Abstract
Objectives
This study aimed to evaluate prognostic value of the combination of monocyte-to-lymphocyte ratio (MLR) with neutrophil-to-lymphocyte ratio (NLR) for predicting long-term major adverse cardiac events (MACE) in patients with non-ST elevated myocardial infarction (NSTEMI) who underwent primary percutaneous coronary intervention (PCI).
Design
Retrospective cohort study.
Setting
Civil Aviation General Hospital, Beijing, China.
Participants
678 patients with NSTEMI undergoing primary PCI between July 2010 and July 2015 were enrolled.
Main outcome measures
The main outcomes were MACE. The cumulative MACE-free survival rates were calculated by Kaplan-Meier analysis and the independent predictors of MACE were assessed by Cox regression analysis.
Results
According to the cut-off values of MLR 0.36 and NLR 2.15, the study population was classified into four groups: low MLR + low NLR group (n=319), low MLR + high NLR group (n=126), high MLR + low NLR group (n=102) and high MLR + high NLR group (n=131). The high MLR + high NLR group had a lower MACE-free survival rate than the other three groups (p logrank <0.001). Both MLR (HR 2.128, 95% CI 1.458 to 3.105) and NLR (HR 1.925, 95% CI 1.385 to 2.676) were independent predictors of long-term MACE. Moreover, the patients in the high MLR + high NLR group had an HR of 4.055 (95% CI 2.550 to 6.448) for long-term MACE, with the low-MLR + low NLR group as reference. Comparisons of receiver operating characteristic curves revealed that the combination of MLR with NLR achieved better performance in differentiating long-term MACE, compared with MLR, NLR, high-sensitivity C reactive protein and brain natriuretic peptide alone, and had similar performance to all other pairwise combinations of the four biomarkers.
Conclusions
Elevated levels of MLR and NLR were independent predictors of long-term MACE in patients with NSTEMI. Moreover, the combination of MLR and NLR could improve the prognostic value in predicting long-term MACE.
Keywords: monocyte-to-iymphocyte ratio, neutrophil-to-iymphocyte ratio, major adverse cardiac events, non-st elevated myocardial infarction
Strengths and limitations of this study.
This is the first study to report the use of monocyte-to-lymphocyte ratio (MLR) in combination with neutrophil-to-lymphocyte ratio (NLR) to predict major adverse cardiac events (MACE) in patients with non-ST elevated myocardial infarction (NSTEMI) undergoing primary percutaneous coronary intervention.
Receiver operating characteristic curves were used to extensively explore and compare the diagnostic efficacies of MLR alone, NLR alone, high-sensitivity C reactive protein alone, brain natriuretic peptide alone and their pairwise combinations in differentiating MACE in patients with NSTEMI.
MLR and NLR, that are non-invasive, simple, economical and feasible biomarkers, possess practical clinical utility in the prediction of prognosis of NSTEMI.
These findings need to be validated in multi-institutional studies with larger sample sizes.
Introduction
Previous studies have verified that inflammatory response plays a vital role in the development of atherosclerosis and cardiovascular diseases.1 2 White blood cells and its subtypes including neutrophils, monocytes and lymphocytes are important immune cells involved in the initiation, formation and destabilisation of atherosclerosis.3 The neutrophil-to-lymphocyte ratio (NLR) has been established as a cost-effective, feasible and reproducible inflammatory biomarker in many cardiovascular disorders, including acute coronary syndrome (ACS), angina pectoris and heart failure.4–6 Elevated NLR has been reported as an independent predictor of major adverse cardiac events (MACE) in patients with ACS.7 Monocytes can recruit to the artery wall, differentiate into macrophages and stimulate the activation of proinflammatory cytokines which play a crucial role at every level of the atherosclerotic process.8 The monocyte-to-lymphocyte ratio (MLR) has emerged as a novel systematic inflammatory marker related to increased cardiovascular risk.9 Recently, MLR has been reported to be associated with adverse clinical outcomes in various cardiovascular diseases.10–12 However, the prognostic value of the combined usefulness of MLR and NLR in non-ST elevated myocardial infarction (NSTEMI) has not been evaluated. The aim of the present study was to investigate the combined usefulness of MLR and NLR in predicting MACE in patients with NSTEMI who underwent primary percutaneous coronary intervention (PCI).
Materials and methods
Study design, setting and participants
This retrospective longitudinal study was performed in the Civil Aviation General Hospital, Beijing, China. A total of 818 consecutive patients with NSTEMI who presented to the emergency department and underwent primary PCI from July 2010 to July 2015 were selected for participation in this study. NSTEMI was defined by typical ischaemia symptoms, elevated level of cardiac troponin-I or creatine kinase-MB and no evidence of ST segment elevation in ECG. We excluded patients who had serious heart failure (New York Heart Association (NYHA)13 class III or IV), rheumatic heart disease, valvular heart disease, congenital heart disease, pulmonary heart disease, active or chronic inflammatory conditions, acute infection, haemodynamic disorders, malignancies, severe renal (estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2) or hepatic (alanine aminotransferase >40 U/L) disease, steroid therapy in the preceding 3 months, history of cerebrovascular events, or incomplete blood cell count or medical records. Hypertension was defined as current use of an antihypertensive medication or, a systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg. Diabetes mellitus was defined as active use of an antidiabetic agent, or fasting plasma glucose level ≥7.0 mmol/L or casual plasma glucose level ≥11.1 mmol/L.
Informed consent was obtained from all patients.
