Abstract
An 82-year-old man with a recurrence of pulmonary pleomorphic carcinoma was treated with pembrolizumab. He achieved partial response after three cycles of pembrolizumab. However, he developed febrile neutropenia. A bone marrow aspiration sample revealed a decrease of mature neutrophils, and anti-neutrophil antibody was detected in blood. Computed tomography scans revealed consolidation in the right lung. Pathological findings in lung biopsy tissue revealed organizing pneumonia. Pembrolizumab-induced agranulocytosis and interstitial lung disease (ILD) were diagnosed. We initiated antibacterial therapy and granulocyte colony-stimulating factor (G-CSF). The neutrophil count immediately increased, and the fever decreased. The improvement of ILD was achieved without using systemic steroids. Moreover, the patient developed ocular myasthenia gravis induced by pembrolizumab. This is the first case report of pembrolizumab-induced agranulocytosis. Agranulocytosis was improved by administration of G-CSF without using systemic steroids. However, further studies are needed to determine the optimal treatment for patients with anti-neutrophil antibody whose tumor has progressed.
INTRODUCTION
Immune checkpoint inhibitors (ICI) targeting the programmed cell death-1 (PD-1), such as nivolumab and pembrolizumab, have been demonstrated to be effective in patients with non-small-cell lung cancer (NSCLC) [1]. PD-1 inhibitor therapy has shown high tolerability in NSCLC patients. However, specific adverse events associated with PD-1 inhibitor therapy occur and are described as immune-related adverse events (irAEs). Although irAEs were reported in a variety of organs, the hematologic toxicity of ICI was lower than that of cytotoxic chemotherapy [1]. Of note, neutropenia was rare [2]. In this case, we are reporting on pembrolizumab-induced agranulocytosis, interstitial lung disease (ILD) and ocular myasthenia gravis (MG) in a patient with pulmonary pleomorphic carcinoma.
CASE REPORT
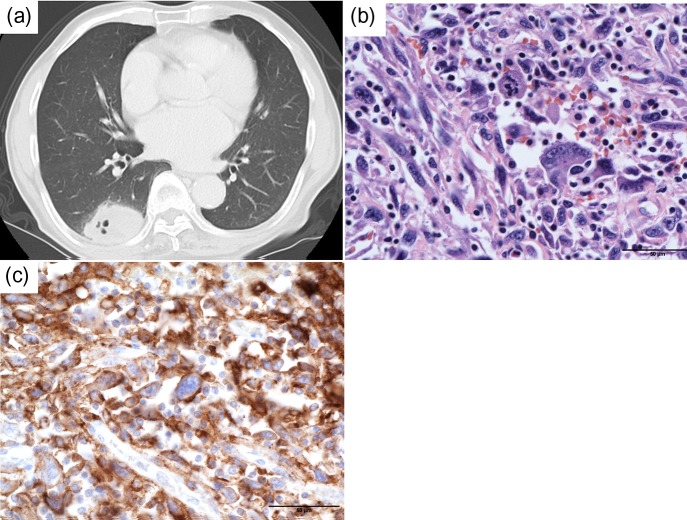
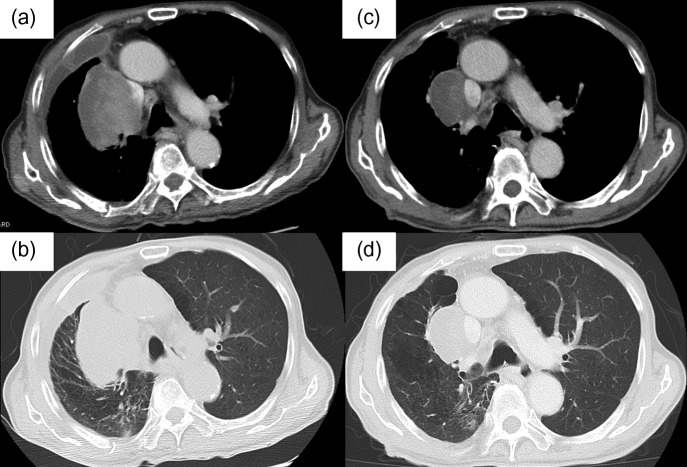
An 82-year-old male former smoker who had smoked for 31 pack-years and quit smoking at 82 years of age was diagnosed with pulmonary pleomorphic carcinoma and underwent a right lower lobectomy with systematic lymph node dissection. He was diagnosed with pulmonary pleomorphic carcinoma (pT3N0M0 stage IIB) (Fig. 1a and b). The surgically resected lung tumor tissue was negative for epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase gene (ALK) rearrangement. Immunohistochemistry (IHC) of the lung tumor tissue, using 22C3 programmed cell death ligand-1 (PD-L1) antibody, revealed a high expression level of PD-L1. The tumor proportion score (TPS) was 75% (Fig. 1c). Two months after surgery, computed tomography (CT) showed a right pulmonary hilar tumor with chest wall infiltration, suggesting recurrence of pulmonary pleomorphic carcinoma (Fig. 2a and b). Therefore, the patient was treated with pembrolizumab. The response to pembrolizumab 200mg/body for 3 weeks was defined as a partial response (PR) after the completion of two cycles of pembrolizumab treatment (Fig. 2c and d). We continued the pembrolizumab treatment.
