Abstract
OBJECTIVE
Phagocyte-derived myeloperoxidase (MPO) and proinflammatory HDL are associated with metabolic syndrome (MetS) and increased cardiovascular disease risk. Therapeutic lifestyle changes (TLCs), such as a Mediterranean diet and exercise, decrease this risk. However, the link among TLCs, HDL, and MPO-mediated oxidative stress remains unclear.
RESEARCH DESIGN AND METHODS
In this study, we characterized changes in cholesterol efflux capacity (CEC), a metric of HDL function; MPO-mediated oxidation; and the HDL proteomic profile in 25 patients with MetS who underwent 12 weeks of TLCs.
RESULTS
After 12 weeks, before significant changes to HDL levels, most MetS components improved as a result of the TLCs. CEC was significantly increased, and HDL MPO oxidation products, 3-chlorotyrosine and 3-nitrotyrosine, were decreased with TLCs. The changes in CEC were inversely related to the unit changes in 3-chlorotyrosine after we controlled for changes in the other MetS components. TLCs did not remodel the HDL proteome.
CONCLUSIONS
In summary, TLCs improved HDL function by inhibiting MPO-mediated oxidative stress even before appreciable changes in HDL levels.
Introduction
Metabolic syndrome (MetS) comprises a cluster of risk factors that portend diabetes and cardiovascular disease (CVD) (1). In the U.S., one-third of the general population and more than half of individuals >50 years of age have MetS (2). The cardiovascular risk posed by MetS can be ameliorated by lifestyle changes such as weight reduction via aerobic exercise and caloric restriction through a balanced diet (3). Specifically, a Mediterranean diet abundant in phytonutrients with a beneficial lipid pattern of increased polyunsaturated fatty acids and reduced saturated fatty acids reduces the risk of CVD mortality (4).
Atherogenic hypertriglyceridemia and low HDL cholesterol levels are hallmarks of MetS, while low HDL levels are associated with cardiovascular mortality and events (5). The primary function of HDL is to retrieve cholesterol esters from macrophages in atherosclerotic lesions for elimination in the liver, a process also known as cholesterol efflux. Cholesterol efflux capacity (CEC) is inversely associated with the incidence and prevalence of cardiovascular events (6). CEC is impaired in patients with MetS independent of glucose tolerance (7). The mechanistic link between CEC and the improved cardiovascular outcomes associated with lifestyle changes in patients with MetS is unknown.
In addition to dyslipidemia, MetS is associated with chronic inflammation and elevated oxidative stress (8). Myeloperoxidase (MPO) is a heme enzyme produced by neutrophils and macrophages in the circulation and the vascular wall. MPO oxidizes protein-bound tyrosine to form a highly specific product, 3-chlorotyrosine. MPO can also cause nitration of tyrosine residues (9). Elevated MPO levels and activity are associated with cardiovascular events and CVD in the general population (10). We found that MPO-induced changes in HDL impair HDL function in many systemic diseases with increased CVD risk (11,12) and elevated MPO levels and activity are seen in patients with MetS (13). Short-term lifestyle changes, including a high-fiber, low-fat diet and daily exercise, decrease MPO levels and activity (14); however, we do not know how these changes impact the effects of diet, physical activity, and weight reduction on HDL or its function in patients with MetS.
The HDL proteome is especially reactive to inflammatory states and dietary lipid composition (15). Diet and exercise modify the HDL profile to an anti-inflammatory pattern in patients with MetS (14,16,17). Similarly, acute exercise and niacin therapy change levels of specific HDL proteins, such as paraoxonase 1 (PON1) and apolipoprotein (apo)A1, in patients with MetS (18). The comprehensive HDL proteome changes in patients with MetS after physical activity and dietary modifications remain uncharacterized. In this study, we investigated changes in HDL function, MPO-oxidized HDL, and the HDL proteome imparted by 12 weeks of moderate exercise and dietary changes in 25 patients with MetS. Using samples collected after 12 weeks of lifestyle changes, we sought to study the functional HDL changes prior to well-characterized improvements in HDL cholesterol levels that eventually occur at 24 weeks as previously demonstrated by our group (19–21). We found that HDL function improved with a reduction in MPO oxidation in response to lifestyle changes even before changes to plasma levels of HDL.
Research Design and Methods
Study Design and Patient Population
This research was designed as a prospective pilot study that enrolled 25 patients with MetS (19). Subjects with MetS were between the ages of 18 and 65 years, with impaired glucose tolerance or impaired fasting glucose as well as two other components of MetS as defined by the updated National Cholesterol Education Program Adult Treatment Panel III: waist circumference ≥102 cm (40 inches) in men and ≥88 cm (35 inches) in women, triglycerides ≥150 mg/dL (1.7 mmol/L) (patients on drug treatment with fibrates or nicotinic acid were presumed to have triglycerides ≥150 mg/dL and low HDL), HDL cholesterol <40 mg/dL (1.0 mmol/L) in men and <50 mg/dL (1.3 mmol/L) in women, blood pressure ≥130/≥85 mmHg, and fasting glucose ≥100 mg/dL (5.5 mmol/L).
