Abstract
OBJECTIVE
Type B insulin resistance due to autoantibodies against the insulin receptor is characterized by diabetes refractory to massive doses of insulin, severe hypercatabolism, hyperandrogenism, and a high mortality rate. We analyzed the efficacy of combined immunosuppressive therapy in the management of this extreme form of diabetes.
RESEARCH DESIGN AND METHODS
We performed a prospective cohort study including patients with confirmed insulin receptor autoantibodies, monitored for median 72 months (25th, 75th interquartile range 25, 88), and treated with rituximab, high-dose pulsed steroids, and cyclophosphamide until remission, followed by maintenance therapy with azathioprine. Remission was defined as the amelioration of the hyperglycemia and discontinuation of insulin and/or normalization of hyperandrogenemia.
RESULTS
All data are given as median (25th, 75th interquartile range). Twenty-two patients aged 42 (25, 57) years, 86.4% women, fulfilled inclusion criteria. At baseline, fasting glucose was 307 (203, 398) mg/dL, HbA1c was 11.8% (9.7, 13.6), total testosterone (women) was 126 (57, 571) ng/dL (normal 8–60), and daily insulin requirement was 1,775 (863, 2,700) units. After 5 (4, 6.3) months, 86.4% (19 of 22) of patients achieved remission, documented by discontinuation of insulin in all patients, normal fasting glucose of 80 (76, 92) mg/dL, HbA1c of 5.5% (5.2, 6), and testosterone (women) of 28 (20, 47) ng/dL. During follow-up of 72 (25, 88) months, 13.6% (3 of 22) of patients developed disease recurrence, occurring 24 (22, 36) months after initial remission, which responded to repeated therapy. None of the patients died.
CONCLUSIONS
Combined immunosuppressive therapy has changed the natural history of this disease, from 54% mortality to a curable form of diabetes and, as such, should be recommended in patients with type B insulin resistance.
Introduction
Type B insulin resistance is a very rare autoimmune disorder caused by a highly specific polyclonal autoantibody against the cell surface insulin receptor. It was first described at the National Institutes of Health (NIH) in a series of publications from 1975 to 1976 (1–3). The autoantibody acts as a partial agonist. At low concentration it elicits a hypoglycemic response, whereas at higher titers, it chronically decreases the cellular response to insulin, resulting in refractory hyperglycemia (4–6). Mortality in type B insulin resistance is as high as 54%, largely related to hypoglycemia (7).
The exact prevalence of type B insulin resistance is unknown, as epidemiologic data are based predominantly on case reports and case series. To the best of our knowledge, to date, only 104 cases of type B insulin resistance have been reported in the literature (7–20). Type B insulin resistance is most commonly observed in women and in African Americans, followed by Asians and Caucasians (20). Affected patients typically present with a hypercatabolic state with dramatic weight loss, hyperglycemia with or without ketoacidosis, and unusually widespread acanthosis nigricans. Less common presentations include hypoglycemia or virilization in women (21,22). The syndrome usually occurs in patients with a background of a rheumatologic illnesses, such as lupus erythematosus, Sjogren disease, or mixed connective tissue disease, but may also occur as a paraneoplastic manifestation of lymphoma or multiple myeloma (7,23,24). The biochemical signature of type B insulin resistance includes markedly elevated fasting insulin concentrations with high insulin–to–C-peptide ratio, hyperadiponectinemia, and low/normal fasting triglyceride concentrations with normal to increased HDL cholesterol (25,26).
The goals of therapy for type B insulin resistance are to 1) reverse the hypercatabolic state, usually with high doses of insulin, and 2) eliminate the autoantibodies with immunosuppressive therapy (25). Elimination of autoantibodies has been attempted using plasmapheresis, plasma exchange, and intravenous Ig (IVIG) (20,27), or with immunosuppressive agents, including mycophenolate mofetil, cyclophosphamide, cyclosporine, azathioprine, and glucocorticoids, without consistent clinical benefit (7,17,25,28–30).
We previously reported initial success of targeted combination therapy with immunosuppressive agents in seven patients with type B insulin resistance (9). This treatment regimen was designed to eliminate the autoantibodies by targeting CD20 B cells with rituximab, decreasing preformed plasma cells using high-dose pulsed steroids, and nonspecific T-cell– and B-cell–directed immunosuppression with cyclophosphamide or cyclosporine. This therapeutic regimen has been proven successful in other autoimmune disorders, such as immune cytopenias associated with chronic lymphocytic leukemia (31), as well as lymphoproliferative disorders such as Waldenström macroglobulinemia (32,33), chronic lymphocytic leukemia (34), and monoclonal Ig deposit–related glomerulopathy (35).
Once clinical remission was achieved, patients received maintenance therapy with azathioprine (9). In the current study, we report continued efficacy of this treatment regimen in a prospective cohort of 22 patients with type B insulin resistance.
Research Design and Methods
We performed a prospective cohort study of patients with type B insulin resistance monitored at the NIH between March 2006 and February 2018. The National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board approved this study (NCT00001987). All patients or their guardians provided written informed consent, and minors provided written assent. Eligible patients had a clinical diagnosis of type B insulin resistance based on classic signs and symptoms, including abrupt onset of extreme insulin resistance or hypoglycemia in the context of a rheumatologic disease or malignancy. In all patients, diagnosis was confirmed by the presence of insulin receptor antibodies.
