Abstract
OBJECTIVE
We assessed whether changes in metabolic syndrome (MetS) severity during the treatment of prediabetes are associated with reduced risk of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD).
RESEARCH DESIGN AND METHODS
We analyzed data from the Diabetes Prevention Program (DPP) for 2,476 adults in 1996–1999 with prediabetes randomized to receive treatment with lifestyle modification, metformin, or placebo for 2–3 years and followed through 2014 for T2DM and CVD outcomes. We calculated effect sizes from baseline in a MetS severity z score (MetS-Z) and the individual MetS components, and assessed relationships between 1-year effect size and incident T2DM and CVD using hazard ratios (HRs) and mediation analysis.
RESULTS
Baseline MetS-Z and its components were associated with risk of incident T2DM and CVD. During year 1 of intervention, MetS-Z and its components decreased most with lifestyle modification, followed by treatment with metformin and placebo. Risk of T2DM within 1–5 years was most strongly associated with 1-year changes in MetS-Z and waist circumference (both HRs for a 1 SD increase = 1.80), whereas the risk of CVD was associated with a 1-year change in MetS-Z, glucose, and systolic blood pressure. In mediation analyses, the effect of lifestyle modification on T2DM risk was mediated by 1-year changes in MetS-Z, waist circumference, glucose, and triglycerides, whereas the effect of metformin was mediated by MetS-Z and glucose.
CONCLUSIONS
Changes in these risk indicators of MetS severity during intervention in the DPP reflect altered disease risk and may help in tracking earlier responses to treatment and in motivating patients.
Introduction
Although 9.4% of Americans have type 2 diabetes mellitus (T2DM)—greatly increasing the risk of comorbidities including cardiovascular disease (CVD) (1)—an additional 26% are at elevated risk of T2DM by virtue of having prediabetes (2). This highlights the importance of using clinical risk indicators to identify high-risk patients who could then be started on preventative treatments and to follow-up for response to these treatments. In addition to lifestyle changes, the American Diabetes Association (ADA) recently stated (3) that some individuals with prediabetes should be considered for treatment with metformin. It is less clear whether risk prediction biomarkers can accurately track reductions in risk during treatment—improvements that could be a compelling means of motivating patients, who may feel empowered by a decrease in score.
One risk indicator relevant to prediabetes is the presence of the metabolic syndrome (MetS), which appears to be driven by excess visceral adiposity and is associated with future T2DM and CVD (4,5). Although MetS has traditionally been characterized based on criteria such as those of the Adult Treatment Panel III (ATP-III), which categorizes MetS among individuals with abnormalities in at least three of the five components (elevated waist circumference [WC], high blood pressure [BP], high triglycerides, low HDL, and high fasting glucose) (6), the dichotomous nature of these criteria makes it difficult to track the response to treatment over time (7), and MetS has been criticized as not providing additional risk information beyond its individual components (8,9). We therefore developed a continuous MetS severity z score (MetS-Z) (10) that is associated with long-term risk for T2DM (11–13) and CVD (13–15), even in models that include the individual MetS components (11,15).
In the current study, we evaluated for temporal changes in MetS severity and the individual MetS components as a biomarker associated with an altered risk of future T2DM and CVD during treatment among participants of the Diabetes Prevention Program (DPP), a randomized controlled trial in individuals with prediabetes who received treatment with usual care, metformin, or intensive lifestyle modification (16). We hypothesized 1) that baseline levels of MetS-Z and MetS components would be associated with a risk for future T2DM and CVD, 2) that MetS severity and individual components would decrease during intervention with lifestyle modification and metformin, 3) that the degree of reduction in MetS severity and its components would be associated with a lowered risk for T2DM and CVD, and 4) that these factors would be significant mediators of any reduced risk noted for these interventions. These data may have implications for providing earlier biomarkers to compare the efficacy of treatments for prediabetes.
Research Design and Methods
DPP Description
The DPP was approved by the Institutional Research Boards of participating institutions, and the current analysis was approved by the Institutional Research Board of the University of Florida. Data from the DPP were provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository (https://repository.niddk.nih.gov/home/). The DPP is registered with ClinicalTrials.gov (NCT00004992), and the methods have been described previously (17). Briefly, between 1996 and 1999, 3,234 individuals (mean age 50.6 years) with impaired glucose intolerance were recruited from 27 research sites and provided informed consent. Impaired glucose tolerance was determined by having fasting glucose <126 mg/dL and glucose levels of 140–199 mg/dL after a 2-h oral glucose tolerance test. Participants in the original trial were excluded if they were taking medications affecting glucose intolerance, were unable to participate in physical activity, or experienced a CVD event in the prior months (16). Further exclusion criteria for the current analysis were as follows: incident T2DM or CVD during year 1; missing data at baseline or year 1 for measures required to calculate MetS severity; and participants randomized to the original troglitazone intervention, which was discontinued (Supplementary Fig. 1). After study enrollment, participants were randomized to one of the following three intervention arms: metformin 850 mg twice daily; placebo twice daily; or an intensive lifestyle modification program with a goal to achieve and maintain ≥7% body weight reduction via a low-calorie, low-fat diet and moderate physical activity ≥150 min/week (e.g., brisk walking). By 24 weeks of follow-up, 50% of the lifestyle group had achieved the goal weight loss (16). For the metformin arm, 71% of participants took ≥80% of the prescribed dose (18).
The original DPP was discontinued early after a mean 2.8 years of intervention because of the efficacy of the lifestyle intervention arm in the reduction of T2DM, the primary end point, with the lifestyle, metformin, and placebo arms having 4.8, 7.8, and 11.0 cases/100 person-years of progression to T2DM.
At baseline and yearly during 3 years of follow-up in the DPP (and yearly thereafter in the DPP Outcomes Study: mean follow-up 13.4 years, SD 4.8 years), participants had in-person visits for the completion of questionnaires and examinations that included measures of WC and BP (18). At these visits, blood was drawn and assessed for CVD risk factors, including triglycerides, HDL, and glucose, as described previously (18).