Women of childbearing age were required to use contraception to prevent pregnancy. Nursing mothers, pregnant women, and patients with preexisting CVD were excluded. Further exclusion criteria included hypoxemic lung or heart disease, established diabetes, severe systemic disease with a recognized complication of neuropathy, and significant neurologic disease. Patients taking drugs that interfere with the uptake or metabolism of catecholamines; patients with a known history of chronic kidney disease; patients with significant hepatic disease; patients with a history of previous kidney, pancreas, or cardiac transplantation; and patients who had taken systemic investigational drugs within 6 months were also excluded.
At the screening visit, the investigators obtained a complete medical history, laboratory testing, and routine treadmill exercise testing, followed by stress imaging before enrollment if necessary, to ensure the absence of CVD. The first visit consisted of a complete physical examination with detailed anthropometric parameters and blood collection to measure fasting glucose, insulin levels, and oxidative stress.
Intervention
In this study, the intervention was the completion of 12 weeks of the Metabolic Fitness Program (Met Fit), collectively referred to as therapeutic lifestyle changes (TLCs) (19). Fitness was assessed at baseline in all participants, and this information was used for the exercise component of the program, which included individual tailoring of the intensity and duration of exercise sessions for each participant. Participants had free access to the exercise facilities and participated in a weekly group exercise session with the exercise physiologist and at least three weekly individual sessions at their convenience with the goal of exercising for a minimum of 180 min/week at 85% maximum heart rate. Participants completed weekly exercise logs that listed weekly cardiovascular exercise, physical activity minutes, and strength training; these were reviewed and analyzed by exercise physiologists.
Patients were asked to attend four 45-min interactive group nutrition education sessions and participate in a calorie-controlled and cardioprotective Mediterranean-style eating plan. Nutrition intake was monitored via phone calls and weekly diet logs, and adherence to the Mediterranean diet was assessed using a previously validated Mediterranean Diet Score (MDS) (22). The MDS is a 10-point scale (ranging from 0 to 9) that incorporates the prominent characteristics of the diet; a higher MDS indicates closer adherence to the diet. A clinical evaluation was conducted 12 weeks after initiation of dietary and exercise TLCs, similar to the evaluation conducted for baseline parameters during the first study visit. All subjects signed a written informed consent document at their screening visit, and the University of Michigan’s institutional review board approved the study.
Quantification of Highly Sensitive and Specific Stable Products of Oxidation in HDL
HDL (d = 1.063–1.210 g/mL) was prepared from plasma by sequential ultracentrifugation. Protein concentration was estimated with the Coomassie protein assay reagent (Thermo Scientific, Rockford, IL). HDL proteins were precipitated and delipidated, and oxidized amino acids were isolated by solid-phase extraction from an acid hydrolysate of HDL proteins. Oxidized amino acids were quantified using isotopically labeled internal standards 13C6 tyrosine, 13C6 3-chlorotyrosine, and 13C6 3-nitrotyrosine by liquid chromatography–electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) with multiple reaction monitoring and positive ion acquisition mode. Labeled precursor amino acid 13C915N1 tyrosine was added to monitor potential internal artifact formation of 3-nitrotyrosine and 3-chlorotyrosine and was noted to be negligible (12).
CEC Assessment
J774 murine macrophages were labeled with 2 µCi/mL 3H cholesterol (PerkinElmer, Waltham, MA) for 24 h in the presence of an acyl-CoA cholesterol acyltransferase (ACAT) inhibitor (Sandoz 58-035; Santa Cruz Biotechnology, Dallas, TX) and equilibrated overnight with 0.3 mmol/L 8-(4-chlorophenylthio)-cyclic AMP to induce ATP-binding cassette A1 (ABCA1) expression. ApoB-depleted serum was obtained from polyethylene glycol precipitation and used as an efflux acceptor (2.8% v/v) for 4 h. Efflux was quantified by liquid scintillation and expressed as a percentage of total cell 3H cholesterol content. All assays were performed in duplicate (12). Efflux was also quantified before and after addition of 10 mmol/L 3-amino-1,2,4-triazole (3-AT) (MPO inhibitor) to the plasma.
HDL Proteomic Analysis
HDL protein (50 µg) was precipitated with 10% trichloroacetic acid and solubilized in 6 mol/L urea and digested overnight at 37°C with trypsin (1:20, wt/wt, trypsin/HDL protein). The tryptic digests are acidified with trifluoroacetic acid, dried in vacuum, resuspended in 0.1% formic acid, and desalted with Waters Sep-Pack C18 filter cartridges (Waters Corporation, Milford, MA) before LC-ESI-MS/MS analysis.
