Abstract
OBJECTIVE
The objective of this study was to examine 1) whether teens’ glycemic control and adherence to type 1 diabetes treatment regimen worsen during the transition from late adolescence to emerging adulthood, and 2) whether teens’ executive function (EF), as measured by performance and self-reported problems with EF, is predictive of these changes (after controlling for general intelligence).
RESEARCH DESIGN AND METHODS
High school seniors with type 1 diabetes (N = 236; mean age 17.74 years) were assessed at three yearly time points. At baseline, during the senior year of high school, participants completed a self-report measure of problems with EF and performance-based measures of EF and general intelligence (IQ). Glycemic control was determined on the basis of results collected from HbA1c assay kits, and teens reported their adherence at all three time points.
RESULTS
HbA1c increased significantly across the three time points and adherence declined. EF performance was not associated with adherence or HbA1c at baseline, nor with changes in adherence over time. However, better EF performance predicted slower increases in HbA1c over time (i.e., slope) while controlling for IQ. Teens’ self-reported problems with EF were associated with worse glycemic control and poorer adherence at baseline (i.e., intercept), but they did not predict changes in either HbA1c or adherence over time (i.e., slope).
CONCLUSIONS
Abilities involved in performance on EF tests may be one resource for maintaining better glycemic control during the transition to emerging adulthood. Assessment of such EF abilities may allow for the identification of individuals who are most at risk for deterioration of glycemic control during this transition.
Introduction
The transition between late adolescence and emerging adulthood has been described as a “high-risk” time for individuals managing type 1 diabetes (1), characterized by higher levels of HbA1c and poorer adherence than for individuals at other developmental time periods (2). Longitudinal studies indicate that glycemic control (3,4) and adherence (5) deteriorate during adolescence. The few studies that have examined longitudinal trajectories extending into early young adulthood suggest these vary greatly across individuals, with some displaying increases in HbA1c during adolescence and young adulthood (6,7), others showing improvements during young adulthood (6,7), and yet others maintaining fairly stable (high or low) levels of glycemic control and adherence across time (6–8). Understanding factors that contribute to such variability may help to identify those who are at highest risk for poor diabetes self-management during this transitional time.
Cognition may be an important factor in understanding adherence and glycemic control trajectories during the transition to emerging adulthood, a factor that to our knowledge has not yet been examined. During emerging adulthood, defined as the period from ages 18 to 25 years (9), teens need to remember, plan, and execute an intensive diabetes treatment regimen, often while moving away from home, entering college or the workforce, and establishing new relationships with peers (10). Cross-sectional research has demonstrated that general cognitive abilities and executive function (EF) abilities such as planning, organization, and working memory may provide adolescents with resources for maintaining better glycemic control and adherence. Specifically, in adolescents, more reported problems with EF (measured on the basis of reports by parents or youths) are associated with poorer adherence and poorer glycemic control (11–15). Previous cross-sectional results from the sample used in this study showed that lower scores on performance-based measures of EF were associated with poor glycemic control during the senior year of high school (15). Cross-sectional findings are supported further by longitudinal research showing that fewer parent-reported problems with EF characterized a low-risk group of adolescents who maintained good glycemic control across a 3-year time frame (16).
What is missing from the literature are longitudinal studies examining how EF specifically (controlling for general cognitive abilities) may be a resource for diabetes management across the transition to emerging adulthood, comparing performance-based measures to self-reported measures of problems with EF. Such research is important for three reasons. First, the vast majority of studies used questionnaires (self- or parent-report) that are purported to assess neurocognitive processes, but such measures are only modestly related to performance-based measures of EF (17,18). Thus, although questionnaires are easier to administer in clinic settings than performance-based measures, it is uncertain whether both are similarly predictive of changes in adherence and glycemic control over time. Second, studies have predominantly used parent-reported EF because of the young age of children, their cognitive impairments, or both (18). During emerging adulthood, teens’ reports may be crucial to identifying those who are at risk, as parents often no longer attend their teens’ health care visits and may be less knowledgeable about teens’ daily lives. Finally, to understand whether EF specifically predicts diabetes management, more general cognitive abilities such as IQ must be statistically controlled, as IQ underlies many daily activities and is associated with both self-report and performance-based measures of EF (19).