Study Outcomes
T2DM and CVD
Incident T2DM was determined as a fasting blood glucose of ≥126 mg/dL or a nonfasting blood glucose of ≥200 mg/dL. Oral glucose tolerance tests were performed at yearly study visits, and fasting glucose measures were obtained on a semiannual basis and for any new symptoms suggestive of diabetes. All T2DM diagnoses required confirmation testing within ∼6 weeks.
Incident CVD was assessed yearly using the question “During the past 12 months, have you had any of the following: heart attack (myocardial infarction [MI], coronary occlusion or coronary thrombosis), stroke, transient ischemic attacks (TIA) or mini-stroke, or carotid endarterectomy or other procedure to open blood vessels in the neck?” Data regarding coronary heart disease status were used to determine whether participants required unblinding of lipid results for possible treatment with lipid-lowering agents. Self-reported data such as these have provided reasonable sensitivity and specificity for MI (89.5% and 98.2%, respectively) and stroke (78.4% and 98.6%, respectively) (19).
Predictors: ATP-III MetS and MetS Severity Z Score
ATP-III MetS
ATP-III MetS was defined by the presence of three or more of the following criteria: elevated WC (≥102 cm for men, ≥88 cm for women); elevated fasting triacylglycerol (≥150 mg/dL); reduced HDL (<40 mg/dL for men, <50 mg/dL for women); elevated BP (≥130 mmHg systolic BP [SBP], ≥85 mmHg diastolic BP, or drug treatment for hypertension); and elevated fasting blood glucose (≥100 mg/dL) (6).
MetS Severity Score
We calculated the MetS-Z values at baseline and yearly follow-ups using sex- and race/ethnicity-based formulas, as described previously (10,20). Briefly, the MetS-Z was derived from the five traditional MetS components (WC, triglycerides, HDL cholesterol, SBP, and fasting glucose) using a factor analysis approach on nationally representative 1999–2010 data from the National Health and Nutrition Examination Survey (NHANES) for adults 20–64 years of age. Because of differences in traditional MetS criteria by race/ethnicity (21–23), the confirmatory factor analysis was performed to determine the weighted contribution of each component to a latent MetS factor on a sex- and race/ethnicity-specific basis. For each of six subgroups based on sex and race/ethnicity (non-Hispanic white, non-Hispanic black, and Hispanic), factor loadings from the five MetS components were determined and used to generate equations for computing a MetS severity score for each subgroup (http://mets.health-outcomes-policy.ufl.edu/calculator/). The resulting score has a standard normal distribution that operates as a “z score” but was not standardized directly from the DPP sample. The MetS severity score was shown to correlate with other MetS risk markers, such as insulin (13) and adiponectin (13), and is predictive of the long-term risk of T2DM (11–13) and CVD (13–15).
Statistical Analysis
All statistical analyses were performed using SAS version 9.4. We excluded individuals who were assigned to troglitazone or had CVD at baseline from all analyses. Because our primary interest was in 1-year changes in MetS severity and how these changes were associated with incident disease, we also excluded individuals in whom CVD or T2DM developed by 1 year or those who had missing MetS severity data at baseline or 1 year. All other individuals were included in all analyses. Descriptive statistics were calculated overall, by intervention group and by disease classification status, which included: no T2DM within 5 years (with or without eventual CVD), and T2DM within 5 years (with or without eventual CVD). The primary interest in the models described below was in examining and comparing the association of the severity and components of MetS with the risk of eventual disease. To allow for direct comparisons, we calculated standardized scores for each of the components at baseline [(value − baseline mean)/baseline SD], and we calculated 1-year “effect sizes” for MetS severity and its components (1-year change/baseline SD). All models thus used these baseline z scores and 1-year effect sizes. We first examined the association of baseline MetS severity (and 1-year change in severity) with the time to T2DM and the time to CVD via Cox proportional hazards models. The proportional hazards assumption was found not to hold for the T2DM model; time-varying hazard ratios (HRs) were thus calculated using incident T2DM within 5 years and beyond 5 years. Baseline MetS severity and 1-year change in severity were included in the same model, adjusting for intervention. Analogous models were fit for each of the components of MetS. As permitted by collinearity, we also fitted a joint model of MetS-Z with individual MetS components (baseline and 1-year changes, after checking for collinearity) to assess whether each of the predictors remained significantly associated with disease outcomes in the same model.
We also were interested in the mediating effect of change in MetS severity and its components on the effect of the intervention on reduced risk of disease. Mediation models assess the possible causal mechanisms underlying the relationship between an independent variable (in this case, the interventions of lifestyle and metformin) and a dependent variable (in this case, disease outcome). Supplementary Fig. 2 displays our conceptual model of the mediation analysis. We used mediation models (24) (PROC CAUSALMED in SAS) to estimate the mediating effect of the 1-year change in MetS severity (and in the components of MetS separately) on the effect of each of the two interventions (lifestyle and metformin vs. placebo) on reduced risk of 1) T2DM within 5 years and 2) T2DM within 5 years including eventual CVD. This mediation analysis was interested in the mechanisms by which the two interventions reduce the risk of disease (25); specifically, we are interested in 1-year changes in MetS severity and in its components and how these changes account for the ultimate reduced risk of disease associated with the outcome. Relative risks were calculated to estimate the total effect (TE) of the intervention. Inclusion of the 1-year change allowed us to estimate the pure indirect effect (PIE), the effect of the intervention on reduced risk of disease through this intermediate 1-year outcome; and the total direct effect (TDE), the effect of the intervention not accounted for by this 1-year change in severity. In evaluating the intervention and its intermediate effect on changes in MetS severity, we ultimately were interested in PIE (and what percentage of the TE was due to the PIE [25]). This type of analysis (and interpretation of the PIE) assumes no measurement error or other bias (26), which admittedly is an assumption that cannot be met here. However, we believe any source of bias will be constant across the measures we are comparing as possible mediators. The mediation models adjusted for baseline values of the potential mediator being examined (e.g., baseline MetS severity).