Tryptic digests of HDL proteins were chromatographically separated using a nano-capillary reverse phase column (Acclaim PepMap C18, 2 μm, 15 cm; Thermo Scientific, San Jose, CA) using a 0.1% formic acid/acetonitrile gradient at 300 L/min. The eluant was subjected to MS/MS in an Orbitrap Fusion Tribrid mass spectrometer (Thermo Scientific). MS1 scans were acquired at 120,000 resolution. Data-dependent collision-induced dissociation MS/MS spectra were obtained with the top-speed option (3 s) after each MS1 scan. Proteins were identified by searching the data against a Homo sapiens database (UniProtKB) using Proteome Discoverer (version 1.4; Thermo Scientific). Search parameters included MS1 mass tolerance of 10 ppm and fragment tolerance of 0.7 Da. Two missed cleavages were allowed; carbamidomethylation of cysteine was considered a fixed modification. Oxidation of methionine and phosphorylation of serine, threonine, and tyrosine were viewed as potential modifications. The Percolator algorithm was used for discriminating between correct and incorrect spectrum identification. Only proteins with two unique peptides identified and present in at least 70% of the patients in both the analysis groups were included for further analysis.
Statistical Analysis
All variables were represented as the mean ± SD. All differences in variables pre-TLC and post-TLC were compared using paired t tests (after log transformation if the data were not normally distributed), and their relationships were explored with Pearson correlation and multivariate linear regression models using SPSS, version 24 (IBM Corporation, Armonk, NY). A P value of 0.05 was considered significant. Data points that were 1.5 times the interquartile range above the third quartile or below the first quartile were removed from further analysis.
For proteomic analysis, the following steps were undertaken. 1) Proteins with at least two unique peptides that were present in 70% of both groups were considered, and missing values were assigned zero. 2) The spectral counts were Log2 transformed. 3) Quantile normalization of the data was performed. 4) Normality was checked at this point. However, only 10 of the 45 proteins in the pre-TLC data and 11 of the 45 proteins in the post-TLC data were normally distributed (21 of 90 proteins when pre- and post-TLC are combined); hence, a nonparametric paired rank sum test was employed. 5) Proteins with P values ≤0.05 were considered significant.
Results
Demographic and Clinical Characteristics of the Study Population
Twenty-five patients with MetS completed this study: 6 men and 19 women with an average age of 49.2 ± 10.8 years and average glycated hemoglobin A1c of 5.88 ± 0.28%. Medication use included ACE inhibitors (6 of 25), β-blockers (5 of 25), calcium channel blockers (3 of 25), diuretics (7 of 25), and statins (6 of 25) and did not change during the duration of the study. All subjects were nonsmokers. After 12 weeks of adherence to the Met Fit program, patients with MetS showed significant reductions in weight, BMI, waist circumference, fasting insulin, diastolic blood pressure, and triglyceride levels (Table 1). The participants dramatically and significantly increased the amount they exercised weekly as a result of the 12-week intervention (Table 1).
Table 1.
Anthropometric and laboratory characteristics of the study participants
| Pre-TLC | Post-TLC | Difference | P | |
|---|---|---|---|---|
| Weight (kg) | 102.8 ± 16.0 | 100.7 ± 14.9 | −3.4 ± 4.8 | 0.002 |
| BMI (kg/m2) | 36.0 ± 4.8 | 34.8 ± 5.3 | −1.2 ± 2.4 | 0.02 |
| Waist circumference (inches) | 42.8 ± 4.5 | 42.0 ± 4 | −0.74 ± 1.4 | 0.02 |
| Systolic blood pressure (mmHg) | 132.6 ± 13.9 | 129.7 ± 14 | −2.84 ± 15.0 | 0.38 |
| Diastolic blood pressure (mmHg) | 77.2 ± 10.6 | 73.1 ± 9.3 | −4.12 ± 8.5 | 0.02 |
| Triglyceride (mg/dL) | 198.4 ± 82.7 | 165.8 ± 67.2 | −33.0 ± 65.2 | 0.02 |
| HDL cholesterol (mg/dL) | 42.3 ± 10.5 | 43.1 ± 10.6 | −0.8 ± 6.0 | 0.51 |
| LDL cholesterol (mg/dL) | 110.8 ± 37.4 | 107.6 ± 23.8 | −3.2 ± 31.3 | 0.62 |
| Fasting glucose (mg/dL) | 101.2 ± 8.9 | 105.2 ± 8 | 4.62 ± 10.4 | 0.04 |
| Fasting insulin (units) | 31.5 ± 15.5 | 24.5 ± 12.5 | −6.96 ± 14.6 | 0.03 |
| Exercise (h/week) | 0.0 ± 0.0 | 2.28 ± 1.7 | 2.28 ± 1.7 | <0.001 |
| MDS | 6.84 ± 2.1 | 7.28 ± 2.2 | 0.44 ± 2.6 | 0.40 |
Data are means ± SD.
