Abstract
Background and Aims
Mucosal appearance on endoscopy is an important indicator of inflammatory burden and determines prognosis in ulcerative colitis (UC). Inflammation induces tryptophan metabolism along the kynurenine pathway (KP) and yields immunologically relevant metabolites. We sought to examine whether changes in serum tryptophan metabolites and tissue expression of KP enzymes are associated with UC endoscopic and histologic disease severity.
Methods
Serum and mucosal samples were prospectively obtained at colonoscopy in patients with UC. Mayo disease activity scores, demographics, smoking status, medications, and outcomes were collected. Serum tryptophan metabolites were analyzed using ultra-high performance liquid chromatography (uHPLC), and gas chromatography-mass spectrometry (GC-MS), and enzyme expression was determined by quantitative real-time polymerase chain reaction. Metabolite and enzyme levels were compared by endoscopic subscore, clinical disease activity, time to surgery, and hospitalization.
Results
This study included 99 patients with Mayo endoscopic subscores 0–3. Kynurenic acid/tryptophan ratio (KYNA/T) and expression of indolamine 2,3-dioxygenase 1 (IDO1), tryptophan 2,3-dioxygenase, kynurinase, and kynurenine monooxygenase correlated positively with endoscopic subscore. Adjusting for age of diagnosis, smoking status, disease extent, and medications yielded significant odds of endoscopic inflammation with increasing KYNA/T (OR 1.0015, P = 0.0186) and IDO1 expression (OR 1.0635, P = 0.0215). The highest tertile ratio of KYNA/T had shorter time to surgery (P = 0.009) and hospitalization (P = 0.01) than the lowest.
Conclusions
Increasing KYNA/T is closely associated with endoscopic inflammation and predictive of disease outcomes in patients with UC. These findings identify this novel metabolic association and further support the role of the KP in regulating mucosal inflammation in UC.
Keywords: ulcerative colitis, tryptophan, mucosal healing
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon in which the immune system of a genetically susceptible host reacts to colonic environmental conditions and causes chronic mucosal inflammation.1 The colonic mucosal appearance on endoscopy is an important determinant of disease prognosis and response to therapy.2, 3
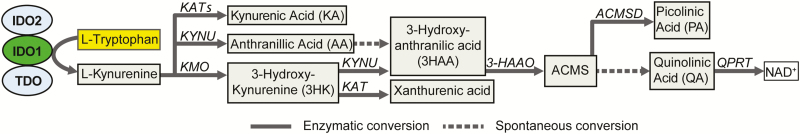
Tryptophan (Trp) is an essential amino acid that is derived from dietary intake of protein rich food.4 Trp is necessary for protein synthesis and the formation of the neurotransmitters serotonin and melatonin. However, more than 90% of dietary Trp is metabolized along the kynurenine pathway (KP).5 Indoleamine 2,3-dioxygenase 1 (IDO1) is the first and rate-limiting enzyme in Trp metabolism along the KP (Fig. 1). IDO1 is highly expressed in macrophages, dendritic cells, and intestinal epithelial cells, and its expression increases in the setting of intestinal inflammation.5–7 IDO1 induces rapid Trp depletion that inhibits T cell growth and immune responsiveness.5
FIGURE 1.
Kynurenine pathway. IDO1 = Indoleamine 2,3-dioxygenase 1, IDO2 = Indoleamine 2,3-dioxygenase 2, TDO = Tryptophan 2,3-dioxygenase, KAT = Kynurenine aminotransferases, KYNU = Kynurinase, KMO = Kynurenine monooxygenase, KYNA = Kynurenic acid, AA = Anthranillic acid, 3HK = 3-hydroxykynurenine, 3HAA = 3-hydroxyanthranilic acid, 3HAAO = 3-Hydroxyanthranilate 3,4-dioxygenase, ACMS = 2-amino-3-carboxymuconic-6-semialdehyde, ACMSD = Aminocarboxymuconate-semialdehyde decarboxylase, PA = Picolinic acid, QA = Quinolinic acid, QPRT = Quinolinate phosphoribosyltransferase, NAD, nicotinamide adenine dinucleotide.
The serum ratio of kynurenine to Trp increases with disease severity in several inflammatory conditions, including Crohn’s disease (CD).8 This may be due to the close relationship between the products of the KP and inflammatory regulation.9 Kynurenine induces T cell death and regulates natural killer T cells.10, 11 Additional downstream metabolites also exert immunologic effects. Kynurenic acid (KYNA) regulates natural killer T cells, attenuates tumor necrosis factor α (TNFα) transcription, and reduces IL-17 and IL-23.12–14 Quinolinic acid and 3-hydroxyanthranilic acid (3HAA) can select for Th2 cells by inducing apoptosis of Th1 cells.15 However, tissue expression levels of enzymes in KP and downstream kynurenine metabolites have not been investigated in UC. This study aims to determine whether changes in serum Trp metabolites and tissue expression of KP enzymes are associated with UC disease severity.
MATERIALS AND METHODS
Patient Recruitment and Sample Acquisition
Patients were enrolled at the time of colonoscopy. Patients were included if they were 18 years or older, had a diagnosis of UC, and a disease extent of at least 20 cm proximal to the anus. Demographic data were collected by questionnaire and included age, sex, age of diagnosis, smoking status, and current medications. A serum sample was obtained before the procedure in BD Vacutainer plastic tubes with silicone-coated interiors and a silica clot activator (catalog no. 367815; BD Biosciences). The patients underwent a standard of care colonoscopy as planned by an attending gastroenterologist at the University of Chicago Medicine. Each examination included documentation of Mayo disease activity score.16 All patients also had 4 biopsies obtained 20 cm from the anal verge and placed in RNA later. Histology from the sigmoid colon was scored as normal, mild, moderate, or severe based on pathologic interpretation.17 Normal was defined as having no features of acute or chronic injury. Mild contained architectural distortion and increased lamina propria lymphocytes. Moderate showed increased lamina propria granulocytes with or without intraepithelial granulocytes without crypt abcesses. Severe included the features of mild and moderate with crypt abcesses or erosions. Patient outcomes were determined by abstracting from the medical records the date of first hospital admission following enrollment and date of total colectomy.