Laboratory Tests
Patients underwent routine hematological, immunological, and biochemical testing after an overnight fast. Glucose and lipid values were determined by a Vista analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). Insulin was analyzed by a chemiluminescence immunoassay on a Siemens Immulite 2500 analyzer. HbA1c was measured by high-performance liquid chromatography. Complete blood count and differential was determined by Cell-Dyn Sapphire (Abbott Diagnostics, Santa Clara, CA). Total testosterone was measured by chemiluminescence immunoassay on a Siemens Immulite 2500 analyzer. The presence of the insulin receptor antibody was confirmed by measuring the ability of patients’ sera to immunoprecipitate recombinant human insulin receptors, as described previously (9). Titers were based on semiquantitative assessment of Western blot band intensities compared with a positive control and were scored by one observer (R.K.S.).
Treatment
Hyperglycemia Management
Patients were treated with regular or concentrated U-500 insulin and insulin sensitizers (metformin), targeting reversal of the hypercatabolic state and preventing diabetic ketoacidosis rather than aiming at achieving euglycemia.
Combined Immunosuppressive Therapy
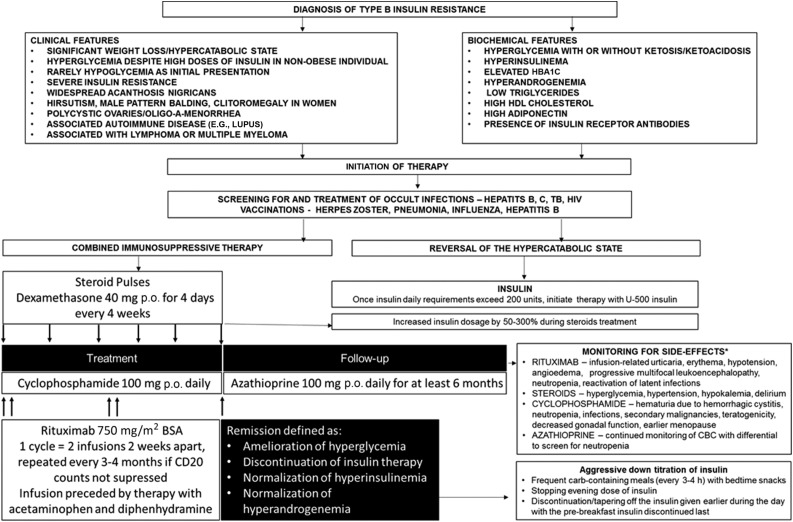
One cycle of rituximab consisted of two doses 2 weeks apart administered at the dose of 750 mg/m2 body surface area. Rituximab cycles were repeated every 3–4 months if remission was not achieved and CD19 counts were not suppressed. Before rituximab infusions, patients received pretreatment with acetaminophen and diphenhydramine to prevent infusion-related reactions. Cyclophosphamide was administered in a dose of 100 mg p.o. daily continuously, until remission was achieved, with doses reduced as needed to avoid neutropenia (absolute neutrophil count <1,000/μL). Glucocorticoids were administered as dexamethasone (40 mg p.o. daily) for 4 days or methylprednisolone (1 g intravenously) for 2 days, repeated after 2 weeks, then every 3–4 weeks until remission was achieved. Once patients achieved remission, maintenance therapy with azathioprine (100 mg daily) was administered for at least 6 months, and the patients were monitored every 6–24 months. If cyclophosphamide or azathioprine was not tolerated, the patients received cyclosporine. The maintenance therapy was introduced based on our historical cohort experience (7), documenting that 8 of 13 deaths occurred in patients who initially obtained remission from either hyper- or hypoglycemia. We therefore hypothesized that continued immunosuppression leading to continued decrement/disappearance of insulin receptor antibodies might be beneficial in this group of patients. The management strategy is depicted in Fig. 1.
Figure 1.
Summary of the management of type B insulin resistance. *The diagram includes the most common adverse side effects, but the spectrum of side effects is not limited to the ones listed. BSA, body surface area; CBC, complete blood count; TB, tuberculosis.
Primary Outcomes
Remission was defined as the amelioration of the hyperglycemia and discontinuation of insulin therapy and/or normalization of hyperandrogenemia.
Statistical Analysis
The baseline demographics and clinical characteristics are summarized using median (25th, 75th interquartile range [IQR]) because some of the continuous variables were not normally distributed. Time to remission was calculated from the date of implementation of therapy until the date of the evidence of remission. The duration of follow-up was calculated from the date of initiation of therapy until the date of the last follow-up evaluation. Clinical and biochemical characteristics before and after treatment were compared using Kruskal-Wallis tests for continuous variables and Fisher exact tests for categorical variables. All analyses were two-tailed tests based on α = 0.05 and conducted using RStudio 3.3.2 software.