Results
Participant Characteristics and MetS Effect Size Over Time
Table 1 displays characteristics of the 2,476 participants in the analytic cohort. The group overall had a relatively high baseline MetS-Z at 0.77 (SD 0.64). The incidence of T2DM between years 1 and 5 was high overall (29.8%) and was lowest in the lifestyle modification intervention, as reported previously (16). The self-reported incidence of CVD over a median time (16.0 years of follow-up; interquartile range = 13.5, 16.0 years of follow-up) was 12.6% overall, with 3.6% and 9.0% among those with and without incident T2DM at 2–5 years, respectively.
Table 1.
Baseline characteristics by intervention group among those with baseline and year 1 follow-up, and ultimate disease status
| Overall | Intervention group |
Eventual disease status* |
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Metformin | Lifestyle | No T2DM within 5 years | T2DM between 1 and 5 years (no CVD) | T2DM between 1 and 5 years (CVD) | CVD, no T2DM within 5 years | ||
| n or n (%) | 2,476 | 788 (31.8) | 851 (34.4) | 837 (33.8) | 1,191 (61.2) | 510 (26.2) | 70 (3.6) | 175 (9.0) |
| Categorical characteristics (% of column) | ||||||||
| Age category (years) | ||||||||
| <40 | 14.5 | 14.3 | 12.7 | 16.4 | 16.1 | 14.7 | 8.6 | 1.7 |
| 40–44 | 14.7 | 13.2 | 15.8 | 15.2 | 14.0 | 15.9 | 5.7 | 5.7 |
| 45–49 | 20.7 | 24.8 | 18.5 | 19.1 | 20.6 | 21.8 | 15.7 | 12.6 |
| 50–54 | 17.7 | 16.6 | 21.5 | 14.7 | 17.0 | 20.6 | 15.7 | 16.6 |
| 55–59 | 12.8 | 13.8 | 11.8 | 12.9 | 12.4 | 12.0 | 22.9 | 18.9 |
| 60–64 | 9.8 | 8.9 | 10.1 | 10.3 | 9.8 | 7.1 | 18.6 | 21.1 |
| ≥65 | 9.9 | 8.4 | 9.8 | 11.5 | 10.1 | 8.0 | 12.9 | 23.4 |
| Male | 31.1 | 29.2 | 34.1 | 29.8 | 29.5 | 28.6 | 57.1 | 44.6 |
| Race/ethnicity | ||||||||
| White | 61.1 | 60.7 | 60.6 | 61.9 | 63.5 | 55.7 | 71.4 | 70.3 |
| African American | 21.6 | 22.5 | 22.8 | 19.6 | 18.5 | 27.5 | 21.4 | 17.1 |
| Hispanic (any race) | 17.3 | 16.9 | 16.6 | 18.5 | 18.1 | 16.9 | 7.1 | 12.6 |
| ATP-III MetS | 69.7 | 71.7 | 68.4 | 69.0 | 63.7 | 79.2 | 82.9 | 69.1 |
| Current smokers | 6.4 | 7.5 | 6.6 | 5.1 | 5.3 | 9.2 | 11.4 | 6.9 |
| Continuous characteristics (mean ± SD) | ||||||||
| MetS-Z | 0.77 ± 0.64 | 0.81 ± 0.62 | 0.7 ± 0.6 | 0.77 ± 0.64 | 0.66 ± 0.63 | 0.99 ± 0.61 | 1.08 ± 0.65 | 0.75 ± 0.64 |
| WC | 105.3 ± 14.4 | 105.2 ± 14.0 | 105.3 ± 14.3 | 105.4 ± 14.9 | 104.0 ± 14.0 | 108.5 ± 15.8 | 112.8 ± 17.0 | 104.7 ± 13.0 |
| SBP | 123.7 ± 14.5 | 123.3 ± 14.2 | 124.1 ± 14.7 | 123.6 ± 14.6 | 122.8 ± 14.0 | 124.9 ± 15.1 | 128.8 ± 15.2 | 126.3 ± 15.9 |
| Glucose | 106.7 ± 7.3 | 106.6 ± 7.1 | 106.8 ± 7.5 | 106.5 ± 7.2 | 104.9 ± 6.3 | 110.2 ± 8.3 | 110.9 ± 8.0 | 106.1 ± 6.9 |
| Triglycerides | 162.2 ± 93.7 | 168.2 ± 94.5 | 157.1 ± 88.1 | 161.9 ± 98.2 | 155.7 ± 90.1 | 168.7 ± 92.1 | 191.1 ± 121.7 | 172.4 ± 102.5 |
| HDL cholesterol | 46.0 ± 12.0 | 45.0 ± 11.6 | 46.3 ± 11.7 | 46.7 ± 12.6 | 47.0 ± 12.0 | 44.6 ± 11.4 | 41.6 ± 11.5 | 45.5 ± 12.8 |
| Total cholesterol | 204.2 ± 35.9 | 204.4 ± 36.4 | 202.9 ± 34.9 | 205.3 ± 36.5 | 203.5 ± 36.9 | 202.7 ± 35.2 | 208.6 ± 34.0 | 207.5 ± 34.2 |
| Follow-up (years) | 13.4 ± 4.8 | 13.5 ± 4.7 | 13.3 ± 4.9 | 13.2 ± 4.9 | 11.7 ± 5.8 | 14.0 ± 4.0 | 15.0 ± 2.4 | 15.3 ± 1.9 |
| Eventual disease (n = 1,946) (n [%]) | ||||||||
| No T2DM within 5 years | 1,191 (61.2) | 346 (54.8) | 415 (61.4) | 430 (67.3) | ||||
| T2DM between 1 and 5 years (no CVD) | 510 (26.2) | 196 (31.1) | 180 (26.6) | 134 (21.0) | ||||
| T2DM between 1 and 5 years (CVD) | 70 (3.6) | 30 (4.8) | 26 (3.9) | 14 (2.2) | ||||
| CVD, no T2DM within 5 years | 175 (9.0) | 559 (9.4) | 55 (8.1) | 61 (9.6) | ||||
*Those individuals in whom T2DM developed after 5 years, without CVD, were not included in subsequent analyses (n = 529); one individual had only 3 years of follow-up with CVD but no T2DM to be determined at 5 years, and was excluded.