Figure 1:
(a) Chest CT scans show the primary tumor before operation. (b) Hematoxylin–eosin staining of surgical specimens show pulmonary pleomorphic carcinoma with spindle cells and giant cells (scale bars 50 μm). (c) Immunohistochemical staining of PD-L1 from surgical specimens show PD-L1 TPS 75%. PD-L1 expression of spindle cells and giant cells was positive (scale bars 50 μm)
Figure 2:
(a, b) Chest CT scans show a right pulmonary hilar tumor after surgery. (c and d) Chest CT scan images obtained after three cycles of pembrolizumab. The response to pembrolizumab was defined as a partial response
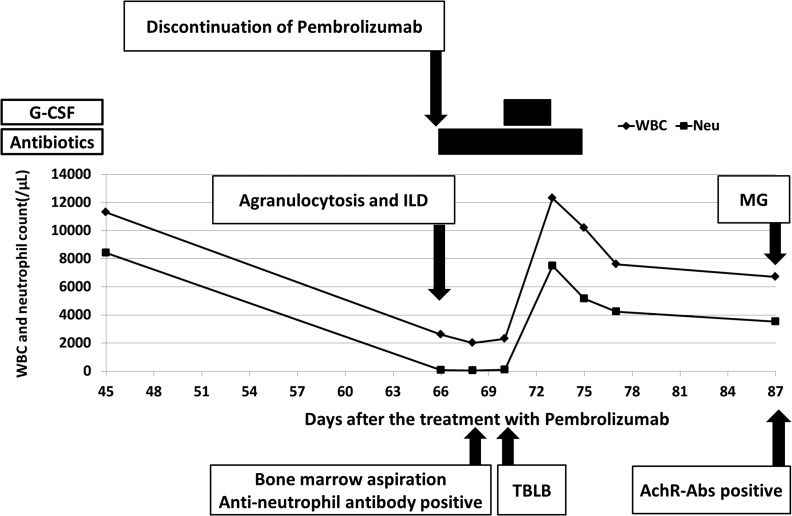
Twenty-two days after the third cycle of pembrolizumab, the patient was hospitalized because of febrile neutropenia. The neutrophil count before initiation of pembrolizumab administration was 5432/μL. The absolute neutrophil count was 68/μL, however, hemoglobin and platelet levels were within normal limits in peripheral blood. He was treated with meropenem 1g three times daily for 9 days, vancomycin 1g twice daily for 6 days and micafungin 150 mg once daily for 5 days. He underwent a bone marrow puncture. The pathological findings revealed a decrease of mature neutrophils without abnormal levels of other types of blood cells and dysplasia of the bone marrow (Fig. 3). Epstein-Barr virus, cytomegalovirus, and human immunodeficiency virus infections were not observed. His titer of antinuclear antibodies (ANA) was 1:40 by a fluorescent antibody method before pembrolizumab administration. He did not receive any medicine other than pembrolizumab. Anti-neutrophil antibody was detected in peripheral blood after the treatment with pembrolizumab. Therefore, the patient was diagnosed with agranulocytosis associated with pembrolizumab treatment. On the fifth hospital day, we began to administer filgrastim 75 μg/body for 4 days.