Trp Metabolites
All reagents used for ultra-high performance liquid chromatography (uHPLC) and gas chromatography-mass spectrometry (GC-MS) were of analytical grade and purchased from Sigma Aldrich (MO, USA) unless otherwise specified. Serum samples were deproteinized with equal volume of 10% (w+v) trichloroacetic acid and filtered through a 0.22 μm PTFE syringe filter (Merck-Milipore, CA, USA). Trp, kynurenine, 3-hydroxykynurenine, 3-hydroxyanthranilic acid, and anthranilic acid quantification was performed using uHPLC in accordance with methods previously described in Jones SP et al.18 KYNA was separately measured using methods described by Lim CK et al.19 Picolinic acid and quinolinic acid are concurrently measured using GC-MS as detailed in Lim CK et al.19 Intra- and interassay coefficient of variation (CV) values were calculated by using standards incorporated in the chromatography steps with CV 5–8%. Analyte levels were expressed in moles/L.
KP mRNA Expression
Tissue was homogenized with the use of a bullet blender (Next Advance) and extracted with an AllPrep DNA/RNA/ miRNA Universal Kit (Qiagen). The cDNA was synthesized from RNA extracted from 85 subjects with the use of a high-capacity cDNA reverse-transcription kit (Life Technologies). Real-time quantitative polymerase chain reaction (qPCR) was performed with Fast SYBR Green Master Mix (Thermo Fisher Scientific) using primers from IDT in a Roche 480 LightCycler. Primer sequences were accessed from PrimerBank (Supplementary Table 1). For IDO1, the thermal profile was as follows: activation at 95°C for 5 minutes followed by 45 amplification cycles (denaturation at 95°C for 10 seconds, annealing from 57°C to 51°C with a 0.25°C decrease/cycle, touch down for 15 seconds, and extension at 72°C for 20 seconds); this was followed by a melt curve (95°C for 5 seconds, 65°C for 1 minute, and 97°C continuous acquisition) and then cooling (40°C for 30 seconds). For indoleamine 2,3-dioxygenase 2 (IDO2), tryptophan 2,3-dioxygenase (TDO), kynurenine aminotransferase (KAT) 1, KAT2, KAT3, KAT4, kynurinase (KYNU), 3-hydroxyanthranilate 3,4-dioxygenase (3-HAAO), and kynurenine monooxygenase (KMO), the thermal profile was as follows: activation at 95°C for 5 minutes followed by 45 amplification cycles (denaturation at 95°C for 10 seconds, annealing at 60°C for 20 seconds, and extension at 72°C for 20 seconds); this was followed by a melt curve (95°C for 5 seconds, 65°C for 1 minute, and 97°C continuous acquisition) and then cooling (40°C for 30 seconds). Results were normalized to beta actin, and comparisons were made between groups using the 2-ΔΔCT method.20
Statistical Methods
Data generated from the above methods were entered into an electronic database, REDCap. All authors had access to the study data and had reviewed and approved the final manuscript. Baseline characteristics for age, age of diagnosis, sex, smoking status, disease extent, and medication use were compared across each group of Mayo endoscopic subscore from 0−3 by use of the Kruskall-Wallis and Pearson’s chi square. Next, each individual metabolite level was compared as a unitless ratio to serum Trp levels. Spearman correlation coefficients were calculated for each metabolite to Trp ratio compared to Mayo endoscopic subscore, total Mayo score, and fold change in KP mRNA expression (fold change compared to normal control tissue). Univariate and multivariate logistic regression using R software version 3.3.021 was then performed to calculate the association between metabolite to Trp ratio and KP mRNA expression with endoscopic inflammation and histologic inflammation. Endoscopic inflammation was defined as Mayo endoscopic subscore of 2 or 3. Histologic inflammation was defined as most inflamed histology identified in biopsies taken at 20 cm from the anal verge.
Ethical Considerations
Patients were prospectively enrolled with informed consent obtained before endoscopy as approved by the University of Chicago Institutional Review Board under the study protocols 11–0202 and 15573A.
RESULTS
Patient Characteristics
There were 100 patients who were selected for analysis in groups of 25 patients per Mayo endoscopic subscore of 0–3 with complete data, mRNA, and serum samples. One patient was subsequently excluded due to a change in diagnosis from UC to CD, leaving 99 patients in the analysis (Table 1). When the groups were compared by Mayo endoscopic score, the average age was younger in individuals with more severe colitis. However, the groups did not differ by extent of disease, age of diagnosis, smoking status, or sex. Medical treatment also varied between groups according to Mayo endoscopic score, with more inflamed patients receiving more corticosteroids and less mesalamine.
TABLE 1:
Population Characteristics of Patients with UC Overall and According to Mayo Endoscopic Subscore.
| Overall n=99 |
0 n=25 |
1 n=25 |
2 n=24 |
3 n=25 |
P | |
|---|---|---|---|---|---|---|
| Age (mean, range) | 43.1 (18–75) | 48.0 (24–72) | 48.4 (23–74) | 38.4 (18–75) | 37.4 (18–61) | 0.003 |
| Male (%) | 55.6% | 60% | 68% | 50% | 44% | NS |
| Age at diagnosis (mean, range) | 29.1 (5–65) | 29.82 (12–62) | 30.1 (5–65) | 28.1 (8–65) | 28.3 (13–59) | NS |
| Currently smoking (%) | 10.10% | 20% | 16% | 0% | 4% | NS |
| Extent of disease | ||||||
| Proctosigmoiditis | 2.0% | 4.0% | 0.0% | 4.2% | 0.0% | |
| Left sided | 33.3% | 28.0% | 40.0% | 29.2% | 36.0% | |
| Pancolitis | 64.7% | 68.0% | 60.0% | 66.7% | 64.0% | NS |
| Medications | ||||||
| 5-ASA | 65.7% | 68.0% | 79.2% | 79.2% | 36.0% | 0.003 |
| Anti-TNFα agents | 23.2% | 20.0% | 20.0% | 20.8% | 32.0 | NS |
| Corticosteroids | 24.2% | 8.0% | 8.0% | 25.0% | 58.3% | <0.001 |
| Immunomodulators | 28.3% | 40.0% | 20.0% | 29.2% | 25.0% | NS |
Means were compared by Kruskall-Wallis test and proportions were compared by Pearson’s chi-square Test.
