Summary
Background
In the UK, gout management is suboptimum, with only 40% of patients receiving urate-lowering therapy, usually without titration to achieve a target serum urate concentration. Nurses successfully manage many diseases in primary care. We compared nurse-led gout care to usual care led by general practitioners (GPs) for people in the community.
Methods
Research nurses were trained in best practice management of gout, including providing individualised information and engaging patients in shared decision making. Adults who had experienced a gout flare in the previous 12 months were randomly assigned 1:1 to receive nurse-led care or continue with GP-led usual care. We assessed patients at baseline and after 1 and 2 years. The primary outcome was the percentage of participants who achieved serum urate concentrations less than 360 μmol/L (6 mg/dL) at 2 years. Secondary outcomes were flare frequency in year 2, presence of tophi, quality of life, and cost per quality-adjusted life-year (QALY) gained. Risk ratios (RRs) and 95% CIs were calculated based on intention to treat with multiple imputation. This study is registered with www.ClinicalTrials.gov, number NCT01477346.
Findings
517 patients were enrolled, of whom 255 were assigned nurse-led care and 262 usual care. Nurse-led care was associated with high uptake of and adherence to urate-lowering therapy. More patients receiving nurse-led care had serum urate concentrations less than 360 μmol/L at 2 years than those receiving usual care (95% vs 30%, RR 3·18, 95% CI 2·42–4·18, p<0·0001). At 2 years all secondary outcomes favoured the nurse-led group. The cost per QALY gained for the nurse-led intervention was £5066 at 2 years.
Interpretation
Nurse-led gout care is efficacious and cost-effective compared with usual care. Our findings illustrate the benefits of educating and engaging patients in gout management and reaffirm the importance of a treat-to-target urate-lowering treatment strategy to improve patient-centred outcomes.
Funding
Arthritis Research UK.
Introduction
Gout is the most common inflammatory arthritis worldwide, and in the UK the prevalence has risen from 1·5% in 1997 to 2·5% in 2012.1 Gout results from sodium urate crystals that form when serum urate persistently exceeds saturation.2 Deposition of sodium urate crystals can cause extremely painful gout flares or attacks, joint damage, and subcutaneous nodules (tophi). Furthermore, gout and hyperuricaemia are associated with various comorbidities,3 increased mortality,4 and reduced quality of life.5 The causes of hyperuricaemia are known, and urate-lowering treatments can maintain serum urate concentrations at less than saturation, which prevents crystal formation and dissolves existing crystals, making gout the only common arthritis where the pathogenic agent can be eliminated. Addressing risk factors for hyperuricaemia (eg, overweight, excessive alcohol intake, high dietary intake of purines and fructose) is advised as well as medical treatment, but alone rarely reduces concentrations of serum urate sufficiently to alter the disease course.6, 7
Despite good understanding of the disease and the availability of curative treatment, gout care remains suboptimum.1, 8 In the UK, gout is managed predominantly in primary care by general practitioners (GPs), but less than half of patients receive urate-lowering therapy.1, 9 In those who do, the dose is usually fixed without titration to achieve a target serum urate concentration9 and adherence is poor.10, 11 Common misconceptions about gout (eg, that it is not a serious condition and that it is self-induced by lifestyle) are important barriers to care8, 12 and, therefore, education of patients is central to management.7, 13 Unfortunately, some physicians share these misconceptions12 and many, because of factors such as work pressures, might have insufficient time to educate patients adequately.
Research in context.
Evidence before this study
Despite increasing prevalence of gout in the UK, various barriers prevent patients from receiving optimum care. Only 40% of gout patients receive urate-lowering therapy that is usually given at a fixed dose without titration to achieve a target serum urate concentration, and adherence is poor. In a search done for a previous proof-of-concept study, we found no long-term randomised controlled trials that had assessed recommended best practice management of gout in a primary care setting. The proof-of-concept study showed that when people with gout are fully informed and involved in management decisions, uptake of urate-lowering therapy is high and adherence under nurse-led care is excellent. The findings led to a 2-year randomised controlled community trial to compare directly nurse-led care and usual care led by general practitioners (GPs).
Added value of this study
Nurse-led care, including providing patients with individualised information and engaging them with care, along with a strategy of treat-to-target urate-lowering therapy, resulted in very high treatment uptake and adherence. Patient-centred outcomes, such as flare frequency, tophi, and quality of life were substantially improved. Adherence was 95% at 2 years, which is greater than in any previously reported clinical studies of gout. We also found that the allopurinol doses needed to achieve target serum urate concentrations were greater than the upper dose of 300 mg/day used by most UK GPs. The nurse-led approach was cost-effective in the short-term and would potentially be cost saving in the long-term.
Implications of all the available evidence
Individualised education and engagement of patients and a treat-to-target strategy are important elements in successful management of gout. The management principles are potentially generalisable to any health professionals who manage people with gout. These findings add to the evidence that refutes the American College of Physicians advice, which seems to ignore patients' involvement in deciding whether urate-lowering therapy should be used, and, when it is used, to not treat to a target serum urate concentration.
In the UK, community-based nurses manage many chronic conditions, and in randomised controlled trials nurse-led care has been similar or superior to physician care.14 We did a proof-of-concept study involving 106 gout patients.15 When the patients were fully informed and involved in decision making, all wanted urate-lowering therapy and, after 1 year of nurse-led care, 92% had serum urate concentrations within the target range (<360 μmol/L [6 mg/dL]), which we based on national and international guidelines and recommendations,7, 13, 16 and had started to show improvements in patient-centred outcomes. Our findings prompted us to do a randomised controlled trial in primary care over a period long enough to show improvements in patient-centred outcomes. The objectives were to compare the efficacy, including patient-centred outcomes, and cost-effectiveness of nurse-led care, reflecting recommended best practice, with usual GP-led care of patients with gout over 2 years.