Figure 3:
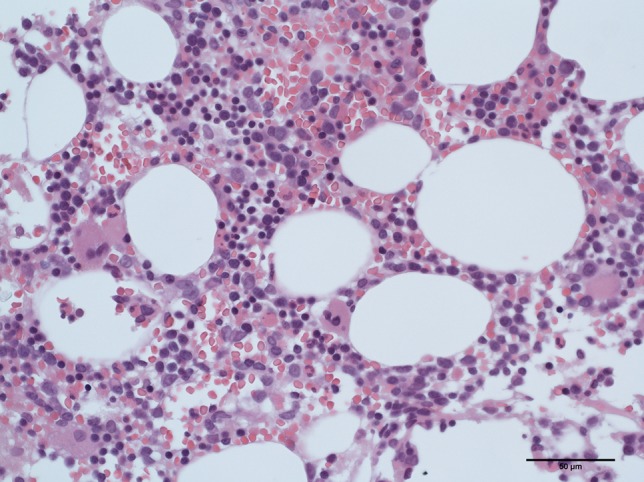
Hematoxylin–eosin staining of bone marrow aspiration clot revealed a decrease of mature neutrophils but normal levels of other types of blood cells (scale bars 50 μm)
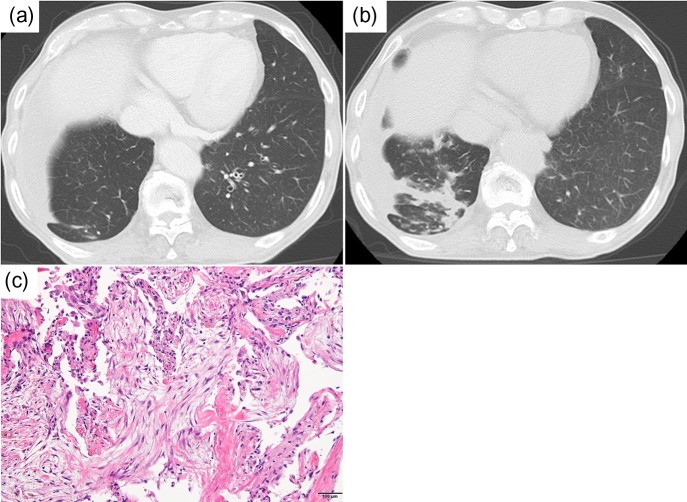
Chest CT scans on admission showed a consolidation in the middle lobe of the right lung (Fig. 4a and b). A sputum culture did not reveal causative bacteria, mycobacteria or fungi in the infection. A blood culture was negative. The patient underwent bronchoscopy because of suspected drug-induced pneumonia, but the bronchoalveolar lavage fluid culture was negative. A pathological examination of transbronchial lung biopsy tissue from the right middle lobe revealed organizing pneumonia showing intraluminal polypoid fibrosis in alveoli (Fig. 4c). The consolidation in the right lower lung field, seen on chest X-ray, did not improve in spite of empiric systemic antibacterial therapy. We diagnosed ILD induced by pembrolizumab. He had no symptoms such as dyspnea, cough or chest pain. We evaluated the condition as grade 1 of immune-related pneumonitis without any medication.
Figure 4:
(a) Chest CT scan images obtained after two cycles of pembrolizumab. (b) Chest CT scans show the presence of an infiltrative shadow after three cycles of pembrolizumab. (c) A pathological examination of transbronchial lung biopsy specimens showed intraluminal polypoid fibrosis in alveoli (hematoxylin–eosin staining) (scale bars 100 μm)
At 7 days after the treatments, the fever had decreased and the neutrophil count had increased to 7503/μL. We discontinued G-CSF administration. The neutrophil count remained within normal limits without G-CSF administration (Fig. 5). The patient was discharged from our hospital 15 days after admission.
Figure 5:
The clinical course in this patient (WBC, white blood cell count; Neu, neutrophil count)
Seven days after discharge, he presented in our hospital with diplopia, although his neutrophil count was normal and ILD was not getting worse. He did not have ocular symptoms before the initiation of pembrolizumab. No other muscle symptoms were found. The other neurological examination was unremarkable. Acetylcholine receptor antibodies (AChR-Abs) were positive. He was diagnosed with ocular MG associated with pembrolizumab treatment and was treated with pyridostigmine 60mg three times a day.
DISCUSSION
In this report, we have presented a patient with pulmonary pleomorphic carcinoma who had agranulocytosis, ILD and ocular MG induced by pembrolizumab. No other case of pembrolizumab-caused agranuloytosis could be found in a review of the literature.
ICI often produce good responses and have lead to durable responses in patients with various types of cancer, including NSCLC. Pembrolizumab prolonged progression free survival and overall survival over that obtained by chemotherapy in untreated advanced NSCLC with PD-L1 expression on at least 50% of tumor cells [1]. The prognosis for pulmonary pleomorphic carcinoma is unfavorable, and a standard therapy has not been established [3]. Recent studies reported that 70.5–90.2% of pulmonary pleomorphic carcinomas showed high expression levels of PD-L1 [3, 4]. Therefore, PD-1 inhibitors can be expected to be effective in patients with pulmonary pleomorphic carcinoma. In this case, the PD-L1 TPS was 75%, and tumors shrunk clearly after the initiation of pembrolizumab treatment.
IrAEs tend to occur 12–16 weeks after the start of immunotherapy and have been reported in a variety of organs. A recent study suggested that a better response to PD-1 inhibitor therapy was associated with early irAEs [5]. In this case, agranulocytosis, ILD and MG developed after 2–3 months, however, the response to pembrolizumab was diagnosed as PR after three cycles of pembrolizumab.