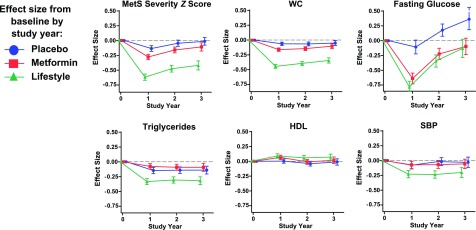
Figure 1 displays the effect size of MetS-Zs and individual components by intervention group during 3 years of follow-up. Unsurprisingly, the placebo group exhibited only a slight decrease in MetS severity effect size from baseline to −0.13 at year 1, rising back to baseline by year 3. In the lifestyle intervention group, MetS severity decreased to −0.62 by year 1, with a partial rebound to −0.42 by year 3. In the metformin group, MetS-Z decreased to −0.23 at year 1 and rebounded to −0.11 by year 3. The mean absolute effects are provided in Supplementary Table 1. These changes were also seen when using a MetS z score derived without glucose (Supplementary Fig. 3A). The prevalence of ATP-III MetS was ∼70% for all groups at baseline, at year 1 it had decreased in all groups (but particularly the lifestyle intervention, at 45%), and by year 3 it was below baseline only in the lifestyle intervention group (Supplementary Fig. 3B).
Figure 1.
Changes in MetS-Z and the individual MetS components over 3 years of intervention. Mean effect sizes (i.e., the change from baseline, divided by the SD at baseline) ±95% CIs for MetS-Z, WC, fasting glucose, triglycerides, HDL cholesterol, and SBP during intervention with lifestyle modification, and treatment with metformin and placebo. The mean absolute effects are provided in Supplementary Table 1.
Figure 1 also displays the effect sizes of the individual MetS components during 3 years of intervention. Between baseline and visit 1, there were significant improvements in all MetS components among those individuals in the lifestyle and metformin groups, while the placebo group only exhibited significant changes in WC, SBP, and triglycerides. By year 3, the lifestyle intervention group remained better than baseline in all components except glucose, while the metformin and placebo groups were only slightly better for WC and triglycerides.
Overall HRs by Intervention and 1-Year MetS Effect Size
Table 2 displays data on HRs for T2DM and CVD. In a combined analysis, both interventions were associated with a reduced risk of incident T2DM from years 1 to 5, with an HR of 0.57 for lifestyle modification and 0.82 for metformin relative to placebo. Neither intervention was associated with altered risk for incident T2DM >5 years or incident CVD.
Table 2.
Analysis of time to diabetes and CVD
| Diabetes survival models* |
CVD survival models |
|||||||
|---|---|---|---|---|---|---|---|---|
| Incident diabetes 1–5 years |
Incident diabetes >5 years |
Model AIC†† | Incident CVD Overall |
|||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | Model AIC†† | ||
| Intervention-only model | 17,269.80 | 3,734.12 | ||||||
| Metformin** | 0.82 (0.68, 0.99) | 0.0367 | 0.93 (0.76, 1.14) | 0.48 | 0.82 (0.60, 1.11) | 0.1985 | ||
| Lifestyle** | 0.57 (0.46, 0.70) | <0.0001 | 1.07 (0.88, 1.30) | 0.50 | 0.87 (0.65, 1.18) | 0.36 | ||
| Individual models for MetS severity and its components (baseline z score and 1-year effect size)† | ||||||||
| MetS severity | 16,949.76 | |||||||
| Baseline | 2.25 (1.97, 2.57) | <0.0001 | 1.46 (1.28, 1.66) | <0.0001 | 1.32 (1.08, 1.62) | 0.0065 | 3,726.96 | |
| 1-Year effect size | 1.80 (1.59, 2.04) | <0.0001 | 1.15 (1.01, 1.30) | 0.0334 | 1.21 (1.01, 1.45) | 0.0363 | ||
| WC | 17,135.20 | 3,731.00 | ||||||
| Baseline | 1.40 (1.30, 1.52) | <0.0001 | 1.12 (1.02, 1.22) | 0.0146 | 1.18 (1.04, 1.34) | 0.0098 | ||
| 1-Year effect size | 1.80 (1.49, 2.17) | <0.0001 | 1.22 (1.01, 1.49) | 0.0412 | 1.17 (0.87, 1.57) | 0.29 | ||
| Glucose | 16,881.32 | 3,732.03 | ||||||
| Baseline | 1.94 (1.81, 2.09) | <0.0001 | 1.41 (1.29, 1.54) | <0.0001 | 1.14 (1.01, 1.29) | 0.0385 | ||
| 1-Year effect size | 1.30 (1.26, 1.34) | <0.0001 | 1.10 (1.03, 1.18) | 0.0078 | 1.07 (1.00, 1.14) | 0.0388 | ||
| HDL | 17,178.38 | 3,728.18 | ||||||
| Baseline | 0.81 (0.74, 0.89) | <0.0001 | 0.85 (0.78, 0.93) | 0.0002 | 0.93 (0.72, 0.95) | 0.0063 | ||
| 1-Year effect size | 0.82 (0.70, 0.96) | 0.0150 | 0.92 (0.79, 1.07) | 0.27 | 0.81 (0.63, 1.03) | 0.0897 | ||
| Triglycerides | 17,184.96 | 3,729.03 | ||||||
| Baseline | 1.20 (1.04, 1.24) | <0.0001 | 1.13 (1.04, 1.24) | 0.0039 | 1.21 (1.06, 1.38) | 0.0043 | ||
| 1-Year effect size | 1.24 (1.10, 1.40) | 0.0004 | 1.07 (0.95, 1.20) | 0.28 | 1.10 (0.91, 1.32) | 0.32 | ||
| SBP | 17,250.30 | 3,717.63 | ||||||
| Baseline | 1.20 (1.10, 1.31) | <0.0001 | 1.11 (1.02, 1.21) | 0.0215 | 1.35 (1.19, 1.53) | <0.0001 | ||
| 1-Year effect size | 1.16 (1.06, 1.28) | 0.0014 | 1.12 (1.02, 1.24) | 0.0177 | 1.16 (1.01, 1.34) | 0.0347 | ||
AIC, Akaike information criterion.