This longitudinal study fills this important gap by examining how both self-reported problems with EF and performance-based measures of EF (while controlling for intelligence) predict longitudinal trajectories of type 1 diabetes management during the transition from late adolescence to early emerging adulthood. We first examine whether HbA1c and adherence systematically change across three annual time points. We anticipated that significant increases in HbA1c and decreases in adherence would occur, but that the rate of deterioration would vary across individuals. Next, we assessed whether teens’ reports of problems with EF and performance-based measures of EF obtained during the senior year of high school (controlling for intelligence) predicted changes in HbA1c and adherence across the three time points. We anticipated that both fewer self-reported problems with EF and higher EF performance would predict slower increases in HbA1c and slower decreases in adherence over time.
Research Design and Methods
Participants
High school seniors with type 1 diabetes were recruited for a 2-year longitudinal, multisite study investigating the transition to early emerging adulthood. Participants were recruited from outpatient pediatric endocrinology clinics during clinic visits or by phone in two southwestern U.S. cities. Youths were eligible if they had been diagnosed with type 1 diabetes for at least 1 year (mean ± SD time since diagnosis 7.35 ± 3.88 years; 94% of teens had diabetes for ≥2 years), spoke English as their primary language, were in their final year of high school, lived with a parent or parental figure at baseline (68.4% lived at home with both parents; 27.1%, with one biological parent; 4.5%, with adoptive parents or grandparents), would be able to have regular contact with parents or the parental figure over the subsequent 2 years, and had no condition that would prohibit study completion (e.g., severe intellectual disability, blindness). Adolescents who had dropped out of high school were eligible if they met all other criteria. Of the 507 qualified individuals we approached, 301 (59%) agreed to participate. Of those who initially agreed, 247 (82%) enrolled in the study. Reasons for not participating included lack of interest (33%) or being too busy during their senior year to participate (34%); 20% declined to give a reason. The institutional review board at one site permitted data collection to allow comparisons between those who did and those who did not participate. We found no differences in HbA1c, time since diagnosis, sex, or pump status (P > 0.05). However, participants were slightly younger (mean ± SD 17.77 ± 0.43 vs. 17.91 ± 0.48 years; t[203] = 2.27; P = 0.02) and were more likely to be Hispanic (21% vs. 11%; χ2 [1] = 3.88; P = 0.049) than nonparticipants.
Procedure
The study had institutional review board approval; parents provided consent and teens provided consent or assent (those who assented provided consent after they turned 18). EF performance and IQ were assessed in the laboratory, and an online survey measured self-reported problems with EF and adherence. Teens were paid $50 for the first two annual assessments and $75 for the third assessment. At each time point, teens reported illness and demographic variables, including their pump status, with whom they were living, and their health insurance. Because extreme hyperglycemia and hypoglycemia can affect cognitive performance (20,21), blood glucose levels were checked before performance measures were completed. If blood glucose levels were outside the range 75–400 mg/dL, participants took steps to normalize blood glucose; testing was rescheduled for one participant who could not bring blood glucose in range. Blood glucose levels during the testing sessions were not related to performance measures of EF and IQ (r < 0.13; all P > 0.11).
Measures
EF
Self-reported problems with EF were measured with the Behavior Rating Inventory of Executive Function–Self-Report. This measure is normed for respondents between 5 and 18 years of age (22), and previous research has demonstrated its high internal consistency (α = 0.72–0.96). Participants rated items (0 = never, 1 = sometimes, 2 = often) to indicate how frequently they experienced each problem over the preceding 6 months. Subscales were combined into a global executive composite score with good reliability (α = 0.95). Age- and sex-corrected t scores taken from the manual (22) were used in analyses.
Performance-based EF was measured through the use of four subtests from the Delis-Kaplan Executive Function System battery (23). We assessed widely recognized components of EF: set maintenance and working memory, assessed with the Trail Making Test (letter–number sequencing condition completion time); response inhibition and cognitive control, assessed with the Color-Word Interference Test (inhibition and inhibition/switching condition completion times); and initiation and generative fluency, assessed with tests of verbal fluency (correct responses for the letter and category conditions) and design fluency (correct responses for each of the three conditions). This assessment battery yielded eight executive scores. The mean of norm-based, age-corrected, scaled scores (as described in the test manual [23]) was computed to generate a single EF performance score. In this sample, the Cronbach α for this composite was 0.83.
General IQ
Teens completed the Vocabulary subtest of the Wechsler Adult Intelligence Scale–Fourth Edition (24), which we used to estimate crystallized IQ (25). This subtest correlates 0.91–0.92 with verbal IQ and 0.79–0.81 with full-scale IQ in this age-group (24). Reported split-half reliability for the Vocabulary subtest is 0.93 for teens aged 16–19 years in the normative sample (24). We analyzed norm-based, age-corrected, scaled scores (24).
