PCR System and the corresponding software (Applied Biosystems). Gene expression was normalized to GAPDH, and the relative quantity of mRNAs was calculated based on the comparative threshold cycle method.34
MicroRNA reverse transcription
qPCR was used to measure the expression of mature miR-711. A unit of 10 ng of total RNA was reverse transcribed using the TaqMan miRNA Reverse Transcription Kit (Applied Biosystems) with miRNA-specific primers. Reverse-transcription reaction products (1.5 μL) were used for qPCR as described above. TaqMan Gene Expression assays for the following miRs were used: mmu-miR-711 (001646); rno-miR-711 (241136_mat); and U6 snRNA (001973; Applied Biosystems).
Cell lysates preparation and western blot
Whole-cell extracts and western blot were prepared/performed as previously described.35 Chemiluminescence was captured on a ChemiDoc Touch Imaging System (Bio-Rad, Hercules, CA), and protein bands were quantified by densitometric analysis using Image Lab Imaging Software (Bio-Rad). The data presented reflect the intensity of the target protein band compared to the control and were normalized based on the intensity of the endogenous control for each sample (expressed in arbitrary units).
Antibodies
The following antibodies were used in this study: Histone H2A.X (ab11175; Abcam); apoptosis-inducing factor (AIF; sc-13116); cytochrome c (sc-13560) and Bim (sc-11425; Santa Cruz Biotechnology, Santa Cruz, CA); cleaved caspase-3 (#9661), cleaved PARP (poly (ADP-ribose) polymerase-1; #9545); phospho-GSK3α/β (glycogen synthase kinase 3α/β; Ser21/9; #9331); forkhead box O3a (FoxO3a; 75D8; #2497); Akt (pan; 11E7; #4685); FAK (#3285); phospho-FAK (Tyr397; #3283); phospho-p53 (Ser15; #9284); p53 (#2524); mitogen-activated protein kinase kinase 1 and 2 (MEK1/2; #8727); Lamin A/C (#4777); cyclooxegenase IV (COX-IV; #4844); phospho-Akt (Ser473; #4060; Cell Signaling Technology; GAPDH (ADI-CSA-335) and α-fodrin (BML-FG6090); active Bax (ALX-804-224-C100; Enzo Life Sciences, Inc., Farmingdale, NY); PUMA (#3041), Noxa (#2437; ProSci Incorporated, Poway, CA); β-actin (A1978); MAP2 (M3696; Sigma-Aldrich); integrin β1 (MAB1965); GFAP (MAB360; Millipore, Billerica, MA); Ang-1 (GTX28451; GeneTex Inc., Irvine, CA); Tie-2 (#AF762); phospho-Tie-2 (#AF2720; R&D Systems); and ionized calcium-binding adaptor molecule 1 (019-19741; Wako Pure Chemical Industries, Osaka, Japan).
Subcellular fractionation
Subcellular fractionation was performed as described previously,35 with some modifications. RCNs were harvested and washed in ice-cold phosphate-buffered saline. Cell suspension was centrifuged at 500g for 15 min at 4°C. Cell pellet was resuspended for 10 min on ice in the digitonin lysis buffer (20 mM of HEPES [pH 7.4], 80 mM of KCl, 1 mM of EDTA, 1 mM of ethylene glycol tetraacetic acid, 1 mM of DTT, 250 mM of sucrose, 200 μg/mL of digitonin and protease inhibitor and phosphatase inhibitor (2, 3) cocktails; Sigma-Aldrich). The lysate was centrifuged at 1000g for 5 min at 4°C to pellet the nuclei. The supernatant was transferred to a new tube and centrifuged again at 12,000g for 10 min at 4°C to pellet the mitochondria. The resulting supernatant, representing the cytosolic fraction, was recovered. Nuclear and mitochondrial lysates were prepared in radioimmunoprecipitation assay buffer (TEKnova, Hollister, CA) with protease inhibitor cocktail (Sigma-Aldrich). Tissue samples were homogenized in a glass dounce after 20 passes with a 20-G needle. All steps were performed on ice. To check the purity of nuclear and cytosolic fractions, we probed pooled nuclear (N), cytosolic (C) fractions and total lysates (T) samples (20 μg of protein per well) with antibodies against the well-established mitochondrial marker, COX-IV, nuclear marker, Lamin A/C, and cytosolic marker, MEK1/2.
Y-maze spontaneous alternation test
The Y-maze measures the willingness of rodents to explore new environments and assesses spatial working memory. Rodents typically prefer to investigate a new arm of the maze rather than returning to one that was visited previously. The Y-maze test was performed on day 14 post-injury as previously described.36
Magnetic resonance imaging procedure
Twenty-four hours after CCI, mice underwent magnetic resonance (MR) imaging (MRI). All experiments were performed on a Bruker BioSpec 7.0 Tesla 30-cm horizontal bore scanner (Bruker BioSpin, Austin, TX), interfaced to a Bruker Paravision 5.1 console. A four-element 1-H surface coil array was used as the receiver and a 72-mm linear-volume coil as the transmitter. Animals were initially anesthetized using isoflurane (3–4% in O2; 1L/min), then placed prone in a Bruker animal bed. The radiofrequency receiver coil was then centered over the brain and the animal bed was moved to the center of the magnet. At all times during the experiment, the animal was under 1–2% isoflurane anesthesia and 1 L/min oxygen administration. An MR-compatible system was used to monitor respiration rate, and body temperature was maintained at 36–37.5°C, using a circulating warm water heater.
A scout image consisting of three slices (one each in the axial, mid-sagittal, and coronal planes) was used to center the mouse's brain in the imaging field of view. A rapid shimming protocol (FASTMAP) was used to reduce external magnetic field inhomogeneity within the region of interest.37 Both proton density- and T2-weighted coronal images were obtained using two-dimensional rapid acquisition with a relaxation enhancement sequence. The sequence parameters were: repetition time/effective echo time (TEeff1/TEeff2) = 3500/18.9/56.8 ms, number of echoes = 4, matrix size = 256 × 256, slice thickness = 0.5 mm, number of averages = 10, field of view = 20 × 20 mm2, in-plane resolution = 78 μm2, number of slices = 32.
MRI data were quantified using the MIPAV application. The MIPAV (Medical Image Processing, Analysis, and Visualization) application enables quantitative analysis and visualization of medical images of numerous modalities, such as positron emission tomography, MRI, computed tomography, or microscopy, and is provided by the Center for Information Technology at the NIH (https://mipav.cit.nih.gov).
Brain lesion volume was measured on the T2-weighted coronal images using a semiautomatic procedure. Hyperintense areas marking the post-traumatic edema at the lesion site were outlined on each image in the series using the “draw level set VOI (volume of interest)” tool of MIPAV. Extended volume was calculated using the “VOI statistics generator” tool. Volume of the skull and overlying skin was determined by modifying the original contours using the “split VOI contour” tool to mark these regions and was subtracted from the extended volume to produce the final lesion volume.
Immunocytochemistry
At 24 h after injury, mice were anesthetized and transcardially perfused with saline, then 4% buffered paraformaldehyde (PFA) solution (Fisher Scientific). Brains were removed, post-fixed in PFA for 24 h, and protected in 30% sucrose. Frozen brain sections (60 and 20 μm) were cut on cryostat and mounted onto glass slides.
Acute neurodegeneration
Selected slides (20 μm) were stained with Fluoro-Jade B (Chemicon), following the manufacturer's protocol. Imaging and analysis were performed as previously described,38 with some modifications, as follows: Image acquisition was performed using a Nikon Ti-E inverted microscope (Nikon Corporation, Tokyo, Japan), at × 10 (Plan APO 10 × NA 0.45 WD) with NIS-Elements AR software. Fluoro-Jade–positive areas of the injured cortex were scanned using the same acquisition parameters and applying the “large-image” and “stiching” tools. Images were analyzed with the Fiji image processing package based on ImageJ (https://fiji.sc/#cite) to quantify the number of Fluoro-Jade–positive degenerating neurons. The same automatic threshold methods and cell-counting parameters were uniformly applied to all sections using the following Macro: [run(“8-bit”); run (“Auto Threshold”, “method = RenyiEntropy white”); set Option (“BlackBackground”, false); run(“Make Binary”); run(“Analyze Particles…”, “size = 20–200 show = Outlines display clear summrize add”)]. For each brain, we selected three separate sections with the highest number of Fluoro-Jade– positive cells and added them to create a composite number representing degenerating neurons.
Statistical analysis
All data have passed the normality test, and one-way analysis of variance (ANOVA) analysis followed by multiple pair-wise comparisons using the Student–Newman–Keuls (SNK) post-hoc test was performed using Prism software (version 6 for Windows; GraphPad Software Inc., La Jolla, CA). Lesion volume and Fluoro-Jade B–positive cell assessments were analyzed by one-tailed unpaired Student's t-test.
Results
Angiopoietin-1 is downregulated in the mouse brain after traumatic brain injury and in in vitro models of neuronal cell death
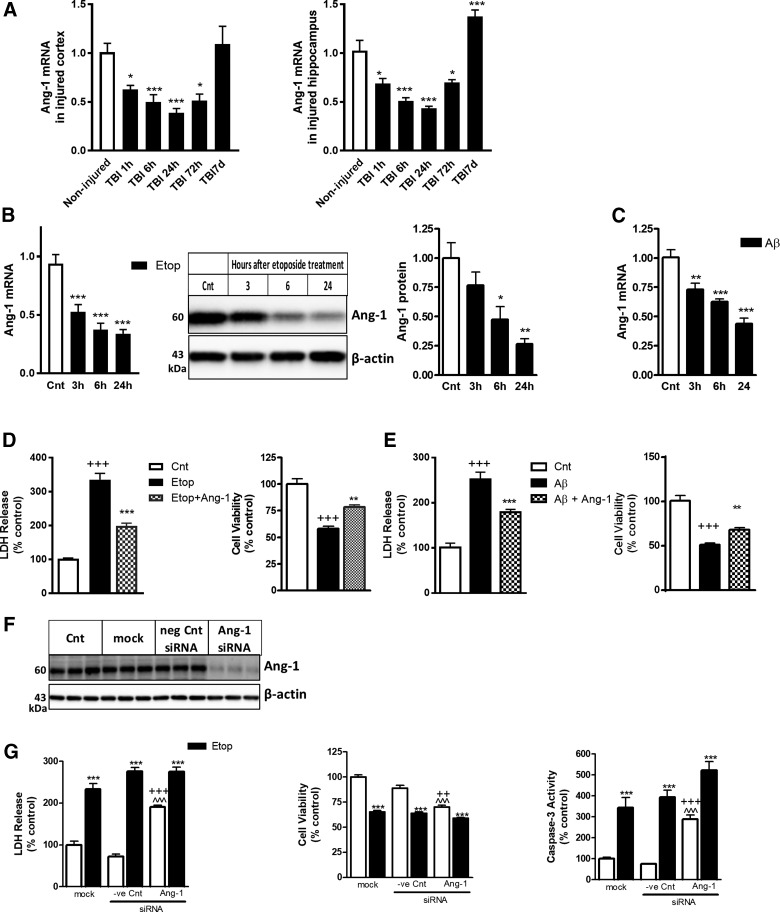
We profiled Ang-1 expression in the injured cortex and hippocampus of mice exposed to moderate-level CCI. In both regions, we observed rapid downregulation of Ang-1 mRNA starting as early as 1 h post-injury, with the lowest level at 24 h; decreased Ang-1 levels persisted through 72 h and returned to control level (cortex) or higher (hippocampus) by 7 days post-injury (Figure 2A). To examine Ang-1 changes during neuronal cell death, we used an in vitro model of primary RCNs whereby apoptosis was induced by etoposide or β-amyloid. Ang-1 levels were determined by qPCR and western blot at different time points. Our data demonstrate significant Ang-1 downregulation at mRNA level as early as 3 h; the magnitude of the decline continued to increase 24 h after apoptosis induction (Fig. 2B,C). Further, etoposide treatment causes a similar pattern of decline in Ang-1 protein levels.