5-ASA = 5-Aminosalicylic Acid Based Medications, TNFα = tumor necrosis factor alpha, NS = Not Significant
Serum KYNA is Associated with Endoscopic and Histologic Disease Activity
The means and standard deviations of the ratios of serum concentration for each Trp metabolite to serum Trp are provided in Table 2. The ratio of KYNA/T correlated positively with the Mayo endoscopic subscore (R = 0.58, P < 0.001) and the total Mayo score (R = 0.47, P < 0.001). The 3HK/T was correlated with total Mayo score (R = 0.31, P = 0.005), but not the endoscopic subscore (R = 0.19, P = 0.11). No other metabolite ratios showed significant correlations with the Mayo endoscopic subscore or total Mayo score.
TABLE 2:
Ratio of Kynurenine Pathway Metabolite to Tryptophan by Mayo Endoscopic Subscore.
| Metabolite | Mayo Endoscopic Subscore | P | |||
|---|---|---|---|---|---|
| 0 n=25 |
1 n=25 |
2 n=24 |
3 n=25 |
||
| Kynurenine (mean, SD) | 19.9 (6.3) | 18.4 (4.0) | 21.1 (6.0) | 22.1 (10.3) | NS |
| Kynurenic acid (mean, SD) a | 9.5 (4.2) | 9.9 (3.8) | 9.9 (1.9) | 16.1 (3.6) | <0.001 |
| 3-HK (mean, SD) * | 13.2 (13.5) | 10.5 (4.3) | 10.9 (6.4) | 18.3 (17.2) | NS |
| 3-HAA (mean, SD) a | 4.7 (2.4) | 5.4 (2.9) | 6.7 (3.7) | 7.2 (6.2) | NS |
| Anthanillic acid (mean, SD) a | 3.6 (2.2) | 3.5 (2.7) | 3.2 (1.5) | 3.7 (2.2) | NS |
| Picolinic acid (mean, SD) * | 10.3 (5.0) | 17.9 (5.3) | 11.0 (4.8) | 23.5 (14.6) | <0.001 |
| Quinolinic acid (mean, SD) a | 21.5 (13.1) | 26.5 (8.7) | 21.3 (15.9) | 34.1 (32.9) | NS |
The values represent the unitless ratio of serum concentration of the kynurenine metabolite divided by the serum concentration of tryptophan and do not have units. a Indicates values that are ×102; SD = standard deviation, 3-HK = 3-Hydroxykynurenine, 3-HAA = 3-Hydroxyanthranillic acid, NS = Not Significant
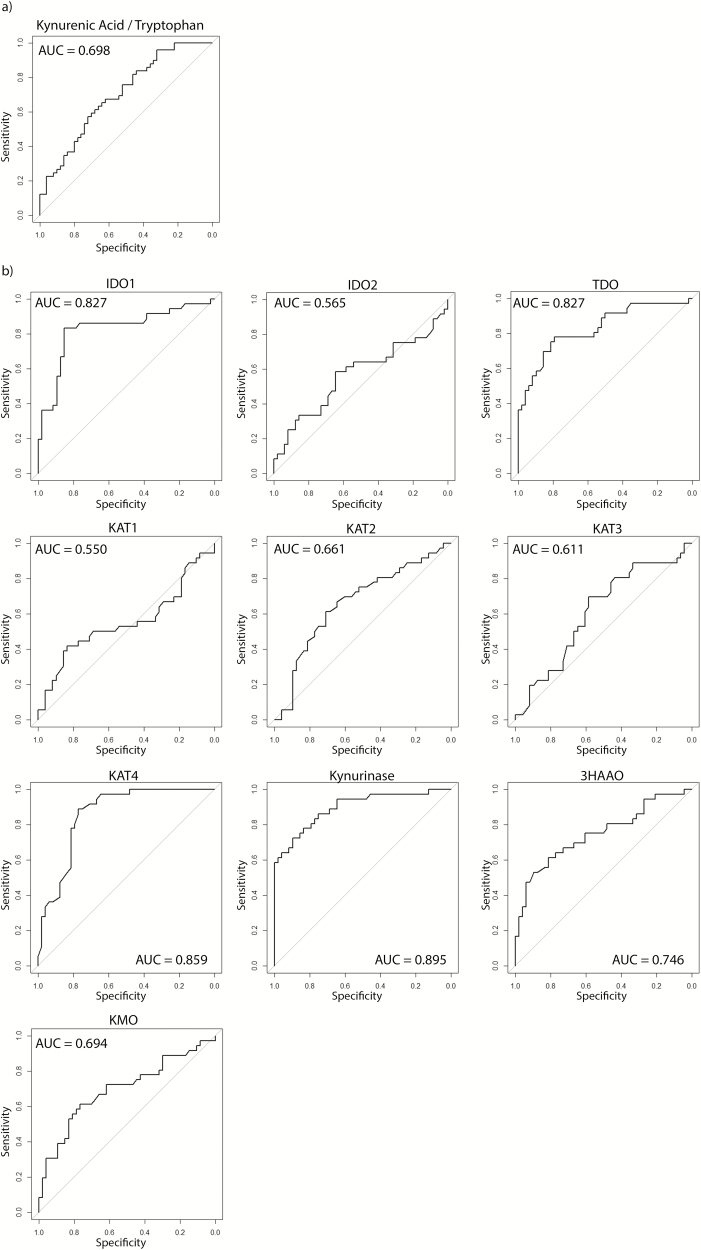
Univariate logistic regression revealed significant odds of moderate to severe endoscopic inflammation (Mayo endoscopic score of 2 or 3) associated with higher KYNA/T and 3HAA/T (Table 3). Multivariate analysis adjusting for age at diagnosis, smoking status, extent of disease, and medication use resulted in significant odds of moderate to severe endoscopic inflammation with higher KYNA/T [OR 1.0015 (95% CI 1.0003–1.0029), P = 0.0186). In addition, univariate analysis showed significant increased odds of histologic inflammation with increasing ratios of KYNA/T , 3HAA/T, and picolinic acid/T , but there were no associations in the multivariate model (Table 3). The greatest area under the curve (AUC) of the receiver operator characteristic (ROC) curve for Mayo endoscopic subscore 2 or 3 was observed with KYNA/T at 0.698 (Fig. 2). No other AUC was greater than 0.6 (Supplementary Fig. 1).
