Abstract
Background
Many women with inflammatory bowel disease (IBD) report changes in symptoms in association with hormonal changes during menses, pregnancy, and hormonal contraceptive use, suggesting a hormonal influence on disease activity. We aimed to identify and characterize IBD symptom fluctuations in women during times of hormonal variation.
Methods
From June 2012 through September 2012, women enrolled in Crohn’s and Colitis Foundation of America Partners , an online Internet cohort of patients with IBD, were invited to participate in this study. Using a 5-point Likert scale, participants were asked to rate symptom changes during their menstrual cycle, pregnancy, the postpartum period, and after menopause. Clinical and demographic differences were assessed using univariate and multivariable methods.
Results
A total of 1,203 female patients with Crohn’s disease (CD) and ulcerative colitis (UC) participated (64% CD, 34% UC). Over half of the women with IBD reported worsening symptoms during menses. Symptom changes were similar between women with CD vs UC, except in pregnancy, where symptom worsening during pregnancy was more commonly seen in UC than CD (P = 0.02). Overall, women reporting symptom worsening were younger at the time of IBD diagnosis (P < 0.01), had lower quality of life (SIBDQ) scores (P < 0.01), and had a higher BMI (25 vs 24) than women without symptom worsening.
Conclusions
Women with IBD report changes in symptom severity during times of hormone fluctuation. Further clarification of the role of hormones in IBD is warranted in order to understand these relationships and to identify potential management strategies for women with IBD and hormonally sensitive gastrointestinal symptoms.
Keywords: inflammatory bowel disease (IBD), Crohn’s disease, ulcerative colitis, hormonal fluctuation
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory disorder, classified as either Crohn’s disease (CD) or ulcerative colitis (UC). Many women with IBD report fluctuation in gastrointestinal symptoms during their menstrual cycle, with both improvement and worsening at different phases.3 As treatment and management of IBD relies, to some extent, on patient-reported symptoms, further understanding of factors contributing to alteration of gastrointestinal symptoms is warranted.
Previous literature has sought to characterize changes in patient-reported symptoms during times of hormone fluctuation. Studies on the effects of pregnancy on IBD have focused on risks of disease relapse and pregnancy outcomes, whereas, symptom changes during pregnancy have not been fully characterized.4–6 However, these studies have been performed in relatively small cohorts, and no single study has comprehensively assessed IBD symptoms during menses, pregnancy, and menopause within the same cohort.
The aim of this study was to survey a large cohort of women with IBD and characterize the influence of states associated with known hormonal fluctuations on symptoms. We sought to clarify whether times of hormonal fluctuation are associated with self-reported symptom changes, and to further clarify demographic and clinical characteristics of women with “hormonally sensitive” IBD symptoms.
METHODS
Study Design and Patient Selection
We performed a cross-sectional study using a large internet-based cohort of patients with IBD (Crohn’s and Colitis Foundation of America [CCFA] Partners). CCFA Partners is an Internet cohort of patients with IBD who complete baseline and twiceyearly online surveys about their disease course and various patient-reported outcomes. Inclusion criteria for this cohort include individuals with UC and CD over 18 years of age, and access to the Internet.8 IBD diagnosis has been validated in a subset of the cohort, with 97% accuracy for both IBD diagnosis and subtype (CD or UC).9
Through CCFA Partners, women with IBD were invited to participate in an online survey between June 2012 and September 2012 through rolling invitations until the intended sample size was achieved.