Methods
Study design and participants
We did a parallel arm, non-blinded randomised controlled trial in 56 East Midlands general practices that represented urban and rural settings around the Nottingham area. Each practice sent a questionnaire to adults (age >21 years) on their database who had a diagnosis of gout. Respondents who reported at least one gout flare in the previous 12 months and indicated willingness for further contact were sent information on the study which explained that nurses successfully manage many long-term conditions in primary care and that the aim of the trial was to assess how well gout would be managed over a 2-year period by specially trained nurses compared with GPs. Patients who were interested in participating returned a reply slip and were telephoned to ensure they fulfilled 1977 American College of Rheumatology gout classification criteria.17 Eligible patients were assessed in their GP surgery by a research nurse. Placement of a study advertisement in two Nottinghamshire newspapers was added to the study protocol as a further recruitment approach. People who responded to these advertisements were sent the questionnaire and those willing to participate underwent telephone screening and assessment in their homes. Exclusion criteria were not meeting the 1977 American College of Rheumatology gout classification criteria, inability to consent, and terminal or severe illness. After a protocol amendment, financial incentives were offered to unresponsive patients in the usual-care group in return for completing the questionnaire and attending the assessment at the end of year 2.
The study was approved by East Midlands Nottingham Research Ethics Committee (12/EM/0044). The University of Nottingham was the study sponsor (reference 11115). The study protocol, including amendments following commencement of the study, and the statistical analysis plan, are available online. All participants gave written informed consent.
Randomisation
A randomisation schedule was created centrally by the Nottingham Clinical Trials Unit, with a secure web-based system. Patients were assigned 1:1 to receive nurse-led care or GP-led usual care in randomly permuted blocks of two, four, or six, stratified by the clinical commissioning groups to which the general practices belonged (n=26). To enrol a patient in the study, a research nurse telephoned the trial coordination office in Academic Rheumatology, University of Nottingham, where a researcher accessed the online system to ascertain the next treatment allocation, which was given to the nurse during the telephone call. Patients were told their allocation by the nurse and their GP was informed by letter.
Intervention
Nurses received training about gout and its management that reflected national and international recommendations and was developed from qualitative research on illness perceptions12 and determination of the key elements to explaining gout that we had established in our previous proof-of-concept study (appendix).15 As part of an individualised package of care, the nurses provided patients with holistic assessment, discussion of illness perceptions, and full information on gout (nature, causes, associations, consequences, and treatment options), and encouraged them to share in decision making.13 Patients were given the Arthritis Research UK gout information booklet. Follow-up assessments and measurement of serum urate concentrations were done as often as required by the nurse. Telephone contact (eg to review serum urate results) could be substituted for face-to-face visits, and home visits were permitted (eg for older patients). Urate-lowering therapy was obtained from Nottingham City Hospital Pharmacy. As recommended,7, 13 first-line treatment was oral allopurinol, started at 100 mg once per day and titrated upwards in 100 mg increments every 3–4 weeks according to serum urate concentrations, to a maximum of 900 mg once per day. As second-line options, oral febuxostat could be started at 80 mg and if required increased to the maximum dose of 120 mg once per day or benzbromarone could be started at 50 mg and titrated up in 50 mg increments to a maximum of 200 mg once per day. Combination urate-lowering therapy (xanthine oxidase inhibitor plus uricosuric) could be used as the final treatment option. Colchicine as prophylaxis against gout flares could be considered. If the nurses had questions about gout management, they could seek advice from a study rheumatologist (MD, FR, or AA). All contacts with participants were logged.
Patients assigned to continue usual GP-led care were given the gout information booklet from Arthritis Research UK. Treatment of flares could be discussed by the research nurse at baseline and at yearly assessments, but if participants enquired about other aspects of management they were advised to ask their GP.
Assessments
At baseline we recorded patients' self-reported age, sex, gout history (age at onset and flare frequency in the previous 12 months), medications, comorbidities, and quality of life (measured with the Short Form [36 item] general Health questionnaire [SF-36]18 and the Gout Assessment Questionnaire, which includes the Gout Impact Scale disease-specific quality of life measure).19 We also assessed patients by clinical examination at baseline to measure body-mass index and subcutaneous tophi (number, sites, and maximum diameter of the largest tophus measured with a Vernier caliper) and did blood tests to measure serum urate concentration and creatinine concentration to estimate glomerular filtration rate.
Patients in both study groups were given diaries in which to record flares.
Outcomes
The primary outcome was the percentage of patients who had achieved serum urate concentrations less than 360 μmol/L at 2 years. Secondary outcomes were other serum urate measures (percentage of patients who had achieved serum urate concentrations <360 μmol/L at 1 year, <300 μmol/L at 1 and 2 years, and group mean serum urate concentrations at 1 and 2 years); frequency of gout flares during years 1 and 2; the percentage of patients with tophi overall; the median number of tophi and the maximum diameter of the largest tophus at 1 and 2 years among patients with tophi at baseline; quality of life (physical and mental components) and Gout Impact Scale at 1 and 2 years; and cost-effectiveness, calculated as cost per quality-adjusted life-year (QALY) gained.
