Abstract
Introduction
Concurrent chemoradiation is the standard therapy for patients with local advanced oesophageal carcinoma unsuitable for surgery. Paclitaxel is an active agent against oesophageal cancer and it has been proved as a potent radiation sensitiser. There have been multiple studies evaluating paclitaxel-based chemoradiation in oesophageal cancer, of which the results are inspiring. However, which regimen, among cisplatin (TP), carboplatin (TC) or fluorouracil (TF) in combination with paclitaxel concurrent with radiotherapy, provides best prognosis with minimum adverse events is still unknown and very few studies focus on this field. The purpose of this study is to confirm the priority of TF to TP or TF to TC concurrent with radiotherapy in terms of overall survival and propose a feasible and effective plan for patients with local advanced oesophageal cancer.
Methods and analysis
ESO-Shanghai 2 is a three-arm, multicenter, open-labelled, randomised phase III clinical trial. The study was initiated in July 2015 and the duration of inclusion is expected to be 4 years. The study compares two pairs of regimen: TF versus TP and TF versus TC concurrent with definitive radiotherapy for patients with oesophageal squamous cell carcinoma (OSCC). Patients with histologically confirmed OSCC (clinical stage II, III or IVa based on the sixth Union for International Cancer Control-tumour, node, metastasis classification) and without any prior treatment of chemotherapy, radiotherapy or surgery against oesophageal cancer will be eligible. A total of 321 patients will be randomised and allocated in a 1:1:1 ratio to the three treatment groups. Patients are stratified by lymph node status (N0, N1, M1a). The primary endpoint is overall survival and the secondary endpoint is progression-free survival and adverse events.
Ethics and dissemination
This trial has been approved by the Fudan University Shanghai Cancer Centre Institutional Review Board. Trial results will be disseminated via peer reviewed scientific journals and conference presentations.
Trial status
The trial was initiated in July 2015 and is currently recruiting patients in all of the participating institutions above.
Trial registration number
Keywords: esophageal squamous cell carcinoma, concurrent chemoradiotherapy, paclitaxel, cisplatin, carboplatin, fluorouracil
Strengths and limitations of this study.
This clinical trial is the first phase III randomised multicentred study comparing these three regimens.
In the randomisation session, patients were stratified by lymph node status (N0, N1, M1a based on the sixth Union for International Cancer Control-tumour, node, metastasis classification).
There is no stratification for different participation centres.
Introduction
Worldwide, oesophageal cancer is the eighth most common cancer, which is responsible for an estimated 455 800 new cases and 400 200 deaths in 2012.1 Since its prognosis is dismal, much effort has been put into improving overall survival through multimodality treatments, which consist of surgery, radiotherapy and chemotherapy.2 Concurrent chemoradiation is the standard non-operative therapy for local advanced oesophageal squamous cell carcinoma (OSCC).3
Paclitaxel is an active agent against oesophageal cancer, with the response rate of 28% in OSCC, and it has been shown to be a potent radiation sensitiser.4 There have been multiple studies evaluating paclitaxel-based chemoradiation in oesophageal cancer, for instance, paclitaxel/fluorouracil (TF) developed at The University of Texas MD Anderson Cancer Center and paclitaxel/cisplatin (TP) developed at Memorial Sloan-Kettering Cancer Center,5 6 with paclitaxel/carboplatin (TC) from Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) trial.7 In many preoperative studies, paclitaxel-based chemoradiotherapy has achieved inspiring effects; the pathological complete response rates of TP-based chemoradiotherapy were 19%–42%8–11 and of TC-based chemoradiotherapy was 49%.7 However, which regimen, among TF-based, TP-based and TC-based definitive chemoradiotherapy, provides best prognosis with minimum adverse events is still unknown and very few studies focus on this field.
RTOG 01135 evaluated two different paclitaxel-based regimens (TP and TF). Eighty-four patients were accrued to this study. Patients in arm A (TF) received induction 5-fluorouracil (5-FU), cisplatin and paclitaxel followed by radiation and concurrent continuous infusion 5-FU and weekly paclitaxel. Patients in arm B (TP) received induction paclitaxel and cisplatin followed by radiation and concurrent weekly cisplatin and 96 hours infusion of paclitaxel. The median survival time was 28.7 months for patients in arm A (TF) and 14.9 months for patients in arm B (TP). Neither arm achieved the hypothesised 1 year survival rate of at least 77.5%. The main deficiency of this study is the small sample size, but the effect of TF group is still inspiring.