Study procedures and laboratory analysis
At the time of admission, venous blood samples were collected from each patient. All haematological and biochemical analyses were performed on fresh whole blood/plasma. Plasma was obtained by centrifuging whole blood samples at 3000 rpm for 5 min. Complete blood counts and biochemical indicators were measured by the core laboratory of the Civil Aviation General Hospital. Complete blood counts were performed using a SYSMEX XE-2100 automated cell counter (Sysmex Corporation, Kobe, Japan). Complete blood counts included haemoglobin, leucocytes, neutrophils, monocytes, lymphocytes and platelets. Biochemical indicators (total cholesterol, triglycerides, creatinine, low-density lipoprotein (LDL), high-density lipoprotein (HDL), creatinine, high-sensitivity C reactive protein (hs-CRP), brain natriuretic peptide (BNP) and Troponin I) were determined using a Hitachi7600 automatic biochemistry analyser (Hitachi, Tokyo, Japan). eGFR was calculated using the Chronic Kidney Disease Epidemiology (CKD EPI) creatinine equation14. MLR was calculated as the ratio of monocyte counts to lymphocyte counts, and NLR was calculated as the ratio of neutrophil counts to lymphocyte counts.
All patients received a loading dose of aspirin (300 mg) and clopidogrel (300 mg) at least 6 hours before PCI, and an intravenous dose of heparin (70–100 U/kg) to maintain an activated clotting time (250–300 s) during the procedure. Primary PCI was performed according to standard clinical practice by experienced cardiologists. A successful PCI was defined as a residual stenosis less than 30% and final thrombolysis in myocardial infarction (MI) II or III flow in the treated artery. Angiographic characteristics were collected for all the patients.
Clinical outcomes
The main outcomes were MACE that happened in-hospital and during the follow-up period, which were defined as a composite of all-cause mortality, cardiac death, stroke, non-fatal MI, target lesion revascularisation (TLR) and target vessel revascularisation (TVR) according to the Academic Research Consortium definition.15 Cardiac death was defined as death resulting from any cardiac-related causes (eg, MI, heart failure, lethally cardiac arrhythmia). Non-fatal MI was defined based on the European Society of Cardiology, American Heart Association, American College of Cardiology and World Heart Federation definitions.16 TLR was defined as repeat revascularisation caused by ≥50% stenosis within the stent or within 5 mm proximal or distal to the stent. TVR was defined as repeat coronary angioplasty or surgical bypass performed within the coronary artery containing the target lesion. Follow-up data were obtained by review of electronic medical records and/or telephone interview with the patients or patients’ primary caregiver.
Statistical analysis
The Kolmogorov-Smirnov test was employed to test the normality of the continuous variables in each group. Continuous variables distributed normally were expressed as mean±SD, while categorical data were expressed as numbers and percentages. We initially used receiver operating characteristic (ROC) curves to determine the ability of MLR and NLR to differentiate MACE. Subsequently optimal cut-off values, and specificity and sensitivity were derived. Based on the optimal cut-off values, participants were assigned to four groups: low MLR + low NLR group, low MLR + high NLR group, high-MLR + low NLR group and high MLR + high NLR group. Continuous data differences between the four groups were compared using one-way analysis of variance followed by Tukey’s post hoc tests, while categorical data were compared by χ2 tests. The MACE-free survival rates according to the cut-off values of MLR and NLR were estimated by the Kaplan-Meier analysis and statistical differences were carried out using the logrank test. Univariate and multivariate Cox regression analyses were carried out to identify the independent predictors of MACE. Variables with p<0.10 in univariate analysis were selected for multivariate Cox regression analysis. We constructed two Cox regression models (model 1 and model 2) with MACE as the dependent variable to investigate the efficacy of MLR and NLR in predicting MACE. Model 1 was to estimate the HR of MLR (low MLR=0 (reference category), high MLR=1) and NLR (low NLR=0 (reference category), high NLR=1) for MACE. Model 2 was to estimate the HR of MLR in combination with NLR for MACE (low MLR + low NLR=0 (reference category), low MLR + high NLR=1, high-MLR + low NLR=2, high MLR + high NLR=3). The effect sizes were expressed as HRs and their 95% CIs. Afterwards, we used ROC curves to evaluate the diagnostic performance of individual biomarkers and their pairwise combinations in predicting long-term MACE. The areas under the curves (AUCs) were compared by Delong’s tests. The statistical significance was considered as a two-tailed p<0.05. Statistical analyses were performed using SPSS V.22.0 (SPSS, Chicago, Illinois, USA).
Patient and public involvement
Patients and public were not involved in the design, recruitment or conduct of this study. There is no plan for the study results to be disseminated directly to participants.
Results
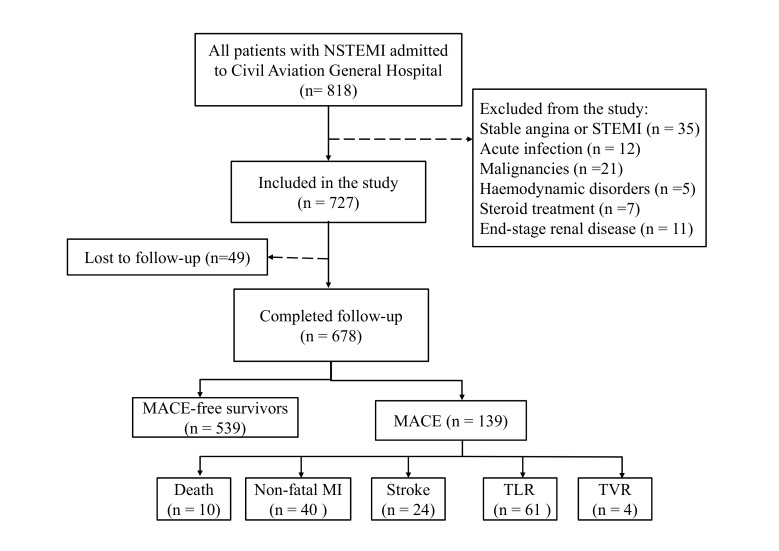
Eight hundred and eighteen patients were screened for inclusion for this study, while 91 (11.12%) patients were excluded because of the exclusion criteria and 49 (6.00%) patients were lost to follow-up. Therefore, a total of 678 (82.89%) patients were included into the analysis, and the median follow-up period was 26 (range: 1–30) months. Figure 1 depicts the clinical layout of the study cohort.