Statistical analysis
We initially calculated that an overall sample size of 724 patients would be needed to show a difference in quality of life between groups in this trial based on two Nottingham observational studies.20, 21 After we obtained data from our proof-of-concept study15 we revised the sample size estimate. For 90% power at a significance level of 0·05 (two-tailed), the sample size needed was 20 to show a difference in the percentage of patients achieving serum urate concentrations less than 360 μmol/L (92% vs 13%),15 166 to assess a difference in the frequency of acute flares (mean difference 2·4 [SD 2·3]),15 and 648 to assess a difference in quality of life (mean difference 0·74 [SD 2·9]).21 Owing to recruitment being slower than expected, we reduced the power to 80% at a significance level of 0·05 (two-tailed), changing the required sample sizes to 16, 124, and 486, respectively. Thus, we aimed to recruit 512 patients, allowing for 10% dropout over 2 years.
We compared groups at baseline and after 1 and 2 years by intention to treat with multiple imputation, assuming that data were missing at random. A Markov chain Monte Carlo method was used to impute missing continuous data, and a fully conditional specification model was used to impute missing dichotomous or categorical data. Missing values were imputed within each group with adjustment for the baseline level of the imputed variable, serum urate concentration, use of urate-lowering treatment, age, and number of flares. We did a per-protocol analysis involving patients who completed 2 years of treatment to test sensitivity.
We used ANCOVA to analyse continuous variables (between-group comparisons and tests for linear trend). Risk ratio (RR) and 95% CIs were calculated for dichotomous data with Poisson regression. We adjusted all analyses for the baseline level of the outcome assessed. A repeated-measures analysis was used to assess linear trends for effect of time from baseline to 1 year and 2 years. We used the GLM procedure in SAS (version 9.4) with three time points (baseline, 1 year, and 2 years) to handle multilevel data and to adjust for cluster effects within individuals.
The cost-effectiveness analysis was done with a National Health Service perspective and a lifetime horizon. A state transition model was constructed based on four serum urate concentration ranges (<360 μmol/L, ≥360 to <480 μmol/L, ≥480 to <600 μmol/L, and ≥600 μmol/L). To reduce stochasticity in the model we assumed that the flare rates per range of serum urate concentrations would be constant within each of the periods 0–6 months, 7–12 months, and 13–24 months. These values were calculated as the number of flares divided by the number of patient-months of follow-up during a period. Beyond 24 months we assumed that the flare rates per range of serum urate concentration would be independent of initial management and set the values to the average nurse-led and usual-care values between 12 and 24 months.
The cost of the management of gout flares was estimated according to the National Institute for Health and Care Excellence (NICE) review of treatment with pegloticase.22 We did a sensitivity analyses in which we lowered the cost per flare (from £341 to £50) and added nurse time for reviewing patients (an extra 30 min per 6 months) in years 3 and 4 (appendix). We also did a post-hoc analysis that made pessimistic assumptions regarding the efficacy of the nurse-led approach, in which the flare rates were increased by 20% in the first 2 years and the split of patients across the serum urate concentration ranges at the end of year 2 was altered (appendix). The model was simplistic and typically unfavourable to the nurse-led treatment, for example, it ignored the effects of tophi, assumed that longer-term flare rates within each serum urate concentration band are independent of treatment strategy, and that crystals did not dissolve in patients with serum urate concentrations maintained below 360 μmol/L. Finally, we did a post-hoc comparison of participants with renal impairment (defined as chronic kidney disease stage 3 [estimated glomerular filtration rate <60 mL/min per 1·73 m2]) and those without renal impairment in the nurse-led group to investigate the percentages of patients taking urate-lowering therapy, with serum urate concentrations less than 360 μmol/L, and with side-effects while taking urate-lowering therapy. We used the χ2 test for comparison between groups.
This study is registered with www.ClinicalTrials.gov, number NCT01477346.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Of 6806 questionnaires sent to patients by GP practices, 2815 (41%) were returned (figure 1). 1605 (57%) of 2815 respondents reported having had gout flares in the previous year, among whom 1071 were willing to be contacted further. Of these, 524 were screened and 505 were randomly assigned to a treatment group. Of the 48 people who responded to the advertisement, 12 were screened and randomised (figure 1). The first participant entered the study on March 21, 2013, and follow-up of the last participant finished on Oct 25, 2016.
Figure 1.
Trial profile
ITT=intention-to-treat. GP=general practitioner. *Patients with a diagnosis of gout who had had at least one gout flare in the previous 12 months. †Unable to commit time, poor health, gout flare more 12 months previously, advised by surgery not to contact, initial appointment was booked but cancelled and could not be rearranged.
Patients' characteristics did not differ significantly at baseline (table 1). Most were middle-aged white men, mean gout duration was 12 years, and 11% of patients had tophi at baseline. 203 (39%) were taking urate-lowering therapy at baseline, of whom 202 were taking allopurinol. The mean dose at baseline was 227 mg/day (SD 101) and only seven (7%) patients were taking more than 300 mg/day. Only around 20% of patients had serum urate concentration less than 360 μmol/L.
Table 1.