Results
Twenty-two patients fulfilled inclusion criteria. Of note, seven patients who were previously reported were also included in this analysis with a longer duration of follow-up data (9). Fasting hyperglycemia was present in 21 of 22 patients, and 1 patient (B35), a 17-year-old African American woman, presented with fasting normoglycemia of 71 mg/dL but with significant hyperandrogenemia, with a testosterone level of 686 ng/dL. None of the included patients had hypoglycemia at initial presentation. Baseline characteristics of the patients are reported in Table 1. The median age at diagnosis was 42 (25, 57) years. Of the enrolled patients, 86% (19 of 22) were women, predominantly of African American ethnicity (86.4% [19 of 22]), (Table 1). Three men included in the study were characterized by significantly older age at presentation than women (63 [62.5–63.5] vs. 33 [22.5–50.5] years; P < 0.0001, respectively).
Table 1.
Baseline characteristics of patients enrolled in the study
| Patients’ characteristics | Baseline data (N = 22) |
|---|---|
| Age, years | 42 (25, 57) |
| Sex | |
| Female | 19 (86.4) |
| Male | 3 (13.6) |
| Ethnicity | |
| African American | 19 (86.4) |
| Caucasian | 1 (4.5) |
| Native American | 1 (4.5) |
| Hispanic | 1 (4.5) |
| Underlying disorder | |
| Lupus | 9 (40.9) |
| Mixed connective tissue disease | 7 (31.8) |
| Sjogren disease | 2 (9.1) |
| Large B-cell lymphoma | 1 (4.6) |
| Undetermined | 3 (13.6) |
Data are presented as median (25th, 75th IQR) or as n (%).
The most common underlying autoimmune diseases were lupus (40.9%) and mixed connective tissue disease (31.8%). In one patient, type B insulin resistance was associated with lymphoma.
Patients were monitored for 72 (25, 88) months. Remission was achieved in 19 of the 22 patients (86.4%) after 5 (4, 6.3) months of therapy comprising a median of 1 (1, 1.5) cycle of rituximab, 5 (2, 6) steroid pulses, and 6 (2.5, 12) months of cyclophosphamide therapy (Table 2). Among three patients who did not achieve remission, one was a 44-year-old African American woman with lupus, who was lost to follow-up after one cycle of rituximab, four steroid pulses, and 5 months of cyclophosphamide therapy; the second patient was a 52-year-old Hispanic woman with lupus, who withdrew from the study after one infusion of rituximab (half of a cycle), one pulse of steroids, and 2 weeks therapy with cyclophosphamide; and the third patient was a 74-year-old Caucasian woman with a history of large B-cell lymphoma, who underwent one cycle of rituximab and two steroid pulses, and was monitored for only 2 months at the time of writing.
Table 2.
Number of treatment cycles and response to therapy
| Data (N = 22) | |
|---|---|
| Remission rate | 19 (86.4) |
| Time to remission, months | 5 (4, 6.3) |
| Rituximab cycles, n* | 1 (1, 1.5) |
| Steroid pulses, n | 5 (2, 6) |
| Duration of therapy, months | |
| With cyclophosphamide | 6 (2.5, 12) |
| With azathioprine | 12 (6, 18) |
| Recurrence rate | 3 (13.6) |
| Death rate | 0 (0) |
Data are presented as median (25th, 75th IQR) or as n (%).
*One cycle consisted of two infusions 2 weeks apart.
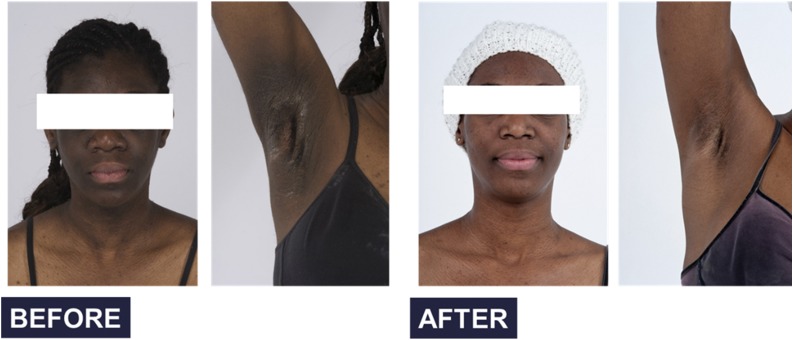
The combined targeted immunotherapy resulted in significant clinical improvement, including reversal of clinical signs and symptoms of hyperandrogenemia and diffuse acanthosis nigricans (Fig. 2), discontinuation of insulin, reversal of the hypercatabolic state, and significant improvement or normalization of biochemical parameters, including fasting glucose, HbA1c, and testosterone in women (Table 3). The median testosterone level in men was 635 (453.5, 789.5) ng/dL, with a reference range for men of 181–758 ng/dL. The upper limit of normal testosterone was exceeded in one of three included men, whose testosterone concentration was 944 ng/dL. His testosterone level decreased to 633 ng/dL after the treatment. Average testosterone levels in men are provided in Table 3.
Figure 2.
Resolution of acanthosis nigricans after the combined targeted immunosuppressive therapy.
Table 3.