Diet and Exercise Improved CEC in Patients With MetS
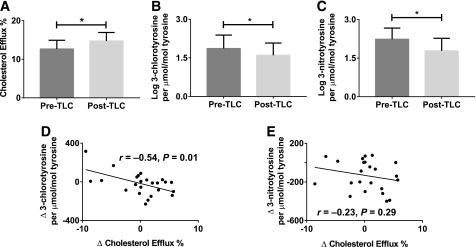
Recent studies suggest that the ability of HDL to accept cholesterol from J774 macrophages better identifies human CVD than measurement of HDL cholesterol levels (23). Therefore, we used an in vitro assay to measure the CEC of apoB-depleted serum from patients with MetS at baseline and after 12 weeks of the dietary and exercise intervention. After the intervention, the patients showed significant improvement in CEC (P < 0.05) (Fig. 1A). We measured CEC with and without MPO inhibitor 3-AT (10 mmol/L) in plasma and found no significant difference in the CEC with and without the addition of 3-AT (n = 12 [data not shown]). These results indicate that MPO inhibition does not alter plasma CEC ex vivo. There was no difference in the change of CEC pre- and post-TLC in the patients who used β-blockers or statins and the rest of the cohort (Supplementary Table 1).
Figure 1.
TLCs improve CEC with reduction of MPO-mediated HDL oxidation in patients with MetS. A: In vitro assay of CEC. B: Quantitation of the MPO oxidation product 3-chlorotyrosine. C: Quantitation of the MPO oxidation product 3-nitrotyrosine. D: Change in CEC with a change in MPO oxidation product 3-chlorotyrosine. E: Change in CEC with change in MPO oxidation product 3-nitrotyrosine. Analyses of the 25 subjects were performed using paired t tests and Pearson correlation analysis (r = correlation coefficient); *P value <0.05.
TLC Decreased MPO-Mediated HDL Oxidation
MPO produces a specific product, 3-chlorotyrosine, and is one of several enzymes that can generate 3-nitrotyrosine. We used a highly sensitive and specific methodology that used LC-ESI-MS/MS to measure these modifications in tyrosine residues of circulating HDL. The levels of 3-chlorotyrosine and 3-nitrotyrosine in HDLs were reduced significantly with 12 weeks of the Mediterranean diet and exercise (P < 0.05) (Fig. 1B and C), indicating decreased MPO activity even before changes in HDL levels are apparent. There was no difference in the change in oxidized tyrosines with TLC in patients on β-blockers or statins versus the rest of the cohort (Supplementary Table 1).
Decreased MPO Activity Was Correlated With Increased CEC
Next, we examined the relationship between changes in CEC and potential predictors of MetS. The changes to specific MPO oxidation product 3-chlorotyrosine were inversely and significantly correlated with changes in CEC (r = −0.54, P = 0.01) (Fig. 1D). This association persisted even after we controlled for changes in 3-nitrotyrosine, HDL cholesterol, weight in kilograms, abdominal circumference, blood pressure, triglycerides, fasting glucose, MDS, and weekly hours of exercise (β = −0.63, SE 0.18, P = 0.002). Changes in 3-nitrotyrosine did not correlate with changes in CEC (r = −0.23, P = 0.29) (Fig. 1E). These results indicated that increases in CEC correlated with decreases in 3-chlorotyrosine, a specific product of MPO, and confirmed a reduction in MPO activity with TLCs.
We performed subgroup analysis by splitting the cohort into two groups: those above and below the mean change in exercise duration (2.3 h/week) and MDS (0.44). As shown in Table 2, there was a strong correlation between change in CEC and 3-chlorotyrosine that was statistically significant in subjects who were above mean in exercise duration (r = −0.63, P = 0.01) and above mean MDS (r = −0.81, P = 0.01). Similar changes were not evident with 3-nitrotyrosine.
Table 2.
More exercise hours and higher MDS reduce MPO-specific HDL oxidation and improve HDL function
| Change in CEC | Change in HDL 3-chlorotyrosine |
Change in HDL 3-nitrotyrosine |
||||
|---|---|---|---|---|---|---|
| n | r | P | n | r | P | |
| Change in exercise in hours above mean | 15 | −0.63 | 0.01 | 14 | −0.03 | 0.92 |
| Change in exercise in hours below mean | 9 | −0.27 | 0.48 | 9 | −0.54 | 0.13 |
| Change in MDS above mean | 11 | −0.81 | 0.01 | 9 | −0.53 | 0.15 |
| Change in MDS below mean | 13 | −0.37 | 0.21 | 14 | 0.02 | 0.94 |
r, Pearson correlation coefficient.
