Abstract
Genetically modified, autologous hematopoietic stem and progenitor cells (HSPCs) represent a new class of genetic medicine. Following this therapeutic paradigm, we are developing a product candidate, designated CD68-ET3-LV CD34+, for the treatment of the severe bleeding disorder, hemophilia A. The product consists of autologous CD34+ cells transduced with a human immunodeficiency virus 1–based, monocyte lineage-restricted, self-inactivating lentiviral vector (LV), termed CD68-ET3-LV, encoding a bioengineered coagulation factor VIII (fVIII) transgene, termed ET3, designed for enhanced expression. This vector was shown capable of high-titer manufacture under clinical scale and Good Manufacturing Practice. Biochemical and immunogenicity testing of recombinant ET3, as well as safety and efficacy testing of CD68-ET3-LV HSPCs, were utilized to demonstrate overall safety and efficacy in murine models. In the first model, administration of CD68-ET3-LV-transduced stem-cell antigen-1+ cells to hemophilia A mice resulted in sustained plasma fVIII production and hemostatic correction without signs of toxicity. Patient-derived, autologous mobilized peripheral blood (mPB) CD34+ cells are the clinical target cells for ex vivo transduction using CD68-ET3-LV, and the resulting genetically modified cells represent the investigational drug candidate. In the second model, CD68-ET3-LV gene transfer into mPB CD34+ cells isolated from normal human donors was utilized to obtain in vitro and in vivo pharmacology, pharmacokinetic, and toxicology assessment. CD68-ET3-LV demonstrated reproducible and efficient gene transfer into mPB CD34+ cells, with vector copy numbers in the range of 1 copy per diploid genome equivalent without affecting clonogenic potential. Differentiation of human CD34+ cells into monocytes was associated with increased fVIII production, supporting the designed function of the CD68 promoter. To assess in vivo pharmacodynamics, CD68-ET3-LV CD34+ cell product was administered to immunodeficient mice. Treated mice displayed sustained plasma fVIII levels and no signs of product related toxicity. Collectively, the findings of the current study support the preclinical safety and efficacy of CD68-ET3-LV CD34+.
Keywords: : lentiviral gene therapy, hematopoietic stem cells, hemophilia A
Introduction
Hematopoietic stem and progenitor cell (HSPC) transplantation gene therapies are being developed for many genetic diseases. Recently, the first gene therapy product in this class was approved by the European Medicines Agency for the treatment of patients with severe combined immunodeficiency due to adenosine deaminase deficiency. Several other product candidates in this class are under clinical testing for treatment of X-linked severe combined immunodeficiency (X-SCID), β-thalassemia, sickle cell disease, Wiskott–Aldrich disease, chronic granulomatous disease, adrenoleukodystrophy, metachromatic leukodystrophy, and others.1–9
Herein, an HSPC transplantation gene therapy product candidate is described for hemophilia A, which is the most common severe congenital bleeding disorder, with an incidence of nearly 1/5,000 male births. The cause of hemophilia A is mutation of the F8 gene, which results in a deficiency of coagulation factor VIII (fVIII) activity in plasma. FVIII is a large plasma glycoprotein and is essential for normal hemostasis due to its role in activated form as a cofactor to the serine protease coagulation factor IX. The F8 gene is located at Xq28 and harbors 26 exons encompassed within 126 kb of nucleotide sequence. The transcribed and processed mRNA is 9,048 nucleotides and encodes a protein of 2,351 amino acids. FVIII has a domain structure designated A1-A2-B-ap-A3-C1-C2, as defined by internal sequence homologies. The function of the B domain is not understood and is not necessary for procoagulant function. Due to the large size and nonessential nature of the B domain, it is often deleted in the context of gene therapy transgenes, which are then designated as B-domain-deleted (BDD) fVIII and possess a transgene size of approximately 4.7 kb. We have further bioengineered the BDD-human fVIII transgene to include amino acids that significantly improve biosynthesis/secretion. The resulting transgene product is designated ET3 (previously HP47) and has demonstrated 10- to 100-fold improved expression, preclinical efficacy, and no evidence of increased immunogenicity when transferred by various gene therapy technologies into hemophilia A mice.10–16
CD68-ET3-LV CD34+ is a product candidate designed for the treatment of patients with severe hemophilia A. It consists of autologous CD34+ cells transduced with a monocyte lineage-restricted, self-inactivating (SIN) lentiviral vector (LV), termed CD68-ET3-LV. Decades of human immunodeficiency virus 1 (HIV-1) research and a rapidly growing list of HSPC-directed gene therapy clinical trials are demonstrating HIV-1 based LV vectors to be safe and efficient vehicles for introducing new genetic material ex vivo into HSPCs. LVs are modified versions of HIV-1 that have most of the viral genes and regulatory sequences removed and replaced with a promoter and therapeutic transgene expression cassette. The resulting vector particles no longer contain the material necessary to replicate upon entry into a target cell and retain primarily the ability to produce the therapeutic transgene product. As a component of several ongoing HSPC-directed LV gene therapy clinical trials, LV integration events are identified using state-of-the-art genomics technology. The relative abundance of each integrant is tracked over time to assess for clonality, a common characteristic of most, if not all, hematopoietic cancers. To date, no evidence of pathogenic insertional mutagenesis by a LV has been observed in >200 subjects treated with LV-modified HSPC or T-cell products in >13 years of treatment.17 For these reasons, an LV platform was adopted to deliver the ET3 transgene to HSPCs. Herein, the results of in vitro and in vivo pharmacology, pharmacodynamic, and toxicology testing of CD68-ET3-LV CD34+ in cell culture and murine xenotransplantation models are described.
Methods
Hemophilia A mice containing a neomycin cassette in exon 16, resulting in a truncated or partially deleted fVIII protein, were obtained from a colony established by Dr. Leon Hoyer,18 and were maintained as a mixed (129S4/SvJae;C57BL/6) background. Transgenic mice expressing enhanced green fluorescent protein (eGFP) from the β-actin promoter on a C57BL/6 genetic background (strain designation: C57BL/6-Tg[Act-eGFP]C14-Y01-FM131 Osb) were a gift from Dr. Masaru Okabe (Osaka University, Osaka, Japan) and are maintained by Dr. David Archer at Emory University. B6.SJL-PtprcaPepcb/BoyCrl, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, and NOD.Cg-Prkdcscid Il2rgtm1WjlTg(PGK1-KITLG*220)441Daw strain mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mouse studies were performed using procedures approved by the Emory University Institutional Animal Care and Use Committee and the Emory University Division of Animal Resources (DAR).
Purification and biochemical characterization of recombinant ET3, recombinant human BDD fVIII, and porcine fVIII
Recombinant human BDD fVIII (HSQ) contains an amino acid sequence identical to Refacto and Xyntha. Recombinant ET3 (ET3i; also called HP47) contains the human A2, C1, and C2 domains, a porcine B domain linker, and specific substitutions of porcine amino acids in the A1 and A3 domains. HSQ, ET3i, and recombinant BDD recombinant porcine (rp) fVIII were expressed from baby hamster kidney (BHK)–derived cells in AIM-V medium, purified by sequential cation-exchange and anion-exchange chromatography, and characterized as described previously with minor modifications (Supplementary Method S1; Supplementary Data are available online at www.liebertpub.com/hum).19 FVIII activity measurement and von Willibrand factor (vWf) binding analysis were performed as described in Supplementary Methods S2 and S3.
Tail-clip bleeding assay experimental protocol
The efficacy of ET3i, human fVIII, or CD68-ET3-LV stem-cell antigen-1+ (Sca-1+) was studied in 8- to 12-week-old male or female E16 hemophilia A mice.20 The primary efficacy variable was normal blood loss 30 min following tail transection. Normal and abnormal blood loss were defined empirically and prospectively as <20 mg/g or >20 mg/g blood loss per body weight, respectively.
ET3i or human fVIII was diluted to the desired concentration into 0.15 M NaCl immediately prior to injection. Mice were injected intravenously by the tail vein with 0.1 mL ET3i or human fVIII and anesthetized with halothane immediately after injection. Five minutes after induction of anesthesia, mice were placed in sternal recumbence, and the tail was placed in a 15 mL centrifuge tube containing saline at 37°C. The tail was transected 4 mm from the distal tip 15 min after injection and placed back into the centrifuge tube containing saline. After 30 min, mice were euthanized, and the tube was weighed to determine blood loss. The up-and-down method was used to estimate the doses of ET3i or human fVIII that prevented abnormal bleeding in 50% of mice (ED50 values) as described previously and in more detail in Supplementary Method S4.21,22
In silico immunogenicity assessment
The Epibase™ In Silico Service (Lonza) entails the identification of potential T-cell epitopes using sequence information, structural bioinformatics, and experimental data while also incorporating the characteristics of known human leukocyte antigen (HLA) receptors and their specific peptide-binding affinities. ET3 and HSQ sequences were provided to Lonza. To assess more accurately the immunogenicity in the fVIII naïve (cross reactive material negative) setting of severe hemophilia A, human fVIII peptide sequences were deleted from the software filter designed to remove native peptides for which immune tolerance should exist.
In vivo immunization protocol
Eight- to ten-week-old male and female mice were used in the experiments. E16 mice were randomized within each cage and selected so that approximately equal numbers of males and females were in each immunogen group. Mice were warmed on a heating pad for 3–4 min to dilate the tail veins before the injections. ET3i and HSQ were thawed and diluted 10 μg/mL in sterile saline for injection immediately before use. Mice received four injections each week of 1 μg ET3i or HSQ by the tail vein as a 0.1 mL bolus. Mice were euthanized, and blood was collected by terminal cardiac puncture into 1/10 volume of 3.8% (w/v) trisodium citrate 7 days after the final injection. Plasma was prepared by centrifugation at 3,000 g for 15 min at 4°C and frozen in 50 μL aliquots. Anti-fVIII Inhibitory and total immunoglobulin G (IgG) titers were determined by modified Bethesda assay and enzyme-linked immunosorbent assay (ELISA) as described in Supplementary Methods S5 and S6, respectively.
Research-grade LV production and viral transcript analysis
Production of research-grade LV encoding ET3 is described in Supplementary Method S7. LV titering was performed using HEK293T/17 cells (American Type Cell Culture [ATCC]; CRL-11268) as described in Supplementary Method S8. Northern blot analysis of RNA obtained from transfected HEK293T/17 LV producer cells is described in detail in Supplementary Method S9.