FIG. 2.
Ang-1 is rapidly downregulated after TBI and in in vitro models of neuronal cell death. (A) qPCR for Ang-1 mRNA in cortex and hippocampus after TBI. Data are presented as fold change compared to uninjured controls. Data represent the mean ± SEM (one-way ANOVA, SNK post-hoc analysis); *p < 0.05; **p < 0.01; ***p < 0.001 versus naïve control (N = 5). (B) RCNs were treated with 50 μM of etoposide (Etop). Cell lysates after 3, 6, and 24 h of treatment were fractioned on SDS-polyacrylamide gel and immunoblotted with antibodies against Ang-1 and β-actin. Protein levels were quantified by densitometry, normalized to β-actin, and presented as fold change compared to control untreated levels. qPCR quantification of Ang-1 expressions in RCNs treated with etoposide. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; *p < 0.05; **p < 0.01; ***p < 0.001 versus cnt (N = 5). (C) RCNs were treated with 50 uM of β-amyloid. qPCR quantification of Ang-1 expressions in RCNs treated with β-amyloid at the times indicated. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; *p < 0.05; **p < 0.01; ***p < 0.001 versus cnt (N = 3). (D) Ang-1 is neuroprotective in etoposide models of neuronal cell death. Neurons were treated with etoposide alone or with etoposide and recombinant Ang-1. Twenty-four hours later, LDH release and cell viability were measured. Data are expressed as percentage of control untreated neurons (cnt). Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; +++p < 0.001 versus cnt; **p < 0.01; ***p < 0.001 versus etoposide treated (N = 3). (E) Ang-1 is neuroprotective in β-amyloid models of neuronal cell death. Neurons were treated with β-amyloid alone or with β-amyloid and recombinant Ang-1. Twenty-four hours later, LDH release and cell viability were measured. Data are expressed as percentage of control untreated neurons (cnt). Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; +++p < 0.001 versus cnt; **p < 0.01; ***p < 0.001 versus etoposide treated (N = 3). (F) Silencing Ang-1 caused increase neuronal cell death and caspase-3 activation. RCNs were transfected with Ang-1, negative control (-ve Cnt) siRNA, or mock transfected. Cells were harvested 24 h after treatment. Whole-cell lysates were fractioned on SDS-polyacrylamide gel and immunoblotted with antibodies against Ang-1 and β-actin. (G) RCNs were transfected with Ang-1, negative control (-ve Cnt) siRNA, or mock transfected. Twenty-four hours after transfection, neurons were treated with etoposide. Twenty-four hours later, LDH release, cell viability, and caspase-3–like activity in cell lysates were measured. Data are expressed as percentage of mock transfected cells (mock). Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; ***p < 0.001 versus mock; ^^^p < 0.001 versus etoposide treated; ++p < 0.01; +++p < 0.001 versus −ve Cnt (N = 3). Ang-1, angiopoietin-1; ANOVA, analysis of variance; LDH, lactate dehydrogenase; mRNA, messengter RNA; qPCR, quantitative real-time polymerase chain reaction; RCN, rat cortical neuron; SDS, sodium dodecyl sulfate; SEM, standard error of the mean; siRNA, small interfering RNA; SNK, Student–Newman–Keuls; TBI, traumatic brain injury.
Angiopoietin-1 is neuroprotective in etoposide and β-amyloid models of neuronal cell death
To test the ability of Ang-1 to attenuate apoptosis mechanisms, RCNs were treated with etoposide or β-amyloid with or without recombinant Ang-1 and examined 24 h later. We observed a significant decrease in etoposide and β-amyloid–induced neuronal cell death (lactate dehydrogenase [LDH] release from dying cells) in RCNs treated with Ang-1. Ang-1 treatment also leads to a corresponding increase in neuronal viability (calcein-AM signal from live cells) after etoposide and β-amyloid treatments (Fig. 2D,E). To confirm protective effect of Ang-1, we examined the effect of siRNA-mediated Ang-1 downregulation. RCNs were transfected with Ang-1 targeting siRNA, and after 24 h, cells were harvested for western blot analysis. siRNA transfection caused a significant attenuation of Ang-1 protein expression (Fig. 2F). Separately, RCNs were treated with etoposide 24 h after transfection with Ang-1 siRNA and examined 24 h later. Silencing of Ang-1 increased neuronal cell death in control (no etoposide) RCNs, as shown by increased LDH release, decreased calcein signal, and elevated caspase-3 activity (Fig. 2G), as compared to untransfected and/or control siRNA-transfected cells. Ang-1 siRNA transfection did not further enhance neuronal cell death after etoposide treatment.
Angiopoietin-1 attenuates molecular mechanisms of neuronal apoptosis
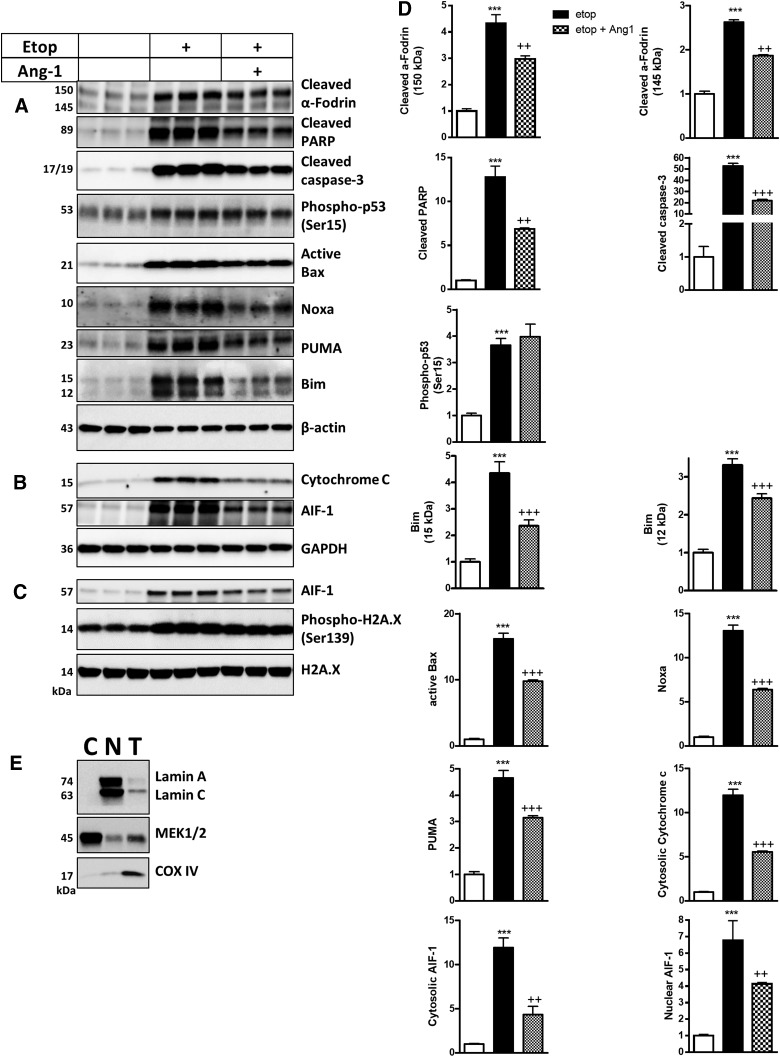
RCNs were treated with etoposide with or without Ang-1 and examined after 24 h. Western blot demonstrated that neurons treated with Ang-1 displayed reduced levels of the markers of apoptosis, including cleaved fragment of caspase-3, cleaved PARP, and cleaved α-fodrin (150/140 kDa), after etoposide treatment (Fig. 3A,D). Western blot demonstrated that early markers of apoptosis, including proapoptotic B-cell lymphoma 2 (Bcl2) family members PUMA, Noxa, Bim, and active Bax protein levels, were significantly increased by etoposide treatment. In contrast, treatment with Ang-1 downregulated the levels of all these proteins (Fig. 3A,D). Etoposide treatment caused upregulation of p53 activation/phosphorylation. Ang-1 treatment did not attenuate activation of p53 (Fig. 3A,D). Etoposide caused DNA damage, resulting in increased histone H2A.X (Ser139) phosphorylation; Ang-1 had no effect on these changes (Fig. 3C). Subcellular fractionation revealed that Ang-1 reduced etoposide-induced release of AIF-1 and cytochrome c from the mitochondria into the cytosol and subsequent translocation of AIF-1 into nucleus (Fig. 3B–D).
FIG. 3.