Biochemical parameters before and after combined targeted immunosuppressive therapy
| Pretherapy | Posttherapy | P value | |
|---|---|---|---|
| Median (25th, 75th IQR) | Median (25th, 75th IQR) | ||
| Fasting glucose (mg/dL) | 307 (203, 397.8) | 79.5 (75.7, 92) | <0.0001* |
| HbA1c (%) | 11.8 (9.7, 13.6) | 5.5 (5.2, 6) | <0.0001* |
| Insulin dose (units/day) | 1,775 (863, 2,700) | 0 | 0.007* |
| Testosterone (ng/dL) | |||
| Women | 126 (57, 571) | 28 (20, 47) | 0.013* |
| Men | 635 (453.5, 789.5) | 499 (432, 566) | 0.04* |
| Triglycerides (mg/dL) | 58 (42, 73) | 55 (43, 81) | 0.87 |
| Total cholesterol (mg/dL) | 129 (119, 145) | 152 (133, 156) | 0.28 |
| LDL cholesterol (mg/dL) | 59.5 (46, 78) | 72 (56, 91) | 0.17 |
| HDL cholesterol (mg/dL) | 58.5 (55, 73) | 60 (47, 79) | 0.66 |
There was normalization of fasting glycemia, significant reduction of HbA1c, reduction of daily insulin dosage to 0 units/day, and normalization of testosterone levels. There were no significant effects on lipids panel. Reference values for androgens in women: testosterone premenopausal women <81 ng/dL, postmenopausal women <63 ng/dL.
*Statistically significant (P < 0.05).
The clinical improvement usually preceded the biochemical remission. Specifically, the reversal of the hypercatabolic state led to a prompt improvement in patients’ clinical status.
During follow-up, three patients had disease relapse with milder manifestations compared with initial presentation. In patient B32, relapse presented as hyperinsulinemia in the context of a lupus flare, which responded to cyclophosphamide only. Patient B42 was previously reported (36). She had a spontaneous remission after her first presentation lasting 22 months, followed by relapse with hyperglycemia, hyperinsulinemia, and hyperandrogenemia, which responded to combined immunosuppressive therapy. Maintenance therapy with azathioprine was not implemented due to low neutrophil count and lack of follow-up. She maintained a second remission lasting for 24 months, then relapsed with hyperinsulinemia only with euglycemia, and was successfully managed with maintenance azathioprine. Patient B38, a 21-year-old African American woman with mixed connective tissue disease, presented initially with generalized acanthosis, weight loss, severe hyperglycemia with hyperinsulinemia, and hyperandrogenemia with a testosterone level of 334 ng/dL. She remitted after 2.5 months of combined immunosuppressive therapy, and remission was maintained for 20 months, when relapse with hyperglycemia and hyperinsulinemia occurred. At that time, given a low-normal CD20 lymphocyte count, she was treated only with cyclophosphamide and steroid pulses, without rituximab, with remission achieved after 1 month of therapy. A second relapse consisting of hyperglycemia and hyperinsulinemia occurred 51 months later and was successfully treated with rituximab, cyclophosphamide, and high-dose steroid pulses. This patient received a high cumulative dose of cyclophosphamide of 31,700 mg, which resulted in persistent leukopenia and reactivation of latent herpes zoster infection.
The efficacy of combination immunosuppression in this cohort was compared with a historical control cohort of 29 patients treated at the NIH in the past with plasmapheresis or single-agent immunosuppression, including cyclophosphamide, cyclosporine, azathioprine, or prednisone. There were no differences in the distribution of underlying autoimmune disease or malignancy between the current and the historical cohorts (P = 0.68). Combination immunosuppressive therapy resulted in a significantly higher remission rate of 86.4% (19 of 22) vs. 41.4% (12 of 29) (P = 0.001). Remission was achieved after a significantly shorter treatment duration with combination immunosuppressive therapy of 5 (4, 6.3) months vs. 30 (10, 44) months with single-agent therapy (P = 0.004). Most importantly, combined immunosuppressive therapy was associated with 0% mortality vs. 44.8% (13 of 29) mortality in the cohort treated with single-agent therapy (P = 0.0002), monitored for similar period of time (P = 0.27).
Adverse Effects of Therapy
No patients experienced infusion-related reactions with rituximab. We did not observe any cases of progressive multifocal leukoencephalopathy after rituximab. Two patients required therapy with lamivudine to prevent reactivation of latent viral hepatitis, and one patient was administered isoniazid to treat latent tuberculosis.
Cyclophosphamide can cause hemorrhagic cystitis. Gross hematuria during cyclophosphamide treatment occurred in one patient in our cohort, which persisted after discontinuation of cyclophosphamide; this patient was subsequently lost to follow-up. Patients with type B insulin resistance may be relatively protected from this adverse effect by hyperglycemia-induced polyuria. Neutropenia with absolute neutrophil count (ANC) <1,500 K/μL was observed in eight patients (38.1%), including one patient with severe neutropenia with an ANC of <500 K/μL who required cessation of cyclophosphamide therapy and treatment with granulocyte-colony stimulating factor. One patient developed lung cancer years after completion of therapy for type B insulin resistance. His cumulative cyclophosphamide dosage was 155,000 mg; however, the association of the lung cancer with cyclophosphamide exposure is unclear because the patient had a history of heavy smoking, with an exposure history of 60 pack-years (37).