CD68-ET3-LV stem-cell antigen-1+ production and testing in hemophilia A mice
Isolation and transduction of Sca-1+ cells were performed as described in Supplementary Method S10. CD68-ET3-LV Sca-1+ cells were transplanted into lethally irradiated hemophilia A mice as described in Supplementary Method S11. Secondary transplantation of whole bone marrow from CD68-ET3-LV Sca-1+-treated mice into naïve secondary hemophilia A recipients was performed as described in Supplementary Method S12. FVIII activity in mouse blood was determined by chromogenic substrate assay (COATEST SP fVIII). Mice were bled through the retro-orbital plexus into one-tenth volume 3.8% trisodium citrate, and plasma was isolated by centrifugation at 1,600 g for 15 min at 4°C. Plasma samples were stored at −80°C until the time of analysis. FVIII activity levels within plasma samples were measured using a commercially available chromogenic substrate assay (COATEST SP fVIII; Diapharma). A log/log standard curve was generated using pooled normal human plasma (FACT). Reactions were performed in 96-well microtiter plates, and the absorbance at 405 nm was determined kinetically using a Versamax plate reader (Molecular Devices). Vector copy number (VCN) analysis was performed using the primers and method described for LV titering in Supplementary Method S8 with the substitution of harvested blood, spleen, or bone marrow cell genomic DNA for HEK293T/17 DNA. Flow cytometry was performed on harvested blood, spleen, or bone marrow cells as described in Supplementary Method S13.
CD68-ET3-LV CD34+ manufacture and testing
Normal human donor mobilized peripheral blood (mPB) CD34+ cells were obtained from a commercial vendor (AllCells) or Cincinnati Children's Hospital Medical Center (CCHMC). In both cases, cells were obtained under approval of an Institutional Review Board. The pre-transduction treatment of the cells is described in detail in Supplementary Method S14.
Full-scale production lots of CD68-ET3-LV were generated using the planned clinical LV production process at both Lentigen Corporation and subsequently CCHMC viral vector core. The initial run was not performed with Good Manufacturing Practice (GMP)-qualified reagents, but did follow the proposed GMP manufacturing processes used in GMP production, and the resulting material is referred to as “process similar” CD68-ET3-LV. The final clinical LV production run was performed by CCHMC under current GMP regulations. More detail regarding the production of process similar and GMP-compliant CD68-ET3-LV are available upon request.
An overview of the ex vivo transduction LV source and analysis used in the current study is provided in Table 1, and the detailed methodology is provided in Supplementary Method S15. Clonogenic potential post transduction was assessed by colony forming cell (CFC) assay and enumeration of individual colony forming units (CFUs) compared to untransduced cells (Supplementary Method S16). Six-week-old NSG Tg (Hu-mSCF) mice were preconditioned for transplantation with 30 mg/kg busulfan via intraperitoneal injection. Each mouse was transplanted via retro-orbital injection with 0.1 mL total volume of cells under isoflurane anesthesia following IACUC guidelines. Blood was collected at specific time points for complete blood count analysis, fVIII antigen measurement, and flow cytometry as described in Supplementary Method S17. Comprehensive blood chemistries, pathology, and histology analysis were performed by the Emory University DAR.
Table 1.
CD68-ET3-LV CD34+ study design
| LV source | Sample size | Treatment cell dose | Post-treatment analyses | Post-treatment follow-up duration |
|---|---|---|---|---|
| CD68-ET3-LV (LTG1434) | 4—LV transduced | 106 | CBC | Up to 16 weeks |
| FVIII activity | ||||
| VCN | ||||
| CD68-ET3-LV (pET68-3) | 7—Mock 16—Untreated 22—LV transduced |
106 | CBC | Up to 52 weeks |
| FVIII activity | ||||
| VCN | ||||
| Chemistries (n = 13) |
LV, lentiviral vector; CBC, complete blood count; FVIII, factor VIII.
Proviral transgene stability was assessed by polymerase chain reaction (PCR) walking, Southern blot analysis, and proviral DNA sequencing as described in Supplementary Methods S18–S20. For CD68-ET3-LV insertion site analysis, two methods were tested that were identical other than the use of enzymatic digestion (fragmentase; New England Biolabs) or mechanical shearing (Covaris ultrasonicator). A detailed description of the method employed and bioinformatic pipeline is provided in Supplementary Method S21.
The procedures described by Modlich et al. were used to assess the immortalization potential of CD68-ET3-LV compared to an oncogenic vector, murine stem-cell virus (MSCV)-SFFV-GFP, which is known to induce murine HSPC immortalization.23 A description of the protocol employed is provided in Supplementary Method S22.
Statistical analysis
Data are reported as the mean ± sample standard deviation. Both parametric and nonparametric statistical methods were utilized in the current study based on a priori assumptions of the expected data distribution, as well as positive or negative results, respectively, from normality testing performed by SigmaPlot software. For head-to-head comparison of normally distributed data, Student's t-test was utilized. For head-to-head comparison of data not assumed to be normally distributed and/or that did not pass normality testing, the Mann–Whitney U-test was performed. For multiple comparisons, both one-way analysis of variance (ANOVA) and Kruskal–Wallis test by ranks were utilized for normally and non-normally distributed data, respectively. For all of the above testing, a p-value of <0.05 was considered statistically significant. When significance was determined by one-way ANOVA or Kruskal–Wallis, a post hoc test incorporating a multiple comparisons post hoc correction was employed (e.g., Dunn's multiple comparisons test).
Results and Discussion
FVIII transgene selection and transgene product characterization
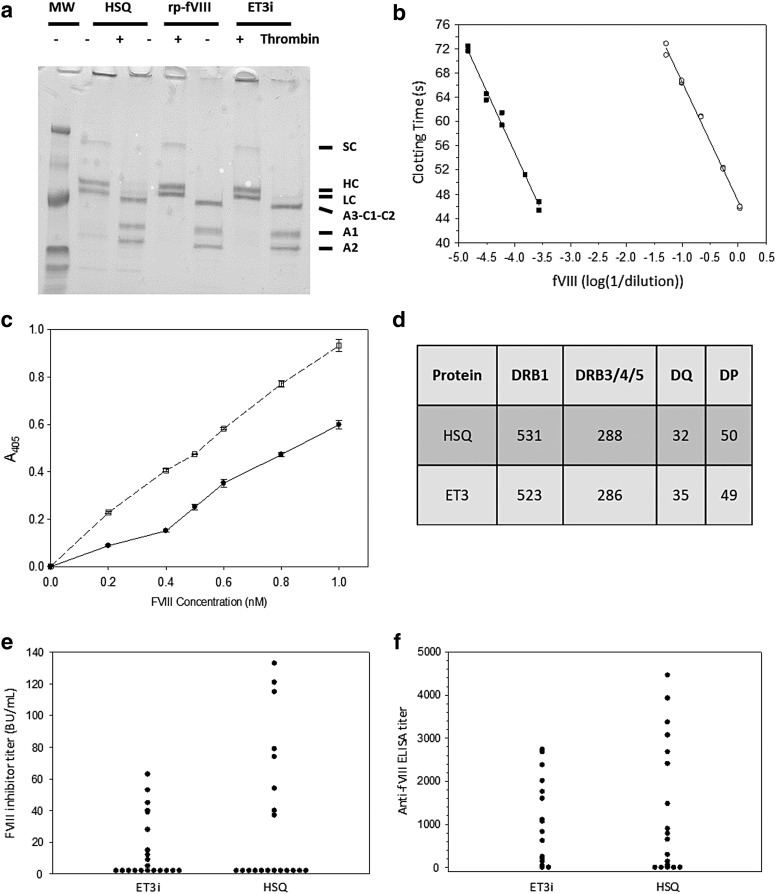
The identification, bioengineering, and in vivo validation of high expression fVIII sequences incorporated into the ET3 transgene using both adeno-associated virus (AAV) vector and LV preclinical testing have been previously described.10–16,19,24–29 The ET3 transgene is defined as a non-codon optimized, BDD form of fVIII transgene encoding specific porcine amino acid substitutions in the A1 and A3 domains (Supplementary Fig. S1). To study the biochemical properties of ET3, a recombinant form, designated ET3i, was expressed from transfected BHK-derived cells. Figure 1a shows sodium dodecyl sulfate–polyacrylamide gel electrophoresis of the purified ET3i, BDD human fVIII (HSQ), and BDD rp fVIII before and after cleavage by thrombin. All three products displayed two major bands corresponding to the heavy and light chains of heterodimeric fVIII. Thrombin produced three bands previously identified as the ∼70 kDa cleaved light chain, and 50 and 40 kDa bands corresponding to the A1 and A2 subunits of activated fVIII.10,11 These results show that ET3i is highly purified and has the same subunit structure as HSQ and recombinant BDD porcine fVIII (BDD rp-fVIII) in non-activated and activated form.
Figure 1.
Recombinant ET3 (ET3i) biochemistry and immunogenicity studies. (a) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of recombinant human BDD fVIII (HSQ), B-domain-deleted (BDD) recombinant porcine (rp) factor VIII (fVIII), and ET3i—2 μg HSQ, rp-fVIII,18 and ET3i were subjected to 4–15% gradient SDS-PAGE under reducing conditions and visualized by Coomassie Blue staining. “+ Thrombin” indicates samples that were treated with 100 nM porcine thrombin for 5 min before analysis. Single chain (SC), heavy chain (HC), light chain (LC), A3-C1-C2, A1, and A2 polypeptides are labeled. The lane designated “MW” contains molecular weight markers of 207, 129, 75, 39.7, 32.1, and 17.5 kDa. (b) fVIII activity assay of ET3i. The clotting times of dilutions of 0.76 μM ET3i (closed squares) into fVIII-deficient plasma were measured and compared to a standard curve made by dilutions of pooled normal human plasma (open circles). The lines represent linear regression fits to the data. (c) Binding of ET3i and HSQ to human von Willibrand factor (vWf). ET3i (open squares) or HSQ (closed circles) were added to human vWF immobilized on microtiter wells, followed by detection of bound fVIII using anti-fVIII monoclonal antibodies as described in the Methods. (d) HSQ and ET3 amino acid sequences were analyzed for predicted T-cell epitopes. The number of critical fVIII epitopes predicted for each human leukocyte antigen gene family are shown. (e and f) Hemophilia A mice received intravenous injections of 1 μg ET3i or HSQ every 4 weeks. One week following the final dose, plasma samples were collected and assayed for anti-fVIII inhibitor titers (e) and anti-fVIII immunoglobulin G titers (f). No significant differences were observed: p = 0.8 and 0.9, respectively, in (e) and (f).