Agranulocytosis is a rare clinical finding, and the incidence of agranulocytosis was reported to be in several cases per million [6]. Drug-induced agranulocytosis tends to appear within 1 year, and the mortality was reported to be 5% [6]. G-CSF was reported to be effective for agranulocytosis when administered together with antibiotics [6]. To date, several cases of agranulocytosis caused by ICI have been reported. Nivolumab-induced agranulocytosis was reported after one to nine cycles of therapy [7, 8]. Ipilimumab-induced agranulocytosis was reported after four cycles of treatment [9]. Although the standard treatment of agranulocytosis caused by immunotherapy has not been established, previous reports have shown that G-CSF, intravenous immunoglobulin and high doses of steroid were effective [7–9]. In this case, viral infections and connective tissue diseases were ruled out by a blood test and physical examination. The patient did not take any other drug than pembrolizumab. Pembrolizumab was considered to be the cause of agranulocytosis according to the findings in the bone marrow aspiration. The neutrophil count increased and remained within normal limits after G-CSF administration. A recent study demonstrated that drug-induced agranulocytosis was induced not only by direct drug toxicity to bone marrow precursors but also by an immune-mediated mechanism [6]. However, the mechanism of pembrolizumab-induced agranulocytosis has not been clarified yet.
Several irAEs can be present simultaneously in different organs [1, 2]. Thus, this patient was affected by agranulocytosis, ILD and ocular MG. The incidence of any-grade ILD by pembrolizumab was reported to be 5.8%, and ILD of grade 3 or 4 was reported to be 2.6% in a recent clinical trial [1]. A recent study showed that MG, including generalized MG and ocular MG induced by an ICI, was relatively rare, however, MG-specific-related mortality was 30.4% [10]. The onset of symptoms of MG was reported to occur 2–12 weeks after the initiation of immunotherapy. It is important to detect symptoms and make a diagnosis of MG as soon as possible. However, the incidence and onset of symptoms of ocular MG alone is unknown. As in this case, patients can be affected by many irAEs. If a patient develops one irAE, one should pay attention to the expression of other irAEs.
In conclusion, we treated pulmonary pleomorphic carcinomas with pembrolizumab with good response. We describe here the first reported case of a patient with pulmonary pleomorphic carcinoma who developed agranulocytosis with anti-neutrophil antibody after pembrolizumab treatment; the patient was found to have ILD and ocular MG. One should watch out for early irAEs if immunotherapy produced a good response. In this patient, agranulocytosis was immediately improved by administration of G-CSF without use of systemic steroids. Further studies are needed to clarify the mechanism of ICI-induced agranulocytosis.
ACKNOWLEDGEMENTS
We are grateful to healthcare professionals who managed this patient.
CONFLICT OF INTEREST STATEMENT
Kaoru Kubota, Masahiro Seike and Akihiko Gemma report honoraria from MSD and Taiho Pharmaceutical, outside the submitted work.
FUNDING
No funding was received to publish this case report.
ETHICAL APPROVAL
Not required.
CONSENT
Written informed consent was obtained from this patient’s family for publication of the study including the patient’s details and accompanying images.
GUARANTOR
Masahiro Seike.
REFERENCES
- 1. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. NEJM 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 2. Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grünwald V, et al. . Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016;45:7–18. [DOI] [PubMed] [Google Scholar]
- 3. Chang YL, Yang CY, Lin MW, Wu CT, Yang PC. High co-expression of PD-L1 and HIF-1α correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer 2016;60:125–35. [DOI] [PubMed] [Google Scholar]
- 4. Kim S, Kim MY, Koh J, Go H, Lee DS, Jeon YK, et al. . Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatousareas. Eur J Cancer 2015;51:2698–707. [DOI] [PubMed] [Google Scholar]
- 5. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. . Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort Study. J Thorac Oncol 2017;12:1798–805. [DOI] [PubMed] [Google Scholar]
- 6. Andrès E, Maloisel F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol 2008;15:15–21. [DOI] [PubMed] [Google Scholar]
- 7. Tabchi S, Weng X, Blasis N. Severe agranulocytosis in a patient with metastatic non-small-cell lung cancer treated with nivolumab. Lung Cancer 2016;99:123–6. [DOI] [PubMed] [Google Scholar]
- 8. Turgeman I, Wollner M, Hassoun G, Bonstein L, Bar-Sela G. Severe complicated neutropenia in two patients with metastatic non-small-cell lung cancer treated with nivolumab. Anticancer Drugs 2017;28:811–4. [DOI] [PubMed] [Google Scholar]
- 9. Akhtari M, Waller EK, Jaye DL, Lawson DH, Ibrahim R, Papadopoulos NE, et al. . Neutropenia in a patient treated with ipilimumab (anti-CTLA-4 antibody). J Immunother 2009;32:322–4. [DOI] [PubMed] [Google Scholar]
- 10. Makarious D, Horwood K, Coward JIG. Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur J Cancer 2017;82:128–36. [DOI] [PubMed] [Google Scholar]