*Proportional hazards assumption violated; Heaviside function at 5 years fit.
**Referent: placebo group.
†Adjusting for intervention; 1-year effect sizes used to allow for comparability across measures.
††AIC provides an estimate of how well a model fits the data, with lower scores reflecting better fit.
Baseline levels of the MetS-Z and each of its components were significantly associated with a risk for T2DM in years 1–5, with an HR of 2.25 per each increasing SD unit of MetS-Z. There were also associations between baseline MetS-Z and each of its components with T2DM after 5 years (HR for MetS-Z = 1.46) and with overall CVD (HR for MetS-Z = 1.32).
In a combined analysis of all participants, adjusted for intervention group, 1-year changes in MetS severity and each of the MetS components were associated with altered risk for T2DM in years 1–5. In the case of MetS severity, each 1-point increase in effect size of MetS-Z between 0 and 1 year resulted in HRs for incident T2DM 1–5 years, T2DM >5 years, and CVD of 1.80, 1.15, and 1.21, respectively. Among the MetS components, this was closely matched by changes in WC, which had HRs for incident T2DM 1–5 years, T2DM >5 years, and CVD of 1.80, 1.22, and 1.17, respectively.
We also assessed a model that included MetS-Z alongside WC and glucose, the two MetS variables most strongly associated with these outcomes in the Cox proportional hazards models and mediation analysis (Supplementary Table 1). In this joint model, 1-year changes in each of these risk indicators (MetS-Z, WC, and glucose) remained significant predictors of incident T2DM at 1–5 years but not incident CVD. The inclusion of other MetS components in the model resulted in excess collinearity.
Mediation Analysis
Because both lifestyle change and metformin treatment were associated with reduced risk of T2DM in years 1–5 relative to the placebo group, we performed mediation analyses to determine the potential role for the MetS-Z and the individual MetS components as potential mediators of this effect (Supplementary Fig. 2 and Table 3). Because of the importance of T2DM as a precursor of CVD, we assessed MetS and its components as potential mediators for both 1–5 year risk for T2DM overall and separately the risk for T2DM with ultimate CVD.
Table 3.
Mediation analysis of lifestyle and metformin interventions (effect on risk of T2DM within 5 years: overall and with/without CVD)
| T2DM ≤5 years** |
T2DM ≤5 years (with CVD during study)† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Excess relative risk (95% CI)†† |
Proportion attributable to intermediate outcome (95% CI)‡ | Excess relative risk (95% CI)†† |
Proportion attributable to intermediate outcome (95% CI)‡ | |||||
| TE | TDE | PIE | TE | TDE | PIE | |||
| Lifestyle intervention mediation analysis | ||||||||
| Intermediate outcomes: 1-year effect size of | ||||||||
| MetS-Z | 0.67 | 0.26 | 0.41 | 61.6% | 1.31 | 0.44 | 0.87 | 66.5% |
| (0.39, 0.95) | (−0.04, 0.56) | (0.25, 0.57) | (29.2%, 94.0%) | (−0.13, 0.08) | (−1.28, 2.15) | (0.05, 1.69) | (0%, 100%) | |
| WC | 0.72 | 0.18 | 0.54 | 75.0% | 1.42 | 0.67 | 0.75 | 52.7% |
| (0.49, 0.95) | (−0.07, 0.43) | (0.36, 0.72) | (45.2%, 100%) | (−0.06, 2.89) | (−1.02, 2.36) | (−0.10, 1.59) | (0%, 100%) | |
| GLU | 0.67 | 0.35 | 0.32 | 48.2% | 1.19 | 0.57 | 0.62 | 52.4% |
| (0.41, 0.92) | (0.08, 0.61) | (0.20, 0.44) | (25.0%, 75.0%) | (−0.22, 2.60) | (−1.00, 2.13) | (0.15, 1.10) | (0%, 100%) | |
| HDL | 0.53 | 0.50 | 0.04 | 6.7% | 1.21 | 1.08 | 0.14 | 11.2% |
| (0.26, 0.81) | (0.22, 0.77) | (−0.00, 0.08) | (0%, 15.1%) | (−0.20, 2.62) | (−0.35, 2.50) | (−0.02, 0.29) | (0%, 29.9%) | |
| TRI | 0.54 | 0.44 | 0.10 | 18.9% | 1.27 | 1.09 | 0.18 | 14.1% |
| (0.27, 0.82) | (0.16, 0.72) | (0.04, 0.16) | (4.4%, 33.3%) | (−0.15, 2.69) | (−0.37, 2.55) | (−0.08, 0.44) | (0%, 40.7%) | |
| SBP | 0.59 | 0.58 | 0.01 | 2.2% | 1.58 | 1.51 | 0.07 | 4.6% |
| (0.31, 0.88) | (0.29, 0.87) | (−0.01, 0.04) | (0%, 6.3%) | (−0.00, 3.17) | (−0.08, 3.10) | (−0.03, 0.18) | (0%, 12.7%) | |
| Metformin mediation analysis | ||||||||
| Intermediate outcomes: 1-year effect size of | ||||||||
| MetS-Z | 0.24 | 0.18 | 0.06 | 25.1% | 0.22 | 0.07 | 0.15 | 68.8% |
| (0.10, 0.38) | (0.05, 0.31) | (0.02, 0.10) | (8.6%, 41.5%) | (−0.39, 0.83) | (−0.56, 0.69) | (0.00, 0.29) | (0%, 100%) | |
| WC | 0.16 | 0.13 | 0.03 | 20.7% | 0.35 | 0.24 | 0.11 | 31.7% |
| (−0.02, 0.34) | (−0.05, 0.31) | (−0.00, 0.07) | (0%, 51.6%) | (−0.33, 1.02) | (−0.44, 0.91) | (−0.01, 0.23) | (0%, 100%) | |
| GLU | 0.20 | 0.09 | 0.11 | 55.2% | 0.32 | 0.12 | 0.20 | 61.0% |
| (0.04, 0.36) | (−0.08, 0.26) | (0.07, 0.16) | (6.0%, 100%) | (−0.37, 1.01) | (−0.48, 0.83) | (0.04, 0.35) | (0%, 100%) | |
| HDL | 0.16 | 0.14 | 0.01 | 9.1% | 0.34 | 0.31 | 0.03 | 9.5% |
| (−0.03, 0.34) | (−0.04, 0.32) | (−0.01, 0.04) | (0%, 27.7%) | (−0.34, 1.02) | (−0.38, 1.00) | (−0.04, 0.10) | (0%, 38.3%) | |
| TRI | 0.15 | 0.15 | −0.01 | 0% | 0.25 | 0.24 | 0.00 | 1.7% |