Baseline characteristics
| Nurse-led care (n=255) | Usual care (n=262) | ||
|---|---|---|---|
| Age (years) | 62·01 (10·81) | 63·69 (11·91) | |
| Women/men | 26 (10%)/229 (90%) | 30 (11%)/232 (89%) | |
| White | 246 (97%) | 255 (97%) | |
| BMI (kg/m2) | 29·78 (5·36) | 29·79 (4·77) | |
| BMI ≥30 kg/m2 | 102 (40%) | 106 (41%) | |
| Comorbidities (self-reported) | |||
| Heart disease | 50 (20%) | 52 (20%) | |
| Hypertension | 137 (54%) | 142 (54%) | |
| Diabetes | 32 (13%) | 37 (14%) | |
| Hyperlipidaemia | 69 (27%) | 93 (36%) | |
| History of renal stones | 15 (6%) | 18 (7%) | |
| Renal function | |||
| eGFR (mL/min per 1·73 m2) | 71·5 (15·9) | 70·2 (15·9) | |
| Chronic kidney disease stage 3* | 58 (23%) | 63 (24%) | |
| Creatinine concentration (μmol/L) | 94·0 (26·3) | 94·7 (24·3) | |
| Age at first gout flare (years) | 50·4 (13·0) | 51·0 (14·7) | |
| Gout disease duration (years) | 11·6 (9·8) | 12·7 (10·6) | |
| Flares in previous year | |||
| Two or more | 203 (80%) | 209 (80%) | |
| Four or more | 97 (38%) | 92 (35%) | |
| Ten or more | 27 (11%) | 19 (7%) | |
| Pain severity during flares† | 8·3 (1·5) | 8·2 (1·6) | |
| Tophi present | 35 (14%) | 23 (9%) | |
| Median (IQR) number of tophi | 2 (1–4) | 2 (1–3) | |
| Diameter of largest tophus (mm) | 16·9 (14·3) | 20·1 (14·0) | |
| Serum urate concentration (μmol/L) | 443·1 (100·5) | 438·9 (98·2) | |
| <360 μmol/L | 57 (22%) | 56 (22%) | |
| Taking urate-lowering therapy | 101 (40%) | 102 (39%) | |
| Allopurinol | 101 (100%) | 101 (99%) | |
| Sulfinpyrazone | 0 | 1 (1%) | |
Data are mean (SD) or n (%) unless stated otherwise. BMI=body-mass index. eGFR=estimated glomerular filtration rate.
eGFR <60 mL/min per 1·73 m2.
Measured with a numerical rating scale, where 0=no pain and 10= severe pain.
Of 517 patients who started the study, 482 (93%) completed 1 year and 441 (85%) completed 2 years (figure 1). A comparison of patients who did and did not remain in the study is shown in the appendix. Retention was higher in the nurse-led group than in the usual-care group (20 vs 46 completed the study, p<0·0001). Two patients in the nurse-led group and eight in the usual-care group died (p=0·0611; figure 1). There were no significant differences between groups in changes of body-mass index or renal function during the study (appendix).
At 2 years, multiple imputation showed that 95% of participants in the nurse-led group had achieved serum urate concentrations less than 360 μmol/L compared with 30% in the usual care group (RR 3·18, 95% CI 2·42–4·18, table 2). A similar difference was seen after 1 year (table 2). Concentrations less than 300 μmol/L at 2 years had been achieved in 88% of patients receiving nurse-led care, compared with 17% in the usual-care group (p<0·0001). The mean serum urate concentrations in the nurse-led group were significantly lower than those in the usual-care group after 1 and 2 years (table 3, Figure 2, Figure 3). The differences between groups at 1 and 2 years reflect a mean decrease of 183 μmol/L in the first 3 months of treatment in the nurse-led group that was sustained compared with reductions of around 10 μmol/L achieved by each of years 1 and 2 in the usual-care group.
Table 2.
Dichotomous efficacy outcomes
| Nurse-led care (n=255) | Usual care (n=262) | Risk ratio (95% CI) | |
|---|---|---|---|
| Serum urate concentration <360 μmol/L | |||
| Baseline | 22·35% | 21·46% | 1·04 (0·72–1·51) |
| 1 year | 94·75% | 26·22% | 3·59 (2·72–4·75) |
| 2 years | 94·88% | 29·71% | 3·18 (2·42–4·18) |
| p for trend within group | <0·0001 | 0·0757 | .. |
| Serum urate concentration <300 μmol/L | |||
| Baseline | 8·63% | 10·34% | 0·83 (0·48–1·46) |
| 1 year | 87·42% | 13·75% | 6·46 (4·46–9·34) |
| 2 years | 88·05% | 17·46% | 5·11 (3·61–7·23) |
| p for trend within group | <0·0001 | 0·0387 | .. |
| Taking urate-lowering therapy | |||
| Baseline | 39·61% | 38·93% | 1·02 (0·77–1·34) |
| 1 year | 96·70% | 46·83% | 2·06 (1·65–2·57) |
| 2 years | 96·10% | 56·13% | 1·71 (1·38–2·11) |
| p for trend within group | <0·0001 | 0·0053 | .. |
| Two or more flares | |||
| Baseline | 79·92% | 79·77% | 1·00 (0·83–1·22) |
| 1 year | 53·99% | 39·82% | 1·36 (1·05–1·77) |
| 2 years | 8·00% | 24·29% | 0·33 (0·19–0·57) |
| p for trend within group | <0·0001 | <0·0001 | .. |
| Four or more flares | |||
| Baseline | 38·04% | 35·11% | 1·08 (0·82–1·44) |
| 1 year | 27·92% | 20·76% | 1·33 (0·92–1·92) |
| 2 years | 1·15% | 12·39% | 0·09 (0·02–0·36) |
| p for trend within group | <0·0001 | <0·0001 | .. |
| Presence of tophi | |||
| Baseline | 13·73% | 8·78% | 1·56 (0·92–2·65) |
| 1 year | 7·06% | 10·15% | 0·53 (0·28–1·02) |
| 2 years | 2·85% | 11·29% | 0·21 (0·08–0·52) |
| p for trend within group | <0·0001 | 0·4145 | .. |
Percentage and risk ratio values were estimated with multiple imputation with the assumption that data were missing at random.