Ang-1 attenuates molecular mechanisms of neuronal cell death. Neurons were treated with etoposide (Etop) alone or with etoposide and recombinant Ang-1 and harvested 24 h after treatment. Whole-cell lysates (A), cytosolic (B), and nuclear fractions (C) were fractioned on SDS-polyacrylamide gel and immunoblotted with antibodies against PUMA, Noxa, Bim (Bim (Bim small (S) (12 kDa) and Bim large (L) (15 kDa) (Bim extra-large isoform (EL) (25 kDa) was weakly detected), active Bax, cleaved fragment of caspase-3, cleaved (PARP), and α-fodrin, β-actin, AIF-1, cytochrome c, GAPDH, and H2A.X. (D) Protein levels were quantified by densitometry, normalized to β-actin, and presented as fold change compared to control untreated levels. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; *p < 0.05; **p < 0.01; ***p < 0.001 versus cnt; ++p < 0.01; +++p < 0.001 versus etoposide treated (N = 3). (E) To check the purity of nuclear and cytosolic fractions, we probed pooled nuclear (N), cytosolic (C) fractions and total lysates (T) samples (20 μg of protein per well) with antibodies against the well-established mitochondrial marker, COX-IV, nuclear marker, Lamin A/C, and cytosolic marker, MEK1/2. AIF-1, apoptosis-inducing factor 1; Ang-1, angiopoietin-1; ANOVA, analysis of variance; Bax, Bcl-2-associated X protein; Bim, Bcl-2 interacting mediator of cell death; COX-IV, cyclooxygenase 4; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MEK1/2, mitogen-activated protein kinase kinase 1 and 2; PARP, poly (ADP-ribose) polymerase-1; PUMA, p53 upregulated modulator of apoptosis; SDS, sodium dodecyl sulfate; SEM, standard error of the mean; SNK, Student–Newman–Keuls.
Angiopoietin-1 attenuates the inhibition of protein kinase B pathway during neuronal cell death
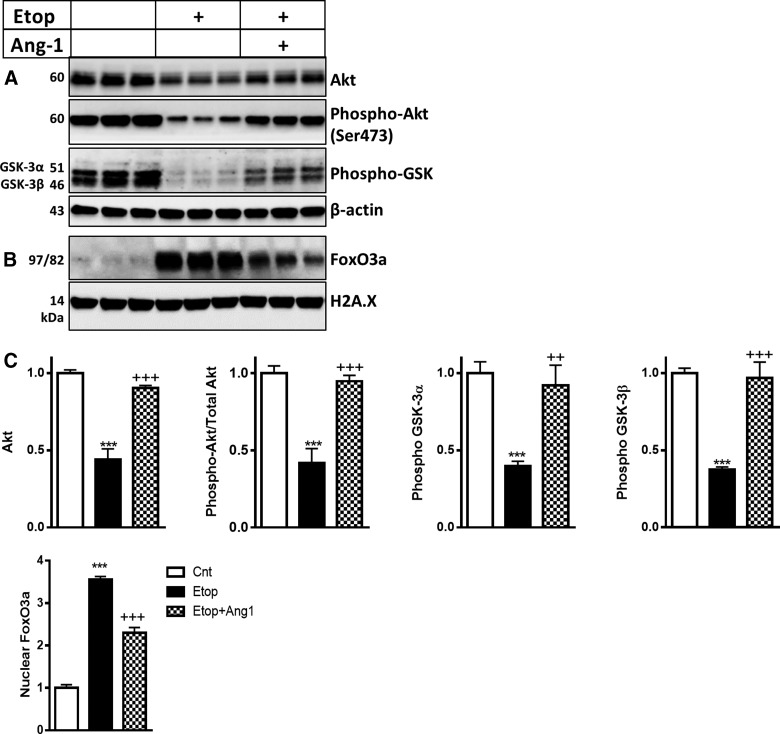
We examined levels of Akt, active/phosphorylated Akt (Ser473), and its downstream targets, such as phosphorylated GSK3α/β and FoxO3a, in an etoposide-induced model of neuronal cell death. Western blot analysis demonstrated that levels of Akt, phosphorylated Akt (Ser473), and phosphorylated GSK3α/β were downregulated after etoposide treatment (Fig. 4A,C). Etoposide treatment also caused increase of FoxO3a translocation in the nucleus (Fig. 4B,C). Treatment of RCNs with Ang-1 attenuated all these changes and reversed the decrease of Akt-specific phosphorylation (normalized to total Akt) after etoposide treatment (Fig. 4A–C).
FIG. 4.
Ang-1 reverses the attenuation of the Akt pathway in neuronal cell death. Neurons were treated with etoposide (Etop) alone or with etoposide and recombinant Ang-1, and harvested 24 h after treatment. Whole-cell lysates (A) and nuclear fractions (B) were fractioned on SDS-polyacrylamide gel and immunoblotted with antibodies against Akt, phospho-Akt (Ser473), phosphor-GSK3α/β β-actin, FoxO3a, and H2A.X. (C) Protein levels were quantified by densitometry, normalized to β-actin (whole cell lysates) or to H2A.X (nuclear fractions), and presented as fold change compared to control untreated levels. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; *p < 0.05; **p < 0.01; ***p < 0.001 versus cnt; ++p < 0.01; +++p < 0.001 versus etoposide treated (N = 3). Akt, protein kinase B; Ang-1, angiopoietin-1; ANOVA, analysis of variance; FoxO3a, forkhead box O3a; GSK3α/β, glycogen synthase kinase 3α/β; SDS, sodium dodecyl sulfate; SEM, standard error of the mean; SNK, Student–Newman–Keuls.
Angiopoietin-1 activates protein kinase B pathway in neurons through Tunica interna endothelial cell kinase 2 and β1-integrin signaling
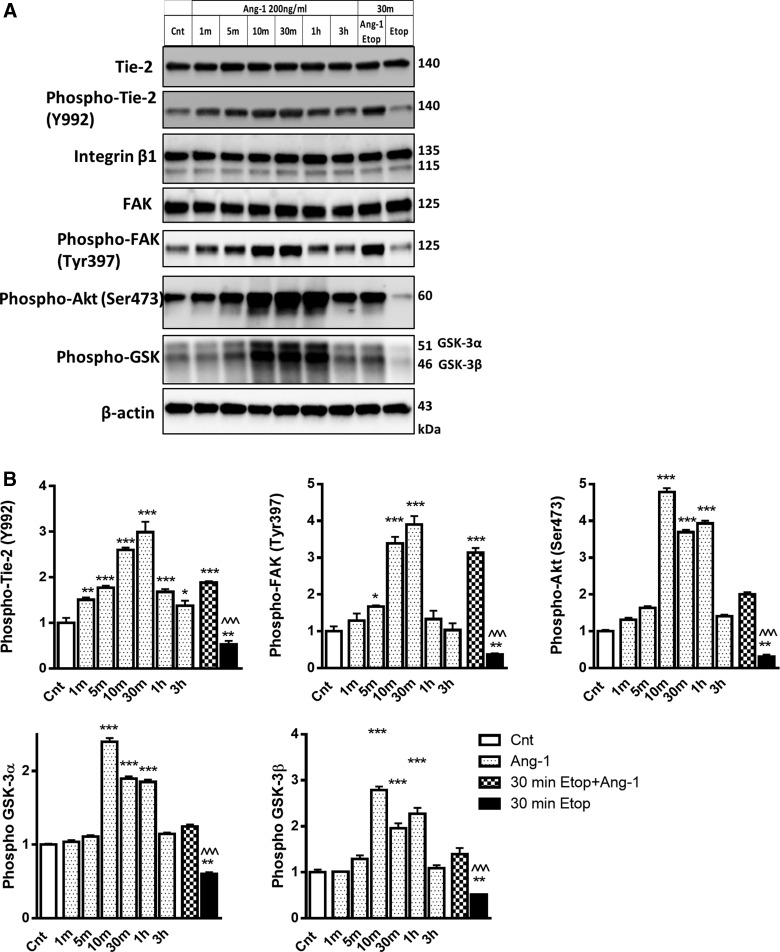
To determine the upstream mechanisms of Ang-1signaling, we examined dynamic changes in activation of Tie-2 and integrin signaling pathways. RCNs were treated with Ang-1 and then levels of Tie-2, phospho-Tie-2 (Y992), integrin β1, FAK, phospho-FAK, phosphorylated Akt, and phosphorylated GSK3α/β were measured at 1, 5, 10, 30 min, 1 h, and 3 h time points. Levels of these proteins were also measured after 30 min of etoposide with or without Ang-1 treatment (Fig. 5). Neither Ang-1 nor etoposide treatments changed levels of Tie-2 and integrin β1 (Fig. 5A). However, Ang-1 caused rapid upregulation of phospho-Tie-2 (Y992) and phospho-FAK as early as 1 and 5 min after Ang-1 treatment, respectively. Peak levels occurred at 30 min for both proteins, and as phospho-Tie-2 remained elevated up to 3 h after Ang-1 treatment, the level of phospho-FAK went back to pre-treatment level within 1 hour (Fig. 5A,B). Ang-1 treatment also caused increase of Akt and GSK3α/β phosphorylation that peaked at 10 min, remained elevated at 1 h, and returned toward control levels by the 3-h time point (Fig. 5A,B). A short 30-min etoposide treatment caused significant downregulation of Tie-2, FAK, Akt, and GSK3α/β and phosphorylation, changes that were significantly reversed by Ang-1 (Fig. 5A,B).
FIG. 5.
Ang-1 activates the Akt pathway in neurons through both Tie-2 and β1-integrin signaling. (A) RCNs were treated with Ang-1 for the indicated time. Whole-cell lysates were fractioned on SDS-polyacrylamide gel and immunoblotted with antibodies against Tie-2, phosphor-Tie2 (Y992), β1-integrin, FAK, phosphor-FAK (Tyr397), phospho-Akt (Ser473), phosphor-GSK3α/β, and β-actin. (B) Protein levels were quantified by densitometry, normalized to β-actin, and presented as fold change compared to control untreated levels. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; *p < 0.05; **p < 0.01; ***p < 0.001 versus cnt; ^^^p < 0.001 versus etoposide treated (N = 3). Akt, protein kinase B; Ang-1, angiopoietin-1; ANOVA, analysis of variance; FAK, focal adhesion kinase; GSK3α/β, glycogen synthase kinase 3α/β; RCNs, rat cortical neurons; SDS, sodium dodecyl sulfate; SEM, standard error of the mean; SNK, Student–Newman–Keuls; Tie-2, Tunica interna endothelial cell kinase 2.