Figure 1.
Flow chart of the study cohort. The flow chart presents the selection criteria of the study and the clinical layout of the study population. MACE, major adverse cardiac events; MI, myocardial infection; NSTEMI, non-STEMI, STEMI, ST elevated myocardial infarction; TLR, target lesion revascularisation; TVR, target vessel revascularisation.
Baseline clinical characteristics
A MLR cut-off value of 0.36 had a sensitivity of 54.74% and a specificity of 73.57%, while an NLR cut-off value of 2.35 had a sensitivity of 77.37% and a specificity of 55.08% for differentiating long-term MACE, via ROC analyses. According to the optimal cut-off values of MLR 0.36 and NLR 2.35, participants were classified into four groups: low-MLR + low NLR group (MLR <0.36, NLR <2.35, n=319), low MLR + high NLR group (MLR <0.36, NLR ≥2.35, n=126), high MLR + low NLR group (MLR ≥0.36, NLR <2.35, n=102) and high MLR + high NLR group (MLR ≥0.36, NLR ≥2.35, n=131). The clinical characteristics were summarised in table 1. The distribution of prior medications and angiographic findings were similar between the four groups. However, patients in the high MLR + high NLR group were older, with higher Killip class and lower ejection fraction, and showed higher levels of white blood cells, monocytes, neutrophils, LDL, hs-CRP, BNP and troponin I, whereas they had lower levels of lymphocytes and haemoglobin.
Table 1.
Baseline clinical characteristics between the four groups based on the cut-off values of MLR and NLR
| Variable | Low MLR + low NLR (n=319) |
Low MLR + high NLR (n=126) |
High MLR + low NLR (n=102) |
High MLR + high NLR (n=131) |
P values |
| Age, years | 61.10±14.38 | 63.63±15.05 | 64.05±15.15 | 65.23±16.34* | 0.036 |
| Male, n (%) | 209 (65.52) | 71 (56.35) | 65 (63.73) | 90 (68.70) | 0.188 |
| Family history, n (%) | 33 (10.34) | 17 (13.49) | 11 (10.78) | 20 (15.27) | 0.463 |
| Hypertension, n(%) | 221 (69.28) | 86 (68.25) | 73 (71.57) | 105 (80.15) | 0.100 |
| Diabetes mellitus, n(%) | 125 (39.18) | 56 (44.44) | 39 (38.24) | 59 (45.04) | 0.522 |
| Dyslipidaemia, n (%) | 114 (35.74) | 51 (40.48) | 35 (34.31) | 49 (37.40) | 0.758 |
| Current smoker, n (%) | 108 (33.86) | 45 (35.71) | 33 (32.35) | 51 (38.93) | 0.702 |
| Killip class (>I) | 192 (60.19) | 86 (68.25) | 69 (67.65) | 99 (75.57)* | 0.015 |
| Ejection fraction (%) | 65.80±10.05 | 66.52±10.11 | 65.26±10.23 | 62.98±10.01* | 0.044 |
| Laboratory parameters | |||||
| Leucocyte, ×109/L | 6.67±1.72 | 6.77±2.03 | 7.06±1.98* | 7.25±2.11* | 0.027 |
| Neutrophil, ×109/L | 4.26±1.07 | 4.69±1.19* | 4.56±1.15* | 5.08±1.27*†‡ | <0.001 |
| Lymphocyte, ×109/L | 2.31±0.53 | 1.98±0.51* | 2.37±0.67† | 2.02±0.64*‡ | <0.001 |
| Monocyte, ×109/L | 0.18±0.09 | 0.22±0.11* | 0.49±0.19*† | 0.46±0.13* † | <0.001 |
| Haemoglobin, g/L | 136.7±30.38 | 129.6±27.57* | 133.7±39.76 | 127.4±36.01* | 0.039 |
| Total cholesterol, mmol/L | 5.70±1.47 | 5.95±1.49 | 5.52±1.35 | 5.69±1.36 | 0.130 |
| Triglycerides, mmol/L | 1.67±0.56 | 1.63±0.54 | 1.77±0.59 | 1.61±0.54 | 0.109 |
| LDL, mmol/L | 2.86±0.95 | 3.10±1.08* | 2.93±1.12* | 3.35±1.06*†‡ | <0.001 |
| HDL, mmol/L | 1.31±0.51 | 1.43±0.57 | 1.37±0.49 | 1.44±0.51 | 0.059 |
| hs-CRP, mg/dL | 1.85±0.71 | 2.14±0.98* | 2.88±0.77* † | 3.13±1.02*†‡ | <0.001 |
| Creatinine, umol/L | 112.38±29.2 | 106.49±30 | 112.72±37.44 | 107.62±34.02 | 0.203 |
| eGFR, ml/min/1.73 m2 | 85.71±29.12 | 81.85±28.71 | 78.92±26.31 | 81.28±27.09 | 0.101 |
| BNP, pg/mL | 265.12±85.39 | 245.58±79.28* | 269.71±76.56* † | 298.73±76.56* †‡ | <0.001 |