The procoagulant activities of purified ET3i and HSQ were measured using a pooled normal human fVIII standard (Fig. 1b). Dilutions of ET3i and HSQ produced regression lines parallel to the standard curve, yielding specific activities of 13,100 IU/mg (2,290 IU/nmol) and 7,920 IU/mg (1,390 IU/nmol), respectively. The specific activity obtained for HSQ is consistent with the 5,500–9,900 IU/mg specific activity range of commercial BDD human fVIII. FVIII binding to vWf is essential to maintaining a normal circulatory half-life. Therefore, the binding of ET3i to vWf at physiologic fVIII concentration was assessed by ELISA and shown to be similar and non-inferior to HSQ (Fig. 1c). In addition, the half-life of the purified and activated product was shown previously to be higher than HSQ, and similar to BDD rp-fVIII.13
Next, the in vivo efficacy of ET3i in hemophilia A mice was compared to full-length human fVIII in a murine hemophilia A bleeding model. Mice received varying doses of ET3i or commercial recombinant human fVIII, and blood loss was measured after tail snipping. Estimates of the doses of ET3i or human fVIII that prevented abnormal bleeding in 50% of mice (ED50 values) were not significantly different (28.9 and 21.9 IU/kg, respectively; p = 0.07; individual data are provided in Supplementary Tables S1 and S2). Based on the specific activities determined for ET3i and HSQ above, the ED50 can be calculated on a per mole basis as 12.6 and 15.8 pmol/kg, respectively. Therefore, ET3i is equally effective in restoring mammalian hemostasis during hemostatic challenge when delivered in a similar fashion to currently available fVIII products.
ET3 immunogenicity studies
Inhibitory antibodies (inhibitors) to fVIII develop in approximately 30% of patients with moderate or severe hemophilia A treated with current human fVIII products.30–33 Inhibitor development is considered the most significant complication in the management of hemophilia A. To address the immunogenicity risk of ET3, both in silico and in vivo studies were performed. Although preclinical assessment of biopharmaceutical immunogenicity has traditionally shown little predictive clinical value, advances in this technology have become available. One example is the recently published study post hoc assessment of a bioengineered recombinant activated factor VII (rfVIIa) product candidate, designated vatreptacog alfa, which was pulled from clinical development during a Phase III clinical trial that uncovered anti-drug antibody (ADA) development in 11% of the patients treated.34 Using in vitro biochemical HLA class II binding data combined with in silico MHC II binding prediction software and cell culture experiments with control and patient peripheral blood mononuclear cell antigen response assays, they were able to demonstrate retrospectively that two of the three amino acid substitutions in the bioengineered molecule were associated with tighter peptide:MHC II binding, efficient peptide display by cultured antigen-presenting cells, and in vitro T-cell recognition. A critical component to the interpretation is the availability of a comparator molecule with a known clinical safety (i.e., immunogenicity) profile, which in the previous case was NovoSeven (rhFVIIa). In >20 years of clinical experience with NovoSeven, no ADA responses have been observed. In silico immunoprofiling of ET3 as well as HSQ, a comparator with known safety profile, was performed by a third-party commercial vendor. Both sequences were analyzed for the presence of putative HLA class II restricted epitopes at the allotype level for 43 DRB1, 8 DRB3/4/5, 22 DQ, and 12 DP (85 HLA class II receptors in total). Within each allotype family, similar numbers of critical epitopes were found, suggesting an equivalent immunogenicity risk (Fig. 1d).
As a second preclinical immunogenicity safety assessment, the immunogenicity of ET3i and HSQ in naïve hemophilia A mice was compared. E16 hemophilia A mice develop an fVIII inhibitor response when infused intravenously with human fVIII using a schedule that mimics therapy in humans in terms of dose frequency and the amount of infused fVIII per unit of body mass. These mice develop a T-helper cell-mediated IgG-specific humoral response against immunodominant fVIII B-cell epitopes that are also recognized by human inhibitory antibodies.18,35–41 Thus, this model is appropriate for preclinical testing of the immunogenicity of fVIII products. FVIII inhibitor levels were measured using a modified Bethesda assay, and no significant difference was revealed between the ET3i and HSQ groups (Fig. 1e). Total anti-fVIII IgG antibodies were measured by ELISA, and no significant difference was revealed between the ET3i and HSQ groups (Fig. 1f). Overall, the results predict that the development of inhibitory anti-fVIII antibodies to ET3i would not be greater than to human fVIII in previously untreated patients with hemophilia A. Therefore, taken together, these data support the conclusion that ET3 is similar to human fVIII with respect to its biologic and immunogenic properties while enabling more efficient biosynthesis, higher specific activity, and an extended active half-life, all properties that support its benefit to gene therapy applications.
CD68-ET3-LV design validation
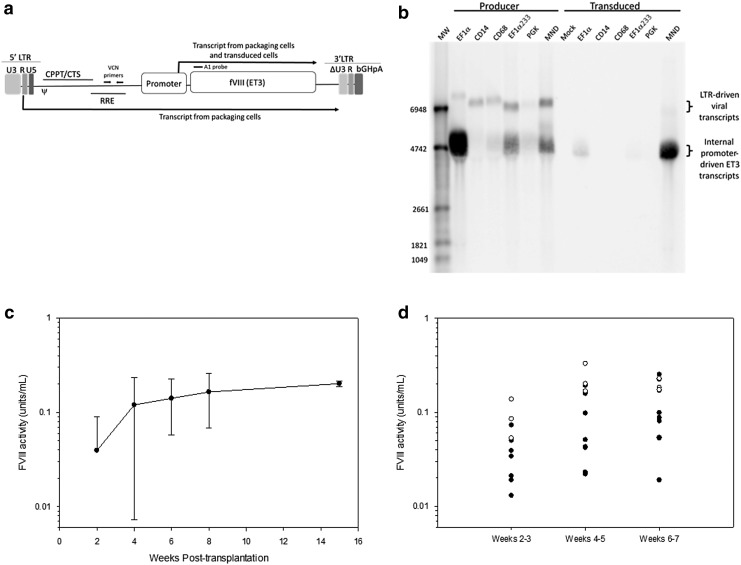
The initial candidate vector was an ET3-encoding LV in which transgene expression was driven from an EF1α promoter (EF1α-ET3-LV). However, manufacture of EF1α-ET3-LV at clinically relevant scales produced LV titers that were insufficient to support clinical studies. ET3 is a secretory protein, and producer cells transfected with an ET3 LV expression plasmid incorporating a constitutively active promoter, such as EF1α, will express ET3 protein into the supernatant during vector production.14 Therefore, it was possible that concurrent ET3 expression was hampering LV manufacture. The addition of ET3i during production of eGFP vectors did not affect recombinant eGFP vector titers nor did removal of the ATG start codon from the ET3 transgene, indicating fVIII expression was not the cause for the low EF1α-ET3 vector titers (data not shown). Therefore, new candidate LV expression plasmid designs were generated and tested. Each of these contained a 5′-LTR, an internal promoter, the ET3 transgene, and a 3′-SIN LTR (Fig. 2a). The internal promoters tested included both constitutive (EF1αs233, engEF1α, eIF4A1, PGK, MND, and hβ-kinesin) and non-constitutive, or lineage-restricted, promoters (CD68, CD14). Figure 2a shows two possible ET3 transgene transcripts produced from an LV expression plasmid in producer cells: one driven from the internal promoter (ET3 transcript), and a longer transcript from the 5′-LTR promoter (viral transcript). Because CD68 and CD14 are myeloid lineage-specific promoters, transgene expression in producer cells transfected with these ET3-LV expression plasmids is largely driven by the 5′-LTR. Therefore, virtually all transcript in producer cells is the packaged form and generated from the 5′-LTR (Fig. 2b). It was observed that the EF1α promoter generates a relatively high level of internally promoted transcript in transfected producer cells. Thus, promoter competition between the 5′-LTR and the internal promoter may contribute to the low vector titers associated with inclusion of the EF1α promoter. Supernatants from HEK-293T/17 cells transfected with LV packaging plasmids and the various ET3-LV expression plasmids were titered on HEK-293T/17 cells, and in repeat experiments the CD68 promoter produced the highest LV titers (Supplementary Table S3). In addition to yielding the highest LV titer, incorporation of the CD68 promoter can be considered a safety modification for two reasons. Restriction of internal promoter activity to differentiated monocyte lineages should reduce both the risk gene dysregulation caused by insertional mutagenesis and toxicity due to diminished transgene product biosynthesis in these sensitive HSPC populations. As expected, transgene expression from the CD68 promoter is not observed in murine Sca-1+ cells (data not shown). Because of the potential safety benefits and ability to manufacture high-titer vector, the CD68 promoter was selected for the lead candidate vector design.
Figure 2.
Development and lead candidate selection of CD68-ET3-LV. (a) A schematic depicting the primary components of the overall self-inactivating (SIN) lentiviral vector (LV) design used in the LV lead optimization studies is depicted. For example, several internal promoters were tested for high-titer vector manufacture and fVIII expression in HEK-293T/17 cells. Transcripts driven by the 5′-LTR and internal promoter that can be generated by the LV expression plasmid during LV production are denoted by the location of the transcription start and direction of transcription. The location of the A1 probe is shown above the diagram as a black bar. (b) Northern blot analysis was performed on transfected LV producer and transduced HEK-293T/17 cells. The A1 probe shown in (a) was used to probe total RNA from producer and transduced HEK-293T/17 cells following agarose gel electrophoresis and blotting to a charged nylon membrane. The expected locations of the LTR-driven viral transcript and the internal promoter-driven ET3 transcript are denoted to the right of the blot image. The internal promoter incorporated in each LV expression plasmid is shown above the blot image. A molecular weight (MW) marker was used to determine transcript sizes. (c) Plasma fVIII activity measurements were made by chromogenic assay on weeks 2, 4, 6, and 8 post transplantation of CD68-ET3-LV Sca-1+ into hemophilia A mice (n = 8). An additional measurement was taken on four of the eight primary transplanted mice at week 15 post transplantation. The other four mice were utilized for secondary bone marrow transplantation analysis. Error bars represent sample standard deviation. (d) Bone marrow was harvested from the primary CD68-ET3-LV Sca-1+ hemophilia A recipients and transplanted into secondary hemophilia A recipients. Plasma fVIII activity measurements were made by chromogenic assay on or between weeks 2 and 3, 4 and 5, and 6 and 7 post primary transplantation of CD68-ET3-LV Sca-1+ into hemophilia A mice (n = 3, open circles) or secondary transplantation of CD68-ET3-LV Sca-1+ primary recipient bone marrow into hemophilia A mice (n = 8, closed circles).