Tunica interna endothelial cell kinase 2 and β1-integrin signaling inhibitors attenuate angiopoietin-1–mediated neuroprotection
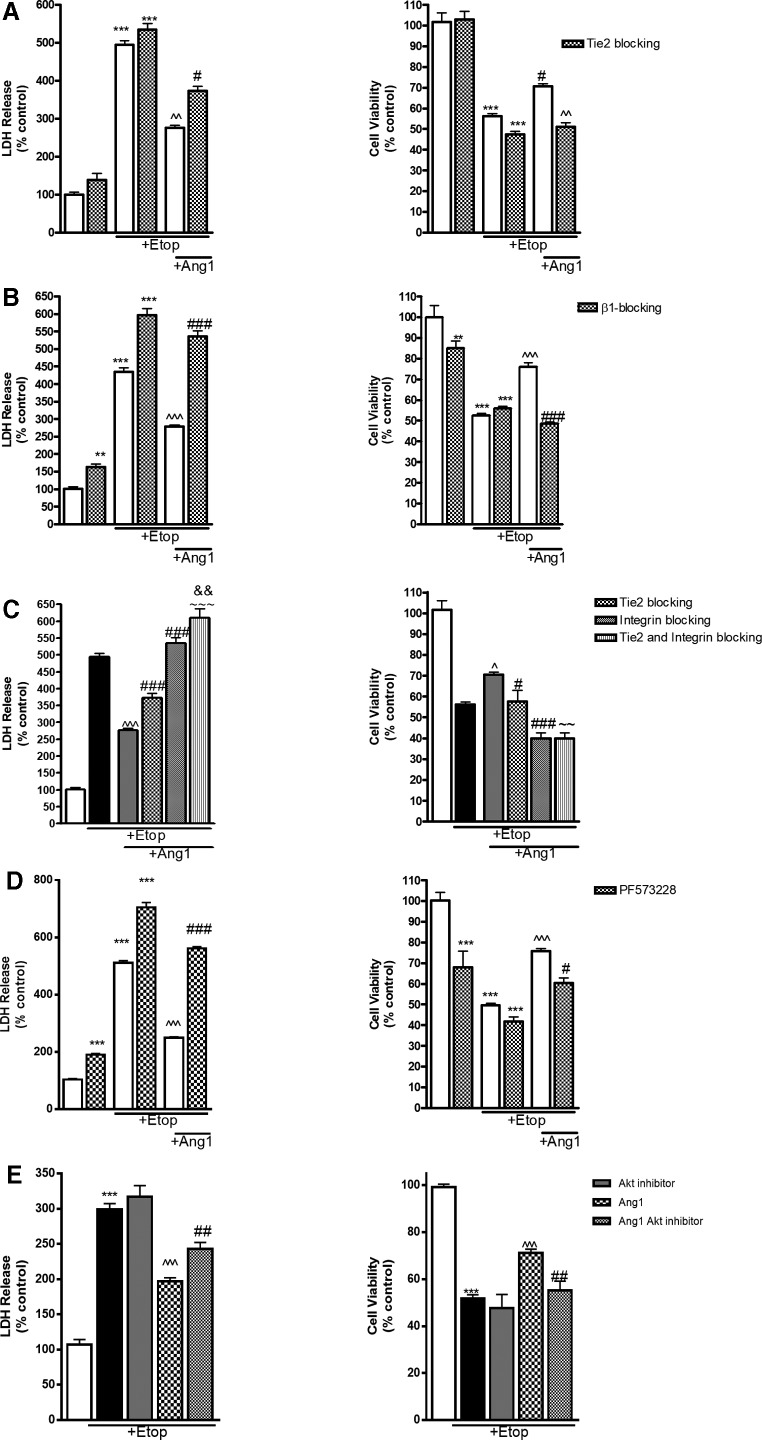
To demonstrate the role of β1-integrin and Tie2 signaling in the activation of Ang-1–mediated Akt prosurvival pathway, RCNs were treated for 30 min with neutralizing antibodies against receptors β1-integrin (5 μg/mL) and Tie2 (50 μg/mL; R&D Systems), or control immunoglobulin G (IgG; 50 μg/mL). Separately, we examined the effect of several inhibitors of receptor signal transduction, including phospholipase C (PLC) inhibitor U73122 (1μM), U73343 (1μM) as control/inactive analog of PLC inhibitor, and FAK inhibitor PF573228 (100 nM). Next, RCNs were treated with etoposide with or without Ang-1. Cell death and viability were analyzed 24 h after treatment. We observed a significant decrease in etoposide-induced neuronal cell death in RCNs treated with Ang-1. Importantly, treatment with β1-integrin and Tie-2 antibodies as well as PF573228 attenuated the neuroprotective effect of Ang-1 (Fig. 6). We did not observe any neuroprotective effect of control IgG (data not shown). Combined treatment with β1-integrin and Tie2 blocking antibodies increased neuronal cell death compared to either β1-integrin and Tie2 blocking antibodies alone, and completely overturned the neuroprotective effect of Ang-1 (Fig. 6C). Interestingly, we did not detect any effects of U73122 and U73122 on neuronal cell death (data not shown).
FIG. 6.
Blocking of Tie-2 and β1-integrin signaling inhibits the neuroprotective effect of Ang-1. RCNs were treated for 30 min with antibodies against Tie2 (50 μg/mL; A); antibodies against β1-integrin (5 μg/mL; B); combination of Tie2 (50 μg/mL) and β1-integrin (5 μg/mL; C) and FAK inhibitor PF573228 (100 nM; D). Next RCNs were treated with Ang-1 and etoposide (Etop) or etoposide alone. Twenty-four hours later, LDH release was measured. Data are expressed as percentage of control untreated neurons. (E) RCNs were treated with etoposide alone; Ang-1 and etoposide and etoposide; and Ang-1 etoposide and Akt inhibitor (2.875 μM). Cell death and cell viability were measured using the LDH and Calcein AM (calcein-acetoxymethyl ester). Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; *p < 0.05; **p < 0.01; ***p < 0.001 versus cnt; ^^p < 0.01; ^^^p < 0.001 versus etoposide treated; #p < 0.05; ##p < 0.01; ###p < 0.001 versus Ang-1 + etoposide treated; ∼∼p < 0.01; ∼∼∼p < 0.001 versus Tie-2 blocking/Ang-1/etoposide treated; &&p < 0.01 versus β1-integrin blocking/Ang-1/etoposide treated (N = 3). Akt, protein kinase B; Ang-1, angiopoietin-1; ANOVA, analysis of variance; FAK, focal adhesion kinase; LDH, lactate dehydrogenase; RCNs, rat cortical neurons; SEM, standard error of the mean; SNK, Student–Newman–Keuls; Tie-2, Tunica interna endothelial cell kinase 2.
Protein kinase B inhibition attenuates the neuroprotective effects of angiopoietin-1
To test the role of Akt activation for Ang-1–mediated neuroprotection, we used a suboptimal dose of an Akt inhibitor (2.875 μM). This intervention in isolation does not induce neuronal cell death nor does it enhance etoposide-induced cell death, but becomes effective when cell survival is primarily dependent on the Akt pathway. Neuronal death after etoposide treatment was reduced by Ang-1 (Fig. 6E). Akt inhibitor attenuated, but did not completely block, the Ang-1 neuroprotective effects.
MicroRNA-711 is upregulated the mouse brain after traumatic brain injury and in in vitro model of neuronal cell death
Ang-1 is a predicted target for miR-711. We performed a detailed expression profiling of miR-711 in the injured cortex, hippocampus, and in an in vitro model of etoposide-induced apoptosis in cortical neurons by qPCR using the same samples that were analyzed for changes in the levels of Ang-1 shown in Figure 2A,B.
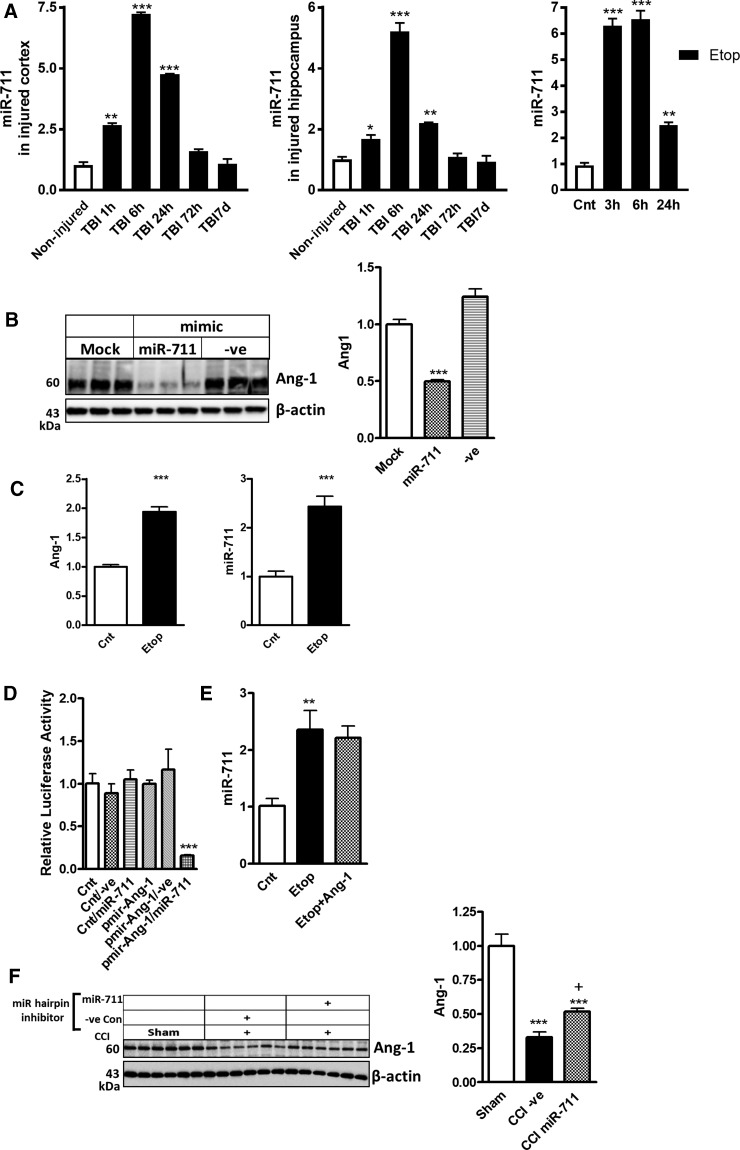
In both cortex and hippocampus, we observed rapid upregulation of miR-711 starting as early as 1 h post-injury, with the highest level at 6 h; the increased miR-711 levels persisted through 24 h and returned to control level by 72 h post-injury (Fig. 7A). To examine miR-711 changes during neuronal cell death, we used an in vitro model of primary RCNs whereby apoptosis was induced by etoposide. miR-711 level was determined by qPCR at different time points. Our data demonstrate significant miR-711 upregulation as early as 3 h (Fig. 7A).