TABLE 3:
Univariate and Multivariate Analysis of KP Metabolites and Enzymes to Inflammation as Assessed by Endoscopy and Histology.
| Metabolites | Mucosal Inflammation Unadjusted OR (95% CI) |
P | Mucosal Inflammation Adjusted OR (95% CI) |
P | Histologic Inflammation at 20cm Unadjusted OR (95% CI) |
P | Histologic Inflammation at 20cm Adjusted OR (95% CI) |
P |
|---|---|---|---|---|---|---|---|---|
| Kynurenine | 1.051 (0.993–1.119) |
NS | 1.050 (0.974–1.143) |
NS | 1.043 (0.986–1.107) |
NS | 1.032 (0.959–1.119) |
NS |
| Kynurenic acid | 1.002 (1.001–1.003) |
0.001 | 1.0015 (1.0003–1.0029) |
0.02 | 1.001 (1.003-1.002) |
0.014 | 1.0007 (0.9995–1.0019) |
NS |
| 3-HK | 1.0002 (0.999–1.001) |
NS | 1.0004 (0.9999–1.0011) |
NS | 1.0003 (1.0000–1.0008) |
NS | 1.0006 (1.0000–1.0013) |
NS |
| 3-HAA | 1.001 (1.0002–1.003) |
0.034 | 1.001 (0.999–1.003) |
NS | 1.0015 (1.0003–1.0028) |
0.022 | 1.0012 (0.9998–1.0029) |
NS |
| Anthranilic acid | 0.999 (0.998–1.002) |
NS | 1.001 (0.998–1.003) |
NS | 1.0002 (0.9982–1.0020) |
NS | 1.0011 (0.9986–1.0036) |
NS |
| Picolinic acid | 1.0004 (0.999–1.001) |
NS | 1.0001 (0.999–1.001) |
NS | 1.0006 (1.0001–1.0011) |
0.033 | 1.0004 (0.9998–1.0011) |
NS |
| Quinolinic acid | 1.0001 (0.9999–1.0003) |
NS | 1.0000 (0.9998–1.0003) |
NS | 1.0001 (0.9999–1.0003) |
NS | 1.0000 (0.9998–1.0003) |
NS |
| Enzymes | ||||||||
| IDO1 | 1.0821 (1.033–1.163) |
0.009 | 1.0635 (1.024–1.141) |
0.022 | 1.0892 (1.041–1.166) |
0.003 | 1.088 (1.040–1.169) |
0.003 |
| IDO2 | 1.3486 (0.989–1.934) |
NS | 0.8409 (0.220–3.010) |
NS | 1.1007 (0.802–1.498) |
NS | 0.415 (0.058–2.168) |
NS |
| TDO | 1.8898 (1.418–2.732) |
<0.001 | 0.7534 (0.212–2.518) |
NS | 1.5869 (1.274–2.102) |
<0.001 | 0.436 (0.098–1.642) |
NS |
| KAT1 | 1.7137 (0.815–3.767) |
NS | 1.7614 (0.390–7.986) |
NS | 1.4490 (0.669–3.167) |
NS | 0.843 (0.145–4.456) |
NS |
| KAT2 | 0.3260 (0.087–0.938) |
NS | 0.7527 (0.223–2.398) |
NS | 0.2875 (0.063–0.941) |
NS | 0.449 (0.103–1.655) |
NS |
| KAT3 | 0.4575 (0.138–1.299) |
NS | 0.8590 (0.244–2.898) |
NS | 0.4069 (0.105–1.268) |
NS | 0.459 (0.095–1.844) |
NS |
| KAT4 | 0.0004 (9.2x10-6-0.008) |
<0.001 | 0.7731 (0.231–2.452) |
NS | 0.0064 (0.0003–0.070) |
<0.001 | 0.455 (0.105–1.688) |
NS |
| KYNU | 4.5554 (2.472–10.226) |
<0.001 | 0.5425 (0.122–2.225) |
NS | 2.2873 (1.620–3.598) |
<0.001 | 0.371 (0.080–1.473) |
NS |
| 3-HAAO | 0.4248 (0.227–0.707) |
0.003 | 1.0184 (0.193–5.023) |
NS | 0.3928 (0.187–0.703) |
0.005 | 0.415 (0.069–1.968) |
NS |
| KMO | 1.7921 (1.255–2.765) |
0.004 | 1.2555 (0.350–4.525) |
NS | 1.6086 (1.166–2.344) |
0.007 | 0.739 (0.160–3.064) |
NS |
Kynurenine metabolites are expressed as unitless ratios of the serum metabolite concentration to the serum tryptophan concentration and do not have units. KP mRNA expression levels were measured by RT-PCR and expressed as fold change compared to normal control. Odds ratios represent the change in odds of moderate to severe endoscopic inflammation (Mayo 2 or 3) or histologic inflammation (moderate or severe) per 1 unit change in the metabolite ratio to tryptophan and 1- fold change in the mRNA expression compared to normal controls, respectively. KP = Kynurenine pathway, IDO = Indoleamine 2,3-dioxygenase, TDO = Tryptophan 2,3-dioxygenase, KAT = Kynurenine aminotransferase, KYNU = Kynurinase, 3-HAAO = 3-Hydroxyanthranilate 3,4-dioxygenase, KMO = Kynurenine monooxygenase, OR = Odds ratio, 95% CI = 95% confidence interval, 3-HK = 3-Hydroxykynurenine, 3-HAA = 3-Hydroxyanthranillic acid, NS = Not Significant
FIGURE 2.