Two models were used to study the pharmacology of the CD68 promoter. Both involved the transduction of Sca-1+ cells, which are a population enriched in murine HSPCs, with LV containing the CD68 promoter sequence as internal promoter element and subsequent transplantation of the transduced Sca-1+ cells into lethally irradiated mice. First, to test the monocyte lineage specificity of the CD68 promoter, wild-type mice (C57BL/6; CD45.1+) were transplanted with congenic hemophilia A (C57BL/6; CD45.2+) Sca-1+ cells transduced with CD68-eGFP-LV. Peripheral blood was obtained, and eGFP expression was compared in myeloid and lymphoid cells by flow cytometry analysis. eGFP+ cells were at least five times higher in frequency in the myeloid compared to the lymphoid compartment (>50% vs. <10%, respectively; Supplementary Fig. S2).
The potential therapeutic efficacy contributed by the CD68 promoter was then tested. In this model, CD68-ET3-LV was used to transduce Sca-1+ cells from eGFP transgenic mice, which express intracellular eGFP ubiquitously, including in all hematopoietic lineages. This model facilitates donor (eGFP+) engraftment tracking by flow cytometry. Similar engraftment of mice transplanted with CD68-ET3-LV- or CD68-eGFP-LV-transduced cells, as judged by recovery of white blood cell, lymphocyte, monocyte, and granulocyte counts, was observed in the two groups (Supplementary Fig. S3). Hemophilia A mice transplanted with CD68-ET3-LV-transduced Sca-1+ cells produced average fVIII levels of 0.165 ± 0.096 and 0.202 ± 0.014 IU/mL (∼20% of normal human level) at 8 and 15 weeks post transplantation, respectively (Fig. 2c). In human hemophilia A, fVIII activity between 5% and 40% of normal is defined as mild hemophilia and is not associated with spontaneous bleeding. As expected, control CD68-eGFP-LV-transduced mice did not have detectable fVIII levels. VCN in these mice were <1 copy/diploid genome equivalent cell (Supplementary Table S4). To confirm stable engraftment of HPSCs expressing the ET3 transgene, bone marrow was harvested from three mice transplanted with CD68-ET3-LV-transduced Sca-1+ cells and transplanted into lethally irradiated hemophilia A mice. White blood cell counts (primarily due to the lymphocyte counts) do not respond as rapidly in the secondary mice compared to the primary mice, but the final engraftment levels were similar (Supplementary Fig. S4). This result is likely due to the lower number of HSPCs in the secondary transplanted animals. Two million Sca-1+ cells were used in the primary transplant compared to approximately 5 × 106 whole bone marrow cells, which have a low percentage (∼10% of the total) of Sca-1+ cells, in the secondary transplants. FVIII levels were detectable in all the secondary transplant recipients (Fig. 2d).
In vitro insertional mutagenesis analysis
To evaluate if CD68-ET3-LV transduction induces clonal expansion of HSPCs, which would be indicative of insertional mutagenesis, three retroviral vectors were evaluated. Process similar CD68-ET3-LV was compared to a positive control vector that was predicted to maintain cell viability 2 weeks after limiting dilution. This vector was created using an ecotropic MSCV-based expression vector containing an internal spleen focus forming virus (SFFV) promoter driving GFP expression. This design was previously shown to induce insertional mutagenesis and in vitro immortalization of murine hematopoietic cells.23 Additionally, a second control was included that was designed using a SIN LV design identical to CD68-ET3-LV, except for substitution of the CD68 promoter and ET3 transgene for an EF1α promoter and encoding eGFP transgene. Sca-1+ cells were harvested from 5-FU-treated mice subjected to 2 days of cytokine stimulation. Then, they were transduced twice with MSCV-SFFV-GFP, CD68-ET3-LV, or EF1α-GFP-LV. After 2 weeks in expansion media, a limiting dilution assay was performed in which the cells were plated on 96-well plates at 100 cells/well. After 2 weeks, the plates were scored. No surviving cells were observed in wells containing CD68-ET-LV- or EF1α-GFP-LV-transduced cells. However, >90% of well with MSCV-SFFV-GFP-transduced cells scored positive.
CD68-ET3-LV manufacture process development
For process development validation, a full-scale production lot of CD68-ET3-LV was generated using the planned clinical LV production process, with the exception that it was not performed with GMP qualified reagents. However, this run did mimic all manufacturing processes used in GMP production, and the resulting material is referred to as “process similar” CD68-ET3-LV and was produced to confirm scalability of production and for comparison to previous manufacture of four lots of EF1α-ET3-LV (Supplementary Fig. S5). These results show that under GMP-compliant manufacturing processes, CD68-ET3-LV titers are higher than EF1α-ET3-LV titers in all lots tested and are an average of at least fivefold higher. Importantly, titers >108 were achieved, which can support the clinical development of this vector.
In vivo testing of CD68-ET3-LV Sca-1+ in hemophilia A mice
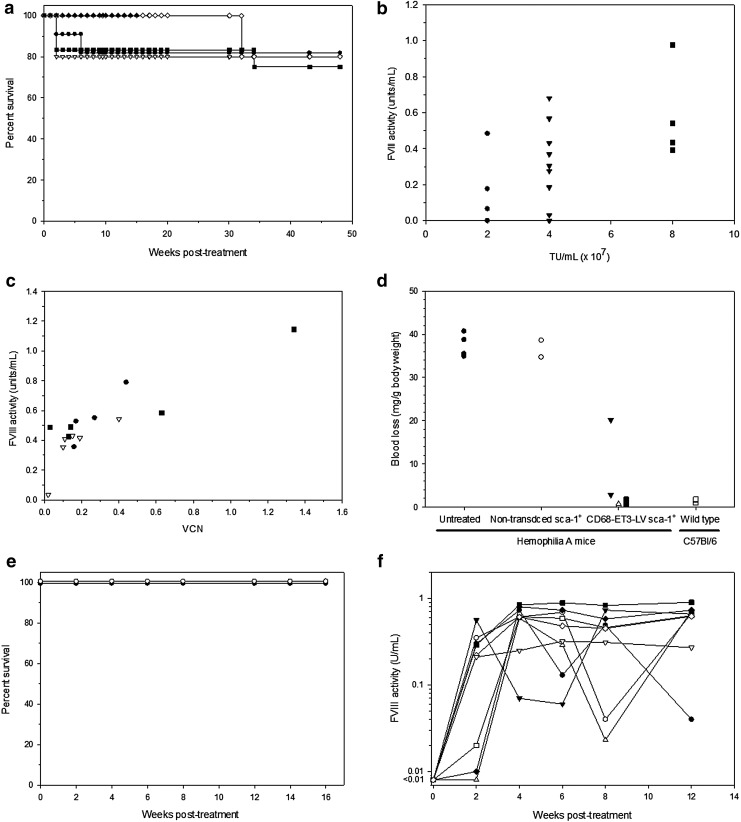
For nonclinical evaluation of CD68-ET3-LV Sca-1+, hemophilia A mice were treated with CD68-ET3-LV Sca-1+ that were generated using varying concentrations of CD68-ET3-LV for ex vivo transduction. The study outline is summarized in Table 1. Survival was followed for approximately 1 year after treatment with CD68-ET3-LV Sca-1+ that were generated using process similar CD68-ET3-LV manufactured by CCHMC and 4 months for CD68-ET3-LV Sca-1+ manufactured by Lentigen. Overall, survival of mice transplanted with CD68-ET3-LV Sca-1+ was similar to the survival of mice transplanted with control, congenic non-transduced Sca-1+ cells (Fig. 3a). In addition to survival analysis, complete blood cell counts were obtained every other week for 8 weeks after treatment to assess hematopoietic reconstitution. All treated animals recovered blood cell counts to the levels of untreated hemophilia A mice within 8 weeks (Supplementary Fig. S6). At 22 weeks after treatment, blood chemistry measurements were made on CD68-ET3-LV Sca-1+-treated hemophilia A mice and compared to reference values provided by Charles River for C57BL/6 mice (Supplementary Table S5). Values obtained for CD68-ET3-LV Sca-1+-treated hemophilia A mice were within the normal range, with the exception of blood urea nitrogen, which was higher in CD68-ET3-LV Sca-1+-treated mice (30.9 ± 4.0 mg/dL) compared to C57BL/6 mice (14.3 ± 5.3 mg/dL; p = 0.012), but not compared to untreated hemophilia A mice (24 ± 2 mg/dL) Thus, this mild elevation appears to be a reflection of the recipient genetic background.
Figure 3.
Murine CD68-ET3-LV Sca-1+ pharmacology. (a) Hemophilia A mice were treated with control, non-transduced Sca-1+ cells (closed circles) or CD68-ET3-LV Sca-1+ generated by transduction at vector concentrations of 2 × 107 TU/mL CCHMC LV (open inverted triangles), 4 × 107 TU/mL CCHMC LV (closed squares), 8 × 107 TU/mL CCHMC LV (open diamonds), or Lentigen LV (closed triangles). Survival was followed to 48 weeks for the mice that received non-transduced Sca-1+ cells or CCHMC LV-generated CD68-ET3-LV Sca-1+ and to 15 weeks for mice that received Lentigen LV-manufactured CD68-ET3-LV Sca-1+. (b) Plasma fVIII activity was measured by chromogenic substrate assay at week 8 post treatment with CD68-ET3-LV Sca-1+ generated by transduction at vector concentrations of 2 × 107 TU/mL CCHMC LV (n = 4, circles), 4 × 107 TU/mL CCHMC LV (n = 10, inverted triangles), or 8 × 107 TU/mL CCHMC LV (n = 5, squares). (c) Blood was collected from hemophilia A mice 12–14 weeks after treatment with CD68-ET3-LV Sca-1+ generated by transduction at vector concentrations of 2 × 107 TU/mL CCHMC LV (n = 4, circles), 4 × 107 TU/mL CCHMC LV (n = 6, inverted triangles), or 8 × 107 TU/mL CCHMC LV (n = 5, squares). From each individual mouse blood sample, VCN and fVIII activity measurements were made on peripheral blood mononuclear cell genomic DNA and plasma samples, respectively. (d) A tail-clip bleeding assay was used 46–52 weeks post treatment to assess hemostatic function. Six experimental groups were analyzed, including untreated hemophilia A mice (closed circles) or non-transduced Sca-1+-treated (open circles) hemophilia A mice, hemophilia A mice treated with CD68-ET3-LV Sca-1+ generated by transduction at vector concentrations of 2 × 107 TU/mL CCHMC LV (n = 2, closed inverted triangles), 4 × 107 TU/mL CCHMC LV (n = 2, open triangles), or 8 × 107 TU/mL CCHMC LV (n = 2, closed squares), and wild-type C57Bl/6 mice (open squares). (e) Hemophilia A mice were treated with cGMP CD68-ET3-LV Sca-1+ (experimental group, closed circles) or non-transduced Sca-1+ cells (experimental control group, open circles), and survival was monitored for 16 weeks. (f) Plasma fVIII activity was measured by chromogenic assay. Each line and symbol represent an individual animal.