Another retrospective multicenter randomised clinical trials from Europe12 showed that the overall survival of TC-based definitive chemoradiotherapy was comparable with cisplatin/5-FU (PF) as definitive concurrent chemoradiotherapy in oesophageal cancer. However, the toxicity rates were lower in the TC group together with higher treatment compliance.
Based on RTOG 0113 and other reports, we designed a clinical trial to confirm the priority of TF to TP and TF to TC concurrent with definitive radiotherapy in terms of overall survival for patients with local advanced OSCC. The trial is a three-arm, multicenter, open-labelled, randomised phase III clinical trial.
Methods and analysis
Patient selection
To be eligible for this study, patient must fulfil all of the following criteria (box 1):
bmjopen-2017-020785supp001.pdf (137.4KB, pdf)
Box 1. Inclusion criteria.
Histologically confirmed oesophageal squamous cell carcinoma.
Clinical stages II, III or IVa based on the sixth Union for International Cancer Control-tumour, node, metastasis classification.
No prior treatments of chemotherapy, radiotherapy or surgery against oesophageal cancer, except for non-curative resection by endoscopic mucosal resection/endoscopic submucosal dissection.
Aged 18–75 years.
Adequate organ functions for chemoradiation therapy: (1) white cell count ≥3×109⁄L; (2) absolute neutrophil counts ≥1.5×109⁄L; (3) haemoglobin ≥10 g⁄dL; (4) platelet ≥100×109⁄L; (5) total bilirubin <1.5 upper limit of normal (ULN); (6) aspartate transaminase ≤2.5 ULN; (7) alanine aminotransferase ≤2.5 ULN and (8) creatinine ≤1.5 ULN.
Eastern Cooperative Oncology Group performance status of 0–2.
Life expectancy ≥3 months, based on the judgement of doctors.
Written informed consent (Supplementary material).
Patients fulfilling any of the following criteria are ineligible for this study (box 2).
Box 2. Exclusion criteria.
Oesophageal perforation or haematemesis.
Synchronous or metachronous malignancies (except for cutaneous (non-melanomas) carcinoma, thyroid papillary carcinoma, phase I seminoma or cervical carcinoma in situ curatively treated and disease free for a minimum of 3 months).
Received thoracic, abdominal or craniocerebral surgery within 30 days.
Enrolled in other clinical trials within 30 days.
Unstable angina and/or congestive heart failure requiring hospitalisation within 6 months.
Severe psychiatric disease.
Pregnancy, lactation or unwillingness to adopt contraception.
Drug addiction.
AIDS based on current Centres for Disease Control and Prevention definition.
History of radiotherapy in the planning area.
Other ineligible conditions according to researchers.
Treatment
The treatment plan is shown in figure 1. Patients receive radiotherapy combined with concurrent chemotherapy. Radiotherapy begins on day 1, concurrent with the beginning of cycle 1 of chemotherapy.
Figure 1.
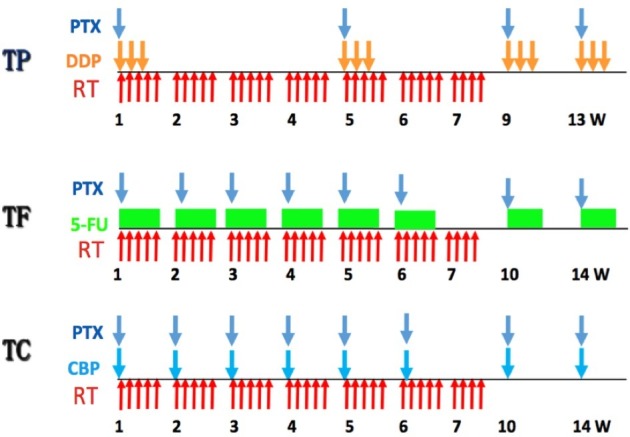
Treatment design of the ESO-Shanghai 2 trial. TP (arm A), TF (arm B) and TC (arm C) are TP-based, TF-based and TC-based definitive chemoradiotherapy, respectively. CBP, carboplatin; DDP, cisplatin; PTX, paclitaxel; w, week; 5-Fu, fluorouracil.
Same radiation therapy will be delivered in all three treatment groups. According to the current clinical practice in China, radiotherapy is delivered with photons (≥6 MV) to a total dose of 61.2 Gy in 34 fractions. Patients will be treated 5 days per week at 1.8 Gy/day. Three-dimensional conformal radiotherapy or intensity-modulated radiotherapy is required. All patient will be positioned in an individualised immobilisation device in the treatment position.