Adherence
Teens’ reports of adherence to their diabetes regimen were measured through the use of the Diabetes Behavior Rating Scale, a 37-item scale assessing self-management behaviors (e.g., meal planning, blood glucose testing, exercise, the amount and timing of insulin) and components of problem-solving (e.g., adjusting insulin doses depending on blood glucose levels or food intake) (26). This measure correlates highly with more time-intensive interview measures (26). In this study, the scale had good reliability (α = 0.84 for teens who use an insulin pump; α = 0.86 for teens who do not use a pump). Scores were computed as proportions ranging from 0 to 1 (higher scores reflected better adherence) (26).
Glycemic Control
HbA1c was measured on the day of cognitive testing and at later time points through the use of mail-in HbA1c kits (provided and processed by CoreMedica Laboratories, accredited by the College of American Pathologists; www.coremedicalabs.com). This approach was chosen over obtaining HbA1c from medical records to ensure that HbA1c was measured on the day of cognitive testing for all participants, that the same procedures were used to measure HbA1c across time points, and that HbA1c measures could be obtained even from those who were not receiving routine care. The kit was completed by the teen at baseline after they received instructions from a trained research assistant, who observed test completion. This measure was highly correlated with HbA1c obtained from point-of-care assays noted in the medical records at baseline (r = 0.74; P < 0.001).
Analytic Plan
Missing data for key study variables ranged from a low of 1% to a high of 12%. To account for missing data, we generated five data sets through multiple imputation (MI) (27) for individuals who provided survey data. We did not impute data for an individual who was missing all data from one time point. The MI procedure included variables beyond those included in the analyses presented here to help ensure that an adequate missing-at-random model was generated. The lowest efficiency was 0.942, suggesting that the MI procedure across five data sets adequately recovered missing data.
Unconditional linear growth curve models of HbA1c and adherence were conducted with the MIXED command in SPSS software version 24 (IBM). Full maximum likelihood was applied to examine changes in HbA1c and adherence across the three time points. We included random effects on the intercept and slope, with baseline coded as 0, such that the intercept represented the mean value of the dependent variable at time 1. Next we created conditional growth curve models to determine whether EF predicted baseline levels (i.e., intercept) and changes in HbA1c or adherence (i.e., slope); we analyzed separate models for EF performance and self-reported problems with EF. All independent variables were centered at their mean to facilitate interactions (e.g., EF predicting slope or a time effect). We examined the conditional effects after controlling for IQ. Time since diagnosis and insulin pump status (0 = no, 1 = yes) measured at baseline were included as covariates in analyses of HbA1c. Because 13% of individuals changed their pump status over time, pump status was analyzed as a time-varying covariate and results were similar to those with pump status controlled only at baseline.
No significant effects were found for sex across all analyses (P > 0.10). Hispanic/Latino participants had higher HbA1c than did Caucasians at baseline (8.97% vs. 8.10%; P < 0.05), but the slope of HbA1c did not differ between the groups. Analyses conducted with and without ethnicity as a covariate yielded no differences in results. Living in the parental home was not associated with either HbA1c or adherence, nor did it alter the trajectories of these variables across time. Because these covariates were either not significant or did not alter results, they were not included in further analyses.
Results
Preliminary Analyses
Consistent with the population at participating clinics, 75.2% of the full sample (N = 247) identified as non-Hispanic white, 14.2% as Hispanic, and 4.8% as African American; the remainder identified as Asian/Pacific Islander, American Indian, or more than one race. Patients were a mean of 17.76 years old (SD = 0.39 year), and 60% were female.
This study included 220 teens with complete survey data at two or more annual assessments and valid scores for EF performance, IQ, and self-reported problems with EF at baseline. Educational background of mothers and fathers was, on average, some college or higher (Table 1). Around 40% of teens were using a pump over time, and about half were living in the parental home at times 2 and 3. The majority of teens reported being either fully or partially covered by their parents’ insurance. The 220 teens included in these analyses (63% female; mean ± SD age 17.77 ± 0.39 years) did not differ from the 16 who were not included (because they completed only a single time point) on primary study variables at baseline, including adherence, self-reported problems with EF, or EF performance (P > 0.4); however, those included had lower HbA1c at baseline than those who completed a single time point (8.23% vs. 8.92%; t = 2.36; P < 0.05).
Table 1.