Eight weeks after CD68-ET3-LV Sca-1+ treatment or administration of non-transduced Sca-1+ cells, plasma fVIII activity levels were determined (Fig. 3b). Sca-1+ cells (2 × 106/mL) were transduced with 2, 4, or 8 × 107 TU/mL (equivalent to multiplicities of infection of 10, 20, and 40). A positive correlation between the LV concentration used for ex vivo transduction of Sca-1+ cells and plasma fVIII activity was observed (Pearson's correlation coefficient 0.526; p = 0.021). Animals in the group that received CD68-ET3-LV Sca-1+ generated using 8 × 107 TU/mL CD68-ET3-LV displayed a mean plasma fVIII level of 0.54 ± 0.25 IU/mL. FVIII activity levels ≥0.5 IU/mL are considered normal in humans.
In addition to fVIII activity, VCN in peripheral blood cells was determined at 12–14 weeks after CD68-ET3-LV Sca-1+ treatment. VCN ranged from 0.02 to 1.34 copies per diploid genome equivalent, with a mean of 0.28 ± 0.34. At the same time as the VCN determination, plasma fVIII activity levels were also measured. Over the entire set of animals, fVIII activity levels ranged from 0.035 to 1.1 IU/mL, with a mean of 0.50 ± 0.24 IU/mL. A positive correlation was observed between VCN and plasma fVIII activity (Fig. 3c; Pearson's correlation coefficient 0.87; p < 0.001). The maximum fVIII activity observed was 1.1 IU/mL at the maximum VCN of 1.4. This is well within the normal human range of 0.5–1.5 IU/mL plasma fVIII activity.
To determine the efficacy of CD68-ET3-LV Sca-1+, the tail transection hemostatic assay was again employed. Figure 3d shows the blood loss from individual mice after transection of the distal 4 mm of tail. Overall, there was no significant difference observed between the CD68-ET3-LV Sca-1+ treatment group (independent of LV concentration) and wild-type mice (p = 0.76). However, there was a significant reduction in blood loss in the CD68-ET3-LV Sca-1+ treatment group compared to untreated or non-transduced Sca-1+-treated hemophilia A mice (p = 0.002). This finding is consistent with previous reports demonstrating that hemophilia A mice bleed abnormally in this model.20,42
Manufacture of clinical LV
A 60L production campaign was initiated using current GMP regulations. CD68-ET3-LV was manufactured using a multistep process, including filtration-based harvest clarification, ion exchange capture/purification, tangential flow filtration-based concentration, and diafiltration/formulation. Approximately 500 mL of clinical product was manufactured with a final titer 4.85 × 108 TU/mL. This material was aliquoted and frozen at −80°C. Aliquots were thawed and used to transduce HEK-293T/17 cell to obtain the LV titer. This product was tested using hemophilia A mice for in vivo safety and efficacy. Transplantation of CD68-ET3-LV Sca-1+ resulted in uniform survival, rapid hematopoietic reconstitution, and induction of therapeutic levels of plasma fVIII activity at low VCN (Fig. 3e and f). Secondary transplantation of bone marrow cells harvested from the non-transduced Sca-1+-treated animals and the CD68-ET3-LV Sca-1+-treated animals into naïve hemophilia A resulted in detectable fVIII levels in the latter but not the former groups and no other differences between the treatment arms (Supplementary Fig. S7). Collectively, these data validate the previous results and continue to support the nonclinical safety and efficacy of CD68-ET3-LV-transduced HSPC-enriched cells.
CD68-ET3-LV CD34+ manufacture and in vitro hematopoietic potential
CD68-ET3-LV CD34+ is an autologous cell product manufactured using the patient's own CD34+ cells. For ethical considerations, normal human donor mPB CD34+ cells were used as a surrogate for hemophilia A patient mPB CD34+ cells during preclinical evaluation. The in vitro effects of CD68-ET3-LV ex vivo transduction of mPB CD34+ cells were determined by measuring cell counts and viability pre and post transduction (Supplementary Tables S6 and S7). Of 33 transductions, only two donors, A4238/6330 and ND15-187, exhibited a decrease in cell viability upon transduction. Donor ND15-187 also displayed even greater reduced cell viability following mock transduction, suggesting differences in cell handling rather than effects of CD68-ET3-LV exposure were responsible for the decrease in cell viability. For all other CD68-ET3-LV CD34+ cell transductions (31/33 total transductions and 10/12 from which independent CD34+ cell donors were utilized), cell viability remained ≥94% post transduction. Overall, the results indicate ex vivo transduction using CD68-ET3-LV under the conditions tested does not acutely affect CD34+ cell viability for the majority of mPB products.
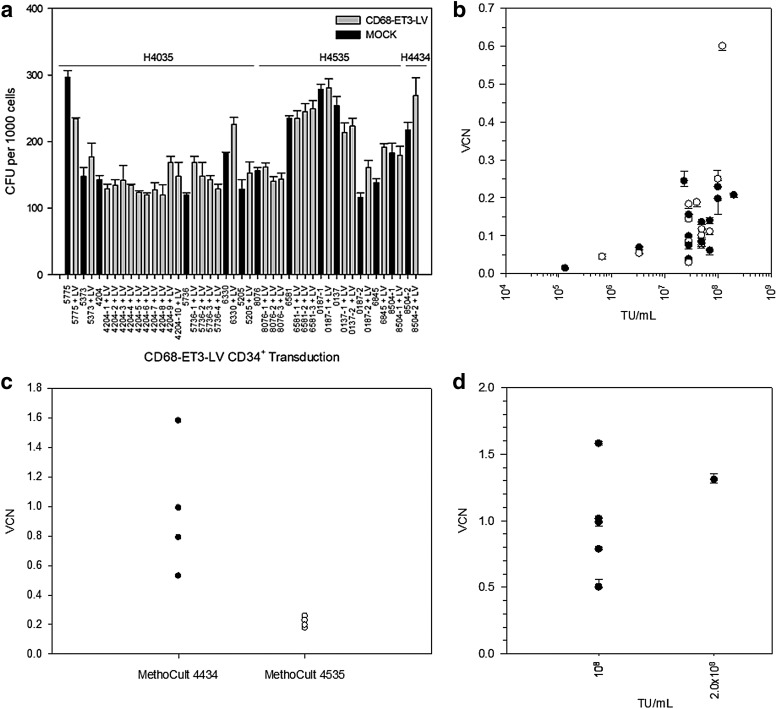
Clonogenic potential of CD68-ET3-LV CD34+ was assessed by semi-solid methylcellulose-based CFC assay and enumeration of CFUs. Three different methylcellulose-based media were used for CFC assays. Both H4035 and H4535 detect CFU-GM, CFU-G, and CFU-M, while the latter is enriched with interleukin (IL)-6. In contrast, H4434 detects a greater repertoire of hematopoietic progenitor cells (BFU-E, CFU-E, CFU-GM, CFU-G, CFU-M, and CFU-GEMM). The MethoCult H4434, H4035, and/or H4535 CFU counts for each non-transduced or CD68-ET3-LV-transduced mPB CD34+ donor cell population are summarized in Fig. 4a. Independent of the CFC assay used, the current findings show CD68-ET3-LV does not affect clonogenic potential, and thus CD68-ET3-LV exposure and genetic modification does not affect mPB CD34+ hematopoietic progenitor cell potential in vitro.
Figure 4.
CD68-ET3-LV CD34+ manufacture and quality assessment. (a) Colony forming units (CFU) were enumerated from mPB CD68-ET3-LV CD34+. Black bars represent non-transduced CD34+ CFU counts, and gray bars represent CD68-ET3-LV CD34+ CFU counts. (b) CD68-ET3-LV CD34+ generated using CCHMC LV (closed circles) or Lentigen LV (open circles) were plated in MethoCult H4035 or H4535, and CFU were isolated in bulk pools from each well. From each CFU pool, genomic DNA was isolated, and vector copy number (VCN) was measured by quantitative polymerase chain reaction (qPCR). (c) CD68-ET3-LV CD34+ were plated in MethoCult H4434 or H4535, and CFU VCN was determined. (d) CD68-ET3-LV CD34+ were generated using CCHMC manufactured CD68-ET3-LV for transduction at 108 TU/mL or 2 × 108 TU/mL starting LV concentration. Subsequently, CD68-ET3-LV CD34+ were plated in MethoCult H4434 and CFU isolated in bulk pools from each well. From each CFU pool, genomic DNA was isolated, and VCN was measured by qPCR.
As a potential efficacy parameter for CD68-ET3-LV CD34+, post-transduction VCN was determined on genomic DNA isolated from CD68-ET3-LV CD34+ CFU colonies harvested from methylcellulose assays. Across the range of LV concentrations tested, there was no difference in VCN observed using MethoCult H4035 or H4535 (Fig. 4b). However, it was found that higher CFU VCN was observed with MethoCult 4434. A comparison of the data obtained using optimized transduction conditions and subsequent MethoCult 4035/4535 or MethoCult 4434 assay demonstrated that significantly different CFU VCNs are obtained for each assay (0.21 ± 0.03 and 0.98 ± 0.39, respectively; p = 0.008), with the higher VCN being obtained with the H4434 product (Fig. 4c and d). Currently, the difference in VCN obtained using MethoCult H4035/4535 versus MethoCult H4434 is not understood, but it is hypothesized that the later assay supports the growth of a larger repertoire of hematopoietic progenitors that are transduced at greater efficiency by CD68-ET3-LV.