Receiver operator characteristic curves for Mayo endoscopic score of either 2 or 3. A) KYNA/T ratio, B) KP mRNA expression levels. IDO1 = Indoleamine 2,3-dioxygenase 1, IDO2 = Indoleamine 2,3-dioxygenase 2, TDO = Tryptophan 2,3-dioxygenase, KAT1 = Kynurenine aminotransferase 1, KAT2 = Kynurenine aminotransferase 2, KAT3 = Kynurenine aminotransferase 3, KAT4 = Kynurenine aminotransferase 4, 3HAAO = 3-Hydroxyanthranilate 3,4-dioxygenase, KMO = Kynurenine monooxygenase.
Mucosal IDO1 is Associated with Mucosal Inflammation and Histologic Disease Activity.
The correlation of each Trp metabolite ratio to the expression of each KP enzyme is shown in Table 4. There were positive correlations between total Mayo score and IDO1 (R = 0.316, P = 0.004), TDO (R = 0.555, P < 0.001), KYNU (R = 0.664, P < 0.001), and KMO (R = 0.372, P = 0.001), whereas there were negative correlations for KAT2 (R = -0.285, P = 0.009), KAT4 (R = -0.610, P < 0.001), and 3-HAAO (R = -0.394, P < 0.001). Similarly, there were positive correlations between Mayo endoscopic subscore and IDO1 (R = 0.233, P = 0.034), TDO (R = 0.501, P < 0.001), KYNU (R = 0.547, P < 0.001), and KMO (R = 0.276, P = 0.012), whereas there were negative correlations for KAT2 (R = -0.244, P = 0.025), KAT4 (R = -0.610, P < 0.001) and 3-HAAO (R = -0.377, P < 0.001). As expression levels of IDO1, TDO, KYNU, and KMO increased, the odds of endoscopic inflammation significantly increased in the univariate analysis. In contrast, increasing expression levels of KAT4 and 3-HAAO in the univariate analysis were associated with decreased odds of endoscopic inflammation. In the multivariate analysis, however, IDO1 expression was the only mRNA expression association that remained significant (OR 1.0635, P = 0.0215) (Table 3). Similar results were seen in a univariate and multivariate analysis of KP mRNA expression with histologic appearance (Table 3). The ROC curves for mucosal KP mRNA expression are depicted in Figure 2. The greatest AUC values were seen with IDO1 (0.827), TDO (0.827), KAT4 (0.859), and KYNU (0.895).
TABLE 4:
Correlation of Kynurenine Pathway Enzyme mRNA Expression Levels and Serum Tryptophan Metabolite Ratios.
| Kynurenine | Kynurenic acid | 3-HK | 3-HAA | Anthranilic acid | Picolinic acid | Quinolinic acid | |
|---|---|---|---|---|---|---|---|
| IDO1 | 0.0688 | -0.0469 |
0.3328 (P = 0.002) |
0.0475 | 0.0942 | 0.0924 | 0.0711 |
| IDO2 |
0.2995 (P = 0.006) |
-0.0528 | 0.0904 | 0.0400 | 0.1111 | -0.0823 | 0.2007 |
| TDO | 0.1452 |
0.3392 (P = 0.002) |
0.0951 | 0.0805 | -0.0018 | 0.1304 | 0.0507 |
| KAT1 | 0.0521 | 0.1760 | -0.1047 | 0.0733 | -0.0690 | -0.0461 | 0.0753 |
| KAT2 | 0.0565 | 0.0245 | -0.0819 | 0.0623 | 0.0320 | -0.0449 | 0.0498 |
| KAT3 | 0.0569 | 0.0613 | -0.0872 | 0.0590 | -0.0080 | -0.0227 | 0.0937 |
| KAT4 | -0.0155 | -0.1916 | -0.1125 | -0.0469 | 0.0021 | -0.0001 | 0.1599 |
| KYNU | 0.1376 | 0.1037 | 0.0391 | 0.1495 | 0.0022 | -0.0509 | -0.0045 |
| 3-HAAO | -0.1069 | -0.1212 | -0.1050 | -0.0415 | 0.1021 | -0.1064 | 0.0120 |
| KMO |
0.2191 (P = 0.046) |
0.0902 | -0.0451 | 0.0679 | 0.0676 | -0.1030 | 0.2102 |
The metabolite values represent the serum concentration of the kynurenine metabolite divided by the serum concentration of tryptophan. The pathway mRNA expression levels are expressed as a fold change from control. IDO = Indoleamine 2,3-dioxygenase, TDO = Tryptophan 2,3-dioxygenase, KAT = Kynurenine aminotransferase, KYNU = Kynurinase, 3-HAAO = 3-Hydroxyanthranilate 3,4-dioxygenase, KMO = Kynurenine monooxygenase, 3-HK = 3-Hydroxykynurenine, 3-HAA = 3-Hydroxyanthranillic acid
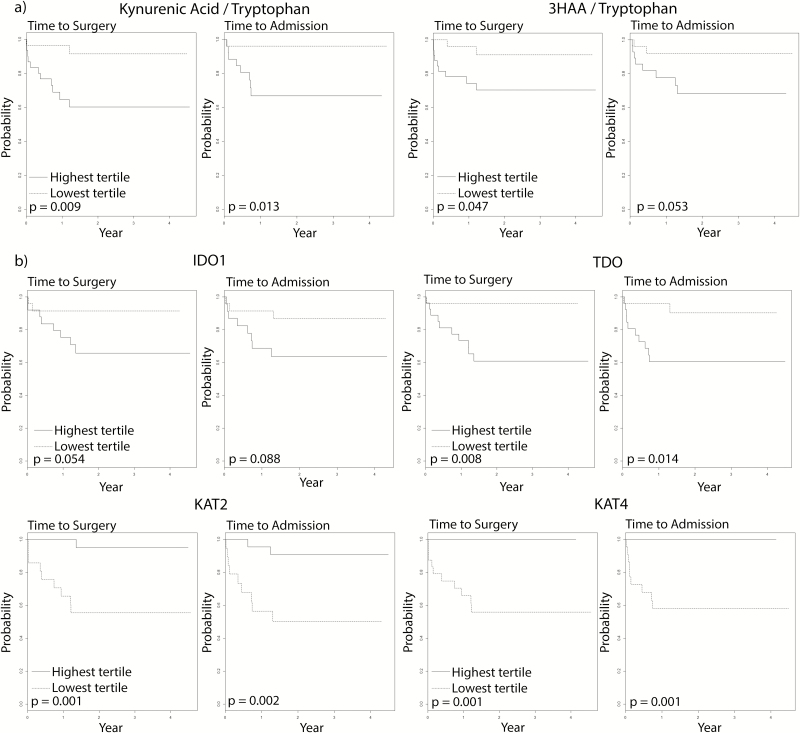
Higher Ratios of KYNA/T Predict Hospitalization and Colectomy.