The pulsed steroid cycles resulted in transient worsening of hyperglycemia in all patients, but insulin doses were adjusted accordingly. In most patients, insulin doses were increased by 50–100% during steroid treatment. One patient required an insulin drip (up to 24,000 units/day, a 300% increase from the predexamethasone insulin dose) to control diabetic ketoacidosis during the first pulsed dexamethasone treatment. With decreasing antibody titers, all patients experienced hypoglycemia episodes without neuroglycopenia, leading to a significant reduction of insulin doses. Due to the prolonged half-life of insulin in this condition, resulting from impaired receptor-mediated degradation, the evening doses of insulin were tapered first to prevent nocturnal hypoglycemia, followed by tapering of afternoon, then morning doses.
Conclusions
In the current study, we confirm our preliminary findings that combination immunotherapy is highly effective in inducing remission of type B insulin resistance. This is the largest prospective cohort study aimed at optimization of therapy for this rare form of difficult-to-control diabetes. The 22 analyzed patients, treated uniformly with combined targeted immunotherapy with rituximab, cyclophosphamide, and high-dose steroid pulses, were compared with our historical cohort of 29 patients, who had a similar duration of follow-up (7,9). We found significantly improved efficacy using the current treatment approach, with induction of remission in 86% of patients undergoing combined immunosuppressive therapy compared with 41% in historical controls. Importantly, we found a significant reduction in mortality—from 54% in the initial NIH report to 0% in the current study (7).
Most published data on the efficacy of different therapeutic regimens in patients with type B insulin resistance derive from case reports. Prior treatment approaches reported to induce remission have included high-dose glucocorticoids and combination of intravenous methylprednisolone with cyclophosphamide in the most severe cases (13). IVIG with high-dose cyclophosphamide for 6 months was reported to induce remission in one patient; remission was maintained for 2 years with leflunomide maintenance therapy (20). In another patient, IVIG was reported to decrease insulin requirements after just 1 day of exposure; the response lasted for 14 days and repeated IVIG was needed to maintain euglycemia (38). There is no information on whether the remission in this patient was long-lasting. Plasmapheresis, which allows rapid mechanical removal of antibodies, has been used in ∼10 patients, with variable and short-lasting responses. Although effects of plasmapheresis are short-lived, it may be beneficial in patients with extremely high insulin receptor antibodies titers, followed by treatment with other immunosuppressive agents such as cyclophosphamide or cyclosporine (27,39).
Rituximab, an anti-CD20 monoclonal antibody, has been used as monotherapy or combined with other agents in type B insulin resistance (10,11,17,18). On one hand, Coll et al. (30) presented a patient who failed to respond to plasmapheresis, methylprednisolone, methotrexate, and mycophenolate mofetil but responded to rituximab infusion. On the other hand, Takei et al. (16) reported efficacy of high-dose steroids combined with cyclosporine in a rituximab-refractory patient. Of note, all of these reports may be subject to significant publication bias, with reporting solely of patients with good response to therapy and not presenting therapeutic failures.
Moreover, patients with type B insulin resistance may have spontaneous remissions of their disease; thus, some published cases may possibly represent spontaneous remission rather than a true response to therapy (7,36). Although the current study is not a randomized controlled trial, we have clearly demonstrated that compared with historical controls, combination therapy is associated with higher remission rates, shorter time to remission, and reduced mortality. However, spontaneous improvement, unrelated to therapy, could possibly have occurred in a subset of patients reported in our study. It is worthwhile to speculate that the therapeutic regimen we have proposed might be effective in other disorders in which pathology is induced by cell-surface receptor antibodies; for example, Graves disease, characterized by thyroid-stimulating hormone receptor antibodies, or myasthenia gravis with acetylcholine receptor antibodies.
Of note, one common cause of death in the historical cohort was hypoglycemia. It is now known that autoantibodies against the insulin receptors act as partial agonists; thus, low titers stimulate insulin signaling, leading to hypoglycemia. Unfortunately, serological confirmation and quantification of the antibodies against the insulin receptor is not commercially available but can be performed in several research laboratories worldwide. Our patients were managed with diets consisting of frequent carbohydrate-containing meals, nighttime snacks, and continued immunosuppression with a maintenance dose of azathioprine administered for a median of 12 months after remission. Rapid tapering of high-dose exogenous insulin was required once insulin sensitivity started to improve. Because insulin is cleared through receptor-mediated degradation, clearance of endogenous and exogenous insulin is impaired in patients with autoantibodies to the insulin receptor. This results in a high risk of nocturnal hypoglycemia, as insulin secreted or exogenously administered during the day continues to act during the night when patients are not consuming oral carbohydrates. To reduce this risk, bedtime doses of insulin were tapered or discontinued once patients’ fasting blood glucose levels approached the normal range, followed by tapering or discontinuation of insulin doses given earlier in the day as insulin resistance continued to improve. We speculate that this approach, combined with continued immunosuppressive therapy with azathioprine to maintain remission, led to a minimized likelihood of severe hypoglycemia with neuroglycopenia compared with the one observed in our historical cohort (7).