Instruments
We developed a 16-item questionnaire with input from clinicians and researchers experienced in the management of women with IBD, hormonal disorders, and survey design. This survey was then evaluated by the CCFA Partners committee to ensure the survey was commensurate with other CCFA Partners surveys. The survey asked respondents to selfassess changes in their baseline disease activity in the historical context of menarche, menses, pregnancy, menopause, and hormone-replacement therapy (HRT). Symptom changes were recorded using a 5-point Likert scale (much worse, somewhat worse, no change, somewhat better, and much better). Disease activity at the time of the survey was assessed using validated instruments: the short Crohn’s Diseases Activity Index (sCDAI) for subjects with CD;10 and the Simple Clinical Colitis Activity Index (SCCAI) for subjects with UC).11 Additionally, disease-specific, health-related quality of life was measured using the short inflammatory bowel disease questionnaire (SIBDQ). The SIBDQ is a 10-item questionnaire with graded responses for each item from 1 (poorest) to 7 (highest). Higher scores suggest a better health-related quality of life. The use of SIBDQ has been previously validated to detect changes in health status among patients with IBD.12 Compliance was assessed by using the Morisky Medication Adherence Scale, and classified as low, medium, and high.8
Statistical Analysis
The primary measure was “hormone sensitivity,” defined by the subjective experience of an extreme change in symptoms (“much better” or “much worse”) associated with at least one of the following hormonal states: menarche, menses, ovulation, pregnancy, menopause, and hormonal contraceptive use. We aimed to identify 400 women with hormonally sensitive IBD symptoms, and we assumed a 1:2 ratio of women with hormonally sensitive symptoms versus those without hormonally sensitive symptoms. Based on this assumption, we aimed for a total sample size of 1,200 for the present study. The population was described using standard descriptive statistics including proportions, means, and standard deviation. Additionally, we assessed symptom severity at defined periods of hormone fluctuation, ie,: menses, ovulation, menopause, and menarche, acknowledging that not all subjects would have experienced all of these events. The populations were stratified for CD and UC to compare disease-type with reported symptom severity. Tests for differences were performed using univariate and multivariate regression methods, using STATA (version 12.0, College Station, TX). Confidence intervals were 95%, and P < 0.05 was considered to be statistically significant.
RESULTS
A total of 1,202 female patients enrolled in the study, including 64% with CD , 34% with UC, and 2% with indeterminate colitis. The median age of respondents was 43 years (interquartile range 31–55), and the mean duration of IBD was 11 years (interquartile range 5 to 21). Further characteristics of the group are listed in Tables 1 and 2.
Table 1.
Characteristics of the Population (N = 1,202)
| Characteristics | N | Percent * |
|---|---|---|
| Age (years) (mean, IQR) | 1202 | 43 (31–55) |
| Type of IBD | ||
| Crohn’s disease | 771 | 64.1 |
| Ulcerative colitis | 405 | 33.7 |
| Indeterminate colitis | 27 | 2.2 |
| Duration of disease (years) (median, IQR) | 1198 | 11 (5–21) |
| BMI (median) | 1185 | 24.4 (21.3–28.7) |
| Ostomy (% yes) | 82 | 6.8 |
| Education | ||
| High school or less | 93 | 8.2 |
| College + | 1039 | 91.8 |
| Smoking (ever) | 446 | 37.1 |
| Disease activity | ||
| sCDAI | 688 | 121 (72–187.5) |
| SCCAI | 402 | 3 (1–5) |
| Quality of life (SIBDQ) (mean, sd) | 1188 | 5.0 (1.1) |
| Medication adherence | ||
| Low | 366 | 37.3 |
| Medium | 335 | 34.2 |
| High | 280 | 28.5 |
| Current medications | ||
| 5-ASA (oral) a | 496 | 41.3 |
| 5-ASA (rectal) a | 103 | 8.6 |
| Biologic anti-TNF b | 411 | 34.2 |
| Thiopurine | 272 | 22.9 |
| Methotrexate | 3 | 3.6 |
| Corticosteroids (oral) | 112 | 9.4 |
| Corticosteroids (rectal) | 71 | 5.9 |
| Entocort | 49 | 4.0 |
| Gynecologist/Hormonal History | ||
| Age at menstruation (mean, sd) | 1184 | 12.9 (3.3) |
| Age at menopause (among those in menopause) (mean) | 458 | 46.4 (8.1) |
| Currently taking supplements (black cohosh, soy, etc) for menopausal symptoms (among those in menopause) | 40 | 8.7 |
| Currently taking HRT for menopausal symptoms (among those in menopause) | 93 | 20.2 |
| Hormonal contraceptives (ever use, % yes) | ||
| Birth control pills | 613 | 51.0 |
| Hormone injection | 44 | 3.7 |
| Hormone patch | 35 | 2.9 |
| Hormone ring | 64 | 5.3 |
| Hormone IUD c | 45 | 3.7 |
| Other hormonal contraception | 10 | 0.8 |
| Never taken hormonal contraception | 96 | 8.0 |
| Pregnancy (% ever) | 674 | 56.3 |
| Pregnancy after IBD diagnosis | 315 | 26.2 |
| Times pregnant after IBD diagnosis | 315 | 2 (1–2), range 1–6 |
| Age at first pregnancy after IBD diagnosis | 272 | 29 (26–32) |
| Breastfeeding among those with pregnancy (% yes) | 197 | 73.0 |
unless otherwise stated.