FIG. 7.
miR-711 downregulates expression of Ang-1. (A) qPCR for miR-711 in cortex and hippocampus after TBI and etoposide (Etop) treatment of RCN was performed as described in Figure 1A,B. (B) miR-711 mimic downregulates Ang-1 expression. RCNs were transfected with miR-711, negative control (-ve Con) mimics, or mock transfected. Neurons were harvested 24 h after transfection. Whole-cell lysates were fractioned on SDS-polyacrylamide gel and immunoblotted with antibodies against Ang-1 and β-actin. Protein levels were quantified by densitometry, normalized to β-actin, and presented as fold change compared to control untreated levels. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; ***p < 0.001 versus mock transfected (N = 3). (C) Etoposide treatment increased levels of miR-711 and Ang-1 within the RISC. Neurons were collected 6 h after etoposide treatment, subjected to RIP with Ago2 antibodies, and samples used for qPCR analysis. qPCR quantification of miR-711 and Ang-1 levels in precipitates after RIP. Data represent the mean ± SEM. Student's t-test; ***p < 0.001 versus untreated RCNs. (D) miR-711 targets 3′-UTRs of Ang-1 mRNA. RCNs were transfected with either control vector (pmirGLO) or pmir-Ang-1 reporter plasmids and co-transfected with either negative control miR (−ve) or miR-711 mimic. Luciferase activity was analyzed 24 h after transfection. Normalized luciferase activities are shown as the percentage relative to cells transfected with plasmid alone, which was set as 100%. Experiments were performed in triplicate. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; ***p < 0.001 versus RCN co-transfected with pmir-Ang-1 reporter plasmid and negative control miR (−ve) mimic (N = 3). (E) Ang-1 does not attenuate etoposide-induced miR-711 upregulation. Neurons were treated with etoposide alone or with etoposide and recombinant Ang-1 for 24 h. Level of miR-711 was quantified by qPCR. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc analysis; **p < 0.01 versus cnt. (F) Central administration of miR-711 hairpin inhibitor after TBI increased the level of Ang-1. Whole-tissue lysates from mouse cortex 24 h after TBI and i.c.v. administration of miR-711 or −ve Con hairpin inhibitors were fractioned on SDS-polyacrylamide gel and immunoblotted with antibodies against Ang-1 and β-actin. Protein levels of were quantified by densitometry, normalized to β-actin, and presented as fold change compared to noninjured controls. Data represent the mean ± SEM. One-way ANOVA. SNK post-hoc test, ***p < 0.001 versus noninjured; +p < 0.05 vs. −ve Con miR inhibitor CCI group (N = 6). Ago2, argonaute 2, RISC catalytic component; Ang-1, angiopoietin-1; ANOVA, analysis of variance; i.c.v., intracerebroventricular; miR, microRNA; mRNA, messengter RNA; qPCR, quantitative real-time polymerase chain reaction; RCN, rat cortical neuron; RIP, RNA-binding protein immunoprecipitation; RISC, RNA-induced silencing complex; SDS, sodium dodecyl sulfate; SEM, standard error of the mean; SNK, Student–Newman–Keuls; TBI, traumatic brain injury; 3′-UTRs, 3′ untranslated regions.
MicroRNA-711 downregulates angiopoietin-1 expression
To investigate whether miR-711 regulates Ang-1 expression, RCNs were transfected with either negative control or miR-711 mimic. Western blot showed that levels of Ang-1 significantly decreased 24 h after transfection with miR-711 mimic (Fig. 7B).
We used RNA-interacting protein immunoprecipitation (RIP) with Ago2 antibodies to confirm the role of miR-711 in silencing Ang-1 through RISC. RCNs were treated with etoposide, and cells were collected after 6 h and subjected to RIP using Ago2 antibodies. qPCR demonstrated significant and similar increases of miR-711 and Ang-1 mRNA in the RISC complex after etoposide treatment (Fig. 7C). To demonstrate the specificity of miR-711 regulation of Ang-1 mRNA, we inserted the 3′-UTRs of mouse Ang-1 3′ of the firefly luciferase gene into pmirGLO plasmid (pmir-Ang-1). pmirGLO (control) and pmir-Ang-1 were co-transfected with either negative control or miR-711 mimics into RCNs. Luciferase activity was reduced in cells transfected with pmir-Ang-1 24 h after transfection with miR-711, but not negative control miR mimic (Fig. 7D). These data demonstrate that miR-711 targets the 3′-UTR of Ang-1 mRNA and inhibits expression of Ang-1.
To test existence of the feed-forward loop Ang-1/miR-711 signal self-amplification, RCNs were treated with etoposide with or without Ang-1. qPCR demonstrated that Ang-1 treatment had no significant impact on the level of miR-711 (Fig. 7E).
Finally, we administered 0.5 nmols of miR-711 hairpin inhibitor or negative control miR hairpin inhibitor by i.c.v. injection in mice 15 min after CCI and examined the expression of Ang-1. TBI followed by negative control miR significantly decreased Ang-1. In contrast, treatment of TBI mice with an miR-711 hairpin inhibitor significantly attenuated the injury-induced Ang-1 downregulation (Fig. 7F).
Central administration of angiopoietin-1 decreased markers of apoptosis 24 h after traumatic brain injury
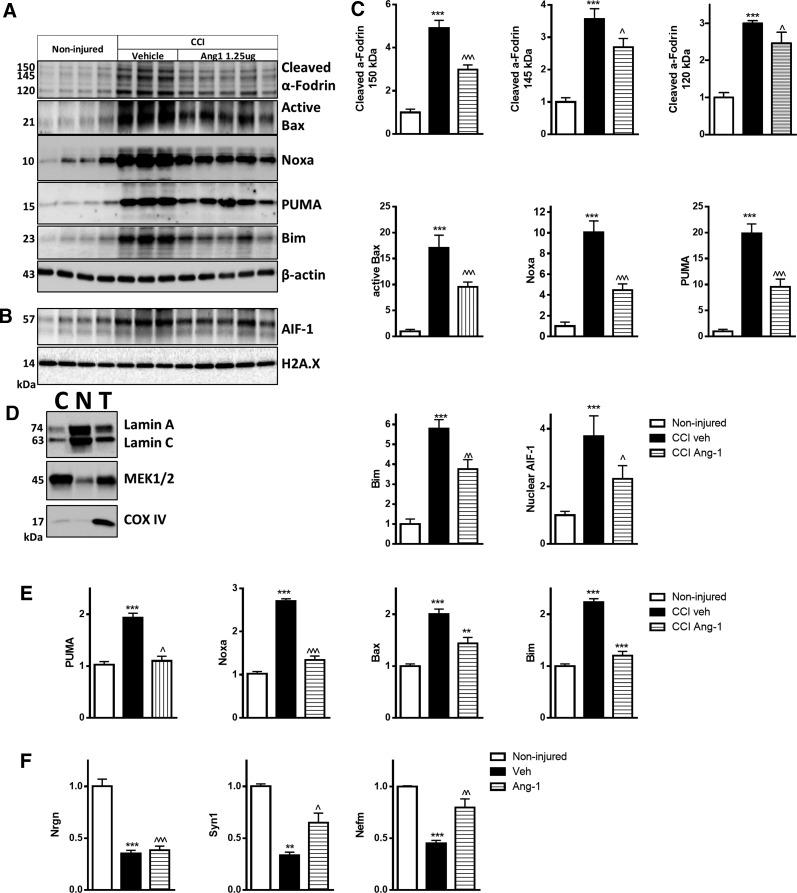
TBI resulted in increased levels of proapoptotic proteins PUMA, Noxa, active Bax, and large isoform of Bim (Fig. 8A,C). Treatment with Ang-1 significantly attenuated these changes (Fig. 8A,C). Ang-1 also attenuated AIF-1 translocation to the nucleus and levels of the cleaved fragments of α-fodrin after TBI (Fig. 8A–C). Neuroprotective effects of Ang-1 after TBI were also detectable in the hippocampus, where qPCR demonstrated that Ang-1 treatment reduced TBI-induced upregulation of Puma, Noxa, Bax, and Bim (Fig. 8E). TBI caused decreased mRNA levels of Nefm, a neuronal marker, as well as the synaptic markers, Syn1 and Nrgn,39,40 in injured cortex. Ang-1 treatment attenuated downregulation of these genes (Fig. 9F). Decreased expression of these neurotypic molecules serves as an index of neuronal damage.41
FIG. 8.
Central administration of Ang-1 attenuates molecular mechanisms of neuronal apoptosis after TBI. (A) Whole-cell lysates from mouse cortex 24 h after TBI and i.c.v. administration of Ang-1 or vehicle (ACSF) were run on SDS-polyacrylamide gel and immunobloted with antibodies against α-fodrin, PUMA, Noxa, Bim (Bim small (S) (12 kDa) and Bim large (L) (15 kDa) isoforms were weakly detected. Bim extra-large isoform (EL; 25 kDa), active Bax, and β-actin. (B) Nuclear fractions were run on SDS-polyacrylamide gel and immunoblotted with antibodies against AIF-1 and H2A.X. (C) Protein levels were quantified by densitometry, normalized to housekeeping proteins, and presented as fold change compared to noninjured controls. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc test; ***p < 0.001 versus noninjured; ^p < 0.05; ^^p < 0.01; ^^^p < 0.001 versus vehicle CCI group (noninjured, N = 4; vehicle CCI, N = 3; ANG-1 CCI, N = 5). (D) To check the purity of nuclear and cytosolic fractions, we probed pooled nuclear (N), cytosolic (C) fractions, and total lysates (T) samples (20 μg of protein per well) with antibodies against the well-established mitochondrial marker, COX-IV, nuclear marker, Lamin A/C, and cytosolic marker, MEK1/2. (E) Injured hippocampus samples from the same animals used for qPCR analysis. qPCR quantification of Puma, Noxa, Bax, and Bim mRNA levels. (F) qPCR quantification of Nefm, Syn1, and Nrgn mRNA levels in injured cortex. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc test; ***p < 0.001 versus noninjured; ^p < 0.05; ^^p < 0.01; ^^^p < 0.001 versus vehicle CCI group (noninjured, N = 4; vehicle CCI, N = 3; ANG-1 CCI, N = 5). ACSF, artificial cerebrospinal fluid; AIF-1, apoptosis-inducing factor 1; Ang-1, angiopoietin-1; ANOVA, analysis of variance; Bax, Bcl-2-associated X protein; Bim, Bcl-2 interacting mediator of cell death; CCI, controlled cortical impact COX-IV, cyclooxygenase 4; i.c.v., intracerebroventricular; MEK1/2, mitogen-activated protein kinase kinase 1 and 2; mRNA, messengter RNA; Nefm, neurofilament medium polypeptide; Nrgn, neurogranin; PUMA, p53 upregulated modulator of apoptosis; qPCR, quantitative real-time polymerase chain reaction; SDS, sodium dodecyl sulfate; SEM, standard error of the mean; SNK, Student–Newman–Keuls; Syn1, synapsin I; TBI, traumatic brain injury.
FIG. 9.