Shotgun Proteomic Analysis Revealed No Changes to HDL Proteome Composition in Patients With MetS After TLCs
Proteins isolated from 23 patients with MetS before and after TLCs were examined using a shotgun proteomics approach. Forty-five HDL proteins were found to be present using stringent criteria including the identification of at least two unique peptides per protein and the presence of the protein in at least 70% of both pre-TLC and post-TLC samples (Table 3 and Supplementary Table 2). The HDL proteome was unaltered with TLCs, suggesting that HDL proteome changes do not significantly contribute to improved HDL function after TLC.
Table 3.
Changes to HDL proteome with TLC in MetS patients
| No. | Protein | Gene | Pre-TLC | Post-TLC | P |
|---|---|---|---|---|---|
| 1 | Actin, cytoplasmic 1 | ACTB | 7.61 ± 5.19 | 5.88 ± 3.53 | 0.46 |
| 2 | α-1-antichymotrypsin | SERPINA3 | 21.82 ± 10.62 | 18.79 ± 11.06 | 0.82 |
| 3 | α-1-antitrypsin | SERPINA1 | 118.76 ± 88.17 | 131.6 ± 92.36 | 0.76 |
| 4 | α-1B-glycoprotein | A1BG | 48.11 ± 26.88 | 49.94 ± 24.11 | 0.52 |
| 5 | α-2-HS-glycoprotein | AHSG | 34.4 ± 19.98 | 36.21 ± 17.21 | 0.86 |
| 6 | α-2-macroglobulin | A2M | 17.31 ± 8.42 | 18.8 ± 21.98 | 0.57 |
| 7 | ApoA-I | APOA1 | 264.48 ± 162.94 | 277.26 ± 169.24 | 1.00 |
| 8 | ApoA-II | APOA2 | 69.78 ± 39.69 | 70.3 ± 35.41 | 0.94 |
| 9 | ApoA-IV | APOA4 | 48.85 ± 26.76 | 54.4 ± 31.96 | 0.69 |
| 10 | ApoB-100 | APOB | 122 ± 102.75 | 162.1 ± 111.76 | 0.88 |
| 11 | ApoC-I | APOC1 | 24 ± 19.61 | 27.55 ± 19.17 | 0.95 |
| 12 | ApoC-II | APOC2 | 19.78 ± 12.54 | 21.04 ± 13.21 | 0.99 |
| 13 | ApoC-III | APOC3 | 31.48 ± 18.41 | 33.57 ± 16.18 | 0.64 |
| 14 | ApoD | APOD | 46.48 ± 22.72 | 55.14 ± 26.86 | 0.88 |
| 15 | ApoE | APOE | 39.04 ± 18.38 | 40.61 ± 20.22 | 0.81 |
| 16 | ApoL1 | APOL1 | 19.37 ± 12.43 | 21.15 ± 13.08 | 0.93 |
| 17 | ApoM | APOM | 19.53 ± 8.73 | 17.7 ± 8.15 | 0.78 |
| 18 | β-2-glycoprotein 1 | APOH | 35.6 ± 22.11 | 38.32 ± 21.83 | 0.73 |
| 19 | Clusterin | CLU | 15.84 ± 6.56 | 15.25 ± 8.52 | 0.72 |
| 20 | Complement C3 | C3 | 62.52 ± 67.14 | 53.4 ± 50.82 | 0.78 |
| 21 | Complement C4-A | C4A | 39.9 ± 31.24 | 38.3 ± 28.07 | 0.98 |
| 22 | Complement C4-B | C4B | 43.55 ± 32.46 | 37.55 ± 27.25 | 0.59 |
| 23 | Fibrinogen α chain | FGA | 34.1 ± 20.45 | 27.9 ± 17.01 | 1.00 |
| 24 | Fibrinogen β chain | FGB | 18.39 ± 13.04 | 15.05 ± 12.38 | 0.78 |
| 25 | Fibrinogen γ chain | FGG | 15.11 ± 10.94 | 14.5 ± 7.56 | 0.65 |
| 26 | Gelsolin | GSN | 23.53 ± 13.29 | 24.18 ± 12 | 0.88 |
| 27 | Haptoglobin | HP | 32.47 ± 25.66 | 32.42 ± 39.45 | 0.54 |
| 28 | Haptoglobin-related protein | HPR | 30.5 ± 19.18 | 31.39 ± 30.05 | 0.56 |
| 29 | Hemopexin | HPX | 63.16 ± 49.57 | 55.79 ± 46.23 | 0.59 |
| 30 | Immunoglobulin heavy constant α 1 | IGHA1 | 19.21 ± 18.25 | 19.94 ± 22.97 | 0.92 |
| 31 | Immunoglobulin heavy constant α 2 | IGHA2 | 7.41 ± 8.84 | 8.56 ± 13.48 | 0.74 |
| 32 | Immunoglobulin heavy constant γ 1 | IGHG1 | 39.05 ± 35.54 | 33.65 ± 41.86 | 0.46 |
| 33 | Immunoglobulin heavy constant γ 2 | IGHG2 | 35.59 ± 39.64 | 25.2 ± 44.26 | 0.75 |
| 34 | Immunoglobulin heavy constant γ 3 | IGHG3 | 28.79 ± 28.21 | 23.8 ± 30.12 | 0.55 |
| 35 | Immunoglobulin heavy constant γ 4 | IGHG4 | 20.22 ± 19.53 | 17.65 ± 25.55 | 0.65 |
| 36 | Immunoglobulin κ constant | IGKC | 31.47 ± 40.57 | 31.42 ± 50.52 | 0.86 |
| 37 | Inter-α-trypsin inhibitor heavy chain | ITIH | 45.83 ± 41.81 | 34.17 ± 38.17 | 0.68 |
| 38 | Phospholipid transfer protein | PLTP | 3.78 ± 1.36 | 4.11 ± 1.24 | 0.88 |