ASA: acetylsalicylic acid.
Anti-TNF: anti-tumor necrosis factor.
IUD: intrauterine device.
Table 2.
Description of Menstruation
| N | Percent | |
|---|---|---|
| Not started | 3 | 0.2 |
| Regular/predictable menses | 469 | 39.0 |
| Irregular menses | 178 | 14.8 |
| No menstrual period for > 1 year (not menopausal) | 67 | 5.6 |
| Post menopausal | 338 | 28.1 |
| Hysterectomy | 129 | 10.7 |
| Currently pregnant | 19 | 1.6 |
IBD Symptoms During Menses
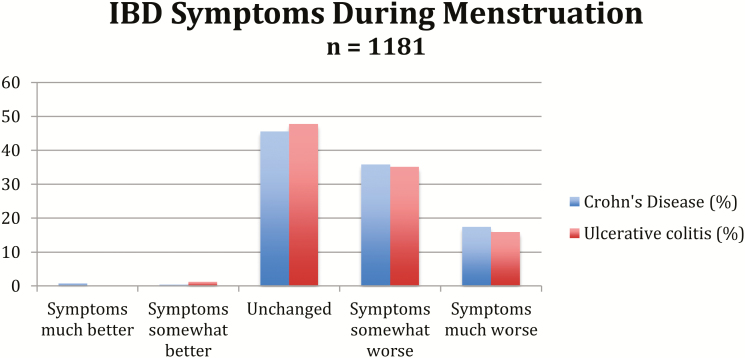
Just over half of women with CD and women with UC reported somewhat worse or much worse symptoms during their menstrual periods (53% of CD respondents, 51% of UC respondents) (Fig. 1). Results were similar when we analyzed a subgroup of women who reported regular and predictable menses, with 67% of women reporting somewhat worse or much worse symptoms during menses (68% of CD respondents, 66% of UC respondents).
FIGURE 1.
IBD Symptoms During Menstruation.
Women who reported worsening of symptoms during menses (n = 619) were younger (26 years vs. 34 years (P < 0.01), and they had an overall decreased quality of life (median SIBDQ 4.9, (IQR 3.5–5.7)), relative to women who reported no symptom changes during menses (median SIBDQ 5.2 (SD 4.3–5.9), P =< 0.01). In addition, women with low medication compliance had worse symptoms during menses (42% and 23% ,respectively, P < 0.01). Of note, reporting of medication compliance was ascertained at the time of survey response using the Morisky Medication Adherence Scale, rather than at reporting of symptoms during menses.
IBD Symptoms Two Weeks Before Menses (Ovulation)
During ovulation (2 weeks before the onset of menses), approximately 79% reported no change in their IBD symptoms (Table 3). Rates of symptom improvement or worsening were similar between women with CD and UC.
Table 3.
What Happens to IBD Symptoms 2 Weeks Before Menstrual Period
| N | percent | |
|---|---|---|
| Symptoms much better | 10 | 0.9 |
| Symptoms somewhat better | 22 | 1.9 |
| Unchanged | 928 | 78.6 |
| Symptoms somewhat worse | 169 | 14.3 |
| Symptoms much worse | 51 | 4.3 |
IBD Symptoms During and After Pregnancy
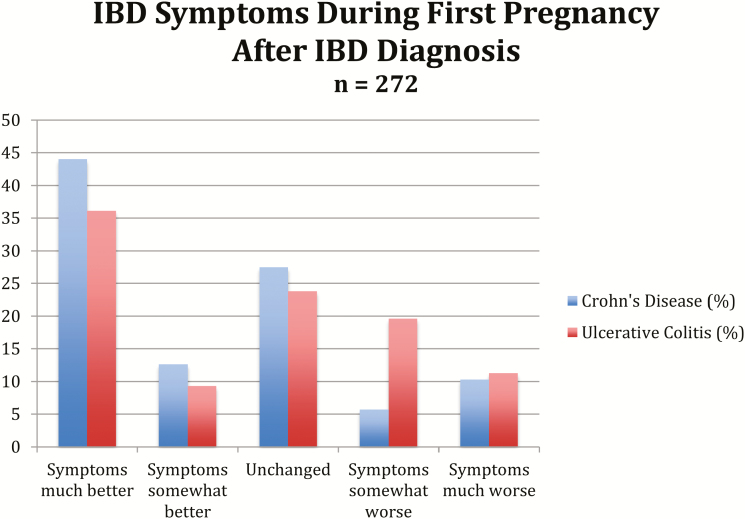
Among women with IBD who had been pregnant at least once after their diagnosis with IBD (n = 270), 52% reported an improvement of symptoms (somewhat better or much better) during pregnancy (57% CD, 44% UC) (Fig. 2). Among women with a prior pregnancy, more women with CD as compared to UC reported improvement in symptoms during pregnancy (59.3% vs 47.8%, P = 0.01 overall chi square), and more women with UC as compared to CD reported worsening of symptoms during pregnancy (32.6% vs 16.8%, P = 0.005). Those women with CD who reported worsening symptoms during pregnancy also reported worse SIBDQ scores at the time of the survey (5.2 vs 4.8, P = 0.03).