The patients required screening for other potential side effects of the combined immunotherapy. Rituximab, cyclophosphamide, and maintenance dose of azathioprine can all cause neutropenia, leading to higher likelihood of severe infections (40,41). In our cohort, neutropenia with ANC <1,500 K/μL was observed in as many as 38.1% of patients. Therefore, obtaining baseline and sequential white blood cell counts with differential during therapy and appropriately educating the patients about signs and symptoms of agranulocytosis is important. Before immunosuppressive therapy was initiated, every patient was screened for potential latent infections, such as tuberculosis, hepatitis B and C, and HIV, which led to a diagnosis of latent hepatitis, requiring lamivudine therapy in two patients, and latent tuberculosis requiring treatment with isoniazid in one patient. Meticulous clinical investigation for signs and symptoms of infection is particularly important in this group of patients, in whom immunosuppression and hyperglycemia create a permissive environment for serious viral-, bacterial-, or fungal-induced complications. One of the very rare but detrimental complications of treatment with rituximab is progressive multifocal leukoencephalopathy (40,41). To our knowledge, there are no data in the literature describing this complication in patients suffering from type B insulin resistance. More common adverse effects of rituximab are infusion-related reactions, such as urticaria and edema, occurring in 1–3% of patients, with most serious complications being angioedema and hypotension (41). We pretreated all patients with acetaminophen and diphenhydramine and did not observe any infusion-related adverse effects.
Hemorrhagic cystitis, due to acrolein deposition in the bladder, is a well-known complication of therapy with cyclophosphamide, which occurs in ∼10–15% of patients requiring it for therapy of autoimmune diseases or myeloablative conditioning (42,43). In our cohort, this complication developed only in one patient, suggesting that likely due to hyperglycemia-induced hyperfiltration, type B insulin resistant patients might be protected from this complication. Therapy with cyclophosphamide has been also associated with an increased risk of secondary malignancies, such as bladder cancer, squamous cell carcinoma of the vagina, and squamous cell skin cancer, with conflicting reports on its association with adenocarcinoma of the lungs (37,43). We observed one case of lung cancer in our cohort; however, its association with exposure to cyclophosphamide is uncertain, as the patient was a heavy smoker with an exposure history of 60 pack-years.
Cyclophosphamide has been also associated with a decrease in gonadal function and teratogenicity (43). Our cohort of patients, consisting predominantly of women of reproductive age, is especially vulnerable to these adverse effects. However, in the context of type B insulin resistance, hyperandrogenemia and the hypercatabolic state per se led to amenorrhea. Once the disease remitted, gonadal function tended to normalize. In fact, one patient with type B insulin resistance in this cohort conceived and delivered a healthy baby shortly after entering remission. Earlier menopause has also been reported in women exposed to cyclophosphamide (43). Despite a relatively long follow-up duration of 6 years, we are unable to assess whether exposure to cyclophosphamide resulted in earlier menopause in our cohort because not all patients reached a perimenopausal age at the time of the study.
To increase the safety of combined immunotherapy, more toxic cyclophosphamide was changed to safer azathioprine as a maintenance therapy, once remission was achieved, leading to a median exposure time to cyclophosphamide of only 6 months.
Combination immunosuppressive therapy not only reversed diabetes and the hypercatabolic state of type B insulin resistance but also resulted in normalization of hyperandrogenism in women. Hyperandrogenemia related to type B insulin resistance is driven by high insulin levels, which, in combination with gonadotropins as cofactors, leads to stimulation of ovarian testosterone production (36). How insulin signals within the ovary to increase androgen production is unclear, because the insulin receptor is blocked by the presence of autoantibodies. It is possible that insulin signals through hybrid insulin/IGF-1 receptors or alternative unknown mechanisms (36). These mechanisms are difficult to study, because in preclinical rodent models, selective insulin receptor deletion in ovarian theca cells prevents hyperandrogenism induced by hyperinsulinemia (44), whereas in humans, germ line insulin receptor mutations result in hyperandrogenism (45).
One of the main features of type B insulin resistance that differentiates it from insulin resistance associated with obesity, type 2 diabetes, or metabolic syndrome, is low-normal triglyceride levels. The elevated triglycerides seen in common, postreceptor insulin resistance occur because insulin continues to stimulate hepatic de novo lipogenesis. By contrast, in receptor-level insulin resistance, including type B insulin resistance, all insulin signaling pathways are blocked, and de novo lipogenesis is not stimulated (46). Consistent with this, we observed low-normal triglyceride levels in our cohort at baseline despite extreme insulin resistance, and triglyceride levels did not significantly change after remission was achieved.
In conclusion, combination therapy with rituximab, cyclophosphamide, and high-dose steroid pulses, followed by maintenance therapy with azathioprine, is a highly effective and relatively safe therapeutic regimen in patients with type B insulin resistance. This treatment has changed the natural history of this disease, from 54% mortality to a curable form of diabetes and should be recommended in this group of patients.