Descriptive statistics for demographics and primary measures
| Baseline |
Year 2 |
Year 3 |
||||
|---|---|---|---|---|---|---|
| Mother’s education, % | ||||||
| High school or less | 13.900 | — | — | |||
| Some college | 42.800 | — | — | |||
| Bachelor’s or higher | 42.500 | — | — | |||
| Father’s education, % | ||||||
| High school or less | 21.300 | — | — | |||
| Some college | 28.800 | — | — | |||
| Bachelor’s or higher | 48.300 | — | — | |||
| Uses pump, % | 43.600 | 45.000 | 51.200 | |||
| Living in parental home, % | 100.000 | 52.510 | 48.470 | |||
| On parents’ insurance, % | 75.000 | 78.600 | 72.000 | |||
| Mean | SD | Mean | SD | Mean | SD | |
|---|---|---|---|---|---|---|
| HbA1c | ||||||
| In % | 08.218 | 1.668 | 08.937 | 1.976 | 09.229 | 2.052 |
| In mmol/mol | 66.000 | 74.000 | 77.000 | |||
| Self-reported EF problems | 54.306 | 10.524 | — | — | ||
| EF performance | 11.312 | 2.038 | — | — | ||
| IQ | 11.512 | 3.317 | — | — | ||
| Adherence | 00.609 | 0.123 | 00.587 | 0.132 | 00.589 | 0.150 |
Scores for self-reported EF problems, EF performance, IQ, and adherence are based on the tests described in research design and methods. Dashes indicate that the variable was only measured at baseline.
Teens had, on average, HbA1c values above American Diabetes Association recommendations (HbA1c ≤7.5% [28]) in all years of the study (Table 1). Teens’ reports of problems with EF and of EF performance and IQ were in the mean range relative to norms (22–24). More self-reported problems with EF were modestly associated with poorer EF performance (r = −0.28; P < 0.01).
EF Performance and Self-Reported Problems With EF Predicting Change in HbA1c
Growth models examining linear change in HbA1c are presented in Table 2. The time effect in the unconditional growth model indicated that HbA1c increased significantly over time, with a 0.507% increase in HbA1c for each year past the senior year of high school (baseline). Significant random effects indicated between-person variability at baseline (intercept) and in changes in HbA1c across time (slopes). Covariate analyses indicated that individuals using insulin pumps had lower HbA1c than those not using pumps.
Table 2.
Growth curve model for HbA1c
| HbA1c growth curve models | β | SE | Random effect | Variance | |
|---|---|---|---|---|---|
| Unconditional model | |||||
| Intercept | 8.267 | 0.108** | 1.368 | 0.216** | |
| Years since diagnosis | 0.038 | 0.025 | |||
| Pump status (0 = MDI, 1 = pump) | −0.844 | 0.196** | |||
| Time (0 = time 1) | 0.507 | 0.072** | 0.347 | 0.101** | |
| Conditional models | |||||
| EF Performancea | |||||
| Intercept | 8.271 | 0.104** | 1.196 | 0.198** | |
| Years since diagnosis | 0.034 | 0.024 | |||
| Pump Status (0 = MDI, 1 = pump) | −0.662 | 0.191** | |||
| Time (0 = Time 1) | 0.502 | 0.071** | 0.340 | 0.097** | |
| IQ | |||||
| On Intercept | −0.096 | 0.038* | |||
| On Slope | 0.048 | 0.026 | |||
| EF Performance | |||||
| On Intercept | −0.066 | 0.060 | |||
| On Slope | −0.096 | 0.043* | |||
| Self-reported EF problemsa | |||||
| Intercept | 8.269 | 0.102** | 1.105 | 0.192** | |
| Years since diagnosis | 0.035 | 0.024 | |||
| Pump status (0 = MDI, 1 = pump) | −0.706 | 0.189** | |||
| Time (0 = time 1) | 0.499 | 0.072** | 0.382 | 0.101** | |
| IQ | |||||
| On Intercept | −0.107 | 0.033** | |||
| On Slope | 0.020 | 0.023 | |||
| Self-reported EF problems | |||||
| On Intercept | 0.029 | 0.010** | |||
| On Slope | 0.002 | 0.007 | |||
MDI, multiple daily injections.
aControlling for IQ.
*P < 0.05; **P < 0.01.