When patients with the highest tertile of values were compared to patients with the lowest tertile of values, higher ratios of KYNA/T were significant predictors of earlier time to colectomy and hospitalization (Fig. 3). A higher ratio of 3HAA/tryptophan was also predictive of a shorter time to colectomy. No other metabolites were predictive of either time to colectomy or time to hospital admission (Supplementary Fig. 2).
FIGURE 3.
Kaplan-Meier analysis for time to colectomy and time to hospital admission. A) Comparing highest and lowest tertiles of select KYNA/T, B) Comparing highest and lowest tertiles KP mRNA expression. IDO1 = Indoleamine 2,3-dioxygenase 1, TDO = Tryptophan 2,3-dioxygenase, KAT2 = Kynurenine aminotransferase 2, KAT4 = Kynurenine aminotransferase 4
KP mRNA Expression Changes Predict Hospitalization and Colectomy.
The highest tertiles of mRNA expression of TDO, KAT2, and KAT4 were predictive of an earlier time to colectomy and hospital admission (Fig. 3). The highest tertiles of mRNA expression of TDO, KAT2, KAT3, and KYNU were also predictive of an earlier time to hospital admission and the highest tertile of 3-HAAO expression was predictive of earlier time to surgery (Supplementary Fig. 3). No other mRNA expression levels were predictive of either time to colectomy or time to hospital admission.
DISCUSSION
Trp is an essential amino acid and precursor molecule for the neurotransmitters serotonin and melatonin. However, following its use for protein synthesis, the majority of metabolized Trp is consumed by the KP.5 Prior studies showed that IDO expression is increased in the setting of intestinal inflammation, and the current study confirmed this association in a larger cohort with objective endoscopic data. We also demonstrated for the first time that IDO1 expression is closely associated with endoscopic and histologic measures of inflammation severity in UC.22, 23 A recent study by Nikolaus and colleagues identified an association between lower serum Trp levels and clinical disease activity in a large cohort CD and UC.24 In that study serum Trp levels were predictive of intestinal resection, but not predictive of flares in disease activity. IDO1 expression was significantly elevated in those patients with clinically active disease, which is in agreement with our observation that IDO1 expression significantly correlates with endoscopic disease activity. A strength of the present study is the multivariate analysis that shows the ratio of the downstream metabolite KYNA to Trp is closely positively associated with the severity of endoscopic inflammation and relevant disease outcomes. Although the functional significance of these changes in inflammatory bowel disease (IBD) is not known, prior studies of IDO and KYNA suggest that these components of the KP exert significant inflammatory and neurologic effects.
The significance of KYNA may derive from its role in promoting inflammatory homeostasis. Previous studies demonstrated that KYNA works as a negative regulator of inflammation in animal models. It can decrease leukocyte recruitment and production of proinflammatory cytokines in splenocytes in vitro.25–28 Notably, there is evidence that it can also negatively regulate TNFα, IL-17, and IL-23.13, 14 A recent publication suggested that KYNA inhibited IL-10 in vitro, IDO1 deficiency was associated with increased IL-10 production, and IL-10 -/- IDO1 -/- mice demonstrated more severe colitis than IDO1 -/- or IL10 -/- mice.29 Thus, it is possible that IDO1, KYNA , and IL-10 work in concert in a feedback mechanism to restrain the immune response in the setting of active UC.
Alterations in Trp and KYNA in the nervous system also may contribute to the pathophysiology of UC and associated neuropsychiatric manifestations of the disease. Trp depletion induces depressive symptoms, and patients with IBD suffer from higher incidence of anxiety and depression.30–33 The prevalence of anxiety and depression also increases with increasing inflammatory activity.32, 34 Prior studies showed that colonic mucosal TNFα production can be increased in the setting of acute psychological stress.35 Although the TNFα inhibiting medication, infliximab, was ineffective in treating depression in a randomized control trial involving a population of patients without IBD, there was a beneficial effect in a subset of patients with depression and elevated C-reactive protein.36 In addition to mood disorders, the enteric nervous system is thought to contribute to symptom manifestations consistent with irritable bowel syndrome.37, 38 KYNA is a potent glutamate receptor antagonist with neuroprotective, anti-epileptic, and psychiatric effects in the central nervous system.5, 39 In addition, KYNA can reduce colonic motility in mouse models of colitis.25 As such, further investigation of this pathway is warranted to assess functional symptoms in addition to inflammatory activity in patients with IBD.
The lack of significant correlation between the KYNA/Tryp ratios and expression of its preceding enzymes suggests a potential alternative source of KYNA production. It is possible that the serum KYNA that was measured was derived from a source other than the gastrointestinal epithelium, including nongastrointestinal endogenous metabolism, dietary intake, and microbial production. In the human body, KYNA is produced by kynurenine aminotransferase isomers 1–4, which are present in the colonic epithelium, liver, kidney, muscle, and omental fat.40–42 If the systemic inflammatory state in UC could induce production from these other tissues, it may lead to KYNA production out of proportion to intestinal activation of the pathway. Alternatively, there are environmental sources of KYNA in common foods such as potatoes, honey, and broccoli, which are rich sources of KYNA in the diet.43–45 Whereas our study did not include a detailed dietary history, it is unlikely that there was greater intake of these foods with increasing UC inflammation. Rather, one would expect the dietary intake to decline with increased disease severity. Lastly, bacteria are capable of converting kynurenine to KYNA. E. coli express the protein aspartate aminotransferase, which converts kynurenine into KYNA.46, 47 Furthermore, pathogenic E. coli are implicated in UC pathophysiology.48 Additional studies show that colonic bacterial populations may be modulated by KYNA.49, 50 This suggests the possibility that serum KYNA levels may reflect gut microbial composition and function, potentially highlighting an important role in microbial regulation of host immune function and symptomatology.