Article Information
Funding. The study was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases. R.K.S. is funded by the Wellcome Trust, grant 210752/Z/18/Z.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.K.-G. contributed to data collection and analysis, preparing the manuscript, and providing clinical care. M.L. contributed to data collection and analysis. E.C. contributed to providing clinical care and ensuring appropriate follow-up. R.K.S. contributed to analysis of the insulin receptor antibodies. R.K.S. and C.G. undertook and analyzed diagnostic immunoprecipitation assays. R.J.B. and P.G. contributed to study design, providing clinical care, and critical review of the manuscript. J.K.-G., R.J.B., and P.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at ENDO 2015, the Endocrine Society's 97th Annual Meeting and Expo, San Diego, CA, 5–8 March 2015.
Footnotes
Clinical trial reg. no. NCT00001987, clinicaltrials.gov.
References
- 1.Flier JS, Kahn CR, Roth J, Bar RS. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science 1975;190:63–65 [DOI] [PubMed] [Google Scholar]
- 2.Kahn CR, Flier JS, Bar RS, et al. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med 1976;294:739–745 [DOI] [PubMed] [Google Scholar]
- 3.Flier JS, Kahn CR, Jarrett DB, Roth J. Characterization of antibodies to the insulin receptor: a cause of insulin-resistant diabetes in man. J Clin Invest 1976;58:1442–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn CR, Baird K, Filier JS, Jarrett DB. Effects of autoantibodies to the insulin receptor on isolated adipocytes. Studies of insulin binding and insulin action. J Clin Invest 1977;60:1094–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore) 2009;88:141–153 [DOI] [PubMed] [Google Scholar]
- 6.Dons RF, Havlik R, Taylor SI, Baird KL, Chernick SS, Gorden P. Clinical disorders associated with autoantibodies to the insulin receptor. Simulation by passive transfer of immunoglobulins to rats. J Clin Invest 1983;72:1072–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81:87–100 [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan L, Sirisena I. Immunosuppressive therapy in treatment of refractory hypoglycemia in type B insulin resistance: a case report. J Endocr Soc 2017;1:1435–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malek R, Chong AY, Lupsa BC, et al. Treatment of type B insulin resistance: a novel approach to reduce insulin receptor autoantibodies. J Clin Endocrinol Metab 2010;95:3641–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iseri K, Iyoda M, Shikida Y, et al. Rituximab for the treatment of type B insulin resistance syndrome: a case report and review of the literature. Diabet Med 2017;34:1788–1791 [DOI] [PubMed] [Google Scholar]
- 11.Kim HN, Fesseha B, Anzaldi L, Tsao A, Galiatsatos P, Sidhaye A. Antibody-mediated extreme insulin resistance: a report of three cases. Am J Med 2018;131:102–106 [DOI] [PubMed] [Google Scholar]
- 12.Zelada H, Gamarra D, Arbañil H, Manrique H. Type B insulin resistance in Peru. Am J Med Sci 2017;353:258–262 [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Zhao J, Li Y, Lv F, Zhang S, Li Y. Successful treatment of type B insulin resistance with mixed connective tissue disease by pulse glucocorticoids and cyclophosphamide. J Diabetes Investig 2017;8:626–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q, Yan Y, Zhao H, Zuo L. A systemic lupus erythematosus patient presenting as type B insulin resistance complicated with cryoglobulinemia. Lupus 2017;26:95–97 [DOI] [PubMed] [Google Scholar]
- 15.Yang GQ, Li YJ, Dou JT, Wang BA, Lu JM, Mu YM. Type B insulin resistance syndrome with Scleroderma successfully treated with multiple immune suppressants after eradication of Helicobacter pylori infection: a case report. BMC Endocr Disord 2016;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takei M, Ishii H, Kawai Y, et al. Efficacy of oral glucocorticoid and cyclosporine in a case of rituximab-refractory type B insulin resistance syndrome. J Diabetes Investig 2015;6:734–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manikas ED, Isaac I, Semple RK, Malek R, Führer D, Moeller LC. Successful treatment of type B insulin resistance with rituximab. J Clin Endocrinol Metab 2015;100:1719–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourron O, Caron-Debarle M, Hie M, et al. Type B insulin-resistance syndrome: a cause of reversible autoimmune hypoglycaemia. Lancet 2014;384:1548. [DOI] [PubMed] [Google Scholar]
- 19.Kang SM, Jin HY, Lee KA, Park JH, Baek HS, Park TS. Type B insulin-resistance syndrome presenting as autoimmune hypoglycemia, associated with systemic lupus erythematosus and interstitial lung disease. Korean J Intern Med 2013;28:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Wang G, Wang J. Type B insulin resistance syndrome induced by systemic lupus erythematosus and successfully treated with intravenous immunoglobulin: case report and systematic review. Clin Rheumatol 2013;32:181–188 [DOI] [PubMed] [Google Scholar]