Clinical protocol simulation study
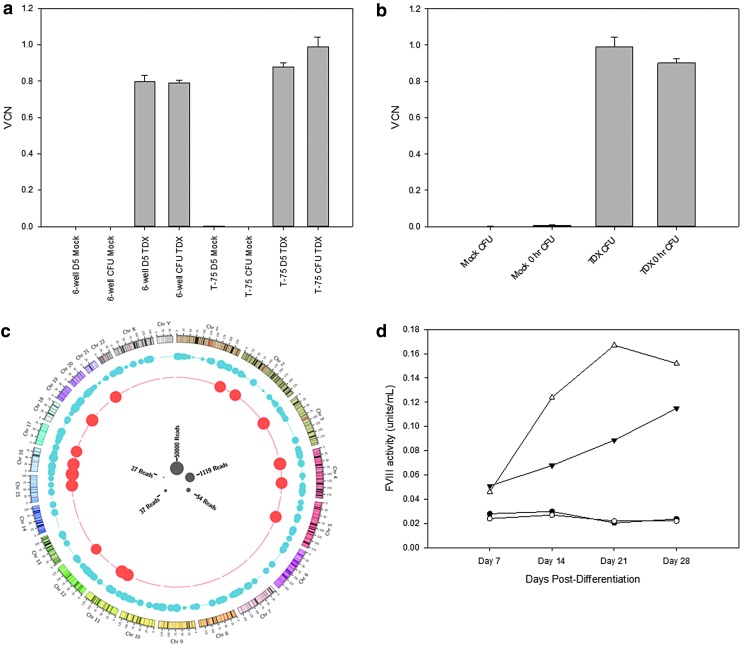
A normal healthy donor underwent apheresis, and the harvested cell product was subjected to mock shipping conditions prior to isolation of mPB CD34+ cells. Isolated mPB CD34+ cells were transduced at a density of 2 × 106 cells/mL with clinical process-like CD68-ET3-LV at 1/10th clinical scale using 108 TU/mL or subjected to mock transduction. A portion of cells were used for post-transduction analysis, and the remainder of the CD68-ET3-LV CD34+ product was cryopreserved for a 49-day cryopreservation stability study. Genomic DNA was isolated from day 5 culture or bulk CFU cell pellets obtained from CD68-ET3-LV CD34+ or mock-transduced CD34+ cells, processed post transduction (prior to cryopreservation) or post thaw after a 49-day cryopreservation period. Subsequently, VCN analysis was performed on the pre- and post-cryopreserved genomic DNA samples. As shown in Fig. 5a, similar transduction efficiencies were observed with the T-75 and six-well plate protocols, supporting process scalability up to 1/10th of the clinical scale. After a 49-day cryopreservation period, cells were thawed following a bedside thaw procedure, and the thawed product was kept at ambient temperature for sampling at 0, 2, and 4 h for reevaluation, a timeline that should be relevant for proposed patient infusion (Supplementary Fig. S8 and Supplementary Table S8). VCN remained consistent over the 4 h post-thaw period. Finally, when comparing the CFU VCN prior to and after cryopreservation, <10% difference in VCN was observed, indicating that VCN is stable over 49 days of cryopreservation (Fig. 5b). Similar to previous studies of mPB CD34+ cell transduction using CD68-ET3-LV, VCN was maintained in the MethoCult H4434 CFC assay, which promotes multi-potential progenitor growth, as well as committed progenitors from hematopoietic stem cells.
Figure 5.
Post-transduction VCN and insertion site analysis of CD68-ET3-LV CD34+. (a) VCN was determined by qPCR analysis of genomic DNA obtained prior to cryopreservation. (b) VCN was determined by qPCR analysis of genomic DNA obtained from CFU prior to or post cryopreservation. Error bars represent sample standard deviation. (c) Depiction of insertional site analysis for all integrations observed in mechanically sheared and enzymatically digested genomic DNA samples from CD68-ET3-LV CD34+. (d) FVIII activity in conditioned culture media was measured during in vitro myeloid differentiation of mock transduced CD34+ cells (closed circles) and CFU (open circles) or CD68-ET3-LV CD34+ cells (closed triangle) and CFU (open triangle) by one-stage coagulation assay.
Proviral transgene stability and insertion site analysis studies
HEK-293T/17 cells and the hematopoietic cell lines K562, U937 and Thp-1 were transduced with CD68-ET3-LV at 9.9 × 106 TU/mL (low LV) and 1.65 × 108 TU/mL (high LV) and cultured for 8 weeks. Proviral integration was assessed by Southern blot analysis using a digoxigenin-labeled probe specific for the ET3 A1 domain of fVIII. Genomic DNA was digested with Afl-II, which cleaves at sites in the 5′-LTR and 3′-LTR transgene cassette, producing an expected 6,895 bp fragment. A single band at 6.9 kb was detected in all the cell lines at each time point tested, demonstrating stability of the transgene for the duration of the culture period (Supplementary Figs. S9–S12).
To assess the proviral integrity of CD68-ET3-LV in the clinical target cell, DNA sequencing was performed on genomic DNA obtained from CD68-ET3-LV-transduced CB CD34+ cells in liquid culture and CFU culture and CD68-ET3-LV-transduced mPB CD34+ cells in CFU culture using a panel of PCR primers spanning the integrated provirus. All primer sets generated the correct size fragment products, except for the plasmid DNA-specific negative control set (Supplementary Figs. S13–S16). After PCR purification, samples were sequenced and verified. Only a single mismatch was identified in a single sample from mPB CD34+ bulk CFU genomic DNA. However, it was located in the non-coding region upstream of the promoter.
To identify the sites of proviral integration within the genomes of transduced hematopoietic cell lines and primary CD34+ cells, two methods were tested for fragmenting genomic DNA: one using enzymatic digestion by fragmentase, and a second using mechanical shearing by ultrasonication. The rationale for testing two methods is that next-generation genomic sequencing depends on breaking the contiguous chromosomal DNA harvested from cells into 500–1,000 bp fragments that can be ligated to sequencing adapters. Linear PCR amplified products were sequenced using the Emory Integrated Genomics Core using Illumina MiSeq. A summary of the samples tested is provided in Supplementary Table S9. DNA sequencing files were then processed using a bioinformatics pipeline developed in-house. First, known LTR and adapter sequences were removed, and the remaining sequence was compared to latest human genome sequence database, which allowed for precise integration site mapping within the human genome. As a positive control for each sample, genomic DNA from a standard cell line containing known integration sites was used. A clonal CD68-ET3-LV-transduced K562 cell line designated Clone 21-7-3 with four known integration sites was also examined, which was generated by transduction with process similar CD68-ET3-LV, and limiting dilution was used to isolate a single clone. Southern blot analysis of genomic DNA from the clone showed four unique integration sites (data not shown). Integration analysis also identified four unique integration sites using both the enzymatic digestion (fragmentase) and mechanical shearing (Covaris) methods and provided the exact location of integrations (Supplementary Tables S10–S21 and Supplementary Figures S17–S27). As expected, non-transduced cells showed no integration events (data not shown). Then, genomic DNA from the clinical target, mPB CD34+ cells, were analyzed. For the analysis shown in Fig. 5c, data were combined from enzymatic digestion and mechanical shearing processed samples. The location and frequency (size of circles) of spiked-in control genomic DNA is shown in red and that of the CD68-ET3-LV CD34+ sample is shown in blue. Table 2 summarizes the genomic locations of proviral integration sites from process similar CD68-ET3-LV-transduced CD34+ cells compared to the data set published by Arens et al. with respect to integration near cancer-related gene sequences.43
Table 2.
Summary of CD68-ET3-LV CD34+ integration site analysis
| Frequency of insertion events | ||||
|---|---|---|---|---|
| Genomic locations of integration events observed | This data set 332 non-redundant | Published data set46 1,717 non-redundant IS | Random data set 106 random genomic positions | |
| Within genes | All genes | 71.15% | 76.50% | 44.61% |
| Cancer genes | 3.16% | 5.10% | 1.49% | |
| <5 kb from TSS | All genes | 16.60% | 12.70% | 10.43% |
| Cancer genes | 0.00% | 0.30% | 0.16% | |
| <50 kb from TSS | All genes | 109.88% | 106.30% | 69.82% |
| Cancer genes | 5.14% | 4.50% | 1.52% | |
| <250 kb from TSS | All genes | 226.88% | 220.40% | 169.38% |
| Cancer genes | 17.39% | 17.60% | 7.50% | |
IS, integration site; TSS, transcription start site.
FVIII expression from monocyte-differentiated CD34+ cells
As anticipated, the monocyte-directed CD68 promoter did not support biosynthesis and secretion of measurable levels of ET3 from cultured CD68-ET3-LV CD34+ cells (data not shown). Therefore, three monocyte differentiation conditions were tested to determine if ET3 expression is increased following in vitro differentiation of CD68-ET3-LV CD34+ into monocytes. Human mPB-CD34+ cells were transduced following the proposed clinical transduction protocol using the GMP qualified CD68-ET3-LV. Post-transduction differentiation of transduced cells into monocytes was tested using three different cocktails of media, serum, and cytokines (Supplementary Table S22). At various times during culture, fVIII activity, cellular morphology, monocyte-specific differentiation markers, and transduction efficiency were monitored. As controls, mock-transduced cells, CD68-GFP-LV, and EF1α-ET3-LV transduced cells were also examined. As expected, a decrease in surface CD34+ expression was observed during culture, indicating progression of progenitor differentiation (Supplementary Tables S23 and S24). Morphology changes also indicated progression toward monocyte differentiation (Supplementary Fig. S28). Total CD68-driven GFP expression did not increase over time, but total MFI increased compared to EF1α-driven GFP expression, which, as expected for the ubiquitous promoter, remained constant. An increase in CD14+/CD16+ double-positive cells was observed during monocyte differentiation and was highest in differentiation media 2 (Supplementary Tables S23 and S24). Similarly, CD68+ expression increased during monocyte differentiation and was greatest in differentiation media 1 and 2, and expression levels were similar for CD68-ET3-LV and GFP transduced cells. FVIII activity in the medium increased over time, which correlated with the differentiation status of the culture (Fig. 5d). All cells were CD45+ at the final harvest (day 28 post monocyte differentiation), confirming human hematopoietic origin. Taken together, these data indicate that differentiation medium 2 supported monocyte differentiation of human mPB-CD34+ cells, CD68-ET3-LV transduction did not affect monocyte differentiation, and differentiated cells efficiently expressed ET3. In addition to a using a continuous liquid culture, cells were first plated in methylcellulose H4535, which supports granulocyte progenitor growth. Fourteen days after plating in H4535, cells were collected from the methylcellulose cultures and further propagated in differentiation medium 2. Differentiation was monitored at each time point of medium exchange, which again over time showed an increase in CD14+/CD16+ double-positive cells, an increase in CD68 expression, morphology changes (data not shown), and an increase in fVIII expression (Fig. 5d).