This study was limited by its observational design and by a lack of dietary data. These observations in human subjects are vulnerable to numerous covariables and the sample size was likely too small to account for all confounding conditions. We attempted to minimize this effect by performing a multivariate, logistic regression analysis that included likely confounding variables including extent of disease burden, age of diagnosis, smoking status, and inflammatory modulating medication use. A detailed medication review did not reveal any patients also taking serotonin-based psychotropic medications, and no patient reported a Trp supplement, so these were not included in the model. Additionally, observational design limits our conclusions about causes and effects, and these results may reflect a correlation with inflammatory burden, rather than identifying a causative pathway. Further study within an animal model system would be useful for elucidating causation and refining our conclusions. Finally, as discussed above, there are dietary sources of KYNA. However, intake of these foods are not likely increased in patients with worsening colonic inflammation. A recent study of Trp levels in patients with IBD did not show a difference in Trp dietary content between patients with active and inactive disease.24
CONCLUSION
In this study, we found that IDO1 expression was increased in the colonic mucosa during active UC and was associated with endoscopic severity. Furthermore, we discovered that the KP metabolite KYNA is also associated with endoscopically active UC when examined as a ratio to Trp. Increasing levels of KYNA relative to Trp are closely associated with endoscopic inflammation and predictive of disease outcomes. These findings support a role for the the KP derived from Trp metabolism in the pathophysiology of UC. Given the significant correlation with mucosal inflammation and its known role in inflammation and psychiatric conditions, the functional consequences of KYNA in this setting merits further study. Overall, our findings support the hypothesis that Trp metabolism along the KP is increased in active UC and identify KYNA as a potential noninvasive biomarker and therapeutic target in ulcerative colitis.
SUPPLEMENTARY DATA
Supplementary data areavailable at Inflammatory Bowel Diseases online.
AUTHOR CONTRIBUTIONS
M. Anthony Sofia, Matthew A. Ciorba, Katherine Meckel, Chai K. Lim, and Gilles J. Guillemin contributed to conducting the study, collecting data, interpreting data, and drafting the manuscript. Christopher R. Weber and Marc Bissonnette contributed to interpreting data and drafting the manuscript. Joel R. Pekow contributed to conducting the study, collecting data, interpreting data, and drafting the manuscript. He has approved the final draft submitted.
Supplementary Material
ACKNOWLEDGMENTS
David Alvarado, PhD for contributing the graphic for Figure 1.
Glossary
Abbreviations:
- 3-HAAO
3-hydroxyanthranilate 3,4-dioxygenase
- 3HAA
3-hydroxyanthranilic acid
- CD
Crohn’s disease
- GC-MS
gas chromatography-mass spectrometry
- IBD
Inflammatory bowel disease
- IDO1
Indoleamine 2,3-dioxygenase 1
- IDO2
indoleamine 2,3-dioxygenase 2
- KAT
kynurenine aminotransferase
- KMO
kynurenine monooxygenase
- KP
Kynurenine pathway
- KYNA
Kynurenic acid
- KYNU
kynurinase
- qPCR
quantitative polymerase chain reaction
- TDO
tryptophan 2,3-dioxygenase
- TNFα
Tumor necrosis factor α
- Trp
Tryptophan
- UC
ulcerative colitis
- uHPLC
ultra-high performance liquid chromatography
Conflicts of Interest: M. Anthony Sofia has served as a consultant for Janssen. Matthew A. Ciorba has served as a consultant for UCB AbbVie, Theravance, Gilead, Pfizer, Incyte, and Takeda. Joel R. Pekow has served as a consultant for Verastem and CVS Caremark, an advisory board member for Janssen, and has received research funding from Takeda and AbbVie. Marc Bissonnette, Gilles J. Guillermin, Chai K. Lim, Katherine Meckel, and Christopher R. Weber have no disclosures to report.,
Supported by: The study was funded in part by the National Institutes of Health grant numbers P30 DK42086, UL1 TR000430, and K08 DK090152 to Joel R. Pekow and RO1DK109384 to Matthew A. Ciorba. The study was funded in part by the Crohn’s and Colitis Foundation of America, grant number #370763 and the Givin’ it all for Guts Foundation (http://givinitallforguts.org/) to Matthew A. Ciorba. The study sponsors were not involved in the study design, collection, analysis, or interpretation of data.
All authors approved the final version of the article, including the authorship list.