| 39 | Serotransferrin | TF | 118.6 ± 104.19 | 111.32 ± 100.3 | 0.58 |
| 40 | Serum albumin | ALB | 1,092.7 ± 761.58 | 1,037.87 ± 731.77 | 0.36 |
| 41 | Serum amyloid A1 | SAA1 | 9.94 ± 13.5 | 10.33 ± 5.84 | 0.52 |
| 42 | Serum amyloid A4 | SAA4 | 79.78 ± 54.93 | 85.48 ± 59.46 | 0.88 |
| 43 | Serum paraoxonase/arylesterase 1 | PON1 | 20.39 ± 12.91 | 25.16 ± 18.49 | 0.70 |
| 44 | Vitamin D–binding protein | GC | 58.94 ± 31.04 | 62.81 ± 23.92 | 0.83 |
| 45 | Vitronectin | VTN | 10.95 ± 5.15 | 10.79 ± 4.74 | 0.53 |
All peptide spectral matches are represented as the mean ± SD and P value of the paired signal rank test.
Conclusions
In this study, CEC in patients with MetS significantly improved with a 12-week intervention of a Mediterranean diet and exercise. Specific markers of MPO activity decreased significantly with the intervention without changes in HDL levels. CEC improvement was predicted by a decrease in a specific MPO product, 3-chlorotyrosine, even when changes in other MetS components are taken into consideration. These changes occurred despite no significant change in HDL cholesterol levels. The HDL proteome did not show significant changes with TLCs, suggesting that the protein cargo of HDL did not account for the improved HDL function. These dynamic changes in HDL function and oxidation state in patients with MetS with short-term diet and exercise changes prior to changes in HDL cholesterol levels are a key finding of this work. More importantly, this sheds light on the mechanisms behind reducing cardiovascular risk with diet and exercise in patients with MetS.
Our study demonstrated that 12 weeks of a Mediterranean diet and exercise improved the CEC of apoB-depleted plasma significantly. Vasudevan et al. (24) found that plasma CEC was elevated in obese patients with MetS compared with control patients and that weight loss over 4–6 weeks reduced these patients’ CEC to levels seen in control subjects prior to changes in HDL cholesterol levels. Their study differs from ours in that the study participants completed varying degrees of exercise and were placed on a highly restrictive, low-calorie diet. The participants in our study were much leaner at the start of the study, engaged in a consistent level of exercise, and adhered to the Mediterranean diet prescribed. Additionally, the study by Vasudevan et al. measured CEC in whole plasma—in contrast to our work, which measured HDL-specific CEC in apoB-depleted plasma. The earliest efflux observed by Vasudevan et al. was predominantly in the HDL fraction, but cholesterol was also transferred over a 2-h period to the apoB-containing portions. CEC was higher in the LDL and VLDL fractions of patients with MetS, and more closely resembled the CEC of control patients after diet and exercise. The HDL fraction showed decreased efflux before the intervention that improved with intervention, which is similar to the findings in our study. In a study by Wang et al. (25), a 16-week, very low-calorie diet reduced CEC in obese patients with insulin-dependent type 2 diabetes. These changes were associated with decreased liver triglyceride content and cholesterol ester transfer protein (CETP) and increased apoA1 but unchanged HDL cholesterol and phospholipid levels. This study population and intervention differed drastically from our study, which may explain why we observed the opposite effect: HDL-associated efflux that increased with a Mediterranean diet and exercise.