| Troponin I, ng/mL | 5.89±2.51 | 7.52±3.52* | 7.65±3.79* | 11.08±4.18* †‡ | <0.001 |
| Medical treatment | |||||
| Aspirin, n(%) | 309 (96.87) | 119 (94.44) | 96 (94.12) | 129 (98.47) | 0.202 |
| Anticoagulant, n(%) | 305 (95.61) | 117 (92.86) | 93 (91.18) | 124 (94.66) | 0.340 |
| Statin, n(%) | 292 (91.54) | 112 (88.89) | 90 (88.24) | 121 (92.37) | 0.589 |
| ACEI or ARB, n(%) | 186 (58.31) | 71 (56.35) | 61 (59.8) | 84 (64.12) | 0.602 |
| Beta-blocker, n(%) | 259 (81.19) | 98 (77.78) | 86 (84.31) | 105 (80.15) | 0.654 |
| Calcium-channel blockers, n(%) | 67 (21.00) | 30 (23.81) | 26 (25.49) | 28 (21.37) | 0.767 |
| Nitrate drugs, n(%) | 257 (80.56) | 96 (76.19) | 75 (73.53) | 106 (80.92) | 0.369 |
| Angiographic findings | |||||
| Number of diseased vessels | 0.145 | ||||
| one vessel, n(%) | 182 (57.05) | 65 (51.59) | 54 (52.94) | 57 (43.51) | |
| two vessels, n(%) | 76 (23.82) | 39 (30.95) | 32 (31.37) | 42 (32.06) | |
| three vessels/left main, n(%) | 61 (19.12) | 22 (17.46) | 16 (15.69) | 32 (24.43) | |
| Number of implanted stents | 1.95±0.79 | 2.01±0.85 | 2.11±0.94 | 2.15±1.01 | 0.125 |
| Total stent length, mm | 39.6±24.1 | 37.2±21.9 | 35.8±26.3 | 41.9±22.2 | 0.190 |
| Stent diameter, mm | 2.59±1.33 | 2.85±1.09 | 2.84±1.27 | 2.68±1.16 | 0.114 |
| Moderate or severe tortuosity, n(%) | 27 (8.46) | 16 (12.70) | 11 (10.78) | 14 (10.69) | 0.575 |
| Moderate or severe calcification, n(%) | 29 (9.09) | 14 (11.11) | 13 (12.75) | 15 (11.45) | 0.706 |
*Compared with the low MLR + low NLR group, p<0.05.
†Compared with the low MLR + high NLR group, p<0.05.
‡Compared with the high MLR + low NLR group, p<0.05.
ACEI, ACE inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C reactive protein; LDL, low-density lipoprotein; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio.
Clinical outcomes
During the median follow-up period of 26 months, long-term MACE were observed in 139 (20.50%) patients. Ten (1.47%) patients died, 40 (5.90%) patients had a non-fatal MI, 24 (3.54%) patients experienced stroke, 61 (9.00%) patients underwent TLR and 4 (0.59%) patients underwent TVR. Overall, the patients in the high MLR + high NLR group had higher MACE rate, compared with the other three groups. The mortality, non-fatal MI, stroke and TLR were significantly higher in patients with high MLR + high NLR, than those with either lower MLR or lower NLR, whereas the four groups had similar TVR (table 2).
Table 2.
Clinical outcomes between the four groups based on the cut-off values of MLR and NLR
| Variable | Low MLR + low NLR (n=319) |
Low MLR + high NLR (n=126) |
High MLR + low NLR (n=102) |
High MLR + high NLR (n=131) |
P values |
| Follow-up 2 years, n(%) | 30 (9.40) | 31 (24.60)* | 28 (27.45)* | 50 (38.17)* †‡ | <0.001 |
| All-cause death, n(%) | 2 (0.63) | 1 (0.79) | 1 (0.98) | 6 (4.58)* | 0.010 |
| Cardiac death, n(%) | 1 (0.31) | 1 (0.79) | 1 (0.98) | 5 (3.82)* | 0.018 |
| Non-fatal MI, n(%) | 9 (2.82) | 8 (6.35) | 9 (8.82)* | 14 (10.69)* | 0.006 |
| Stroke, n(%) | 4 (1.25) | 6 (4.76)* | 5 (4.9)* | 9 (6.87)* | 0.017 |
| TLR, n(%) | 14 (4.39) | 16 (12.70)* | 12 (11.76)* | 19 (14.50)* | 0.001 |
| TVR, n(%) | 1 (0.31) | 0 (0) | 1 (0.98) | 2 (1.53) | 0.335 |
*Compared with the low MLR + low NLR group, p<0.05.
†Ccompared with the low MLR + high NLR group, p<0.05.
‡Compared with the high MLR + low NLR group, p<0.05.
MI, myocardial infarction; MLR, monocyte-to lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; TLR, target lesion revascularisation; TVR, target vessel revascularisation.