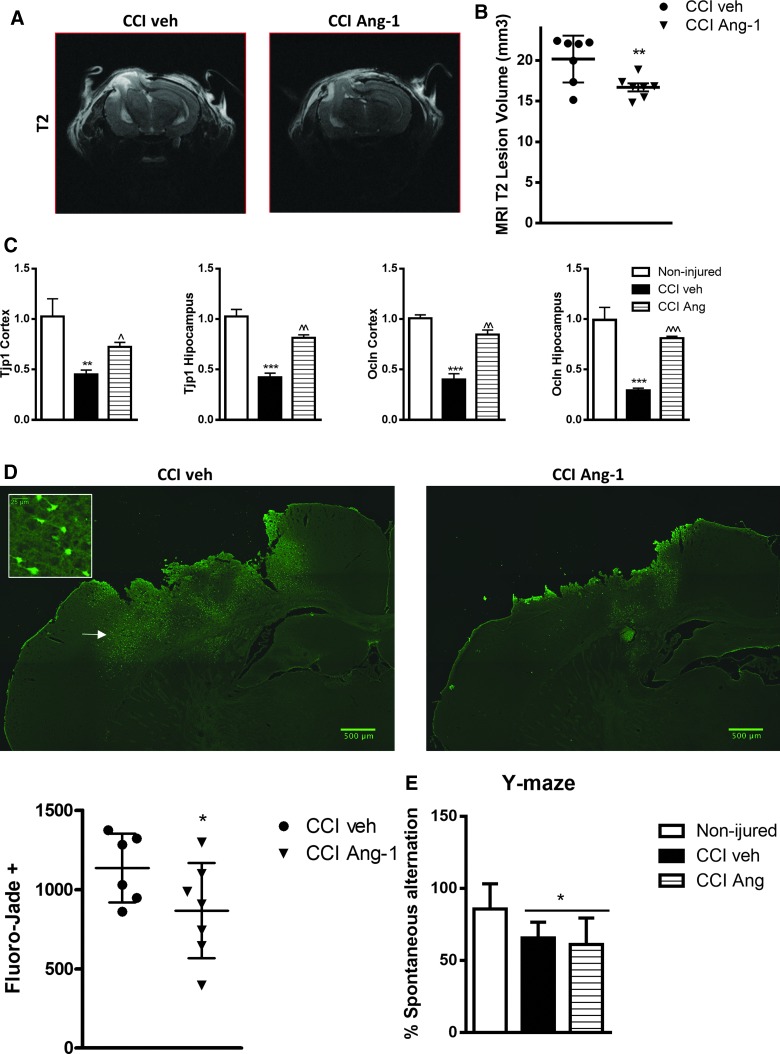
Treatment with Ang-1 significantly reduced neurodegeneration in 24 h after TBI. (A and B) Post-injury TBI-induced edema/lesion volume was quantified by T2-weighted MRI at 24 h after CCI. Treatment with Ang-1 significantly reduced the lesion volume compared to the CCI veh group (p < 0.01; CCI veh, N = 7; CCI Ang-1, N = 7). (C) qPCR quantification of Tjp1 and Ocln mRNA levels in injured cortex and hippocampus. Data represent the mean ± SEM. One-way ANOVA, SNK post-hoc test; ***p < 0.001 versus noninjured; ^p < 0.05; ^^p < 0.01; ^^^p < 0.001 versus vehicle CCI group (noninjured, N = 4; vehicle CCI, N = 3; ANG-1 CCI, N = 5). (D) Fluoro-Jade B staining at 24 h post-injury of the injured hemispheres is displayed; magnified area whose localization is indicated by arrow illustrates the neuronal morphology/origin of the Fluoro-Jade–positive cells. Injury-induced neurodegeneration, indicated by higher number of Fluoro-Jade B–positive cells, was significantly attenuated by Ang-1 treatment (p < 0.05; CCI veh, N = 6; CCI Ang-1, N = 7). Analysis by one-tailed unpaired Student's t-test. Individual values for the experimental animals are displayed. (E) Treatment with Ang-1 did not improve TBI-induced long-term cognitive function deficits. Noninjured mice showed intact spatial working memory function and performed significantly better than the 50% chance level (N = 8). CCI veh and Ang-1 CCI groups showed significantly reduced percentages of spontaneous alternation (*p < 0.05; N = 10). No significant differences were observed between veh and Ang-1 groups. One-way ANOVA, SNK post-hoc test. Ang-1, angiopoietin-1; ANOVA, analysis of variance; CCI, controlled cortical impact; MRI, magnetic resonance imaging; mRNA, messengter RNA; Ocln, occludin; qPCR, quantitative real-time polymerase chain reaction; SEM, standard error of the mean; SNK, Student–Newman–Keuls; TBI, traumatic brain injury; Tjp1, tight junction protein 1. Color image is available online at www.liebertpub.com/neu
Treatment with angiopoietin-1 reduced lesion volume and neurodegeneration 24 h after traumatic brain injury
TBI-induced acute edema/contusion volume was quantified by MRI 24 h after CCI. Treatment with Ang-1 significantly reduced the volume of hyperintense T2-weighted signal compared to the CCI vehicle (veh) group (Fig. 9A,B; p < 0.01; CCI veh = 20.20 ±1.087 mm3 and CCI Ang-1 = 16.71 ± 0.4928 mm3, mean ± standard error of the mean [SEM]). To determine the specific effects of Ang-1 treatment on CCI-induced acute neurodegeneration, we performed Fluoro-Jade B staining (marker of neuronal degeneration) at 24 h post-injury (Fig. 9D). Analysis showed that the number of injury-induced Fluoro-Jade B–positive cells was significantly attenuated by Ang-1 treatment (Fig. 9B,D; p < 0.05; CCI veh = 1136 ± 88.72 cells and CCI Ang-1 = 866.9 ± 113.5 mm3; mean ± SEM).
Treatment with angiopoietin-1 attenuated the decrease of tight junction protein expression 24 h after traumatic brain injury
We examined the effect of Ang-1 on expression of tight junction proteins (TJs) after TBI by qPCR. TBI decreased mRNA levels of ZO-1 gene (TJP1) and Occludin (Ocln) in injured cortex and hippocampus. Ang-1 treatment attenuated the downregulation of these genes (Fig. 9C). Decreased expression of these genes may lead to brain–blood barrier (BBB) disruption, contributing to edema formation.42
Treatment with angiopoietin-1 did not significantly attenuate chronic injury outcomes
The Y-maze spontaneous alternation test was performed on day 14 post-injury to assess spatial working memory (Fig. 9E). Both vehicle-treated and Ang-1–treated TBI mice showed a significant reduction of spontaneous alternation compared to controls. But Ang-1–treated TBI mice did not show increased spontaneous alternation compared to the vehicle-treated TBI group. To assess long-term motor function recovery after TBI, Ang-1–treated TBI and vehicle-treated TBI mice were tested on the beam walk task.24 We observed significant differences between the control and TBI groups on all days post-injury. However, administration of Ang-1 did not improve motor recovery after TBI when compared to the vehicle-treated TBI group (data not shown). Lesion volume estimation of missing tissue at 28 days after TBI has been performed as described.38,43 We did not observe significant differences in TBI-induced lesion in Ang-1– versus vehicle-treated experimental animals (data not shown).
Discussion
Changes in expression of angiogenic genes in response to central nervous system (CNS) injury have complex effects on outcome. Vascular endothelial growth factor (VEGF) is rapidly upregulated after brain ischemic or contusion injury and increased levels persist for several weeks—promoting angiogenesis and recovery, but also resulting in BBB leakage, post-traumatic vasogenic edema, and an inflammatory response leading to secondary injury.44 Ang-1 expression displays a different profile, rapidly decreasing after cerebral ischemia followed by prolonged upregulation starting 72 h post-ischemia.45,46 Interestingly, Ang-1 is not downregulated in the blood of patients after TBI.47,48 There is no evidence that the brain and, specifically, neurons are a main source of Ang-1 in the blood. Thus, Ang-1 changes in the brain after TBI may not match Ang-1 systemic changes. The profile and mechanisms of Ang-1 changes in the blood after experimental and clinical TBI should be topic of future investigations. It has been suggested that Ang-1 protects the vasculature against leakage, opposing VEGF effects of on vascular permeability.49 Thus, the early VEGF/Ang-1 imbalance may contribute to the pathobiology of acute cerebral ischemia.49 In contrast, the late upregulation of both Ang-1 and VEGF may promote repair- supporting processes such as angiogenesis and vascular remodeling.49 We observed a similar Ang-1 profile after experimental TBI, with rapid downregulation of Ang-1 expression in injured cortex and hippocampus during the first 3 days after TBI followed by an increase by 7 days. Ang-1 and -2 are expressed by many cell types, including endothelial cells, leukocytes, macrophages, platelets, and astrocytes.49,50 The neuroprotective effects resulting from modulation of the acute Ang-1 downregulation, as observed by us, may, in part, reflect attenuation of trauma-induced downregulation of TJs and BBB disruption, consistent with the observed reduction in acute post-traumatic contusion/edema volume identified by T2-weighted MRI analysis.
Because TBI causes activation of neuronal cell death pathways,51 their potential modulation by Ang-1 was a focus of our investigation. Previous studies have showed that Ang-1 inhibits apoptosis in endothelial cells5,6 and in zinc or serum deprivation-induced models of neuronal cell death.9,10 DNA damage, including DNA breaks produced by oxidative injury, is a key inducer of neuronal cell death after TBI27 and results in rapid phosphorylation of H2A.X.30 Etoposide is an anticancer drug that produces DNA breaks and neuronal caspase-dependent and -independent apoptosis.28 We show that rapid Ang-1 decline is an important feature of apoptosis in etoposide and β-amyloid neuronal in vitro models and after TBI; Ang-1 administration attenuates neuronal cell death in these models and supports basal neuronal viability. Future studies should examine Ang-1 neuroprotection in other models of neuronal cell death in vitro and in vivo. We demonstrate, both in vitro and after TBI, that Ang-1 administration attenuates the upregulation of proapoptotic Bcl-2 molecules as well as caspase-dependent and -independent apoptosis pathways. These findings suggest that downregulation of Ang-1 may be an important element of the neuronal cell death program. Upregulation of proapoptotic Bcl2 family molecules in apoptosis is mediated by molecules such as p5352,53 and forkhead box, class O (FoxO) transcription factors.17 We demonstrated increased p53 Ser15 phosphorylation (activation) after etoposide treatment in primary neurons, consistent with our previous observations.18,54,55 However, Ang-1 treatment did not impact these changes, indicating that its attenuation of apoptosis likely occurs downstream and/or in parallel to p53 activation. In contrast, Ang-1 neuroprotective effects appear to be, at least in part, attributed to inhibition of FoxO3a proapoptotic activity.