Table 3.
Continuous efficacy outcomes
| Nurse-led care (n=255) | Usual care (n=262) | Mean difference (95% CI) | ||
|---|---|---|---|---|
| Serum urate concentration (μmol/L) | ||||
| Baseline | 443·07 (100·50) | 438·85 (98·17) | 4·22 (−12·97 to 21·40) | |
| 1 year | 250·56 (60·59) | 427·87 (103·65) | 178·86 (164·80 to 192·92) | |
| 2 years | 251·52 (72·15) | 421·13 (109·62) | 170·98 (154·37 to 187·58) | |
| p for trend within group | <0·0001 | 0·0647 | ||
| Number of tophi | ||||
| Baseline | 2 (1–4) | 2 (1–3) | 0·28 (−2·89 to 3·45) | |
| 1 year | 1 (1–3) | 1 (1–2) | 2·19 (0·77 to 3·61) | |
| 2 years | 1 (1–1) | 1 (1–2) | 2·06 (0·94 to 3·19) | |
| p for trend within group | 0·0010 | 0·3784 | .. | |
| Diameter of largest tophus (mm) | ||||
| Baseline | 16·89 (14·08) | 20·09 (13·25) | −3·20 (−10·73 to 4·32) | |
| 1 year | 7·53 (11·34) | 16·54 (16·27) | 7·18 (1·08 to 13·28) | |
| 2 years | 3·29 (7·89) | 13·61 (15·06) | 8·77 (3·75 to 13·79) | |
| p for trend within group | <0·0001 | 0·1478 | .. | |
| SF-36 score | ||||
| Physical component | ||||
| Baseline | 35·64 (14·20) | 35·48 (14·29) | 0·16 (−2·31 to 2·62) | |
| 1 year | 40·46 (14·10) | 36·54 (14·21) | 3·82 (1·88 to 5·76) | |
| 2 years | 41·01 (16·71) | 37·43 (14·80) | 3·48 (1·20 to 5·75) | |
| p for trend within group | <0·0001 | 0·1371 | .. | |
| Mental component | ||||
| Baseline | 51·44 (10·47) | 52·81 (10·35) | 1·37 (0·43 to 3·17) | |
| 1 year | 53·46 (8·99) | 54·01 (9·33) | 0·21 (−1·14 to 1·56) | |
| 2 years | 52·92 (14·34) | 54·02 (9·26) | 0·22 (−1·62 to 2·07) | |
| p for trend within group | 0·1582 | 0·1658 | .. | |
| Gout Impact Scale score | ||||
| Gout concern overall | ||||
| Baseline | 71·56 (23·61) | 68·51 (23·14) | 3·31 (−0·71 to 7·33) | |
| 1 year | 48·78 (25·05) | 57·79 (26·53) | 10·66 (6·39 to 14·93) | |
| 2 year | 37·54 (24·97) | 53·62 (27·02) | 17·54 (13·15 to 21·94) | |
| p for trend within group | <0·0001 | <0·0001 | .. | |
| Unmet gout treatment need | ||||
| Baseline | 44·33 (21·81) | 43·19 (21·62) | 1·14 (−2·62 to 4·90) | |
| Year-1 | 25·62 (18·16) | 36·29 (18·81) | 11·00 (7·71 to 14·30) | |
| Year-2 | 21·03 (15·93) | 33·71 (19·67) | 12·88 (9·58 to 16·19) | |
| p for trend within group | <0·0001 | <0·0001 | .. | |
Data in groups are mean (SD) or median (IQR). Values were calculated with multiple imputation with the assumption that data were missing at random. SF-36=Short Form (36 item) Health Survey.
Figure 2.
Mean (95% CI) serum urate concentrations throughout the study
Data in the usual-care group were only available at baseline, 1 year, and 2 years but serum urate monitoring data recorded in follow-up visits were available in the nurse-led group.
Figure 3.
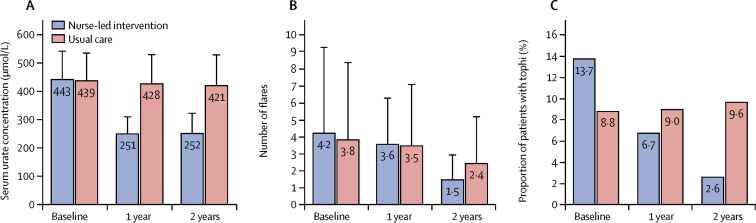
Serum urate concentration, number of flares and presence of tophi at baseline, 1 year, and 2 years
(A) Mean (95% CI) serum urate concentration. (B) Mean (95% CI) number of flares. (C) Proportion of patients with any tophi.