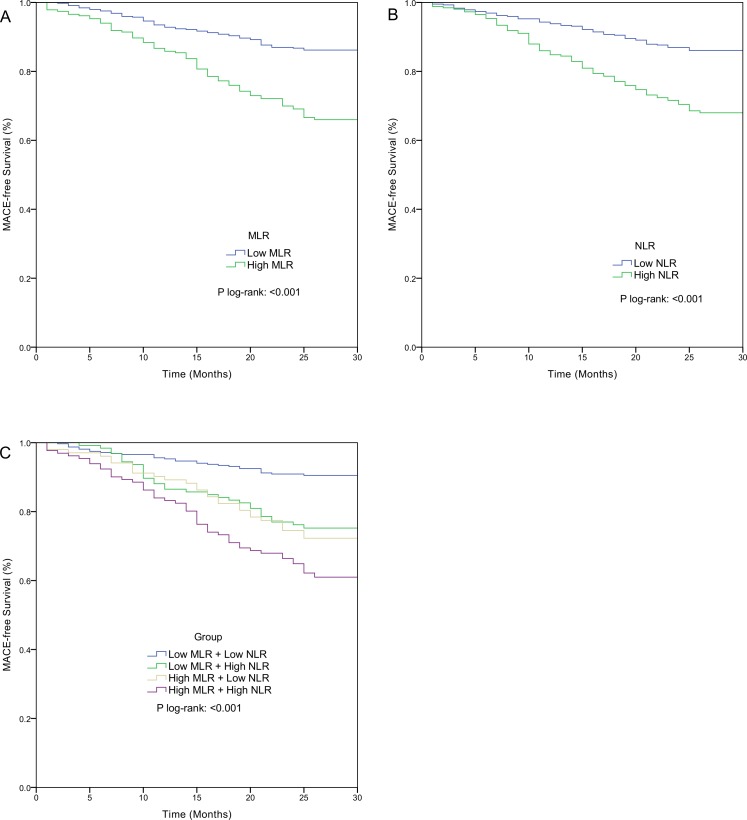
Kaplan-Meier curves based on the cut-off values of MLR and NLR, are shown in figure 2A and figure 2B, respectively. Significantly increased long-term MACE rates were observed in patients with high MLR (33.48% vs 13.71%, p<0.001, figure 2A) and in patients with high NLR (31.52% vs 13.78%, p<0.001, figure 2B). The Kaplan-Meier MACE-free curve based on the combined markers is shown in figure 2C. The MACE rates were significantly different among the four groups (p<0.001) and patients in the high MLR + high NLR group had the highest MACE rate.
Figure 2.
Kaplan-Meier cumulative MACE-free curves in patients with NSTEMI (A) according to the cut-off value of MLR; (B) according to the cut-off value of NLR; (C) according to MLR combined with NLR. MACE, major adverse cardiac events; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; NSTEMI, non-ST elevated myocardial infarction.
Independent predictors of long-term MACE
Univariate and multivariate COX regression analyses were used to determine the independent predictors of long-term MACE in patients with NSTEMI undergoing primary PCI. In univariate Cox analysis, leucocytes, neutrophils, lymphocytes, monocytes, MLR, NLR, LDL, hs-CRP, BNP and troponin I were found to be significantly associated with long-term MACE (see online supplementary table S1). After adjusting for covariates, both MLR (HR 2.128, 95% CI 1.458 to 3.105, p<0.001) and NLR (HR 1.925, 95% CI 1.385 to 2.676, p<0.001) were found to be significant predictors of long-term MACE in multivariate Cox regression. Moreover, the combination of MLR and NLR was found to be an independent predictor of long-term MACE (HR 4.055, 95% CI 2.550 to 6.448, p<0.001 for patients with high MLR + high NLR vs patients with low MLR + low NLR). In addition to MLR and NLR, hs-CRP and BNP were also independent predictors of long-term MACE in patients with NSTEMI undergoing primary PCI (table 3). The details of multivariate Cox regression analyses are presented in online supplementary table S2.
Table 3.
Independent predictors of long-term major adverse cardiac events in patients with non-ST elevated myocardial infarction (NSTEMI) by multivariate Cox regression analyses
| Variable | HR | 95% CI | P values |
| Model 1 | |||
| MLR | |||
| Low MLR, MLR <0.36 | Ref | ||
| High MLR, MLR ≥0.36 | 2.128 | 1.458 to 3.105 | <0.001 |
| NLR | |||
| Low NLR, NLR <2.15 | Ref | ||
| High NLR, NLR ≥2.15 | 1.925 | 1.385 to 2.676 | <0.001 |
| hs-CRP | 1.747 | 1.173 to 2.601 | 0.006 |
| BNP | 1.950 | 1.156 to 3.290 | 0.012 |
| Model 2 | |||
| Combination of MLR and NLR | |||
| Low MLR + low NLR | Ref | - | - |
| Low MLR + high NLR | 2.732 | 1.417 to 5.268 | 0.003 |
| High MLR + low NLR | 3.004 | 1.519 to 5.940 | 0.002 |
| High MLR + high NLR | 4.055 | 2.550 to 6.448 | <0.001 |
| hs-CRP | 1.576 | 1.058 to 2.349 | 0.025 |
| BNP | 1.874 | 1.137 to 3.088 | 0.014 |
BNP, brain natriuretic peptide; hs-CRP, high-sensitivity C reactive protein; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio.