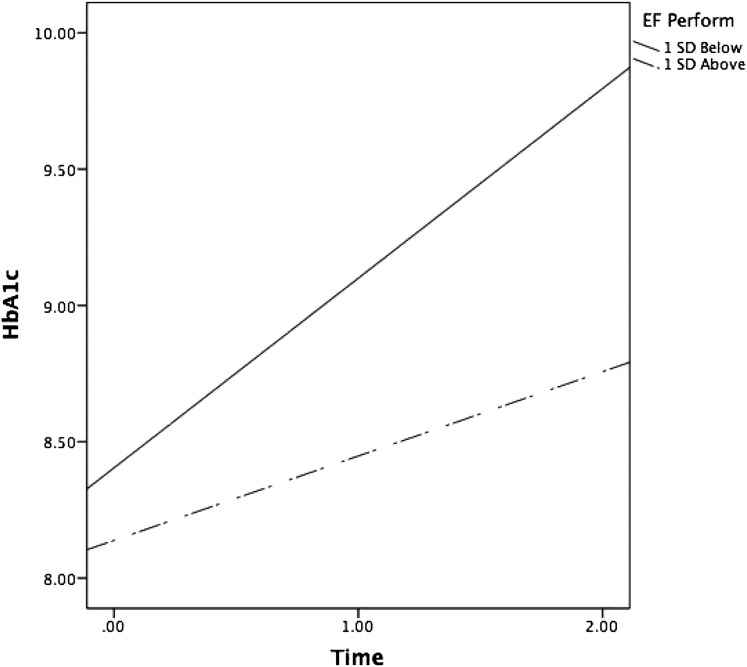
Conditional growth models indicated that higher IQ was associated with lower HbA1c at baseline. Further, EF performance predicted change in HbA1c (EF performance on slope effect; Table 2), indicating that EF performance predicted significantly different slopes in HbA1c across time. As displayed in Fig. 1, individuals with higher EF performance at baseline experienced slower increases in HbA1c across the three time points (plotted 1 SD above and below the mean EF performance). Simple slope analyses revealed that both slopes were significantly different from zero, indicating that both those with better and those with poorer EF performance experienced increases in HbA1c across the three time points. As predicted, however, those with better EF performance deteriorated slower (slope of 0.361%) than those with poorer EF performance (slope of 0.622%).
Figure 1.
Changes in HbA1c over time as a function of EF performance (EF Perform; 1 SD above and below the mean).
A similar analysis was conducted using self-reported problems with EF. As displayed in Table 2, both higher IQ and fewer self-reported problems with EF were associated with lower HbA1c at baseline. However, self-reported problems with EF did not predict changes in HbA1c across time.
EF Performance and Self-Reported Problems With EF Predicting Change in Adherence
Growth models examining linear change in adherence are presented in Table 3. The unconditional growth model indicated that adherence decreased significantly over time, with a very small decrease of 0.012 for each year past the senior year of high school. Random effects were statistically significant, indicating between-person variability at baseline (intercept) and in changes in adherence across time (slope). Neither IQ nor EF performance predicted intercepts or slopes for adherence across time.
Table 3.
Growth Curve Model for Adherence
| Components of adherence growth curve models | Adherence |
Random effect | Variance | |
|---|---|---|---|---|
| β | SE | |||
| Unconditional model | ||||
| Intercept | 0.604 | 0.008** | 0.010 | 0.001** |
| Time (0 = time 1) | −0.012 | 0.005* | 0.002 | 0.001** |
| Conditional models | ||||
| EF performancea | ||||
| Intercept | 0.604 | 0.008** | 0.010 | 0.001** |
| Time (0 = time 1) | −0.011 | 0.005* | 0.002 | 0.000** |
| IQ | ||||
| On Intercept | 0.000 | 0.003 | ||
| On Slope | 0.001 | 0.002 | ||
| EF performance | ||||
| On Intercept | 0.005 | 0.005 | ||
| On Slope | 0.000 | 0.003 | ||
| Self-reported EF problemsa | ||||
| Intercept | 0.604 | 0.008** | 0.009 | 0.001** |
| Time (0 = time 1) | −0.010 | 0.005 | 0.002 | 0.000** |
| IQ | ||||
| On Intercept | 0.000 | 0.002 | ||
| On Slope | 0.001 | 0.001 | ||
| Self-reported EF problems | ||||
| On Intercept | −0.004 | 0.001** | ||
| On Slope | 0.000 | 0.000 | ||
All units are proportions as adherence ranges from 0–1.
aControlling for IQ.
*P < 0.05; **P < 0.01.
A similar analysis was conducted using self-reported problems with EF. As displayed in Table 3, greater self-reported problems with EF were associated with lower adherence at baseline. No variable predicted slopes of adherence across time.