Use of urate-lowering therapy increased in both groups but was significantly higher in the nurse-led group than in the usual-care group at 1 and 2 years (table 2). In the nurse-led group, based on multiple imputations, 97% and 96% of patients were taking urate-lowering therapy at 1 year and 2 years, respectively, compared with 47% and 56%, respectively, in the usual-care group. Most patients were taking allopurinol (84% vs 96%). Doses of allopurinol were greater than 300 mg/day in 80% patients in the nurse-led group at 1 year and in 79% at 2 years, compared with 11% and 10%, respectively, in the usual-care group (appendix). The mean dose at 2 years in the nurse-led group was 460 mg/day compared with 230 mg/day in the usual-care group. Among the patients taking other urate-lowering therapy, febuxostat was being taken by more patients in the nurse-led group than in the usual-care group at 2 years (14% vs 3%). Only four patients in the nurse-led group and one in the usual-care group were receiving uricosurics at 2 years and none required combination urate-lowering therapy. Only three participants in the nurse-led group elected to receive colchicine prophylaxis against gout during the titration phase of urate-lowering therapy.
In the nurse-led group 24 (10%) patients discontinued urate-lowering therapy, among whom 12 did so due to one or more side-effects (rash or pruritus n=4; reduced estimated glomerular filtration rate n=4; gastrointestinal upset n=2; arthralgia n=2; systemic upset, including fatigue and sweating n=2; reduced libido n=1; and breast tenderness, flushes, and cramps n=1) and the remainder because of no perceived benefit and wish for different treatment (n=8), no response to urate-lowering therapy after titration (n=3), and cessation during chemotherapy (n=1). All side-effects resolved within 1 week of stopping treatment. All 24 patients wished to receive an alternative urate-lowering regimen and were successfully taking treatment by the end of year 1, including two patients who had second regimen changes.
Equivalent surveillance and monitoring of side-effects were not available in the usual care group. Two (1%) participants reported side-effects attributed to allopurinol that led to discontinuation, and neither was started on another urate-lowering regimen. A post-hoc comparison of patients with and without renal impairment in the nurse-led group showed no difference after 2 years among those taking urate-lowering therapy (50 [94%] of 58 with renal impairment vs 171 [97%] of 191 without renal impairment), those achieving serum urate concentrations less than 360 μmol/L (50 [94%] vs 168 [95%]) or less than 300 μmol/L (46 [87%] vs 156 [89%]), or those who had had side-effects associated with allopurinol (eight [14%] vs 15 [8%]).
Flare frequency reduced gradually from baseline to 2 years in both groups (p<0·0001 for trend, table 2, figure 3). The risk of having two or more flares per year was higher in the nurse-led group than in the usual-care group at 1 year but had become much lower after 2 years (table 2). A similar pattern was observed for risk of four or more flares (table 2).
The risk of having any tophi was similar in the two treatment groups at baseline, but number of patients with tophi reduced from 35 (14%) at baseline to six (3%) at 2 years in the nurse-led group, whereas no change was seen in the usual-care group (table 2, figure 3). The median number of tophi fell in the nurse-led group (p<0·0010 for trend, table 3) and at years 1 and 2 the numbers of tophi were lower than in the usual-care group (p<0·0001 and p=0·0090, respectively; table 3). The mean diameter of the largest tophus was significantly smaller among patients in the nurse-led group than among those in the usual-care group at years 1 and 2 (table 3).
In a post-hoc per-protocol analysis, the relationship between serum urate concentrations and clinical outcomes was assessed in tertiles of serum urate concentrations at 2 years in all patients who completed the study (lowest <247 μmol/L, middle 248–365 μmol/L, and highest >365 μmol/L). The percentages of participants experiencing flares during year 2 were 7%, 10%, and 29%, respectively, for two or more (p<0·0001 for trend) and 1%, 3%, and 14%, respectively, for four or more (p<0·0001 for trend), and the percentages of patients with tophi at the end of year 2 were 2%, 6%, and 10%, respectively (p=0·0050 for trend).
The SF-36 physical component, but not the mental component, significantly improved in the nurse-led group but did not change in the usual-care group (table 3). Mean scores were significantly better in the nurse-led group than in the usual-care group at 1 year (p<0·0010) and 2 years (p=0·0027). Gout Impact Scale scores improved significantly in both groups, for the overall and unmet needs components (table 3) but were better in the nurse-led group at 1 and 2 years (both p<0·0001).
Participants in the nurse-led group attended a mean of 9·3 visits (SD 2·2) and had a mean of 8·3 (3·2) telephone calls about gout, mostly occurring in the first year of treatment, and mainly in the first 6 months of treatment (appendix). Patients in the usual-care group visited GPs a mean of 0·6 times (SD 1·4) specifically for gout during the 2-year study period.
When assessed per protocol, results for all primary and secondary outcomes did not differ qualitatively from those in the intention-to-treat dataset (appendix).
The cost per QALY gained for nurse-led care at 2 years was £5066 and was modelled to be £286 at 3 years. We calculated that nurse-led care would produce 2% more health than usual care and cost £1726 less per QALY gained at 5 years, and would produce 3% more health gain and cost £2783 less per QALY gained at 10 years. If the cost of gout flares were decreased, the cost per QALY gained would rise to £6144 at 3 years, £3578 at 5 years, and £2425 at 10 years, but remained cost-effective given the NICE threshold of £20 000 (appendix). The inclusion of additional nurse time did not substantially alter the cost per QALY gained value at 3 years (increase £520), and at 5 years and beyond the nurse-led approach led to cost savings. Even with the model of extremely unfavourable efficacy, the cost per QALY gained was £5011 at 3 years and £648 at 5 years, and became cost saving at 10 years (appendix).