bmjopen-2018-023459supp001.pdf (208.7KB, pdf)
Diagnostic efficacy of MLR in combination with NLR in differentiating MACE
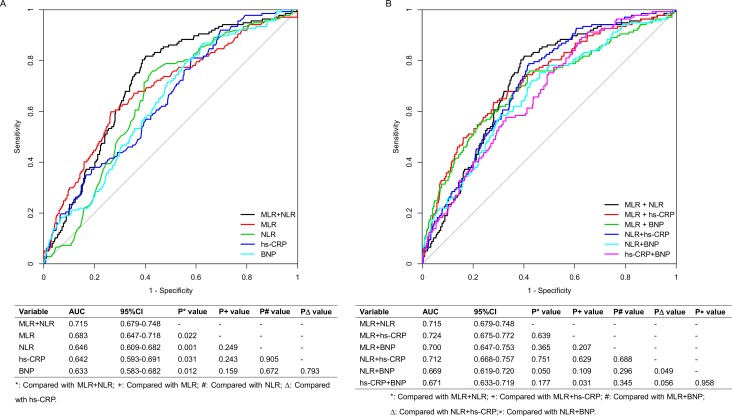
ROC curves were used to evaluate and compare the predictive performance of MLR in combination with NLR with (1) MLR, NLR, hs-CRP and BNP alone. (2) All other pairwise combinations of the four biomarkers, for differentiating long-term MACE. Figure 3A shows that MLR in combination with NLR (AUC 0.715, 95% CI 0.679 to 0.748) achieved better performance in predicting long-term MACE, than MLR (AUC 0.683, 95% CI 0.647 to 0.718), NLR (AUC 0.646, 95% CI 0.609 to 0.682), hs-CRP (AUC 0.642, 95% CI 0.593 to 0.691) and BNP alone (AUC 0.633, 95% CI 0.583 to 0.682) (all p values <0.05), whereas there was no statistically significant difference among the four individual biomarkers in AUC values. Additionally, MLR in combination with NLR performed similarly to all other pairwise combinations of the four biomarkers (all p values ≥0.05, figure 3B).
Figure 3.
Receiver operating characteristic curves showing area under the curve (AUC) for (A) MLR in combination with NLR (MLR + NLR), MLR alone, NLR alone, hs-CRP alone and BNP alone; (B) MLR + NLR, MLR in combination with hs-CRP (MLR + hs CRP), MLR in combination with BNP (MLR + BNP), NLR in combination with hs-CRP (NLR + hs CRP), NLR in combination with BNP (NLR + BNP) and hs-CRP in combination with BNP (hs-CRP + BNP), for long-term MACE in patients with NSTEMI. BNP, brain natriuretic peptide; hs-CRP, high-sensitivity C reactive protein; MACE, major adverse cardiac events; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; NSTEMI, non-ST elevated myocardial infarction.
Discussion
In this study, 139 of 678 patients (20.50%) presented with MACE during the follow-up period. The MACE rate in this study was comparable with that of the previous study (21.62%).17 The novel finding of the present study was that elevated MLR and NLR were independently associated with adverse clinical outcomes in patients with NSTEMI. Moreover, the study demonstrated for the first time that the combination of MLR with NLR has stronger predictive potential for long-term MACE in patients with NSTEMI undergoing primary PCI, compared with individual MLR or NLR.
Many compelling studies have clearly indicated that NLR can be a reliable prognostic factor for short-term and long-term adverse outcomes in patients with ACS undergoing PCI.18 19 Neutrophils, the most abundant leucocytes in the circulation, are actively involved in atherogenesis and plaque destabilisation.20 21 Several mechanisms can probably explain the pivotal role of neutrophils in atherosclerosis: (1) Neutrophils can infiltrate coronary atherosclerotic plaques and the infarcted myocardium, and mediate tissue damage by releasing matrix-degrading enzymes and reactive oxygen species. (2) Increases in neutrophil counts can aggravate endothelial dysfunction, modulate microvascular permeability and contribute to foam cell formation. (3) Neutrophils can promote endothelial erosion, weaken fibrous cap and accelerate neointima formation which contribute to plaque destabilisation.22–25 Lymphocytes are an integral part of the immune system, which participate in every phase of atherosclerosis. Lymphocytopenia, resulting from increased lymphocyte apoptosis, contributes to atherosclerotic plaque growth, lipid core development, plaque destabilisation, postinfarct cardiac remodelling and progression.3 26 Lower lymphocyte count was reported to be an early marker of acute myocardial infraction, and was associated with worse cardiovascular outcomes.27 28 Obviously, it could be concluded that NLR, a composite marker of neutrophils and lymphocytes, can provide prognostic value in patients with ACS. In agreement with previous evidence, our study confirmed the prognostic role of increased NLR in patients with NSTEMI.
MLR, a novel haematological marker, has recently been reported to be a prognostic factor in many diseases, especially in various malignancies.29 30 To date, just a few studies have attempted to elucidate the impact of MLR on cardiovascular disease. In our previous studies, MLR had the potential to assess coronary lesion severity,9 and identify the vulnerable plaques in patients with stable angina.31 Siva et al showed that increased MLR level was associated with higher mortality in patients with acute heart failure.10 Kiris et al reported that elevated MLR level was independently associated with a higher risk of 6-month mortality in patients with STEMI undergoing primary PCI.11 Gijsberts et al found that MLR significantly improved mortality prediction in patients with coronary angiography.12 Thus, a high MLR was associated with adverse cardiac clinical outcomes, though fewer studies have been performed for MLR and cardiac prognosis, compared with those for NLR. Monocytes play an essential role in every stage of atherosclerosis32, which can recruit to the artery wall, differentiate into macrophages and stimulate activating the secretion of proinflammatory cytokines.8 Compared with neutrophils, monocytes can produce higher levels of cytokines.33 Recent pathological studies have found that monocytes can replace neutrophils and become the prominent infiltrating leucocytes within 48 hours of the onset of myocardial ischaemia.34 On the other hand, MI may liberate haematopoietic stem and progenitor cells from bone marrow niches which could increase the availability of monocytes.35 Therefore, MLR, being an integrated reflection of two important immune cells, could be a potential prognostic factor for ACS, and the present study confirmed this hypothesis. Our results revealed that MLR was an independent predictor of long-term MACE and had comparable diagnostic ability as NLR for long-term MACE in patients with NSTEMI undergoing primary PCI. Compared with STEMI, NSTEMI is much more common and tends to have increased mortality in the year following MI.36 Furthermore, we evaluated the combined usefulness of MLR and NLR for predicting long-term MACE in patients with NSTEMI undergoing primary PCI. Our results showed that the combination of MLR with NLR was an independent predictor, more predictive than individual markers, in predicting long-term MACE in patients with NSTEMI.