FIGURE 2.
IBD Symptoms During First Pregnancy After IBD Diagnosis.
IBD Symptoms During Menopause
Four hundred and fifty-six women reported being post-menopausal, of whom 24% were taking HRT. About two thirds of postmenopausal women (n = 295) reported no change in symptoms due to menopause itself, while 16% reported improvement in symptoms during and after menopause (Table 4).
Table 4.
What Happens to IBD Symptoms After Menopause Among Those in Menopausewith CD/UC
| Crohn’s disease | Ulcerative colitis | |||
|---|---|---|---|---|
| n | percent | n | percent | |
| Symptoms much better | 20 | 6.7 | 10 | 6.4 |
| Symptoms somewhat better | 29 | 9.7 | 14 | 8.9 |
| Unchanged | 195 | 65.2 | 100 | 63.7 |
| Symptoms somewhat worse | 30 | 10.0 | 12 | 7.6 |
| Symptoms much worse | 25 | 8.4 | 21 | 13.4 |
Women with worsened symptoms after menopause were older at the time of receiving an IBD diagnosis compared to those who did not report worsened symptoms (44 years old at time of diagnosis vs 32 years old, P < 0.01). Among postmenopausal women reporting worse symptoms, disease worsening was independent of smoking and BMI.
IBD Symptoms While on Exogenous Hormones
Women were asked to identify whether they had taken hormone supplementation during their disease course, including HRT and hormonal contraceptives. Six hundred and thirteen women reported ever using hormonal contraceptives, and 93 reported using HRT for menopausal symptoms. Reasons for the use of hormonal contraceptive included pregnancy prevention (60%), regulation of menses (21%), dysmenorrhea (13%), and to improve IBD symptoms (2.3%).
Among those using HRT at time of survey response, 61% reported no change in IBD symptoms with HRT, while 31% stated they “didn’t know” whether HRT influenced symptoms. Among those who were current or prior users of hormonal contraceptives, 83% reported no change in IBD symptoms, whereas 3% reported improvement, and approximately 7% reported worsening of symptoms from hormonal contraceptive use, identified as symptoms “somewhat worse” and symptoms “much worse”. (Tables 5 and 6).
Table 5.
What Happens to IBD Symptoms After Taking HRT/Supplements Among Those in Menopause (n = 111)
| Crohn’s disease | Ulcerative colitis | |||
|---|---|---|---|---|
| n | percent | n | percent | |
| Symptoms much better | 6 | 7.9 | 2 | 5.7 |
| Symptoms somewhat better | 3 | 4.0 | 1 | 2.9 |
| Unchanged | 67 | 88.2 | 32 | 91.4 |
| Symptoms somewhat worse | 0 | 0 | 0 | 0 |
| Symptoms much worse | 0 | 0 | 0 | 0 |
Table 6.
What Happens to IBD Symptoms After Starting Birth Control Pills (n = 612)
| Crohn’s disease | Ulcerative colitis | |||
|---|---|---|---|---|
| n | percent | n | percent | |
| Symptoms much better | 12 | 3.1 | 6 | 2.6 |
| Symptoms somewhat better | 26 | 7.0 | 15 | 6.6 |
| Unchanged | 319 | 83.3 | 187 | 81.7 |
| Symptoms somewhat worse | 14 | 3.7 | 14 | 6.1 |
| Symptoms much worse | 11 | 2.9 | 7 | 3.1 |
Hormone Sensitivity
In a separate analysis, we classified 413 women (34%) with hormonally sensitive symptoms, reporting that symptoms were much better or much worse at a time of hormonal fluctuation. These individuals with hormonally sensitive symptoms had lower overall SIBDQ scores (Table 7). Additionally, we found that women with hormonally sensitive symptoms tended to be younger at the time of IBD diagnosis (28 years vs 31 years P < 0.01) and had IBD for a longer duration of time (13 years vs 9 years P < 0.01) compared to those who were not hormonally sensitive.