Conclusions
The findings of this study support the view of the transition to early emerging adulthood as a “high-risk” time for diabetes management (1). HbA1c increased substantially during early emerging adulthood. This increase is clinically concerning given that at baseline teens were already well above American Diabetes Association–recommended HbA1c values. Substantial variability existed in the slopes of HbA1c, with HbA1c in some individuals increasing more rapidly than in others, consistent with the growing literature on variability in glycemic control across the transition to emerging adulthood (5–7). Teens also reported lower adherence during their senior year than was reported in a sample of 12- to 18-year-olds (0.61 vs. 0.75 out of 1.00) (26), with further modest declines in adherence across time.
This study is to our knowledge the first to demonstrate that better scores on a performance-based measure of EF predict slower increases in HbA1c over time, even when controlling for intelligence. Our participants scored within the mean range for EF relative to national norms, indicating that EF performance even within the normal range is important in understanding changes in HbA1c across time. EF performance likely would be more predictive if examined in individuals with scores in the clinically impaired range (29). The fact that the performance-based measure predicted change even when controlling for intelligence provides evidence that EF specifically, rather than cognition more generally, is predictive of HbA1c changes across time. Although performance-based EF scores predicted changes in HbA1c across time, the slope of HbA1c still varied significantly. Such findings suggest that other factors are involved in changes in HbA1c during emerging adulthood, such as health care appointments and changes in parental involvement or other support—many of which we did not have access to in this study (e.g., severe hypoglycemic episodes, diabetic ketoacidosis). These factors will be important to include in future research.
Self-reported problems with EF were associated with HbA1c and adherence at baseline, but not with changes in HbA1c or adherence across the 2-year period. The modest correlation between EF performance and self-reported problems with EF in this study is consistent with the broader literature about younger children and those with developmental disorders (18). The neurocognitive processes needed for execution of self-reported versus performance-based tasks likely differ, as functional MRI research demonstrates that such measures activate different neuroanatomical regions (30). Self-report measures of EF problems have been described as tapping typical behavior in real-world contexts and reflect factors other than EF abilities, including contextual factors such as available resources (e.g., support from parents) or the complexity of one’s daily life. By contrast, performance-based EF measures reflect underlying neurocognitive capacity assessed under constrained, structured conditions (18), and thus they are relatively nonconfounded by contextual factors. The results suggest that it is the extent to which emerging adults can engage in EF optimally across contexts that allows them to maintain good glycemic control throughout emerging adulthood. The discrepancy between self-reported EF performance and problems with EF could be due to teens underestimating or misremembering their problems with EF. Scores from self-report and performance-based measures of EF clearly represent different constructs and should not be interpreted as commensurate (17,18).
Neither teens’ reported EF performance nor problems with EF were predictive of changes in adherence across time; this perhaps is attributable to the limited changes in self-reported adherence across the 2 years. The large increases in HbA1c and the very modest declines in adherence indicate that emerging adults’ perceptions of adherence do not correspond with their HbA1c values. Young emerging adults may be adhering in the same manner as they did during adolescence but without the same beneficial result for HbA1c. Contextual changes in early emerging adulthood, including the reduced involvement of parents, may alter the efficacy of their adherence behaviors. In addition, emerging adults’ judgments of their adherence are only modestly related to more objective measures such as blood glucose tests (31). Future research on individuals in this age range would benefit from objective measures such as records downloaded from glucometers, continuous glucose monitors, or insulin pumps, which were not available for this sample.
The current findings have numerous clinical implications. First, teens’ EF performance may be a useful indicator of who is at increased risk for deteriorations in glycemic control during the transition out of high school. The fact that only EF performance—rather than self-reported problems with EF—was predictive is important, as most research has used self- or parent-report measures such as the Behavior Rating Inventory of Executive Function. Although traditional measures of EF performance cannot realistically be administered by medical personnel, the field of clinical neuropsychology is actively developing computer-administered tests that could be adapted for clinical use (32). Currently, however, batteries that are partially (e.g., the National Institutes of Health Executive Abilities: Measures and Instruments for Neurobehavioral Evaluation and Research or the Cognition Battery from the NIH Toolbox [33,34]) or fully (e.g., the Cambridge Neuropsychological Test Automated Battery [35]) computerized are primarily appropriate for use in research. Growing evidence that performance measures of cognition (including EF) contribute to glycemic control, and that diabetes itself has a deleterious impact on cognition (36), suggest that neuropsychological evaluations may be beneficial in identifying those who are at risk for poor health outcomes. Such evaluations, together with tailored feedback, have been associated with improved patient outcomes in a variety of settings (37).