We quantified MPO activity by demonstrating a decrease in the MPO products 3-chlorotyrosine and 3-nitrotyrosine in the HDL fraction after TLCs. The decrease in these products was strongly correlated with improvement in CEC, implying an association between increased exercise and a healthier diet, decreased MPO action, and improved CEC. An earlier study demonstrated that MPO levels decreased after 3 weeks of a low-fat diet and aerobic exercise in 31 obese men, 15 of whom had MetS (14). Similarly, an extended 6-month intervention of caloric restriction and aerobic exercise decreased MPO levels in 25 women with MetS compared with levels in those with no intervention (26). In 15 female patients with MetS, a 15-week exercise intervention decreased MPO activity independent of weight loss (27). In a similar study, plasma MPO activity markers were elevated in 25 patients with MetS compared with control subjects and decreased after a 24-week dietary and exercise intervention (19). A key finding of our work is that both exercise and the diet contribute to improved HDL function. In subgroup analysis, decreases in 3-chlorotyrosine, a specific marker for MPO oxidation, correlated with improved CEC, strongly suggesting that more exercise hours or higher MDS reduce MPO-specific HDL oxidation and improve HDL function. However, substantial statistical power might be lost with this subgroup analysis in our small cohort, which might miss small effect sizes for 3-nitrotyrosine. Finally, our study showed that even moderate weight loss significantly reduced oxidative stress and increased CEC even before improvement in HDL levels.
The use of lipid-modulating drugs like β-blockers and statins did not alter the change in CEC compared with CEC in the group not on these medications. This is consistent with studies that showed that statin therapy did not alter CEC, but the combination with niacin improved CEC (28). Similarly, the use of a β-blocker alone, as shown in prior studies, did not alter CEC in our study (29). Statins are known to decrease MPO activity in plasma, but our study did not detect any difference between the statin and nonstatin groups with TLCs (30). However, substantial statistical power might be lost with subgroup analysis in our small cohort, which might result in missing small effect sizes.
HDL proteome changes were not detected in our study with TLCs, as our study might not have been adequately powered to detect changes with our small sample size. Changes in HDL oxidation with lifestyle modification suggest the possibility of targeting HDL oxidation and function for future studies. The utility of these measurements to serve as surrogate markers for the effectiveness of TLC or adherence to TLC needs to be further explored in larger cohorts. Our study is limited by small sample size but strengthened by individual data on the HDL oxidation products and HDL function. The HDL proteome changes were not significant and may require a larger cohort to identify subtler differences. Shotgun proteomics involves data-dependent acquisition that may not capture differences in nonabundant proteins; thus, a multiple reaction monitoring–based quantitative assay might be a more suitable option once we have identified the requisite targets. We did not, for example, find significant differences in other proteins associated with CVD (e.g., apoE, complement 3, PON1, clusterin, or apoC-IV) with our intervention (31).
In summary, this work revealed an essential role of MPO in modulating CEC in patients with MetS with 12 weeks of TLCs. The study examined the early mechanisms behind improved inflammatory and lipid patterns established with exercise and a healthy diet in patients with MetS. MPO activity has been implicated in HDL dysfunction in MetS, and its reduction with TLCs points to a potential role of MPO in determining CVD risk in this population. Our patients showed no demonstrable change in HDL cholesterol levels, indicating that the mechanism leading to reduced oxidation by MPO is earlier than, and independent of, HDL cholesterol improvement after TLCs. The association of CEC with 3-chlorotyrosine levels confirms a well-known association of MPO activity with CEC, a relationship that leads to improvement in CEC with TLCs. This relationship remained unaffected by changes in any of the other MetS parameters, suggesting that the increase in CEC is unique to this intervention. Improved HDL function before changes to HDL cholesterol levels further supports the hypothesis that HDL cholesterol levels alone do not accurately reflect changes in CVD risk in patients with MetS. Further studies on the relevance of the altered protein profile associated with improvements in CEC may reveal therapeutic targets and answer questions regarding alteration of CEC with MPO activity.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the help provided by Vetalise Cheofor (Division of Nephrology, Department of Internal Medicine, University of Michigan) in performing the experiments.
Funding. This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (P30-DK-081943 and P30-DK-089503), and the National Heart, Lung, and Blood Institute, National Institutes of Health (K08-HL-130944 and R01-HL-129778).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.V.M. designed and conducted the experiments and wrote the manuscript. L.L., J.B., and Y.G. conducted the experiments and reviewed the manuscript. G.M. conducted the statistical analysis and reviewed the manuscript. M.J. coordinated data collection analysis and reviewed the manuscript. Y.E.C. designed the experiments and reviewed the manuscript. R.P.-B. recruited the subjects, designed the study, and reviewed the manuscript. S.P. designed the study and experiments, acquired funding, and wrote the manuscript. S.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00907127, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0049/-/DC1.