Table 7.
Disease Activity Between Hormone Sensitive and Hormone Insensitive
| Individuals (n = 1075) | |||
|---|---|---|---|
| Severity Score | Hormone Sensitive Average (SD) |
Hormone Insensitive Average (SD) |
P-value |
| SIBDQ | 4.7 (1.2) | 5.0 (1.1) | <0.01 |
Additionally, women with UC who had hormonally sensitive symptoms were more likely to have higher clinical disease activity scores, but this was not seen among women with CD (Table 8).
Table 8.
Disease Activity Between Hormone Sensitive and Hormone Insensitive
| Individuals (n = 1075) | |||
|---|---|---|---|
| Severity Score | Hormone Sensitive Average (IQR) |
Hormone Insensitive Average (IQR) |
P-value |
| CD (SCDAI) | 128 (79–205) | 107 (72–184) | 0.64 |
| UC (SCCAI) | 4 (2–6) | 3 (1–5) | <0.01 |
DISCUSSION
We aimed to clarify associations of symptom changes in women with IBD during several different times of hormonal fluctuations. We found that many women have hormonally sensitive symptoms at various times of hormonal changes, defined as having significant changes in their symptoms at various phases of hormonal fluctuation. Over half of women had worsening of symptoms during menses. In general, symptom changes were not different among women with CD versus. UC, with the exception of more significant reported disease worsening during pregnancy in women with UC relative to CD among all women reporting worsening of symptoms during pregnancy (Fig. 2). In contrast, pregnancy was associated with overall improved symptoms in the majority of women with IBD, regardless of disease subtype.
Approximately one-third of our cohort met criteria for having hormonally sensitive symptoms, ie, reported extreme symptom changes (much better or much worse) at a time of hormonal fluctuation. This finding suggests the need to ask about symptom changes at the time associated with hormonal fluctuations in all women with IBD, and additional effort to consider this association when differentiating hormonally-related symptom fluctuation from disease exacerbation in the clinical setting. Although we asked women to identify symptoms related to their IBD, it is unclear whether self-reported changes in gastrointestinal symptoms truly reflect worsening inflammatory symptoms, or perhaps hormonally-triggered motility disturbances leading to gastrointestinal manifestations.
Many of our findings are consistent with previously published literature, although some key differences are noted. Several studies have found that women with CD were more likely to report worsening of symptoms at the time of menses than women with UC. Several studies found higher reported diarrheal symptoms during the menstrual cycle among women with CD compared to those with UC.3,13,14 In contrast, we found that both CD and UC were associated with symptom worsening during menses, which may be a function of assessing a larger sample size compared to other studies. Consistent with prior literature, our cohort did not report a significant change in symptoms after menopause.15 Previous studies have demonstrated that estrogen surges during the menstrual cycle appear to cause decreases in gut motility, increased gut permeability, and heightened pain perception.16 During menopause, the fluctuations in hormones, estrogen in particular, are more gradual than they are in premenopausal women.17 We hypothesize that times of hormonal surge, rather than depletion as seen in the postmenopausal period, is a contributing factor to worsening GI symptoms.
The use of exogenous hormones including HRT and hormonal contraceptives were not associated with changes in reported symptom severity in our cohort, similar to findings previously published.18
Women with UC who met the criteria for having hormonally sensitive symptoms were more likely to have higher disease activity scores, unlike women with CD who had hormonally sensitive symptoms. This finding differs from prior work by Saha et al, who demonstrated that women with CD who reported dysmenorrhea symptoms, including diarrhea and abdominal pain, were more likely to have higher disease activity scores.19 These findings need to be interpreted cautiously in light of the self-reported nature of our study, which may more directly correlate with endoscopic disease activity scores in patients with UC relative to CD.20,21
Previous studies have reported disparate findings with regard to the effect of pregnancy on symptoms in IBD. We found that more than half of the women reported improvement of symptoms during pregnancy. Notably, while the majority of women reported improved symptoms, there was a higher proportion of women with UC compared to women with CD who reported worsening of symptoms. This discrepancy of symptoms during pregnancy between women with UC and CD has been previously noted in a survey of 209 women, where those with UC had increased chances of relapse of disease, unlike those with CD.4 Other studies have focused on rates of disease recurrence and/or relapse during pregnancy, and several studies, including a metaanalysis by Abhyankar et al that demonstrated an increased risk for IBD relapse during pregnancy, influenced by disease activity at time of conception.6 These studies must be interpreted with caution, however, as several represent findings before the introduction of biologic therapy for treatment of IBD.