Akt can attenuate apoptosis through phosphorylation/inhibition of caspases, proapoptotic Bcl2 family molecules, and selected transcription factors.17 Akt phosphorylates/inactivates the FoxO3a transcription factor by inhibiting its translocation to the nucleus and transcription of proapoptotic proteins such as Bim and PUMA.56 Akt also phosphorylates/inactivates GSK3α/β, which may also promote FoxO3a activity and PUMA expression.57 We previously demonstrated that neuronal apoptosis may be attributed to Akt inactivation followed by sequential activation of GSK3α/β, FoxO3a, and proapoptotic Bcl2 family molecules.18 We showed that treatment with Ang-1 reversed the etoposide-induced decrease of Akt-specific phosphorylation, increasing its activity and inhibiting its proapoptotic targets. Thus, Ang-1 downregulation may suppress the Akt neuroprotective pathway after neuronal injury, consistent with previous observations that Ang-1 attenuates neuronal apoptosis induced by serum deprivation through activation of the PI3K/Akt pathway.9
Earlier studies have suggested that Ang-1 signals through Tie-2 and Integrin receptors.9,10 Ang-1–mediated phosphorylation of Tie-2 leads to activation of the PI3K/Akt pathway. We identified Tie-2 receptors in neurons and demonstrate that Ang-1 causes phosphorylation/activation of Tie-2 at Tyr992 whereas etoposide inhibits Tie-2 phosphorylation, likely through Ang-1 downregulation. Recombinant Ang-1 rescues Tie-2 phosphorylation after etoposide treatment. Blocking of Tie-2 using antibodies inhibits the neuroprotective effect of Ang-1 after etoposide treatment. Akt signaling can be also activated by β1-integrin, promoting cell survival and inhibiting apoptosis.30 Ang-1 promotes PC12 cell survival and induces neurite outgrowth in a β1-integrin–dependent manner.30 Phosphorylation/activation of FAK on Tyr397 is a key step of Ang1/β1-integrin signaling pathways.30,58,59 We demonstrate that Ang-1 leads to rapid FAK phosphorylation in primary neurons and that the rapid profiles of Tie-2 and FAK phosphorylation correlate with the Ang-1–induced phosphorylation of downstream Akt pathway molecules. Ang-1 also rescued FAK activation after etoposide treatment. Previous studies have also shown that Ang-1 neuroprotection against zinc toxicity is mediated by the Ang-1/integrin/FAK pathway and includes PLC-mediated hydrolysis of PIP2.10
We demonstrate that blocking β1-integrin as well as inhibiting FAK reversed the neuroprotective effect of Ang-1 after etoposide treatment. However, we observed no changes when a PLC inhibitor was used (data not shown), suggesting that PLC did not play a significant role in our model. Importantly, combined blockade of both Tie-2 and β1-integrin significantly enhanced neuronal cell death compared to individual interventions and completely inhibited the neuroprotective effect of Ang-1, indicating that both receptors contribute to Ang-1 neuroprotective effects. Inhibition of Akt activity attenuates Ang-1 effects, indicating the importance of this pathway for Ang-1 mechanisms. Interestingly, Akt inhibition only partially reverses Ang-1 neuroprotection, suggesting the potential involvement of alternative mechanisms such as the ras/mitogen-activated protein kinase pathway.60
The role of miRs in modulation of neuronal cell death has been described for various models of CNS injury.18,54,55 Previously, we demonstrated that miR-711 is upregulated in cortex after TBI and in in vitro models of neuronal apoptosis and showed that miR-711 induces neuronal cell death by directly targeting Akt mRNA.20
It has been shown that upregulation of miR-711 correlated with downregulation of Ang-1 in ischemia reperfusion–injured heart grafts and hypoxia/reperfusion treated primary cardiomyocytes, suggesting that miR-711 may target Ang-1.61 We demonstrate rapid upregulation of miR-711 in the injured cortex and after etoposide treatment of RCNs (consistent with our previous data20) as well as in the injured hippocampus. Importantly we show that upregulation of miR-711, in all these cases, coincided with downregulation of Ang-1. Our mRNA target prediction analysis identified Ang-1 as a target for miR-711 not only in rodents, but also in humans. We demonstrated that miR-711 can specifically target the 3′-UTR of Ang-1 mRNA and inhibit its expression. miR-711 mimics downregulated Ang-1 expression in neurons and increased levels of miR-711, and that Ang-1 mRNA coprecipitates with the RISC complex after etoposide treatment. Moreover, administration of an miR-711 hairpin inhibitor rescued Ang-1 decline after TBI. Although miR-targeted gene feed-forward loop self-amplification may occur,62 Ang-1 administration had no significant effect on miR-711 levels in neuronal apoptosis, indicating that the former may act downstream of the latter.
Our data show that Ang-1 not only attenuates the activation of key apoptotic pathways and neuronal degeneration, but also reduces edema/contusion volume (T2-weighted MRI) in the acute phase post-TBI. This latter effect is consistent with Ang-1–mediated induction of gene expression for multiple TJs, thus reducing BBB disruption/permeability.42,63 However, Ang-1 treatment failed to affect long-term lesion volume and functional outcomes (Y-maze spontaneous alteration test and beam walk task). It is possible that different Ang-1 dosing and/or delivery profiles may improve its efficacy. Another alternative is that Ang-1 alone may be insufficient to influence the critical number of secondary injury pathways required for sustained neuroprotection after TBI.
Conclusions
Overall, our data identify Ang-1 as an important target for miR-711 in neuronal cell death post-injury. The ability of miR-711 to independently downregulate both elements of the Ang-1/Akt neuroprotective axis reinforces its important role in neuronal apoptosis. Administration of Ang-1, especially in the acute period after TBI, has significant neuroprotective effects, acting both directly on neurons and possibly by limiting the vascular permeability effects of Ang-1/VEGF imbalance.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01 NS091191 to A.I.F. and R01 NS096002 to B.A.S and B.S.
The authors are grateful for the technical support provided by Dr. Junfang Wu, Xiaoyi Lin, Dr. Asit Kumar, and Marie Hanscom.
Authors Disclosure Statement
No competing financial interests exist.
References
- 1.Davis S., Aldrich T.H., Jones P.F., Acheson A., Compton D.L., Jain V., Ryan T.E., Bruno J., Radziejewski C., Maisonpierre P.C., and Yancopoulos G.D. (1996). Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87, 1161–1169 [DOI] [PubMed] [Google Scholar]
- 2.Partanen J., Armstrong E., Makela T.P., Korhonen J., Sandberg M., Renkonen R., Knuutila S., Huebner K., and Alitalo K. (1992). A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol. Cell. Biol. 12, 1698–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dallabrida S.M., Ismail N., Oberle J.R., Himes B.E., and Rupnick M.A. (2005). Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ. Res. 96, e8–e24 [DOI] [PubMed] [Google Scholar]
- 4.Carlson T.R., Feng Y., Maisonpierre P.C., Mrksich M., and Morla A.O. (2001). Direct cell adhesion to the angiopoietins mediated by integrins. J. Biol. Chem. 276, 26516–26525 [DOI] [PubMed] [Google Scholar]
- 5.Kwak H.J., So J.N., Lee S.J., Kim I., and Koh G.Y. (1999). Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 448, 249–253 [DOI] [PubMed] [Google Scholar]
- 6.Zhao J., Chen L., Shu B., Tang J., Zhang L., Xie J., Liu X., Xu Y., and Qi S. (2015). Angiopoietin-1 protects the endothelial cells against advanced glycation end product injury by strengthening cell junctions and inhibiting cell apoptosis. J. Cell. Physiol. 230, 1895–1905 [DOI] [PubMed] [Google Scholar]
- 7.Kim I., Kim H.G., So J.N., Kim J.H., Kwak H.J., and Koh G.Y. (2000). Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ. Res. 86, 24–29 [DOI] [PubMed] [Google Scholar]
- 8.Papapetropoulos A., Fulton D., Mahboubi K., Kalb R.G., O'Connor D.S., Li F., Altieri D.C., and Sessa W.C. (2000). Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J. Biol. Chem. 275, 9102–9105 [DOI] [PubMed] [Google Scholar]
- 9.Valable S., Bellail A., Lesne S., Liot G., Mackenzie E.T., Vivien D., Bernaudin M. and Petit E. (2003). Angiopoietin-1-induced PI3-kinase activation prevents neuronal apoptosis. FASEB J. 17, 443–445 [DOI] [PubMed] [Google Scholar]
- 10.Lim J.S., Koh G.Y., and Koh J.Y. (2015). Angiopoietin-1 blocks neurotoxic zinc entry into cortical cells via PIP2 hydrolysis-mediated ion channel inhibition. Neurobiol. Dis. 81, 203–213 [DOI] [PubMed] [Google Scholar]
- 11.Han S., Arnold S.A., Sithu S.D., Mahoney E.T., Geralds J.T., Tran P., Benton R.L., Maddie M.A., D'Souza S.E., Whittemore S.R., and Hagg T. (2010). Rescuing vasculature with intravenous angiopoietin-1 and alpha v beta 3 integrin peptide is protective after spinal cord injury. Brain 133, 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin H.Y., Lee Y.J., Kim H.J., Park C.K., Kim J.H., Wang K.C., Kim D.G., Koh G.Y., and Paek S.H. (2010). Protective role of COMP-Ang1 in ischemic rat brain. J. Neurosci. Res. 88, 1052–1063 [DOI] [PubMed] [Google Scholar]
- 13.Kosacka J., Nowicki M., Kloting N., Kern M., Stumvoll M., Bechmann I., Serke H., and Bluher M. (2012). COMP-angiopoietin-1 recovers molecular biomarkers of neuropathy and improves vascularisation in sciatic nerve of ob/ob mice. PLoS One 7, e32881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths-Jones S., Grocock R.J., van Dongen S., Bateman A., and Enright A.J. (2006). miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34, D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei P., Li Y., Chen X., Yang S., and Zhang J. (2009). Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 1284, 191–201 [DOI] [PubMed] [Google Scholar]