- 21.Taylor SI, Dons RF, Hernandez E, Roth J, Gorden P. Insulin resistance associated with androgen excess in women with autoantibodies to the insulin receptor. Ann Intern Med 1982;97:851–855 [DOI] [PubMed] [Google Scholar]
- 22.Taylor SI, Grunberger G, Marcus-Samuels B, et al. Hypoglycemia associated with antibodies to the insulin receptor. N Engl J Med 1982;307:1422–1426 [DOI] [PubMed] [Google Scholar]
- 23.Braund WJ, Naylor BA, Williamson DH, et al. Autoimmunity to insulin receptor and hypoglycaemia in patient with Hodgkin’s disease. Lancet 1987;1:237–240 [DOI] [PubMed] [Google Scholar]
- 24.Chan JC, Zhu SQ, Ho SK, Cockram CS. Hypoglycaemia and Hodgkin’s disease. Br J Haematol 1990;76:434–436 [DOI] [PubMed] [Google Scholar]
- 25.Willard DL, Stevenson M, Steenkamp D. Type B insulin resistance syndrome. Curr Opin Endocrinol Diabetes Obes 2016;23:318–323 [DOI] [PubMed] [Google Scholar]
- 26.Semple RK, Halberg NH, Burling K, et al. Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes 2007;56:1712–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page KA, Dejardin S, Kahn CR, Kulkarni RN, Herold KC, Inzucchi SE. A patient with type B insulin resistance syndrome, responsive to immune therapy. Nat Clin Pract Endocrinol Metab 2007;3:835–840 [DOI] [PubMed] [Google Scholar]
- 28.Kawashiri SY, Kawakami A, Fujikawa K, et al. Type B insulin resistance complicated with systemic lupus erythematosus. Intern Med 2010;49:487–490 [DOI] [PubMed] [Google Scholar]
- 29.Chon S, Choi MC, Lee YJ, et al. Autoimmune hypoglycemia in a patient with characterization of insulin receptor autoantibodies. Diabetes Metab J 2011;35:80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coll AP, Thomas S, Mufti GJ. Rituximab therapy for the type B syndrome of severe insulin resistance. N Engl J Med 2004;350:310–311 [DOI] [PubMed] [Google Scholar]
- 31.Kaufman M, Limaye SA, Driscoll N, et al. A combination of rituximab, cyclophosphamide and dexamethasone effectively treats immune cytopenias of chronic lymphocytic leukemia. Leuk Lymphoma 2009;50:892–899 [DOI] [PubMed] [Google Scholar]
- 32.Simon L, Baron M, Leblond V. How we manage patients with Waldenström macroglobulinaemia. Br J Haematol 2018;181:737–751 [DOI] [PubMed] [Google Scholar]
- 33.Kapoor P, Ansell SM, Fonseca R, et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) guidelines 2016. JAMA Oncol 2017;3:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meunier G, Ysebaert L, Nguyen-Thi PL, et al. First-line therapy for chronic lymphocytic leukemia in patients older than 79 years is feasible and achieves good results: a FILO retrospective study. Hematol Oncol 2017;35:671–678 [DOI] [PubMed] [Google Scholar]
- 35.Perry M, Delarche A, Ribes D, et al. Rituximab-cyclophosphamide-dexamethasone is highly effective in patients with monoclonal Ig deposit-related glomerulopathy and indolent non-Hodgkin lymphomas. Am J Hematol 2014;89:969–973 [DOI] [PubMed] [Google Scholar]
- 36.Brown RJ, Joseph J, Cochran E, Gewert C, Semple R, Gorden P. Type B insulin resistance masquerading as ovarian hyperthecosis. J Clin Endocrinol Metab 2017;102:1789–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum 1995;38:1120–1127 [DOI] [PubMed] [Google Scholar]
- 38.Tran HA, Reeves GE. Treatment of type B insulin resistance with immunoglobulin: novel use of an old therapy. Med J Aust 2009;190:168. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson JW, Bremell T, Eliasson B, Fowelin J, Fredriksson L, Yu ZW. Successful treatment with plasmapheresis, cyclophosphamide, and cyclosporin A in type B syndrome of insulin resistance. Case report. Diabetes Care 1998;21:1217–1220 [DOI] [PubMed] [Google Scholar]
- 40.Mok CC. Current role of rituximab in systemic lupus erythematosus. Int J Rheum Dis 2015;18:154–163 [DOI] [PubMed] [Google Scholar]
- 41.Tian J, Luo Y, Wu H, Long H, Zhao M, Lu Q. Risk of adverse events from different drugs for SLE: a systematic review and network meta-analysis. Lupus Sci Med 2018;5:e000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furst DE, Tseng CH, Clements PJ, et al.; Scleroderma Lung Study . Adverse events during the Scleroderma Lung Study. Am J Med 2011;124:459–467 [DOI] [PubMed] [Google Scholar]
- 43.Teles KA, Medeiros-Souza P, Lima FA, Araújo BG, Lima RA. Cyclophosphamide administration routine in autoimmune rheumatic diseases: a review. Rev Bras Reumatol Engl Ed 2017;57:596–604 [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Divall S, Nwaopara A, et al. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes 2014;63:1270–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musso C, Cochran E, Moran SA, et al. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 2004;83:209–222 [DOI] [PubMed] [Google Scholar]
- 46.Semple RK, Sleigh A, Murgatroyd PR, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 2009;119:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]