Guarantor of the article: Joel R. Pekow
REFERENCES
- 1.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- 2.Meucci G, Fasoli R, Saibeni S, et al. ; IG-IBD Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: a prospective, multicenter study. Inflamm Bowel Dis. 2012;18:1006–10. [DOI] [PubMed] [Google Scholar]
- 3.Frøslie KF, Jahnsen J, Moum BA, et al. ; IBSEN Group Mucosal healing in inflammatory bowel disease: results from a norwegian population-based cohort. Gastroenterology. 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins TA, Nguyen JC, Polglaze KE, et al. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20. [DOI] [PubMed] [Google Scholar]
- 6.Ciorba MA. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol. 2013;29:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherayil BJ. Indoleamine 2,3-dioxygenase in intestinal immunity and inflammation. Inflamm Bowel Dis. 2009;15:1391–6. [DOI] [PubMed] [Google Scholar]
- 8.Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: correlation with crohn’s disease activity. Inflamm Bowel Dis. 2012;18:1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm (Vienna). 2012;119:197–209. [DOI] [PubMed] [Google Scholar]
- 10.Terness P, Bauer TM, Röse L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song H, Park H, Kim YS, et al. L-kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. Int Immunopharmacol. 2011;11:932–8. [DOI] [PubMed] [Google Scholar]
- 12.Fallarini S, Magliulo L, Paoletti T, et al. Expression of functional GPR35 in human iNKT cells. Biochem Biophys Res Commun. 2010;398:420–5. [DOI] [PubMed] [Google Scholar]
- 13.Tiszlavicz Z, Németh B, Fülöp F, et al. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-α (TNF-α) production by mononuclear cells, HMGB1 production by monocytes and HNP1-3 secretion by neutrophils. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:447–55. [DOI] [PubMed] [Google Scholar]
- 14.Salimi Elizei S, Poormasjedi-Meibod MS, Wang X, et al. Kynurenic acid downregulates Il-17/1l-23 axis in vitro. Mol Cell Biochem. 2017;431:55–65. [DOI] [PubMed] [Google Scholar]
- 15.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 17.Weber CR, Nalle SC, Tretiakova M, et al. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SP, Franco NF, Varney B, et al. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. Plos One. 2015;10:e0131389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim CK, Essa MM, de Paula Martins R, et al. Altered kynurenine pathway metabolism in autism: implication for immune-induced glutamatergic activity. Autism Res. 2016;9:621–31. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2012. ISBN 3-900051-07-0. http://www.R-project.org/ [Google Scholar]
- 22.Ferdinande L, Demetter P, Perez-Novo C, et al. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int J Immunopathol Pharmacol. 2008;21:289–95. [DOI] [PubMed] [Google Scholar]
- 23.Wolf AM, Wolf D, Rumpold H, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113: 47–55. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaus S, Schulte B, Al-Massad N, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153:1504–16.e2. [DOI] [PubMed] [Google Scholar]
- 25.Varga G, Erces D, Fazekas B, et al. N-methyl-D-aspartate receptor antagonism decreases motility and inflammatory activation in the early phase of acute experimental colitis in the rat. Neurogastroenterol Motil. 2010;22:217–25, e68. [DOI] [PubMed] [Google Scholar]
- 26.Małaczewska J, Siwicki AK, Wójcik RM, et al. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes - in vitro and ex vivo studies. Cent Eur J Immunol. 2016;41:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barth MC, Ahluwalia N, Anderson TJ, et al. Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J Biol Chem. 2009;284:19189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maes M, Mihaylova I, Ruyter MD, et al. The immune effects of TRYCATS (tryptophan catabolites along the IDO pathway): relevance for depression - and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol Lett. 2007;28:826–31. [PubMed] [Google Scholar]
- 29.Metghalchi S, Ponnuswamy P, Simon T, et al. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab. 2015;22:460–71. [DOI] [PubMed] [Google Scholar]
- 30.Ravindran AV, Griffiths J, Merali Z, et al. Influence of acute tryptophan depletion on mood and immune measures in healthy males. Psychoneuroendocrinology. 1999;24:99–113. [DOI] [PubMed] [Google Scholar]
- 31.Luo XP, Mao R, Chen BL, et al. Over-reaching beyond disease activity: the influence of anxiety and medical economic burden on health-related quality of life in patients with inflammatory bowel disease. Patient Prefer Adherence. 2017;11:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikocka-Walus A, Knowles SR, Keefer L, et al. Controversies revisited: A systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752–62. [DOI] [PubMed] [Google Scholar]
- 33.Mittermaier C, Dejaco C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. 2004;66:79–84. [DOI] [PubMed] [Google Scholar]
- 34.Persoons P, Vermeire S, Demyttenaere K, et al. The impact of major depressive disorder on the short- and long-term outcome of crohn’s disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22:101–10. [DOI] [PubMed] [Google Scholar]
- 35.Mawdsley JE, Macey MG, Feakins RM, et al. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology. 2006;131:410–9. [DOI] [PubMed] [Google Scholar]
- 36.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. [DOI] [PubMed] [Google Scholar]
- 38.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–34. [DOI] [PubMed] [Google Scholar]
- 39.Schwarcz R, Stone TW. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology. 2017;112:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costa CV, Ragazzi E, Caparrotta L, et al. Liver and kidney kynurenine aminotransferase activity in different strains of rats. Adv Exp Med Biol. 1999;467:629–35. [DOI] [PubMed] [Google Scholar]
- 41.Schlittler M, Goiny M, Agudelo LZ, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. 2016;310:C836–40. [DOI] [PubMed] [Google Scholar]
- 42.Favennec M, Hennart B, Caiazzo R, et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring). 2015;23:2066–74. [DOI] [PubMed] [Google Scholar]
- 43.Turski MP, Turska M, Zgrajka W, et al. Presence of kynurenic acid in food and honeybee products. Amino Acids. 2009;36:75–80. [DOI] [PubMed] [Google Scholar]
- 44.Paluszkiewicz P, Zgrajka W, Saran T, et al. High concentration of kynurenic acid in bile and pancreatic juice. Amino Acids. 2009;37:637–41. [DOI] [PubMed] [Google Scholar]
- 45.Turski MP, Kamiński P, Zgrajka W, et al. Potato- an important source of nutritional kynurenic acid. Plant Foods Hum Nutr. 2012;67:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuc D, Zgrajka W, Parada-Turska J, et al. Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids. 2008;35:503–5. [DOI] [PubMed] [Google Scholar]
- 47.Han Q, Fang J, Li J. Kynurenine aminotransferase and glutamine transaminase K of escherichia coli: identity with aspartate aminotransferase. Biochem J. 2001;360:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilarczyk-Zurek M, Strus M, Adamski P, et al. The dual role of escherichia coli in the course of ulcerative colitis. BMC Gastroenterol. 2016;16:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dolecka J, Urbanik-Sypniewska T, Skrzydło-Radomańska B, et al. Effect of kynurenic acid on the viability of probiotics in vitro. Pharmacol Rep. 2011;63:548–51. [DOI] [PubMed] [Google Scholar]
- 50.Kuc D, Rahnama M, Tomaszewski T, et al. Kynurenic acid in human saliva–does it influence oral microflora?Pharmacol Rep. 2006;58:393–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.