References
- 1.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24:683–689 [DOI] [PubMed] [Google Scholar]
- 2.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA 2015;313:1973–1974 [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka K, Tango T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med 2012;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesse-Guyot E, Ahluwalia N, Lassale C, Hercberg S, Fezeu L, Lairon D. Adherence to Mediterranean diet reduces the risk of metabolic syndrome: a 6-year prospective study. Nutr Metab Cardiovasc Dis 2013;23:677–683 [DOI] [PubMed] [Google Scholar]
- 5.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707–714 [DOI] [PubMed] [Google Scholar]
- 6.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371:2383–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annema W, Dikkers A, de Boer JF, et al. Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: the CODAM study. Sci Rep 2016;6:27367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab 2013;39:99–110 [DOI] [PubMed] [Google Scholar]
- 9.Hazen SL, Hsu FF, Gaut JP, Crowley JR, Heinecke JW. Modification of proteins and lipids by myeloperoxidase. Methods Enzymol 1999;300:88–105 [DOI] [PubMed] [Google Scholar]
- 10.Bergt C, Pennathur S, Fu X, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A 2004;101:13032–13037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaisar T, Tang C, Babenko I, et al. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res 2015;56:1519–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivekanandan-Giri A, Slocum JL, Byun J, et al. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis 2013;72:1725–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Fonseca LJ, Nunes-Souza V, Guedes Gda S, Schettino-Silva G, Mota-Gomes MA, Rabelo LA. Oxidative status imbalance in patients with metabolic syndrome: role of the myeloperoxidase/hydrogen peroxide axis. Oxid Med Cell Longev 2014;2014:898501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts CK, Won D, Pruthi S, et al. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol (1985) 2006;100:1657–1665 [DOI] [PubMed] [Google Scholar]
- 15.O’Reilly M, Dillon E, Guo W, et al. High-density lipoprotein proteomic composition, and not efflux capacity, reflects differential modulation of reverse cholesterol transport by saturated and monounsaturated fat diets. Circulation 2016;133:1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rector RS, Warner SO, Liu Y, et al. Exercise and diet induced weight loss improves measures of oxidative stress and insulin sensitivity in adults with characteristics of the metabolic syndrome. Am J Physiol Endocrinol Metab 2007;293:E500–E506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sang H, Yao S, Zhang L, et al. Walk-run training improves the anti-inflammation properties of high-density lipoprotein in patients with metabolic syndrome. J Clin Endocrinol Metab 2015;100:870–879 [DOI] [PubMed] [Google Scholar]
- 18.Taylor JK, Plaisance EP, Mahurin AJ, Mestek ML, Moncada-Jimenez J, Grandjean PW. Paraoxonase responses to exercise and niacin therapy in men with metabolic syndrome. Redox Rep 2015;20:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennathur S, Jaiswal M, Vivekanandan-Giri A, et al. Structured lifestyle intervention in patients with the metabolic syndrome mitigates oxidative stress but fails to improve measures of cardiovascular autonomic neuropathy. J Diabetes Complications 2017;31:1437–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casella-Filho A, Chagas AC, Maranhão RC, et al. Effect of exercise training on plasma levels and functional properties of high-density lipoprotein cholesterol in the metabolic syndrome. Am J Cardiol 2011;107:1168–1172 [DOI] [PubMed] [Google Scholar]
- 21.Thompson PD, Cullinane EM, Sady SP, et al. Modest changes in high-density lipoprotein concentration and metabolism with prolonged exercise training. Circulation 1988;78:25–34 [DOI] [PubMed] [Google Scholar]
- 22.Rumawas ME, Dwyer JT, McKeown NM, Meigs JB, Rogers G, Jacques PF. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J Nutr 2009;139:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasudevan M, Tchoua U, Gillard BK, Jones PH, Ballantyne CM, Pownall HJ. Modest diet-induced weight loss reduces macrophage cholesterol efflux to plasma of patients with metabolic syndrome. J Clin Lipidol 2013;7:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Snel M, Jonker JT, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases plasma CETP and increases apolipoprotein AI levels without improving the cholesterol efflux properties of HDL. Diabetes Care 2011;34:2576–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh EG, Bang SY, Kim SH, et al. Therapeutic lifestyle modification program reduces plasma levels of the chemokines CRP and MCP-1 in subjects with metabolic syndrome. Biol Res Nurs 2013;15:48–55 [DOI] [PubMed] [Google Scholar]
- 27.Farinha JB, Dos Santos DL, Bresciani G, et al. Weight loss is not mandatory for exercise-induced effects on health indices in females with metabolic syndrome. Biol Sport 2015;32:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronsein GE, Hutchins PM, Isquith D, Vaisar T, Zhao XQ, Heinecke JW. Niacin therapy increases high-density lipoprotein particles and total cholesterol efflux capacity but not ABCA1-specific cholesterol efflux in statin-treated subjects. Arterioscler Thromb Vasc Biol 2016;36:404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesnik P, Dachet C, Petit L, et al. Impact of a combination of a calcium antagonist and a beta-blocker on cell- and copper-mediated oxidation of LDL and on the accumulation and efflux of cholesterol in human macrophages and murine J774 cells. Arterioscler Thromb Vasc Biol 1997;17:979–988 [DOI] [PubMed] [Google Scholar]
- 30.Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003;108:426–431 [DOI] [PubMed] [Google Scholar]
- 31.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 2007;117:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.