The results of this study should be interpreted in the context of some limitations. First, although we used a composite of eight EF performance–based scores, we did not comprehensively assess all aspects of the EF construct, especially emotional control and decision making (19). Second, we assessed EF performance at only one time point. Although the longitudinal design of the study provides some assurance that EF performance did affect changes in HbA1c, emerging adulthood is a time when EF is still developing. Because deteriorations in HbA1c and the frequency of severe hypoglycemia may have consequences for cognitive function (36), additional research reassessing EF during emerging adulthood is necessary in order to understand the bidirectional associations between EF and glycemic control (36). Third, the majority of teens in the sample had parents who had attained at least some college education, which may limit generalizability to teens who have fewer advantages and whose EF may have been negatively affected by low socioeconomic status (38). Finally, by design, our participants were enrolled during their senior year of high school (ages 17 and 18 years), which limits the generalizability of the findings to those of other ages.
In sum, this study demonstrates that EF is a resource for good glycemic control across the high-risk time of emerging adulthood. The challenges that emerging adults with low EF face may be compounded by the concomitant decline in parental involvement that often occurs during this time. Early emerging adults (especially those with low EF abilities) may benefit from multiple supports for diabetes management, such as text-based reminders (39) and the support and assistance that parents, friends, and health care providers may provide, in order to facilitate their adherence behaviors and maintain good glycemic control during this transitional time (40).
Article Information
Acknowledgments. The authors thank the physicians and staff at the Primary Children’s Hospital Diabetes Program, Mountain Vista Medicine, and Children’s Medical Center Dallas and the teens and parents who participated in this study.
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (grant no. R01 DK092939). C.A.B. and D.J.W. are co–primary investigators on the grant.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.A.B., D.J.W., Y.S., and J.B. designed the study. C.A.B., J.B., and A.M. analyzed data. Y.S., S.L.T., and A.H.L. performed the literature search and acquired data. P.C.W. and M.M. facilitated clinic recruitment. C.A.B., D.J.W., Y.S., S.L.T., J.B., A.M., A.H.L., P.C.W., and M.M. wrote and critically revised the manuscript and approved the manuscript for submission. C.A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. These data were presented at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
A.H.L. is currently affiliated with the University of Nevada, Reno, Reno, NV.
References
- 1.Weissberg-Benchell J, Wolpert H, Anderson BJ. Transitioning from pediatric to adult care: a new approach to the post-adolescent young person with type 1 diabetes. Diabetes Care 2007;30:2441–2446 [DOI] [PubMed] [Google Scholar]
- 2.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 3.Rausch JR, Hood KK, Delamater A, et al. . Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care 2012;35:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hood KK, Beavers DP, Yi-Frazier J, et al. . Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health 2014;55:498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King PS, Berg CA, Butner J, Butler JM, Wiebe DJ. Longitudinal trajectories of parental involvement in type 1 diabetes and adolescents’ adherence. Health Psychol 2014;33:424–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwandt A, Hermann JM, Rosenbauer J, et al.; DPV Initiative . Longitudinal trajectories of metabolic control from childhood to young adulthood in type 1 diabetes from a large German/Austrian registry: a group-based modeling approach. Diabetes Care 2017;40:309–316 [DOI] [PubMed] [Google Scholar]
- 7.Helgeson VS, Vaughn AK, Seltman H, Orchard T, Libman I, Becker D. Featured article: trajectories of glycemic control over adolescence and emerging adulthood: an 11-year longitudinal study of youth with type 1 diabetes. J Pediatr Psychol 2018;43:8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilliard ME, Wu YP, Rausch J, Dolan LM, Hood KK. Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. J Adolesc Health 2013;52:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol 2000;55:469–480 [PubMed] [Google Scholar]
- 10.Hanna KM, Weaver MT, Stump TE, Guthrie D, Oruche UM. Emerging adults with type 1 diabetes during the first year post-high school: perceptions of parental behaviors. Emerg Adulthood 2014;2:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duke DC, Harris MA. Executive function, adherence, and glycemic control in adolescents with type 1 diabetes: a literature review. Curr Diab Rep 2014;14:532–542 [DOI] [PubMed] [Google Scholar]
- 12.McNally K, Rohan J, Pendley JS, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care 2010;33:1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goethals ER, de Wit M, Van Broeck N, et al. . Child and parental executive functioning in type 1 diabetes: their unique and interactive role toward treatment adherence and glycemic control. Pediatr Diabetes 2018;19:520–526 [DOI] [PubMed] [Google Scholar]