| (−0.04, 0.33) | (−0.03, 0.33) | (−0.02, 0.01) | (0%, 5.8%) | (−0.39, 0.89) | (−0.40, 0.88) | (−0.03, 0.04) | (0%, 15.4%) | |
| SBP | 0.17 | 0.17 | −0.00 | 0% | 0.05 | 0.08 | −0.03 | 0% |
| (−0.02, 0.35) | (−0.01, 0.35) | (−0.02, 0.01) | (0%, 6.4%) | (−0.44, 0.53) | (−0.40, 0.56) | (−0.15, 0.09) | (0%, 100%) | |
Effect on the risk of T2DM within 5 years: overall and with/without CVD, adjusting for baseline value of corresponding 1-year intermediate outcome (Supplementary Fig. 2). GLU, fasting glucose; TRI, fasting triglycerides.
**Among 2,177 with no disease by 1 year, T2DM developed in 580 within 5 years.
†Among the 1,261 eligible participants, 70 of these participants developed both T2DM within 5 years and CVD in the overall study.
††Excess relative risk of placebo relative to the intervention = relative risk − 1 = [risk (placebo)/risk (intervention)] − 1.
‡Proportion attributable to intermediate outcome = PIE/TE.
Lifestyle Modification
The proportion of reduction in T2DM in the lifestyle intervention attributable to a 1-year change in MetS severity (compared with placebo) was 61.6%. Because of the wide CIs, this was similar overall to the attributable proportion seen for WC (75.0%) and glucose (48.2%).
Metformin Intervention
The proportion of reduction in T2DM in the metformin intervention attributable to the 1-year change in MetS severity (compared with placebo) was 25.1%. Again because of wide CIs, this was similar overall to the attributable proportion seen for glucose (55.2%).
For participants with incident T2DM at 1–5 years in whom CVD also developed (n = 70), neither MetS-Z nor any of the MetS components had a significantly associated attributable proportion, with wide CIs, although MetS-Z, WC, and glucose all had high point estimates for the effect seen in both lifestyle and metformin interventions.
Conclusions
Although risk indicators can be helpful for identifying individuals at high risk for future disease based on baseline measures—in some cases forming the basis for initiating treatment (27)—it has been less clear whether changes in these markers after treatment were an accurate representation of alterations in an individual’s risk (28). In this analysis of participants in the DPP, we found that, with respect to baseline measures, the levels of each of the MetS components as well as a MetS-Z were, not surprisingly, associated with future T2DM and CVD—with baseline MetS-Z and glucose being the most strongly linked to diabetes incidence. However, the degree of change in MetS components and the MetS z score during the first year of lifestyle modification or metformin treatment of prediabetes in the DPP was also associated with a lower risk for future T2DM—with these 1-year changes in MetS severity, glucose level, and SBP also being linked to reduced risk of CVD. Moreover, in mediation analyses, changes in MetS severity and glucose were associated with a significant amount of the effect of both lifestyle modification and metformin treatment on reduced odds of T2DM, with changes in WC being a strong mediator of lifestyle modification. Overall, these data suggest that risk indicators of metabolic disarray such as these—potentially used via an electronic medical record system (29)—can be followed over time to document both baseline risk and subsequent alterations in risk during treatment, providing the potential to identify the effects of treatments earlier, or to motivate patients toward greater adherence and improved outcomes (30).
There has been ongoing debate as to whether MetS as a concept provided added risk prediction beyond its individual components (31) or was worth “no greater than the sum of its parts” (8). Indeed, in analyzing data from the Cardiovascular Health Study, Mozaffarian et al. (9) reported that the dichotomous ATP-III MetS criteria predicted cardiovascular mortality only in those individuals with elevated fasting glucose, diabetes, or hypertension. The MetS z score that we used here serves as a continuous biomarker of the severity of metabolic derangement and in prior studies was associated with future CVD and T2DM, even in models that include the individual MetS components (11,15), with further associations with CVD risk once T2DM has developed (32). Thus, although abnormalities in risk factors such as hyperglycemia, hypertension, and dyslipidemia within a given patient continue to need to be addressed individually, there may be additional risk from processes underlying MetS (e.g., inflammation, oxidative stress, and cellular dysfunction) (4,5) that would benefit from a broader approach such as lifestyle modification. Prior observational data from population-based studies showed that changes in MetS severity in a 3- to 4-year period were associated with alterations in disease risk (33); the current data from a randomized controlled trial of lifestyle modification and metformin help to establish that this risk reduction is also true for treatment-induced changes in MetS severity.