- 16.Liu D.Z., Tian Y., Ander B.P., Xu H., Stamova B.S., Zhan X., Turner R.J., Jickling G., and Sharp F.R. (2010). Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J. Cereb. Blood Flow Metab. 30, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez-Mateos E.M., Engel T., Merino-Serrais P., McKiernan R.C., Tanaka K., Mouri G., Sano T., O'Tuathaigh C., Waddington J.L., Prenter S., Delanty N., Farrell M.A., O'Brien D.F., Conroy R.M., Stallings R.L., DeFelipe J., and Henshall D.C. (2012). Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat. Med. 18, 1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabirzhanov B., Zhao Z., Stoica B.A., Loane D.J., Wu J., Borroto C., Dorsey S.G., and Faden A.I. (2014). Downregulation of miR-23a and miR-27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl-2 proteins. J. Neurosci. 34, 10055–10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Addo-Quaye C., Miller W., and Axtell M.J. (2009). CleaveLand: a pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 25, 130–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabirzhanov B., Stoica B.A., Zhao Z., Loane D.J., Wu J., Dorsey S.G., and Faden A.I. (2016). miR-711 upregulation induces neuronal cell death after traumatic brain injury. Cell Death Differ. 23, 654–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bazarian J.J., Blyth B., Mookerjee S., He H., and McDermott M.P. (2010). Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma 27, 527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox G.B., Fan L., Levasseur R.A., and Faden A.I. (1998). Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma 15, 599–614 [DOI] [PubMed] [Google Scholar]
- 24.Loane D.J., Pocivavsek A., Moussa C.E., Thompson R., Matsuoka Y., Faden A.I., Rebeck G.W., and Burns M.P. (2009). Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 15, 377–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakovlev A.G., Ota K., Wang G., Movsesyan V., Bao W.L., Yoshihara K., and Faden A.I. (2001). Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J. Neurosci. 21, 7439–7446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoica B.A., Loane D.J., Zhao Z., Kabadi S.V., Hanscom M., Byrnes K.R., and Faden A.I. (2014). PARP-1 inhibition attenuates neuronal loss, microglia activation and neurological deficits after traumatic brain injury. J. Neurotrauma 31, 758–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark R.S.B., Chen M., Kochanek P.M., Watkins S.C., Jin K.L., Draviam R., Nathaniel P.D., Pinto R., Marion D.W., and Graham S.H. (2001). Detection of single- and double-strand DNA breaks after traumatic brain injury in rats: comparison of in situ labeling techniques using DNA polymerase I, the Klenow fragment of DNA polymerase I, and terminal deoxynucleotidyl transferase. J. Neurotrauma 18, 675–689 [DOI] [PubMed] [Google Scholar]
- 28.Pietrzak M., Smith S.C., Geralds J.T., Hagg T., Gomes C., and Hetman M. (2011). Nucleolar disruption and apoptosis are distinct neuronal responses to etoposide-induced DNA damage. J. Neurochem. 117, 1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabirzhanov B., Stoica B.A., Hanscom M., Piao C.S., and Faden A.I. (2012). Over-expression of HSP70 attenuates caspase-dependent and caspase-independent pathways and inhibits neuronal apoptosis. J. Neurochem. 123, 542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogakou E.P., Pilch D.R., Orr A.H., Ivanova V.S., and Bonner W.M. (1998). DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 [DOI] [PubMed] [Google Scholar]
- 31.Jimenez-Mateos E.M., Bray I., Sanz-Rodriguez A., Engel T., McKiernan R.C., Mouri G., Tanaka K., Sano T., Saugstad J.A., Simon R.P., Stallings R.L., and Henshall D.C. (2011). miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am. J. Pathol. 179, 2519–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehmsmeier M., Steffen P., Hochsmann M., and Giegerich R. (2004). Fast and effective prediction of microRNA/target duplexes. RNA 10, 1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buzza M.S., Netzel-Arnett S., Shea-Donohue T., Zhao A., Lin C.Y., List K., Szabo R., Fasano A., Bugge T.H., and Antalis T.M. (2010). Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc. Natl. Acad. Sci. U. S. A. 107, 4200–4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak K.J., and Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 35.Stoica B.A., Movsesyan V.A., Knoblach S.M., and Faden A.I. (2005). Ceramide induces neuronal apoptosis through mitogen-activated protein kinases and causes release of multiple mitochondrial proteins. Mol. Cell. Neurosci. 29, 355–371 [DOI] [PubMed] [Google Scholar]
- 36.Wu C.-H., Hung T.-H., Chen C.-C., Ke C.-H., Lee C.-Y., Wang P.-Y., and Chen S.-F. (2014). Post-injury treatment with 7,8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling. PLoS One 9, e113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruetter R. (1993). Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. 29, 804–811 [DOI] [PubMed] [Google Scholar]
- 38.Kabadi S.V., Stoica B.A., Loane D.J., Luo T., and Faden A.I. (2014). CR8, a novel inhibitor of CDK, limits microglial activation, astrocytosis, neuronal loss, and neurologic dysfunction after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 34, 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellwig K., Kvartsberg H., Portelius E., Andreasson U., Oberstein T.J., Lewczuk P., Blennow K., Kornhuber J., Maler J.M., Zetterberg H., and Spitzer P. (2015). Neurogranin and YKL-40: independent markers of synaptic degeneration and neuroinflammation in Alzheimer's disease. Alzheimers Res. Ther. 7, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lista S., and Hampel H. (2017). Synaptic degeneration and neurogranin in the pathophysiology of Alzheimer's disease. Expert Rev. Neurother. 17, 47–57 [DOI] [PubMed] [Google Scholar]
- 41.O'Callaghan J.P. (1988). Neurotypic and gliotypic proteins as biochemical markers of neurotoxicity. Neurotoxicol. Teratol. 10, 445–452 [DOI] [PubMed] [Google Scholar]
- 42.Jiao H., Wang Z., Liu Y., Wang P. and Xue Y. (2011). Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. 44, 130–139 [DOI] [PubMed] [Google Scholar]
- 43.Rosen G.D., and Harry J.D. (1990). Brain volume estimation from serial section measurements: a comparison of methodologies. J. Neurosci. Methods 35, 115–124 [DOI] [PubMed] [Google Scholar]
- 44.Skold M.K., von Gertten C., Sandberg-Nordqvist A.C., Mathiesen T., and Holmin S. (2005). VEGF and VEGF receptor expression after experimental brain contusion in rat. J. Neurotrauma 22, 353–367 [DOI] [PubMed] [Google Scholar]
- 45.Lin T.N., Wang C.K., Cheung W.M., and Hsu C.Y. (2000). Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemia-reperfusion. J. Cereb Blood Flow Metab. 20, 387–395 [DOI] [PubMed] [Google Scholar]
- 46.Lin T.N., Nian G.M., Chen S.F., Cheung W.M., Chang C., Lin W.C., and Hsu C.Y. (2001). Induction of Tie-1 and Tie-2 receptor protein expression after cerebral ischemia-reperfusion. J. Cereb. Blood Flow Metab. 21, 690–701 [DOI] [PubMed] [Google Scholar]
- 47.Gong D., Zhang S., Liu L., Dong J., Guo X., Hao M., Tu Y., Diao Y., and Zhang J. (2011). Dynamic changes of vascular endothelial growth factor and angiopoietin-1 in association with circulating endothelial progenitor cells after severe traumatic brain injury. J. Trauma 70, 1480–1484 [DOI] [PubMed] [Google Scholar]
- 48.Genet G.F., Johansson P.I., Meyer M.A., Solbeck S., Sorensen A.M., Larsen C.F., Welling K.L., Windelov N.A., Rasmussen L.S., and Ostrowski S.R. (2013). Trauma-induced coagulopathy: standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J. Neurotrauma 30, 301–306 [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z.G., Zhang L., Tsang W., Soltanian-Zadeh H., Morris D., Zhang R., Goussev A., Powers C., Yeich T., and Chopp M. (2002). Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 22, 379–392 [DOI] [PubMed] [Google Scholar]
- 50.Nourhaghighi N., Teichert-Kuliszewska K., Davis J., Stewart D.J., and Nag S. (2003). Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab. Invest. 83, 1211–1222 [DOI] [PubMed] [Google Scholar]
- 51.Stoica B.A., and Faden A.I. (2010). Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics 7, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeffers J.R., Parganas E., Lee Y., Yang C., Wang J., Brennan J., MacLean K.H., Han J., Chittenden T., Ihle J.N., McKinnon P.J., Cleveland J.L., and Zambetti G.P. (2003). Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4, 321–328 [DOI] [PubMed] [Google Scholar]
- 53.Yakovlev A.G., Di Giovanni S., Wang G., Liu W., Stoica B., and Faden A.I. (2004). BOK and NOXA are essential mediators of p53-dependent apoptosis. J. Biol. Chem. 279, 28367–28374 [DOI] [PubMed] [Google Scholar]
- 54.Selvamani A., Sathyan P., Miranda R.C., and Sohrabji F. (2012). An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One 7, e32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redell J.B., Zhao J., and Dash P.K. (2011). Altered expression of miRNA-21 and its targets in the hippocampus after traumatic brain injury. J. Neurosci. Res. 89, 212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akhter R., Sanphui P., and Biswas S.C. (2014). The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in beta-amyloid-induced neuron death. J. Biol. Chem. 289, 10812–10822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ambacher K.K., Pitzul K.B., Karajgikar M., Hamilton A., Ferguson S.S., and Cregan S.P. (2012). The JNK- and AKT/GSK3beta- signaling pathways converge to regulate Puma induction and neuronal apoptosis induced by trophic factor deprivation. PLoS One 7, e46885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J., Kim K.E., Choi D.K., Jang J.Y., Jung J.J., Kiyonari H., Shioi G., Chang W., Suda T., Mochizuki N., Nakaoka Y., Komuro I., Yoo O.J., and Koh G.Y. (2013). Angiopoietin-1 guides directional angiogenesis through integrin alphavbeta5 signaling for recovery of ischemic retinopathy. Sci. Transl. Med. 5, 203ra127. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz M.A., and Ginsberg M.H. (2002). Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65–E68 [DOI] [PubMed] [Google Scholar]
- 60.Peters K.G., Kontos C.D., Lin P.C., Wong A.L., Rao P., Huang L., Dewhirst M.W., and Sankar S. (2004). Functional significance of Tie2 signaling in the adult vasculature. Recent Prog. Horm. Res. 59, 51–71 [DOI] [PubMed] [Google Scholar]
- 61.Zhou L., Zang G., Zhang G., Wang H., Zhang X., Johnston N., Min W., Luke P., Jevnikar A., Haig A., and Zheng X. (2013). MicroRNA and mRNA signatures in ischemia reperfusion injury in heart transplantation. PLoS One 8, e79805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Q., Qin H., Zhao Q., and He X.X. (2015). Emerging role of transcription factor-microRNA-target gene feed-forward loops in cancer. Biomed. Rep. 3, 611–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hori S., Ohtsuki S., Hosoya K., Nakashima E., and Terasaki T. (2004). A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J. Neurochem. 89, 503–513 [DOI] [PubMed] [Google Scholar]