- 14.Berg CA, Wiebe DJ, Suchy Y, et al. . Individual differences and day-to-day fluctuations in perceived self-regulation associated with daily adherence in late adolescents with type 1 diabetes. J Pediatr Psychol 2014;39:1038–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchy Y, Turner SL, Queen TL, et al. . The relation of questionnaire and performance-based measures of executive functioning with type 1 diabetes outcomes among late adolescents. Health Psychol 2016;35:661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohan JM, Rausch JR, Pendley JS, et al. . Identification and prediction of group-based glycemic control trajectories during the transition to adolescence. Health Psychol 2014;33:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAuley T, Chen S, Goos L, Schachar R, Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? J Int Neuropsychol Soc 2010;16:495–505 [DOI] [PubMed] [Google Scholar]
- 18.Toplak ME, West RF, Stanovich KE. Practitioner review: do performance-based measures and ratings of executive function assess the same construct? J Child Psychol Psychiatry 2013;54:131–143 [DOI] [PubMed] [Google Scholar]
- 19.Suchy Y. Executive Funtioning: A Comprehensive Guide for Clinical Practice. New York, NY, Oxford University Press, 2015 [Google Scholar]
- 20.Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol 2004;10:36–52 [DOI] [PubMed] [Google Scholar]
- 21.Weinger K, Jacobson AM. Cognitive impairment in patients with type 1 (insulin-dependent) diabetes mellitus: incidence, mechanisms and therapeutic implications. CNS Drugs 1998;9:233–252 [Google Scholar]
- 22.Guy SC, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function—Self-Report Version: Professional Manual. Lutz, FL, PAR, Inc., 2004 [Google Scholar]
- 23.Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System: Examiner’s Manual. San Antonio, TX, Psychological Corporation, 2001 [Google Scholar]
- 24.Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX, Pearson, 2008 [Google Scholar]
- 25.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th ed. New York, NY, Oxford University Press, 2013 [Google Scholar]
- 26.Iannotti RJ, Nansel TR, Schneider S, et al. . Assessing regimen adherence of adolescents with type 1 diabetes. Diabetes Care 2006;29:2263–2267 [DOI] [PubMed] [Google Scholar]
- 27.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol 2009;60:549–576 [DOI] [PubMed] [Google Scholar]
- 28.Chiang JL, Kirkman MS, Laffel LMB, Peters AL; Type 1 Diabetes Sourcebook Authors . Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suchy Y, Queen TL, Huntbach B, et al. . Iowa gambling task performance prospectively predicts changes in glycemic control among adolescents with type 1 diabetes. J Int Neuropsychol Soc 2017;23:204–213 [DOI] [PubMed] [Google Scholar]
- 30.Faridi N, Karama S, Burgaleta M, et al. . Neuroanatomical correlates of behavioral rating versus performance measures of working memory in typically developing children and adolescents [published correction appears in Neuropsychology 2015;29:91]. Neuropsychology 2015;29:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg CA, Butner JE, Turner SL, Lansing AH, King P, Wiebe DJ. Adolescents’, mothers’, and fathers’ reports of adherence across adolescence and their relation to HbA1c and daily blood glucose. J Behav Med 2016;39:1009–1019 [DOI] [PubMed] [Google Scholar]
- 32.Parsons TD, McMahan T, Kane R. Practice parameters facilitating adoption of advanced technologies for enhancing neuropsychological assessment paradigms. Clin Neuropsychol 2018;32:16–41 [DOI] [PubMed] [Google Scholar]
- 33.Zelazo PD, Anderson JE, Richler J, et al. . NIH Toolbox Cognition Battery (CB): validation of executive function measures in adults. J Int Neuropsychol Soc 2014;20:620–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer JH, Mungas D, Possin KL, et al. . NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc 2014;20:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994;5:266–281 [DOI] [PubMed] [Google Scholar]
- 36.Kirchhoff BA, Jundt DK, Doty T, Hershey T. A longitudinal investigation of cognitive function in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes 2017;18:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sieg EK, Brook M, Mai Q. The utility of neuropsychological consultation in complex medical inpatients with suspected neurocognitive impairment at risk for poor post-hospitalization outcomes. Clin Neuropsychol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hackman DA, Gallop R, Evans GW, Farah MJ. Socioeconomic status and executive function: developmental trajectories and mediation. Dev Sci 2015;18:686–702 [DOI] [PubMed] [Google Scholar]
- 39.Mulvaney SA, Anders S, Smith AK, Pittel EJ, Johnson KB. A pilot test of a tailored mobile and web-based diabetes messaging system for adolescents. J Telemed Telecare 2012;18:115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helgeson VS, Palladino DK, Reynolds KA, Becker DJ, Escobar O, Siminerio L. Relationships and health among emerging adults with and without type 1 diabetes. Health Psychol 2014;33:1125–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]