The definition of volumes will be in accordance with the 1993 ICRU Report #50 and 1999 ICRU Report #62.
The gross target volume (GTV) is defined as all known involved field, which detected by endoscopic ultrasound, barium swallow or CT scan (whichever is larger). The regional lymph nodes that have diameters more than 1 cm (0.5 cm for lymph nodes at tracheoesophageal groove) or that have been histologically proven metastatic after puncture are included in GTV.
The superior and inferior borders of the clinical target volume (CTV) are 3 cm beyond the primary tumour along the oesophagus. The lateral, anterior and posterior borders of the field are the same as GTV.
The superior, inferior, anterior, posterior and lateral borders of planning target volume (PTV) are 1 cm beyond CTV. Field next to the spinal cord could be slightly adjusted in order to reduce the exposure of spinal cord.
As for target volume, tissue inhomogeneity correction is adopted and it is required that more than 99% PTV receive 95% prescription dose and more than 95% PTV receive 99% or more prescription dose. Highest and lowest point dose inside PTV should be recorded.
When making the treatment plan, we should take normal organ dose restrictions into consideration as the following order (table 1).
Table 1.
Contour regulation and dose restriction of risk organs
| Risk organ | Contour regulation | Dose restriction |
| Spinal cord | All the layers of CT scan have to be contoured and the margin of vertebra tube can be regarded as that of planning organ at risk volume. | Highest point dose less than 45 Gy. |
| Lung | It is allowed to use automatic tools in the delineation of margin of lungs. (Trachea and bronchia must be contoured manually.) | The volume of lung (planning target volume excluded) receiving 20 Gy or higher has to be less than 30% of the total lung volume, and the mean dose has to be less than 15 Gy. |
| Heart | The superior margin of heart consists of right atrium and right ventricle, pulmonary artery trunk, ascending main aorta and superior vena cava excluded. The inferior margin is at the level of heart apex. | The mean dose has to be less than 40 Gy. |
Chemotherapy
Patients are randomly assigned to receive one of the three therapies.
Arm A (TP)
Patients in arm A receive four courses of TP every 4 weeks. Details are as follows:
Paclitaxel: 175 mg/m2/day, intravenously guttae (IVGTT) over 3 hours, day 1; cisplatin: 25 mg/m2/day, IVGTT, day 1–3.
Arm B (TF)
Patients in arm B receive six courses of TF concurrent with radiotherapy every week and two courses of TF consolidation chemotherapy every 4 weeks. Details are as follows:
Concurrent: paclitaxel 50 mg/m2/day, IVGTT over 3 hours, day 1; 5-FU 300 mg/m2, continuous intravenous infusion (CIV) 96 hours, day 1–4.
Consolidation: paclitaxel 175 mg/m2/day, IVGTT over 3 hours, d1; 5-FU 1800 mg/m2, CIV 72 hours, day 1–3.
Arm C (TC)
Patients in arm C receive six courses of TC concurrent with radiotherapy every week and two courses of TC consolidation chemotherapy every 4 weeks. Details are as follows:
Concurrent: paclitaxel 50 mg/m2/day, IVGTT over 3 hours, day 1; carboplatin area under the curve (AUC)=2, IVGTT, day 1.
Consolidation: paclitaxel 175 mg/m2/day, IVGTT over 3 hours, day 1; carboplatin AUC=5, IVGTT, day 1.
Patients receive premedication to prevent allergic reaction and significant nausea or vomiting as indicated.
Dose modifications
Radiotherapy interruption
If following toxicity is observed, radiotherapy has to be delayed until toxicity is no more than grade 2.
White cell count <2.0×109/L or absolute neutrophil count (ANC) <1.0×109/L.
Platelet <50×109/L.
Grade 3 or higher non-haematological toxicity.
If following toxicity is observed, radiotherapy has to be delayed until complete recovery.
Mediastinal or thoracic infection with fever over 38.5°C.
It is allowed to suspend at most 2 weeks or radiotherapy will be terminated.
Chemotherapy interruption and dose modifications
If following toxicity is observed on day 1, chemotherapy has to be delayed until toxicity is no more than grade 1.
ANC <1.5×109/L.
Platelet <100×109/L.
Grade 2 or higher non-haematological toxicity, except for nausea, vomiting and alopecia.