Discussion
Nurse-led care of people with gout in the UK community can achieve high uptake of urate-lowering therapy and adherence over 2 years. 95% of participants in the nurse-led group achieved the recommended target serum urate concentration of less than 360 μmol/L,15 and patient-centred outcomes, including flare frequency, presence of tophi, and quality of life, improved significantly compared with those in the usual-care group. This study strongly reaffirms the importance of education and engagement of patients in disease management and the usefulness of a treat-to-target strategy. As well as clinical benefits to patients with gout, the cost per QALY gained was far below the standard NICE threshold of £20 000 and started to save costs at 5 years. A similar pattern was seen even in an extremely unfavourable efficacy model and when the cost of managing gout flares was substantially reduced.
To our knowledge, this is the first randomised controlled trial to compare nurse-led gout care with usual GP-led care. The key differences from usual care in the nurse-led approach in this study were the time spent explaining gout and making the explanations individualised and easy to understand, addressing illness perceptions, and involving patients in shared decision making. Although education and engagement are thought to be professional responsibilities and core elements of care, they are often suboptimum in gout management,23 which possibly reflects misconceptions and poor understanding of gout by physicians8, 12 and time constraints on health-care delivery. The role of non-physician health-care professionals in providing gout education has been examined in two US pilot studies. One assessed pharmacist-assisted management of urate-lowering therapy for 100 patients referred by primary care physicians24 and the other assessed nurse-delivered education plus pharmacist telephone calls in 45 patients referred to hospital-based rheumatologists.25 As with our pilot study of nurse-led care,15 the two US studies reported good acceptability and benefits to patients from education irrespective of the health professionals involved. In a later US randomised controlled trial involving 77 patients, pharmacist-led, telephone-based management of urate lowering therapy was compared with usual care led by primary physicians.26 At 26 weeks, 13 (35%) of 37 patients in the pharmacist-led group compared with five (14%) of 40 in the physician-led group had serum urate less than 360 μmol/L.
Strengths of our study are that it was a randomised controlled trial with a usual-care comparator and that it was done in the community, where most gout patients are managed. Additionally, we followed up patients for 2 years and the study was powered to show differences between groups in patient-centred outcomes. The patients included were typical of those encountered in clinical practice, with many having associated comorbidities (eg, being overweight or obese or having cardiovascular disease) and around 20% had renal insufficiency (chronic kidney disease stage 3), yet were able to achieve optimum clinical outcomes with nurse-led care. Furthermore, retention in this group was 91% at 2 years and adherence to urate-lowering therapy was the best reported in clinical trials of gout. These findings probably reflect the importance of education and engagement of patients, but also regular follow-up and positive patient–practitioner interactions. A qualitative study to assess the various elements of care in relation to outcomes has been done in 30 of the patients in this study who were sampled 18–26 months after study end, and results will be published separately.
Several aspects of nurse-delivered treat-to-target urate-lowering therapy deserve emphasis. First, allopurinol doses were higher in the nurse-led group than in the usual-care group (mean 460 mg/day vs 230 mg/day), and in the UK overall, where prescriptions rarely exceed 300 mg/day.9 These higher doses were needed to improve outcomes. Suboptimum dosing in the usual-care group might reflect physician inertia, lack of a treat-to-target strategy, poor monitoring of serum urate concentrations,9, 27, 28, 29 and concerns over safety of and adherence to urate-lowering therapy.12 Febuxostat was a successful alternative urate-lowering therapy, uricosurics were needed infrequently, and no patient needed combined therapy. Second, only three participants elected to receive prophylaxis against gout flares during the titration phase at the start of urate-lowering therapy.7, 13 This low uptake might explain the slightly higher flare frequency in the nurse-led group in year 1 than in the usual-care group, but adherence was not affected, which suggests that prophylaxis might not be needed if up titration is done slowly.15 Third, 99% of patients in the nurse-led group, when fully informed about treatment options, wanted to start urate-lowering therapy. This finding challenges the recommendations to reserve such treatment for patients with severe gout. Some guidelines that support full education about gout, including urate-lowering therapy, at time of diagnosis7, 13 are moving towards early definitive treatment rather than waiting until gout is severe. A similar paradigm has been applied to rheumatoid and other inflammatory arthritides. Finally, the American College of Physicians has advised doctors to not use treat-to-target urate-lowering therapy because they found no evidence of benefits to patient.30 However, their treat-to-avoid-symptoms strategy, which ignores serum urate concentrations and engagement of patients, is not clearly explained, and the approach is not supported by evidence.31 If that strategy is taken to approximate the usual-care situation in our GP-led group, our study supports treat to target.7, 13, 16, 32 The improved patient-centred outcomes we saw in the nurse-led group at 2 years and clear relationships between tertiles of serum urate concentration at the end of the study and flare frequency in year 2 and presence of tophi at the end of the study further refute the American College of Physicians advice.
This study has limitations. First, recruitment might have been subject to selection bias if participating practices were interested in gout. Likewise, if people concerned about gout were more likely to participate, the population might have been subject to response bias. Nevertheless, the proportion of patients taking urate-lowering therapy at baseline (39%) is representative of the UK,1, 9 and neither bias would diminish or enhance between-group differences. Second, blinding of the intervention was not possible, which might have affected the behaviour of GPs and participants in the usual-care group (eg, GPs more actively managing the gout of patients known to be in the study or patients feeling encouraged to consult more after receiving the gout information from a nurse and booklet at baseline). Certainly, use of urate-lowering therapy increased from 39% to 56% in the usual-care group, but allopurinol doses remained low and mean serum urate concentrations did not improve. Therefore, any behaviour changes did not importantly affect results and, if anything, would have diminished between-group differences. Third, because crystal identification was not required for the diagnosis of gout, some patients might have been misclassified. This diagnostic method is, however, rarely used by GPs and should not affect between-group differences. Misclassification might also have occurred through patients self-reporting flares without requirement for specific clinical criteria or assessment and irrespective of any modification by rapid self-treatment. Again, though, this factor should not have altered between-group differences. Fourth, although the study lasted 2 years, longer observation would be useful to allow sufficient time to eliminate all sodium urate crystals and assess people through to true remission. Fifth, the study was done in just one UK region, and generalisability of the findings remains to be determined. Similarly, this care model might not suit countries with less well established nurse-led care. However, providing that patients are fully informed and engaged and a treat-to-target strategy is used for urate-lowering therapy, our findings are likely to be generalisable to any practitioner who delivers the care. Finally, we trained research nurses rather than practice-based nurses. This caveat will be addressed in a future research-to-implementation study.