A number of mechanisms explaining the relationship between worsening GI symptoms and menstruation have been suggested. One theory suggests that elevated prostaglandin production during menses may be responsible for increased contraction of colonic smooth muscle.22 Additional studies have demonstrated the influence of estrogen surge on gut permeability, gut motility, and pain perception as described above.16 Other studies have demonstrated protective effects of pregnancy on rheumatoid arthritis, multiple sclerosis, and psoriasis, with proposed mechanisms including immunomodulation of T cell activity during pregnancy, thereby reducing inflammation-driven symptoms in these women.23 The molecular basis of IBD involves a complex interplay between inflammatory modulators and key cytokines including prostaglandins and variations in T cell subsets. A pivotal cytokine in IBD is TNFα, which has been shown to be down-regulated in pregnancy, itself a physiological state of immune tolerance in some respects.24 It may be that for some women with IBD, alterations in these profiles may lead to improved (or worsened) disease activity as a result of normal pregnancy driven changes in immunity.
Our study has several limitations. Patient responses to surveys may be influenced by recall bias. Our survey asked participants to report symptoms at various times of hormonal fluctuation, including possible remote episodes such as pregnancy or during the premenopausal period. Prior work has demonstrated that recall of symptoms in the premenstrual period is particularly subject to recall bias and influenced by social stressors, anxiety, and depression.14,25–27 Additionally, our survey also included data such as BMI and SIDBDQ at the time of survey participation, which may not reflect these values at the time of reporting historical symptoms. Additionally, we recruited self-motivated and highly-educated participants who were already participating in the CCFA Partners cohort, which may limit the generalizability of our results. Our surveys relied on subjective reporting of symptoms without objective assessments to correlate with true biologic disease activity. As demonstrated in studies by Kane et al, women with irritable bowel syndrome also report GI symptoms during menses, and it is difficult to objectively attribute changes in GI symptoms to IBD disease activity based solely on the patient’s experience.3 Although e we assessed medication adherence using the Morisky Medication Adherence Scale at the time of survey response, we did not ask participants about medication adherence during specific times of hormonal fluctuation such as pregnancy or during menses, which may be historical in nature and may influence symptom reporting by our participants. When women were asked within our survey about use of exogenous hormones, we did not differentiate between vaginal HRT versus oral therapy, which may also influence these results.
Another limitation within our survey tool was the lack of piloting before implementation of this survey to members of the CCFA, however, it was iteratively developed with input by experienced clinicians and survey design specialists. Finally, we did not ask participants if they had undergone hysterectomy or oophorectomy, and hence the inclusion of such participants also may bias our results.
Despite these limitations, our study demonstrates that women with IBD report symptom changes during times of hormone fluctuation, particularly during menses and pregnancy. In particular, women with UC experience a greater likelihood of worsening symptoms than women with CD. Additionally, women with lower SIBDQ scores and more severe disease appear to be more susceptible to these fluctuations. Women with IBD should be asked about their symptom changes during times of hormonal fluctuation, to better elucidate cycles of disease activity. Further investigations to clarify the true associations and pathogenesis of hormonally influenced changes in IBD symptoms and disease activity are warranted.
Conflict of Interest: There are no relevant financial disclosures to report.
This work has been presented in part as a poster presentation at Digestive Disease Week, 2013 (Orlando, FL, USA).
Supported by grants from the Crohn’s and Colitis Foundation of America and the National Institutes of Health (P30 DK034987).