Nevertheless, another finding of these analyses is that although the MetS z score provided the best risk prediction based on baseline values and was a mediator of the effects of metformin, the overall change in WC (as an estimate of visceral obesity) provided overall risk prediction similar to that of the change in MetS-Z. This is not unexpected given the central role that visceral obesity plays in driving insulin resistance and the abnormalities associated with MetS (34). Dysfunction of hypertrophied visceral adipocytes causes an increase in the release of cytokines, chemokines, and free fatty acids, and a decrease in the secretion of adiponectin, all of which ultimately contribute to insulin resistance in other tissues (4,5). These data suggest that a reduction in WC associated with lifestyle changes but not with metformin treatment was a mediator of the reduced risk of future T2DM. Although WC is still not widely measured in clinical care (35), this analysis supports the importance of tracking changes in WC as a simple indicator of risk.
Intervention with metformin (relative to lifestyle modification) produced less dramatic decreases in the 1- to 5-year incidence of T2DM and in MetS severity, with the change in MetS-Z from 0 to 1 year most prominently related to changes in glucose, which itself was a powerful potential mediator of the effect of metformin, although 0- to 1-year changes in glucose overall had a lower HR for the 1- to 5-year risk of T2DM than the seen for MetS-Z. This decrease in MetS-Z with metformin may relate to recent recommendations by the ADA for the consideration of treatment with metformin for some cases of prediabetes (3). This is important because the intensive lifestyle changes attained during the DPP are often difficult to attain in usual clinical practice (36), whereas treatment of prediabetes using metformin is much more easily achieved. As a potential mediator of the effect of metformin and lifestyle changes, MetS-Z could represent a biomarker to track an individual’s response to clinical treatment combining these interventions.
Whereas we assessed 1-year changes in MetS severity and other factors as indicators of risk, the interventions during the DPP continued for at least 2–3 years. That the correlations and potential mediation reported here were present after only 1 year of intervention supports the idea that relatively early clinical follow-up for these factors in individual patients may provide accurate estimates of the ongoing change in risk. Although the effect of these interventions on incident T2DM risk were seen only in the 5 years after treatment initiation, the overall first-year changes in MetS severity and the MetS components remained linked to T2DM beyond 5 years, demonstrating more durable associations with T2DM as an outcome. It also should be noted that the DPP trial was discontinued early after only 3 years because of the impressive decrease in the incidence of T2DM due to the intensive lifestyle intervention (16). As continuous measures associated with the primary outcome, the use of these 1-year changes in MetS-Z, WC, and glucose as surrogate measures of risk could have provided estimates of the need for discontinuation even earlier than performed in the original study.
Reported incident CVD was less common in this study compared with T2DM. Indeed, although the lifestyle intervention resulted in more durable decreases in microvascular outcomes after 15 years of follow-up (37), no effects on macrovascular outcomes have been reported for the interventions. Nevertheless, we noted that these 1-year reductions in MetS-Z (and also glucose) during intervention were associated with lower HRs for subsequent CVD, emphasizing the possible links between early response to therapy and ultimate CVD. In addition, because of the importance of T2DM as a risk factor for CVD (1), we separately assessed changes in these factors as mediators of any effect of intervention on T2DM that subsequently progressed to CVD. Although participant numbers for incident CVD were low and CIs were wide, there appeared to be potential for changes in multiple MetS-related factors to predict reductions in such incidence.
The motivation to change can be difficult, with patients frequently misunderstanding factors that increase their risk for future disease (38). The use of risk-prediction tools such as the Framingham Risk Score may improve patient motivation to change (39), although long-term data are lacking. Additionally, epidemiology-based algorithms that use longitudinal cohort data to derive disease prediction are not designed to follow risk over time, and therefore often lack the ability to reinforce the benefits of treatment over time (28). For example, predicted risk according to the American Heart Association Atherosclerotic CVD Pooled Cohort algorithm increases after the initiation of treatment with a BP-lowering agent because individuals to whom the BP-lowering medications were prescribed in the derivation cohorts were generally of poorer health (28). The use of risk indicators such as those reported here—where progress can be followed as markers of improvements in risk—may help to overcome some difficulties in patients’ understanding of current risk-scoring systems (38).
Although these data reflect changes seen during a well-characterized randomized controlled trial, we recognize that there are limitations to this analysis. The participants in the DPP were fairly advanced in their state of prediabetes, with 29.8% progressing to T2DM over years 1–5. This likely represents a greater progression than seen in individuals with prediabetes as defined by other criteria (e.g., elevated fasting glucose), potentially exaggerating the HRs related to decreased risk of T2DM. We assessed the risk for T2DM in 1–5 years (as the intervention effects on reducing incident T2DM did not persist past that point) (40), which is shorter than the 10-year time frame frequently assessed in many studies. Additionally, we lacked data regarding adjudicated CVD outcomes and instead relied on participant report, which can lead to the overestimation of CVD; in this sense, the implications of the study may be stronger for diabetes than for CVD. Nevertheless, the self-report of events such as MI and stroke from previous studies still has high positive predictive values (19), and the overestimation of incident CVD from misclassification would have been expected to bias us toward the null in relating the MetS severity score to CVD prediction. Finally, these data are from a cohort that was initially recruited in the late 1990s with different clinical practice patterns, and 1-year change in these MetS markers may not be as relevant to clinical care today, limiting generalizability.
In conclusion, we found that during intervention with lifestyle modification and metformin, 1-year changes in MetS severity, glucose, and WC were associated with reductions in risk for future T2DM and/or CVD. These measures had further clinical relevance for T2DM incidence, serving as possible mediators of the effect of lifestyle, with MetS-Z and glucose also being possible mediators of metformin. This was all in addition to significant associations based on baseline values. These factors may serve as important biomarkers of metabolic disarray to identify individuals at highest risk and track the response to treatment with important implications about changes in risk, with potential importance in motivating patients.