It is allowed to delay at most 2 weeks or chemotherapy will be terminated.
Chemotherapy dose modifications are based on the greatest toxicity during the last cycle. Any patients who need to make chemotherapy dose modifications will receive the modified dose in the following cycles.
If modifications are needed, dose of paclitaxel, cisplatin, carboplatin and 5-FU will decrease by 25% from the planned dose for the first time and 50% for the second time. It is allowed to make dose modifications at most twice or chemotherapy will be terminated. Details are as follows:
Dose modification of paclitaxel
Febrile neutropenia (ANC <0.5×109/L and fever over 38.3° C or over 38.0°C for 1 hour).
Grade 2 or higher peripheral neuropathy.
Dose modification of cisplatin and carboplatin
Febrile neutropenia (ANC <0.5×109/L and fever over 38.3° C or over 38.0° C for 1 hour).
Grade 2 or higher peripheral neuropathy.
Serum creatinine >3 upper limit of normal.
Dose modification of 5-FU
Febrile neutropenia (ANC <0.5×109/L and fever over 38.3°C or over 38.0° C for 1 hour).
Grade 3 or higher mucositis.
The adverse events will be evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE V.4.0). All adverse events, occurring during the course of the trial, which is from randomisation until 28 days after the end of treatment, regardless of relatedness to study medication, will be recorded. Adverse events occurring later than 28 days after the end of treatment will only be recorded if they are considered relevant.
Randomisation
After the confirmation of eligibility criteria, patients will be randomly allocated in a 1:1:1 ratio to the three treatment groups by a central randomisation centre (Fudan University Shanghai Cancer Center, Shanghai, China). Patients will be stratified by lymph node status (N0, N1, M1a). The SAS was used to generate a random permutation sequence and produce patient randomisation numbers. The data centre registers the enrollment, assigns a unique identification number to every participant and replies to the respective investigators.
Sample size calculation and statistical analysis
This three-arm randomised trial is designed to confirm whether TF is superior to TP or TC concurrent with radiotherapy in terms of overall survival. According to RTOG 0113 and other reports, median survival time of TF concurrent with radiotherapy for oesophageal cancer is 28.7 months, while TP 14.9 months5and TC 17.4 months13. According to the Schoenfeld and Richter’s method, the sample size of 107 patients per arm (154 events in total) is required to warrant a power of 80% at a two-sided α level of 0.025 for the comparison between TP and TF with relatively smaller difference, assuming an accrual period of 48 months, a minimum follow-up period of 24 months and a dropout rate of 10%.14 15 The total sample size is planned as 321 patients (107 patients in each arm, a total of 231 events).
The median overall survival will be estimated with Kaplan-Meier method, and log-rank test will be used to compare the overall survival among treatment arms. We will conduct a subgroup analyses to test whether the treatment effects differ among subgroups (N0, N1, M1a).
Endpoints
The primary endpoint is overall survival in all randomised patients. Overall survival is defined as time from the date of randomisation until death. The secondary endpoint is progression-free survival (PFS) and adverse events. PFS is defined as the time from the date of randomisation to the date of progression or to the date of death, whichever occurs first and disease progression will be evaluated according to RECIST V.1.1. Adverse events will be evaluated according to the National Cancer Institute CTCAE V.4.0.
Data collection
Participants will be seen at hospitals or contacted by telephone and letters from randomisation to the last treatment cycle, then at months 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 54 and 60 after last treatment. Research staffs at the hospitals will be expected to complete trial case report forms.
Interim analysis
We plan to conduct two interim analyses. The first interim analysis will be conducted independently from the study group when half of the planned number of patients are enrolled and the second interim just after the planned patient accrual is completed. If the superiority of one of the test arms (TF arm superior to TP arm or TC arm) is demonstrated with an adjusted α level, the study will be terminated.
In general, the interim reports will contain the following information:
Patient accrual rate with a projected completion date (while the study is still accruing).
Total patients accrued.
Distributions of important pretreatment and prognostic baseline variables.
The frequencies and severity of adverse events by treatment arm.
Compliance rates of treatment delivery.
Observed results with respect to the primary and secondary endpoints.
Patient and public involvement
Neither patients nor public will be involved in the design, recruitment, outcome measures and conduct of the study. Trial results will be disseminated via peer-reviewed scientific journals and conference presentations rather than specifically notified to a single patient.