In addition, our study found that hs-CRP and BNP were also independent predictors of long-term MACE in patients with NSTEMI undergoing primary PCI. hs-CRP and BNP were classical biomarkers correlated with cardiovascular risk and prognosis. A recent meta-analysis of 14 studies concluded that elevated hs-CRP could predict the risk of cardiovascular mortality in the general population.37 Cho KI et al showed that an increased hs-CRP level was a significant independent predictor of long-term adverse events in patients with NSTEMI/unstable angina.17 BNP has been established as a biomarker in vascular diseases used for monitoring disease progression. Porapakkham et al performed a meta-analysis of eight randomised clinical trials and indicated that BNP could be used for guiding the treatment of chronic heart failure,38 while Klok et al conducted a meta-analysis of 13 studies and revealed the prognostic value of BNP in patients with pulmonary embolism.39 In patients with NSTEMI, Fukazawa et al showed that an increased concentration of BNP at admission was closely associated with poor prognosis.40 Our study suggested that in addition to MLR and NLR, hs-CRP and BNP are also independent prognostic factors of long-term MACE in patients with NSTEMI undergoing primary PCI. Besides, compared with hs-CRP and BNP, the combined usefulness of MLR and NLR had a higher HR for predicting long-term MACE in patients with NSTEMI. ROC curves revealed that MLR in combination with NLR was superior to either MLR, NLR, hs-CRP or BNP alone in predicting long-term MACE, and it had similar performance to all other pairwise combinations of the four biomarkers. Moreover, the measurement of MLR and NLR could be more cost-effective and easily accessible in clinical practice, which would possess practical clinical utility in the prediction of prognosis of NSTEMI.
This study had several limitations. First, this study comprised a modest sample size which may introduce selection bias. This single-centre study lacks external validation. Thus, these findings need further multi-institutional validation with larger samples. Second, we evaluated MLR and NLR on admission to the hospital, but didn’t assess their dynamic changes during the follow-up period. Third, inflammatory biomarkers such as myeloperoxidase, interleukin 6 and tumour necrosis factor were not analysed in our patients. Finally, several scoring systems, for example, the HEART Score,41 have been developed to risk-stratify patients with ACS and have been to be associated with patients’ prognosis. It would be of interest to investigate the additive value of MLR/NLR to the scoring systems, but this is beyond the scope of this study. Notwithstanding these limitations, this study first reported the prognostic value of the combination of MLR with NLR in patients with NSTEMI.
In conclusion, the combined usefulness of MLR with NLR gains a prognostic value in patients with NSTEMI, which could be used to identify the high-risk patients with poor outcomes and adjust their treatment accordingly. These findings provide a new perspective on the non-invasive, simple, economical and feasible biomarkers in predicting long-term MACE in patients with NSTEMI.
Supplementary Material
Footnotes
Contributors: ZF made the major contribution to the conception and design of the study, and drafted the manuscript. YL and HJ contributed to the data acquisition and performed the statistical analyses. XJ revised the manuscript. XJ is the guarantor of the study. All authors read and approved the final manuscript prior to the submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Ethical Committee of Civil Aviation General Hospital (CAGH7EC100201). The study was designed and performed in accordance with the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Raw data can be obtained by contacting the corresponding author.
References
- 1. Murtagh BM, Anderson HV. Inflammation and atherosclerosis in acute coronary syndromes. J Invasive Cardiol 2004;16:377–84. [PubMed] [Google Scholar]
- 2. Nahrendorf M, Swirski FK. Immunology. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science 2015;349:237–8. 10.1126/science.aac7801 [DOI] [PubMed] [Google Scholar]
- 3. Horne BD, Anderson JL, John JM, et al. . Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005;45:1638–43. 10.1016/j.jacc.2005.02.054 [DOI] [PubMed] [Google Scholar]
- 4. Arbel Y, Finkelstein A, Halkin A, et al. . Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012;225:456–60. 10.1016/j.atherosclerosis.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 5. Gul M, Uyarel H, Ergelen M, et al. . Predictive value of neutrophil to lymphocyte ratio in clinical outcomes of non-ST elevation myocardial infarction and unstable angina pectoris: a 3-year follow-up. Clin Appl Thromb Hemost 2014;20:378–84. 10.1177/1076029612465669 [DOI] [PubMed] [Google Scholar]
- 6. Kurtul A, Yarlioglues M, Duran M, et al. . Association of neutrophil-to-lymphocyte ratio with contrast-induced nephropathy in patients with non-st-elevation acute coronary syndrome treated with percutaneous coronary intervention. Heart Lung Circ 2016;25:683–90. 10.1016/j.hlc.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 7. Azab B, Zaher M, Weiserbs KF, et al. . Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 2010;106:470–6. 10.1016/j.amjcard.2010.03.062 [DOI] [PubMed] [Google Scholar]
- 8. Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 2011;31:1506–16. 10.1161/ATVBAHA.110.221127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ji H, Li Y, Fan Z, et al. . Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord 2017;17:90 10.1186/s12872-017-0507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silva N, Bettencourt P, Guimarães JT. The lymphocyte-to-monocyte ratio: an added value for death prediction in heart failure. Nutr Metab Cardiovasc Dis 2015;25:1033–40. 10.1016/j.numecd.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 11. Kiris T, Çelik A, Variş E, et al. . Association of lymphocyte-to-monocyte ratio with the mortality in patients with st-elevation myocardial infarction who underwent primary percutaneous coronary intervention. Angiology 2017;68:707–15. 10.1177/0003319716685480 [DOI] [PubMed] [Google Scholar]