CD68-ET3-LV CD34+ testing in murine xenotransplantation models
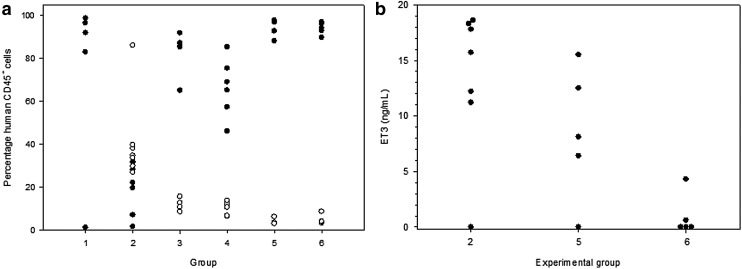
As a final pharmacology/toxicology study, CD68-ET3-LV CD34+ xenotransplantation into immunodeficient mice was performed. Although NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl /SzJ) mice have been used to study human hematopoiesis in a nonclinical system,44,45 several transgenic modifications have been made to this model, including the NOD.Cg-Prkdcscid Il2rgtm1WjlTg(PGK1-KITLG*220)441Daw strain, commonly referred to as “NSG Tg(Hu-mSCF),” which expresses human membrane bound stem-cell factor (mSCF). This model supports improved CD34+ engraftment and was used to test the effects of CD68-ET-LV transduction on CD34+ cell engraftment.46 To assess the in vivo safety and efficacy of CD68-ET3-LV-CD34+, NSG Tg(Hu-mSCF) mice were transplanted with CD68-ET3-LV CD34+ generated using two independent lots of CD68-ET3-LV or non-transduced CD34+ cells. A description of the treatment groups, as well as the individual animals, is provided in Supplementary Table S25. The percentages of human myeloid and lymphoid CD45+ cells present in peripheral blood were determined 7–9 weeks after treatment (Fig. 6a). Human CD34+ cells were transduced ex vivo with CD68-ET3-LV manufactured by Lentigen (groups 1 and 2) or CCHMC (groups 3, 4, and 5). Control, non-transduced CD34+ cells also were administered to NSG Tg(Hu-mSCF) mice (group 6). Overall, there was no significant difference in the engraftment levels of human CD45+ cells when comparing non-transduced CD34+ cells and CD68-ET3-LV CD34+ generated using the two lots of CD68-ET3-LV. However, group 2 animals displayed lower engraftment than groups 5 and 6 (p = 0.002 and 0.005, respectively). Of note, the group 2 mice also displayed higher myeloid engraftment than groups 5 and 6 (p < 0.001 and 0.004, respectively). The cause of this finding is not known. However, the simplest explanation is individual donor CD34+ variability as group 2 animals were treated with CD68-ET3-LV CD34+ generated using a different donor cell population than the other groups. In contrast, groups 5 and 6 mice were treated with CD68-ET3-LV CD34+ generated using the same starting CD34+ cell population.
Figure 6.
Murine CD68-ET3-LV CD34+ pharmacology. (a) Human CD34+ cells were transduced ex vivo with CD68-ET3-LV and transplanted into NSG Tg(Hu-mSCF) mice (groups 1–5). Control, non-transduced CD34+ cells also were administered to NSG Tg(Hu-mSCF) mice (group 6). Human cell engraftment was determined at 7–9 weeks post treatment by immunostaining peripheral blood mononuclear cells with anti-human CD45+ and performing flow cytometric analysis to determine the percentage of human lymphocytes (closed circles) and myeloid cells (open circles). (b) Peripheral blood was obtained 5–6 weeks post treatment, and plasma ET3 antigen levels were determined using an ET3-specific enzyme-linked immunosorbent assay.
ET3 levels were measured using an ELISA that incorporates ET3-specific murine monoclonal antibodies that do not cross-react with murine fVIII. Although it was possible to measure ET3 antigen levels, ET3 activity levels could not be determined because of the high background signal from endogenous fVIII in NSG Tg(Hu-mSCF) mice. The mean ET3 antigen levels in CD68-ET3-LV CD34+ treated mice were 13.4 ± 6.6 and 8.5 ± 6 ng/mL for the first and second production lots of CD68-ET3-LV (Fig. 6b). These levels were higher than levels measured in non-transduced CD34+-treated NSG Tg(Hu-mSCF) mice (0.98 ± 1.9 ng/mL). As described above, the specific activity of highly purified recombinant ET3 (i.e., ET3i) is 13,100 IU/mg. Therefore, it is estimated that the fVIII activity levels associated with the measured antigen levels are 0.18 and 0.11 IU/mL for the two CD68-ET3-LV CD34+ treatment groups, respectively. Both CD68-ET3-LV CD34+ treatment groups displayed ET3 levels that would be expected to provide therapeutic benefit in the setting of severe hemophilia A. These results are notable because myeloid engraftment is not robust in NSG Tg(Hu-mSCF) mice, and the CD68 promoter is myeloid specific. So, fVIII levels are predicted to be low in this model.
Approximately 8 weeks post treatment, mice were bled, and samples were delivered to the Emory DAR for blood chemistry analysis (Supplementary Table S26). Chemistry values were then matched to control or CD68-ET3-LV CD34+ treatment groups. Overall, there were no significant differences in the values for albumin, total bilirubin, alanine amino transferase, amylase, phosphate, creatinine, glucose, total protein, globulin, or potassium levels. Significant differences were observed for Ca+ and Na+, but all mice were within the normal range. Blood urea nitrogen was also significantly different due to one outlier, which was above normal levels. However, all other blood chemistry values for this animal were normal. Alkaline phosphatase values were mildly but significantly lower for CD68-ET3-LV CD34+-treated mice compared to controls (p < 0.001). Eight months after CD68-ET3-LV CD34+ or non-transduced CD34+ treatment, mice in groups 3–6 (CCHMC LV groups) were transported to the Emory DAR for necropsy. Necropsy uncovered no significant findings for control or treated mice. Additionally, there was no significant difference in the percentage of human CD45+ cell engraftment in mice transplanted with CD68-ET3-LV CD34+ compared to non-transduced CD34+ cells.
Conclusions
CD68-ET3-LV CD34+ is a genetically modified autologous cell product whose mechanism of action involves hematopoietic stem-cell engraftment, differentiation into blood cell lineages, and secretion of coagulation fVIII into the bloodstream. This report describes the results of in vitro and in vivo pharmacology, pharmacodynamic, and toxicology testing of CD68-ET3-LV CD34+ in cell culture and murine xenotransplantation models believed to provide a rationale for clinical testing. ET3i and the ET3 transgene product have been studied extensively for more than a decade in biochemical in vitro cell culture models and in vivo gene therapy studies incorporating hemophilia A mice.10–16,29,47 In every system tested, ET3 displays superior biosynthetic efficiency over BDD human fVIII, which should equate to higher potency of the CD68-ET3-LV CD34+ product candidate over an otherwise comparable CD68-BDDhfVIII-LV CD34+ candidate. In clinical gene therapy, other than safety, potency is the key desirable property because viral vector dose limiting toxicities typically result from viral-derived and not transgene-derived vector components. Herein, it is demonstrated that highly purified ET3i has the same subunit structure as HSQ and BDD porcine fVIII in non-activated and activated form. ET3i also binds vWf indistinguishably from BDD human fVIII, which suggests that ET3i would have pharmacokinetic characteristics similar to existing commercial non-extended half-life fVIII products and clinical gene therapy candidates. As ET3 contains porcine amino acid substitutions in the A1 and A3 domains, immunogenicity is a relevant concern. However, the results presented from a naïve hemophilia A mouse immunogenicity testing model predict that the development of inhibitory anti-fVIII antibodies to ET3i would not be greater than to human fVIII in previously untreated patients with hemophilia A. All clinical hemophilia A gene therapy testing has occurred in patients previously treated with human fVIII products and no history of inhibitory antibodies. Thus, the preclinical studies presented do not completely recapitulate this clinical setting, but no preclinical model exists for this setting, since hemophilia A mice uniformly develop antibodies to infused human fVIII products. Previously, the ability of MSCV-porcine fVIII vector transduced Sca-1+ cells transplanted under myeloablative and non-myeloablative conditioning to eradicate inhibitors and provide sustained plasma fVIII production has been demonstrated.25,27,28 Additionally, this approach has been demonstrated to provide robust tolerance (or immune non-responsiveness) to subsequently infused recombinant fVIII, which was not observed following liver-directed AAV gene therapy in hemophilia A mice.15,28
Overall the data collected from ET3i analysis suggest the ET3 transgene and its product are similar to BDD human fVIII. Although no direct comparisons to CD68-HSQ-LV Sca-1+ or CD34+ product candidate were made in the current study, a substantial body of work describing similar comparisons in cell culture and in vivo gene therapy models supports the prediction that CD68-ET3-LV CD34+ would be superior to a comparable therapeutic candidate containing a human fVIII transgene.10–16,29,47 Beyond incorporation of the ET3 transgene, another unique and clinically untested aspect of the CD68-ET3-LV is the inclusion of the CD68 promoter. The CD68 gene promoter directs monocyte/macrophage-specific expression. Although the promoter lacks a classical TATA box, it contains other protein-binding sites consistent with preferential monocyte/macrophage gene expression.48 CD68 promoter has been used to achieve constitutive expression of IL-10 specifically in macrophages.49 Aside from the theoretical safety obtained by driving transgene expression away from stem-cell population and into mature hematopoietic, and mostly myeloid, lineages, inclusion of the CD68 appears to support high-titer LV manufacture. The relatively high titers associated with the CD68 promoter containing LV appear to be at least partly due to the low levels of internally promoted transcripts in producer cells and reduced promoter competition. Despite restriction to mostly the monocytic compartment, CD68-ET-LV displays sufficient potency to drive therapeutic levels of fVIII production in both murine hemophilia A Sca-1+ and human CD34+ xenotransplantation models at low levels of engraftment similar to those being observed in other HSC-LV products. Again, this result supports the clinical efficacy of CD68-ET3-LV CD34+.
Through rigorous testing in two preclinical models, CD68-ET3-LV CD34+ and the CD68-ET3-LV Sca-1+ surrogate product candidates displayed a strong safety profile. One aspect of the utilization of CD68-ET3-LV CD34+ not specifically addressed in the current study is the conditioning regimen required for product engraftment. Previous studies demonstrated successful engraftment and therapeutic levels of fVIII expression following multiple conditioning regimens, including myeloablative and non-myeloablative doses of gamma irradiation or combinations of alkylating agents and immunosuppressants.25–28 Unlike CD68-ET3-LV CD34+, which has not been clinically studied, the acute safety and long-term toxicity of available conditioning agents are well established. Thus, it should be possible to select a conditioning regimen for clinical investigation of CD68-ET3-LV CD34+ that takes into careful consideration the risk–benefit profile in severe hemophilia A patient populations globally. Based on recent reports and an ongoing clinical trial in X-SCID patients, it appears likely that non-genotoxic, targeted agents such as anti-stem and progenitor cell monoclonal antibodies and antibody–drug conjugates will become available, thereby further improving the safety profile of many LV CD34+-based gene therapy products.50,51
Supplementary Material
Acknowledgments
This work was supported by funding from the National Institutes of Health/National Heart, Lung and Blood Institute (G.D., C.B.D., P.L., and H.T.S.) and Hemophilia of Georgia (H.T.S., C.B.D., and P.L.). We would like to thank Dr. Bagirath Gangadharan for assistance with some of the ET3i studies. We also thank Dr. Boro Dropulic, formerly of Lentigen Corporation, for early collaborative work on LV design and manufacture and Bill Swaney (CCHMC) for process similar and GMP LV manufacture.