Ethics and dissemination
This trial has been approved by all participating centres including Fudan University Shanghai Cancer Center Institutional Review Board (Ethics Committee of Fudan University Shanghai Cancer Center: No. 1505146–13). Written informed consent will be obtained from all participants. Serious adverse events will be reported to the safety desk of the trial, the Data and Safety Monitoring Board and trial sites. Trial results will be disseminated via peer reviewed scientific journals and conference presentations.
Participating institutions (from east to west)
Fudan University Shanghai Cancer Center, Huadong Hospital Affiliated to Fudan University, Fudan University Shanghai Cancer Center Minhang Branch, Affiliated Hospital of Jiangnan University, Fujian Province Cancer Hospital, Jiangsu Province Cancer Hospital, The First Affiliated Hospital of Xiamen University, Jiangxi Province Cancer Hospital, Shanxi Province Cancer Hospital, Hainan Province People’s Hospital, Gansu Province Cancer Hospital.
Supplementary Material
Footnotes
Contributors: DA was responsible for drafting the manuscript. YC, QL, JZ, JD, HZ, WR, KW, MF, HY, ZZ, WZ and LL were responsible for the collection of previous study and putting forward the conception. XZ, YL, JY, JZ, QL, HL, JC, SW, JF, JL, GH and HB were responsible for designing the details of the study. KZ was responsible for all aspects of trial design, the protocol and trial conduct. All authors have read and approved this manuscript.
Funding: The study was supported by 2015 Prospective Clinical Research Fund of Fudan University Shanghai Cancer Center.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Fudan University Shanghai Cancer Center Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Torre LA, Bray F, Siegel RL, et al. . Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52. 10.1056/NEJMra035010 [DOI] [PubMed] [Google Scholar]
- 3. Herskovic A, Martz K, al-Sarraf M, et al. . Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8. 10.1056/NEJM199206113262403 [DOI] [PubMed] [Google Scholar]
- 4. Ajani JA, Ilson DH, Daugherty K, et al. . Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 1994;86:1086–91. 10.1093/jnci/86.14.1086 [DOI] [PubMed] [Google Scholar]
- 5. Ajani JA, Winter K, Komaki R, et al. . Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol 2008;26:4551–6. 10.1200/JCO.2008.16.6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schnirer II, Komaki R, Yao JC, et al. . Pilot study of concurrent 5-fluorouracil/paclitaxel plus radiotherapy in patients with carcinoma of the esophagus and gastroesophageal junction. Am J Clin Oncol 2001;24:91–5. 10.1097/00000421-200102000-00018 [DOI] [PubMed] [Google Scholar]
- 7. Shapiro J, van Lanschot JJB, Hulshof M, et al. . Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–8. 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 8. Safran H, Gaissert H, Akerman P, et al. . Paclitaxel, cisplatin, and concurrent radiation for esophageal cancer. Cancer Invest 2001;19:1–7. 10.1081/CNV-100000068 [DOI] [PubMed] [Google Scholar]
- 9. Bains MS, Stojadinovic A, Minsky B, et al. . A phase II trial of preoperative combined-modality therapy for localized esophageal carcinoma: initial results. J Thorac Cardiovasc Surg 2002;124:270–7. 10.1067/mtc.2002.122545 [DOI] [PubMed] [Google Scholar]
- 10. Urba SG, Orringer MB, Ianettonni M, et al. . Concurrent cisplatin, paclitaxel, and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer 2003;98:2177–83. 10.1002/cncr.11759 [DOI] [PubMed] [Google Scholar]
- 11. Lin CC, Hsu CH, Cheng JC, et al. . Concurrent chemoradiotherapy with twice weekly paclitaxel and cisplatin followed by esophagectomy for locally advanced esophageal cancer. Ann Oncol 2007;18:93–8. 10.1093/annonc/mdl339 [DOI] [PubMed] [Google Scholar]
- 12. Honing J, Smit JK, Muijs CT, et al. . A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol 2014;25:638–43. 10.1093/annonc/mdt589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haj Mohammad N, Hulshof MCCM, Bergman JJ, et al. . Acute toxicity of definitive chemoradiation in patients with inoperable or irresectable esophageal carcinoma. BMC Cancer 2014;14 10.1186/1471-2407-14-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lakatos E. Designing complex group sequential survival trials. Stat Med 2002;21:1969–89. 10.1002/sim.1193 [DOI] [PubMed] [Google Scholar]
- 15. Lakatos E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics 1988;44:229-41 10.2307/2531910 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-020785supp001.pdf (137.4KB, pdf)