REFERENCES
- 1.Boroujerdi L, Long MD, McGovern DP, et al. . Inflammatory bowel disease symptom severity is influenced by hormone fluctuations in many women with IBD. Gastroenterology. 2013;144:S632. [Google Scholar]
- 2.Boroujerdi L, Long MD, McGovern DP, et al. . Symptom worsening during pregnancy and lactation is associated with age, body mass index, and disease phenotype in women with inflammatory bowel disease. Gastroenterology. 2013;144:S753–4. [Google Scholar]
- 3.Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93:1867–72. [DOI] [PubMed] [Google Scholar]
- 4.Castiglione F, Pignata S, Morace F, et al. . Effect of pregnancy on the clinical course of a cohort of women with inflammatory bowel disease. Ital J Gastroenterol. 1996;28:199–204. [PubMed] [Google Scholar]
- 5.Ng SW, Mahadevan U. Management of inflammatory bowel disease in pregnancy. Expert Rev Clin Immunol. 2013;9:161–73; quiz 174. [DOI] [PubMed] [Google Scholar]
- 6.Abhyankar A, Ham M, Moss AC. Meta-analysis: the impact of disease activity at conception on disease activity during pregnancy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane S, Lemieux N. The role of breastfeeding in postpartum disease activity in women with inflammatory bowel disease. Am J Gastroenterol. 2005;100:102–5. [DOI] [PubMed] [Google Scholar]
- 8.Long MD, Kappelman MD, Martin CF, et al. . Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflamm Bowel Dis. 2012;18:2099–106. [DOI] [PubMed] [Google Scholar]
- 9.Randell RL, Long MD, Cook SF, et al. . Validation of an internet-based cohort of inflammatory bowel disease (CCFA partners). Inflamm Bowel Dis. 2014;20:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thia K, Faubion WA Jr, Loftus EV Jr, et al. . Short CDAI: development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm Bowel Dis. 2011;17:105–11. [DOI] [PubMed] [Google Scholar]
- 11.Bennebroek Evertsz F, Nieuwkerk PT, Stokkers PC, et al. . The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis. 2013;7:890–900. [DOI] [PubMed] [Google Scholar]
- 12.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–8. [PubMed] [Google Scholar]
- 13.Parlak E, Dagli U, Alkim C, et al. . Pattern of gastrointestinal and psychosomatic symptoms across the menstrual cycle in women with inflammatory bowel disease. Turk J Gastroenterol. 2003;14:250–6. [PubMed] [Google Scholar]
- 14.Bernstein MT, Graff LA, Targownik LE, et al. . Gastrointestinal symptoms before and during menses in women with IBD. Aliment Pharmacol Ther. 2012;36:135–44. [DOI] [PubMed] [Google Scholar]
- 15.Kane SV, Reddy D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1193–6. [DOI] [PubMed] [Google Scholar]
- 16.Meleine M, Matricon J. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World J Gastroenterol. 2014;20:6725–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin North Am. 2004;33:637–59. [DOI] [PubMed] [Google Scholar]
- 18.Gawron LM, Goldberger A, Gawron AJ, et al. . The impact of hormonal contraception on disease-related cyclical symptoms in women with inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1729–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha S, Midtling E, Roberson E, et al. . Dysmenorrhea in women with Crohn’s disease: a case-control study. Inflamm Bowel Dis. 2013;19:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins PD, Schwartz M, Mapili J, Zimmermann EM. Is endoscopy necessary for the measurement of disease activity in ulcerative colitis?Am J Gastroenterol. United States 2005;100:355–61. [DOI] [PubMed] [Google Scholar]
- 21.Jones J, Loftus EV Jr, Panaccione R, et al. . Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. United States 2008;6:1218–24. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe BM. Prostaglandins and serotonin in diarrheogenic syndromes. Adv Exp Med Biol. 1978;106:285–95. [DOI] [PubMed] [Google Scholar]
- 23.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasef NA, Ferguson LR. Inflammatory bowel disease and pregnancy: overlapping pathways. Transl Res. 2012;160:65–83. [DOI] [PubMed] [Google Scholar]
- 25.Marvan ML, Cortes-Iniestra S. Women’s beliefs about the prevalence of premenstrual syndrome and biases in recall of premenstrual changes. Health Psychol. 2001;20:276–80. [DOI] [PubMed] [Google Scholar]
- 26.Wong LP. Attitudes toward menstruation, menstrual-related symptoms, and premenstrual syndrome among adolescent girls: a rural school-based survey. Women Health. 2011;51:340–64. [DOI] [PubMed] [Google Scholar]
- 27.Negriff S, Dorn LD, Hillman JB, Huang B. The measurement of menstrual symptoms: factor structure of the menstrual symptom questionnaire in adolescent girls. J Health Psychol. 2009;14:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]