- 12. Gijsberts CM, Ellenbroek GH, Ten Berg MJ, et al. . Routinely analyzed leukocyte characteristics improve prediction of mortality after coronary angiography. Eur J Prev Cardiol 2016;23:1211–20. 10.1177/2047487315621832 [DOI] [PubMed] [Google Scholar]
- 13. Hurst JW, Morris DC, Alexander RW. The use of the New York Heart Association’s classification of cardiovascular disease as part of the patient’s complete Problem List. Clin Cardiol 1999;22:385–90. 10.1002/clc.4960220604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, et al. . CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–16. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cutlip DE, Windecker S, Mehran R, et al. . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 16. Paiva L, Providência R, Barra S, et al. . Universal definition of myocardial infarction: clinical insights. Cardiology 2015;131:13–21. 10.1159/000371739 [DOI] [PubMed] [Google Scholar]
- 17. Cho KI, Ann SH, Singh GB, et al. . Combined usefulness of the platelet-to-lymphocyte ratio and the neutrophil-to-lymphocyte ratio in predicting the long-term adverse events in patients who have undergone percutaneous coronary intervention with a drug-eluting stent. PLoS One 2015;10:e0133934 10.1371/journal.pone.0133934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park JJ, Jang HJ, Oh IY, et al. . Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2013;111:636–42. 10.1016/j.amjcard.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 19. Sawant AC, Adhikari P, Narra SR, et al. . Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol J 2014;21:500–8. 10.5603/CJ.a2013.0148 [DOI] [PubMed] [Google Scholar]
- 20. Amulic B, Cazalet C, Hayes GL, et al. . Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012;30:459–89. 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- 21. Strydom N, Rankin SM. Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun 2013;5:304–14. 10.1159/000350282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naruko T, Ueda M, Haze K, et al. . Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation 2002;106:2894–900. 10.1161/01.CIR.0000042674.89762.20 [DOI] [PubMed] [Google Scholar]
- 23. Abou-Raya A, Abou-Raya S. Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun Rev 2006;5:331–7. 10.1016/j.autrev.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 24. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011;17:796–808. 10.1038/nm.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res 2012;110:875–88. 10.1161/CIRCRESAHA.111.257535 [DOI] [PubMed] [Google Scholar]
- 26. Blum A. Role of lymphocytes in heart disease. Circulation 1998;98:1590 10.1161/circ.98.15.1587/c [DOI] [PubMed] [Google Scholar]
- 27. Tamhane UU, Aneja S, Montgomery D, et al. . Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol 2008;102:653–7. 10.1016/j.amjcard.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 28. Tang TT, Yuan J, Zhu ZF, et al. . Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol 2012;107:232 10.1007/s00395-011-0232-6 [DOI] [PubMed] [Google Scholar]
- 29. Nishijima TF, Muss HB, Shachar SS, et al. . Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev 2015;41:971–8. 10.1016/j.ctrv.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 30. Gu L, Li H, Chen L, et al. . Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget 2016;7–42. doi:10.18632/oncotarget.7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan Z, Ji H, Li Y, et al. . Relationship between monocyte-to-lymphocyte ratio and coronary plaque vulnerability in patients with stable angina. Biomark Med 2017;11:979–90. 10.2217/bmm-2017-0235 [DOI] [PubMed] [Google Scholar]
- 32. Auffray C, Sieweke MH, Geissmann F, et al. . Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009;27:669–92. 10.1146/annurev.immunol.021908.132557 [DOI] [PubMed] [Google Scholar]
- 33. Ramsay SC, Maggs JA, Ketheesan N, et al. . Relative uptake of technetium 99m stannous colloid by neutrophils and monocytes is altered by gram-negative infection. Nucl Med Biol 2005;32:101–7. 10.1016/j.nucmedbio.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 34. Freytes DO, Santambrogio L, Vunjak-Novakovic G. Optimizing dynamic interactions between a cardiac patch and inflammatory host cells. Cells Tissues Organs 2012;195:171–82. 10.1159/000331392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dutta P, Courties G, Wei Y, et al. . Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–9. 10.1038/nature11260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fine DH. Methods for diagnosing and treating a heart condition in a patient: Google Patents. 2009;22:7. [Google Scholar]
- 37. Li Y, Zhong X, Cheng G, et al. . Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017;259:75–82. 10.1016/j.atherosclerosis.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 38. Porapakkham P, Porapakkham P, Zimmet H, et al. . B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch Intern Med 2010;170:507–14. 10.1001/archinternmed.2010.35 [DOI] [PubMed] [Google Scholar]
- 39. Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008;178:425–30. 10.1164/rccm.200803-459OC [DOI] [PubMed] [Google Scholar]
- 40. Fukazawa S, Teruya K, Uemura T, et al. . The relationship between long-term changes in plasma B-type natriuretic peptide levels and electrocardiographic findings. Environ Health Prev Med 2008;13:156–61. 10.1007/s12199-008-0027-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marcoon S, Chang AM, Lee B, et al. . HEART score to further risk stratify patients with low TIMI scores. Crit Pathw Cardiol 2013;12:1–5. 10.1097/HPC.0b013e31827377e1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023459supp001.pdf (208.7KB, pdf)