Author Disclosure
P.L. is inventor on a patent application describing ET3. C.B.D., P.L., and H.T.S. are co-founders of Expression Therapeutics and own equity in the company. Expression Therapeutics owns the intellectual property associated with ET3. G.D. is an employee of Expression Therapeutics and owns equity in the company. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. A.S. has no relevant conflicts of interest.
References
- 1.Aiuti A, Biasco L, Scaramuzza S, et al. . Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott–Aldrich syndrome. Science 2013;341:1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiuti A, Cattaneo F, Galimberti S, et al. . Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 2009;360:447–458 [DOI] [PubMed] [Google Scholar]
- 3.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. . Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009;326:818–823 [DOI] [PubMed] [Google Scholar]
- 4.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. . Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000;288:669–672 [DOI] [PubMed] [Google Scholar]
- 5.Negre O, Eggimann AV, Beuzard Y, et al. . Gene Therapy of the beta-hemoglobinopathies by lentiviral transfer of the beta(A(T87Q))-globin gene. Hum Gene Ther 2016;27:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeil JA, Hacein-Bey-Abina S, Payen E, et al. . Gene therapy in a patient with sickle cell disease. N Engl J Med 2017;376:848–855 [DOI] [PubMed] [Google Scholar]
- 7.Kang HJ, Bartholomae CC, Paruzynski A, et al. . Retroviral gene therapy for X-linked chronic granulomatous disease: results from Phase I/II trial. Mol Ther 2011;19:2092–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sessa M, Lorioli L, Fumagalli F, et al. . Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, Phase 1/2 trial. Lancet 2016;388:476–487 [DOI] [PubMed] [Google Scholar]
- 9.Biffi A, Montini E, Lorioli L, et al. . Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013;341:1233158. [DOI] [PubMed] [Google Scholar]
- 10.Brown HC, Wright JF, Zhou S, et al. . Bioengineered coagulation factor VIII enables long-term correction of murine hemophilia A following liver-directed adeno-associated viral vector delivery. Mol Ther Methods Clin Dev 2014;1:14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown HC, Zakas PM, George SN, et al. . Target-cell-directed bioengineering approaches for gene therapy of hemophilia A. Mol Ther Methods Clin Dev 2018;9:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doering CB, Denning G, Dooriss K, et al. . Directed engineering of a high-expression chimeric transgene as a strategy for gene therapy of hemophilia A. Mol Ther 2009;17:1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doering CB, Healey JF, Parker ET, et al. . Identification of porcine coagulation factor VIII domains responsible for high level expression via enhanced secretion. J Biol Chem 2004;279:6546–6552 [DOI] [PubMed] [Google Scholar]
- 14.Johnston JM, Denning G, Doering CB, et al. . Generation of an optimized lentiviral vector encoding a high-expression factor VIII transgene for gene therapy of hemophilia A. Gene Ther 2013;20:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lytle AM, Brown HC, Paik NY, et al. . Effects of fVIII immunity on hepatocyte and hematopoietic stem cell-directed gene therapy of murine hemophilia A. Mol Ther Methods Clin Dev 2016;3:15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakas PM, Brown HC, Knight K, et al. . Enhancing the pharmaceutical properties of protein drugs by ancestral sequence reconstruction. Nat Biotechnol 2017;35:35–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGarrity GJ, Hoyah G, Winemiller A, et al. . Patient monitoring and follow-up in lentiviral clinical trials. J Gene Med 2013;15:78–82 [DOI] [PubMed] [Google Scholar]
- 18.Qian J, Borovok M, Bi L, et al. . Inhibitor antibody development and T cell response to human factor VIII in murine hemophilia A. Thromb Haemost 1999;81:240–244 [PubMed] [Google Scholar]
- 19.Doering CB, Healey JF, Parker ET, et al. . High level expression of recombinant porcine coagulation factor VIII. J Biol Chem 2002;277:38345–38349 [DOI] [PubMed] [Google Scholar]
- 20.Bi L, Lawler AM, Antonarakis SE, et al. . Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet 1995;10:119–121 [DOI] [PubMed] [Google Scholar]
- 21.Dixon WJ. The up-and-down method for small samples. J Am Stat Assoc 1965;60:967–978 [Google Scholar]
- 22.Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 1991;15:47–50 [DOI] [PubMed] [Google Scholar]
- 23.Modlich U, Bohne J, Schmidt M, et al. . Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood 2006;108:2545–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown HC, Gangadharan B, Doering CB. Enhanced biosynthesis of coagulation factor VIII through diminished engagement of the unfolded protein response. J Biol Chem 2011;286:24451–24457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doering CB, Gangadharan B, Dukart HZ, et al. . Hematopoietic stem cells encoding porcine factor VIII induce pro-coagulant activity in hemophilia A mice with pre-existing factor VIII immunity. Mol Ther 2007;15:1093–1099 [DOI] [PubMed] [Google Scholar]
- 26.Gangadharan B, Parker ET, Ide LM, et al. . High-level expression of porcine factor VIII from genetically modified bone marrow-derived stem cells. Blood 2006;107:3859–3864 [DOI] [PubMed] [Google Scholar]
- 27.Ide LM, Gangadharan B, Chiang KY, et al. . Hematopoietic stem-cell gene therapy of hemophilia A incorporating a porcine factor VIII transgene and nonmyeloablative conditioning regimens. Blood 2007;110:2855–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ide LM, Iwakoshi NN, Gangadharan B, et al. . Functional aspects of factor VIII expression after transplantation of genetically-modified hematopoietic stem cells for hemophilia A. J Gene Med 2010;12:333–344 [DOI] [PubMed] [Google Scholar]
- 29.Spencer HT, Denning G, Gautney RE, et al. . Lentiviral vector platform for production of bioengineered recombinant coagulation factor VIII. Mol Ther 2011;19:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lusher JM, Arkin S, Abildgaard CF, et al. . Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. Kogenate Previously Untreated Patient Study Group. N Engl J Med 1993;328:453–459 [DOI] [PubMed] [Google Scholar]
- 31.Bray GL, Gomperts ED, Courter S, et al. . A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. The Recombinate Study Group. Blood 1994;83:2428–2435 [PubMed] [Google Scholar]
- 32.Lusher JM, Lee CA, Kessler CM, et al. . The safety and efficacy of B-domain deleted recombinant factor VIII concentrate in patients with severe haemophilia A. Haemophilia 2003;9:38–49 [DOI] [PubMed] [Google Scholar]
- 33.Kreuz W, Ettingshausen CE, Zyschka A, et al. . Inhibitor development in previously untreated patients with hemophilia A: a prospective long-term follow-up comparing plasma-derived and recombinant products. Semin Thromb Hemost 2002;28:285–290 [DOI] [PubMed] [Google Scholar]
- 34.Lamberth K, Reedtz-Runge SL, Simon J, et al. . Post hoc assessment of the immunogenicity of bioengineered factor VIIa demonstrates the use of preclinical tools. Sci Transl Med 2017;9. [DOI] [PubMed] [Google Scholar]
- 35.Hoyer LW, Qian J. Characterization of the immune response to factor VIII using hemophilia A* mice. Haematologica 2000;85:100–102 [PubMed] [Google Scholar]
- 36.Reipert BM, Ahmad RU, Turecek PL, et al. . Characterization of antibodies induced by human factor VIII in a murine knockout model of hemophilia A. Thromb Haemost 2000;84:826–832 [PubMed] [Google Scholar]
- 37.Hausl C, Maier E, Schwarz HP, et al. . Long-term persistence of anti-factor VIII antibody-secreting cells in hemophilic mice after treatment with human factor VIII. Thromb Haemost 2002;87:840–845 [PubMed] [Google Scholar]
- 38.Sasgary M, Ahmad RU, Schwarz HP, et al. . Single cell analysis of factor VIII-specific T cells in hemophilic mice after treatment with human factor VIII. Thromb Haemost 2002;87:266–272 [PubMed] [Google Scholar]
- 39.Meeks SL, Healey JF, Parker ET, et al. . Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood 2007;110:4234–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markovitz RC, Healey JF, Parker ET, et al. . The diversity of the immune response to the A2 domain of human factor VIII. Blood 2013;121:2785–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batsuli G, Deng W, Healey JF, et al. . High-affinity, noninhibitory pathogenic C1 domain antibodies are present in patients with hemophilia A and inhibitors. Blood 2016;128:2055–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connelly S, Andrews JL, Gallo AM, et al. . Sustained phenotypic correction of murine hemophilia A by in vivo gene therapy. Blood 1998;91:3273–3281 [PubMed] [Google Scholar]
- 43.Arens A, Appelt JU, Bartholomae CC, et al. . Bioinformatic clonality analysis of next-generation sequencing-derived viral vector integration sites. Hum Gene Ther Methods 2012;23:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa F, Yasukawa M, Lyons B, et al. . Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 2005;106:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shultz LD, Lyons BL, Burzenski LM, et al. . Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005;174:6477–6489 [DOI] [PubMed] [Google Scholar]
- 46.Takagi S, Saito Y, Hijikata A, et al. . Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood 2012;119:2768–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.European Medicines Agency—Committee for Medicinal Products for Human Use. Reflection Paper on Immune Tolerance Induction in Haemophilia A Patients with Inhibitors, EMA/CHMP/BPWP/153137/2011, 2013. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/03/WC500140787.pdf (last accessed September17, 2018)
- 48.Jiang Z, Shih DM, Xia YR, et al. . Structure, organization, and chromosomal mapping of the gene encoding macrosialin, a macrophage-restricted protein. Genomics 1998;50:199–205 [DOI] [PubMed] [Google Scholar]
- 49.Lang R, Rutschman RL, Greaves DR, et al. . Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J Immunol 2002;168:3402–3411 [DOI] [PubMed] [Google Scholar]
- 50.Czechowicz A, Kraft D, Weissman IL, et al. . Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 2007;318:1296–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palchaudhuri R, Saez B, Hoggatt J, et al. . Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat Biotechnol 2016;34:738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.