Supplementary Material
Article Information
Funding. This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant 1R01-HL-120960 (M.D.D. and M.J.G.). The DPP was conducted by the DPP Research Group and was supported by the NIDDK, the General Clinical Research Center Program, the National Institute of Child Health and Human Development, the National Institute on Aging, the Office of Research on Women’s Health, the Office of Research on Minority Health, the Centers for Disease Control and Prevention, and the ADA. The data from the DPP were supplied by the NIDDK Central Repositories.
This manuscript was not prepared under the auspices of the DPP and does not represent analyses or conclusions of the DPP Research Group, the NIDDK Central Repositories, or the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.D.D. participated in the design of the research and the analysis of the data, was responsible for the write-up of the research, and had primary responsibility for the final content. S.L.F. participated in the design, analysis, and interpretation of the research. M.J.G. participated in the design, analysis, interpretation, and write-up of the research. M.J.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
Clinical trial reg. nos. NCT00004992 and NCT00038727, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1079/-/DC1.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association [published correction appears in Circulation 2017;135:e646; 136:e196]. Circulation 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017. Atlanta, GA, Centers for Disease Control and Prevention, 2017 [Google Scholar]
- 3.American Diabetes Association 5. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S51–S54 [DOI] [PubMed] [Google Scholar]
- 4.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014;371:2237–2238 [DOI] [PubMed] [Google Scholar]
- 5.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition 2013;29:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Daniels SR, et al.; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 7.Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation 2007;115:2316–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn R, Buse J, Ferrannini E, Stern M; American Diabetes Association; European Association for the Study of Diabetes . The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005;28:2289–2304 [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Kamineni A, Prineas RJ, Siscovick DS. Metabolic syndrome and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med 2008;168:969–978 [DOI] [PubMed] [Google Scholar]
- 10.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism 2014;63:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurka MJ, Golden SH, Musani SK, et al. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: the Atherosclerosis Risk In Communities Study and Jackson Heart Study. Diabetologia 2017;60:1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia 2015;58:2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBoer MD, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes 2016;40:1353–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of metabolic syndrome as a predictor of cardiovascular disease between childhood and adulthood: the Princeton Lipid Research Cohort Study. J Am Coll Cardiol 2015;66:755–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBoer MD, Gurka MJ, Golden SH, et al. Independent associations between metabolic syndrome severity and future coronary heart disease by sex and race. J Am Coll Cardiol 2017;69:1204–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Diabetes Prevention Program Research Group The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes [published correction appears in Diabetes Care 1999;22:1389]. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratner R, Goldberg R, Haffner S, et al.; Diabetes Prevention Program Research Group . Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care 2005;28:888–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–1103 [DOI] [PubMed] [Google Scholar]
- 20.Gurka MJ, Ice CL, Sun SS, Deboer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol 2012;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 2008;196:696–703 [DOI] [PubMed] [Google Scholar]
- 22.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-Hispanic black adolescents: an analysis of NHANES 1999-2008. Diabetes Care 2011;34:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the ability of metabolic syndrome criteria to predict elevations in fasting insulin levels in adolescents. J Pediatr 2011;159:975–981.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014;25:749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafeman DM, Schwartz S. Opening the Black Box: a motivation for the assessment of mediation. Int J Epidemiol 2009;38:838–845 [DOI] [PubMed] [Google Scholar]
- 26.Hafeman DM. “Proportion explained”: a causal interpretation for standard measures of indirect effect? Am J Epidemiol 2009;170:1443–1448 [DOI] [PubMed] [Google Scholar]
- 27.Stone NJ, Robinson JG, Lichtenstein AH, et al.; 2013 ACC/AHA Cholesterol Guideline Panel . Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med 2014;160:339–343 [DOI] [PubMed] [Google Scholar]
- 28.Lloyd-Jones DM, Huffman MD, Karmali KN, et al. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among medicare patients: the Million Hearts Longitudinal ASCVD Risk Assessment Tool: a special report from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2017;69:1617–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheitel MR, Kessler ME, Shellum JL, et al. Effect of a novel clinical decision support tool on the efficiency and accuracy of treatment recommendations for cholesterol management. Appl Clin Inform 2017;8:124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamnan P, Simmons RK, Sharp SJ, Griffin SJ, Wareham NJ. Cardiovascular risk assessment scores for people with diabetes: a systematic review. Diabetologia 2009;52:2001–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golden SH, Folsom AR, Coresh J, Sharrett AR, Szklo M, Brancati F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the Atherosclerosis Risk in Communities Study. Diabetes 2002;51:3069–3076 [DOI] [PubMed] [Google Scholar]
- 32.Gurka MJ, Guo Y, Filipp SL, DeBoer MD. Metabolic syndrome severity is significantly associated with future coronary heart disease in type 2 diabetes. Cardiovasc Diabetol 2018;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurka MJ, Filipp SL, Pearson TA, DeBoer MD. Assessing baseline and temporal changes in cardiometabolic risk using metabolic syndrome severity and common risk scores. J Am Heart Assoc 2018;7:e009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887 [DOI] [PubMed] [Google Scholar]
- 35.Bauduceau B, Vachey E, Mayaudon H, et al. Should we have more definitions of metabolic syndrome or simply take waist measurement? Diabetes Metab 2007;33:333–339 [DOI] [PubMed] [Google Scholar]
- 36.Agency for Healthcare Research and Quality 2010 National Healthcare Quality Report; AHRQ Publication No. 11-0004. Rockville, MD, Agency for Healthcare Research and Quality, 2010 [Google Scholar]
- 37.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll C, Naylor E, Marsden P, Dornan T. How do people with type 2 diabetes perceive and respond to cardiovascular risk? Diabet Med 2003;20:355–360 [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Gonzalez AA, Aguilo A, Frontera M, et al. Effectiveness of the Heart Age tool for improving modifiable cardiovascular risk factors in a Southern European population: a randomized trial. Eur J Prev Cardiol 2015;22:389–396 [DOI] [PubMed] [Google Scholar]
- 40.Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study [published correction appears in Lancet 2009;374:2054]. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.