Community-based nurse-led care involving education and engagement of patients and a treat-to-target strategy for urate-lowering therapy achieved target serum urate concentrations and improved patient-centred outcomes in more than 90% of patients with gout. Compared with usual GP-led care, this model was cost-effective and potentially cost-saving, and merits further consideration in the UK. Our results highlight the importance and success of individualised education and care of patients, a principle that should be considered by any health-care professional who manages people with gout.
Data sharing statement
Deidentified patients' data can be requested by researchers for use in independent scientific research and will be provided following review and approval of the research proposal (including statistical analysis plan) and completion of a data sharing agreement with the University of Nottingham. Data requests can be made anytime from 9 months after the publication of this trial for up to 5 years (extendable). Requests should be sent to the corresponding author.
Acknowledgments
Acknowledgments
This work was supported by Arthritis Research UK [grant number 19703]. We thank members of the Nottingham Rheumatology Patient and Public Involvement group for advice during development of the study, the participating practices (appendix); Terence O'Neill, University of Manchester; George Nuki, University of Edinburgh; Edward Roddy, University of Keele; and Malcolm Coy for participation on the independent steering committee.
Contributors
MD, HR, MS, and WZ conceived and designed the study. HR was trial manager, organised all financial aspects of the trial, recruited the practices, and was principal liaison with the funder, research ethics committee, and National Health Service authorities. MD and WJ trained the research nurses in gout care. LD oversaw development of the trial database and randomisation procedure. WJ, DA, CB, and SD provided the care and did the data collection for the nurse-led group. MD, AA, and FR authorised trial prescribing and advised nurses on clinical issues. AS, MD, and WZ did the clinical data analysis and interpretation. RH and MS did the economic analysis. All authors critically revised the work for intellectual content, approved the final version, and agreed to be accountable for the work. MD is overall guarantor for the study.
Declaration of interests
MD and AA have received research funding from AstraZeneca for the Sons of Gout study. MD has received consultation fees from AstraZeneca, Grunenthal, and Mallinckrodt. WZ has received consultation fees from AstraZeneca and Grunenthal. The other authors declare no competing interests.
Supplementary Material
References
- 1.Kuo C-F, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74:661–667. doi: 10.1136/annrheumdis-2013-204463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhana A, Lee G, Dalbeth N. Factors influencing the crystallization of monosodium urate: a systematic literature review. BMC Musculoskelet Disord. 2015;16:296. doi: 10.1186/s12891-015-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis. 2016;75:210–217. doi: 10.1136/annrheumdis-2014-206410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Effect of allopurinol on all-cause mortality in adults with incident gout: propensity score-matched landmark analysis. Rheumatology. 2015;54:2145–2150. doi: 10.1093/rheumatology/kev246. [DOI] [PubMed] [Google Scholar]
- 5.Chandratre P, Roddy E, Clarson L, Richardson J, Hider SL, Mallen CD. Health-related quality of life in gout: a systematic review. Rheumatology. 2013;52:2031–2040. doi: 10.1093/rheumatology/ket265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland R, McGill NW. Comprehensive dietary education in treated gout patients does not further improve serum urate. Intern Med J. 2015;45:189–194. doi: 10.1111/imj.12661. [DOI] [PubMed] [Google Scholar]
- 7.Richette P, Doherty M, Pascual E. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 8.Doherty M, Jansen TL, Nuki G. Gout: why is this curable disease so seldom cured? Ann Rheum Dis. 2012;71:1765–1770. doi: 10.1136/annrheumdis-2012-201687. [DOI] [PubMed] [Google Scholar]
- 9.Annemans L, Spaepen E, Gaskin M. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000-2005. Ann Rheum Dis. 2008;67:960–966. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437–443. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehlin M, Ekstrom EH, Petzold M, Stromberg U, Telg G, Jacobsson LT. Factors associated with initiation and persistence of urate-lowering therapy. Arthritis Res Ther. 2017;19:6. doi: 10.1186/s13075-016-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer K, Carr A, Doherty M. Patient and provider barriers to effective management of gout in general practice: a qualitative study. Ann Rheum Dis. 2012;71:1490–1495. doi: 10.1136/annrheumdis-2011-200801. [DOI] [PubMed] [Google Scholar]
- 13.Hui M, Carr A, Cameron S. The British Society for Rheumatology guideline for the management of gout. Rheumatology. 2017;56:e1–20. doi: 10.1093/rheumatology/kex156. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Gonzalez NA, Tandjung R, Djalali S, Rosemann T. The impact of physician-nurse task shifting in primary care on the course of disease: a systematic review. Hum Resour Health. 2015;13:55. doi: 10.1186/s12960-015-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees F, Jenkins W, Doherty M. Patients with gout adhere to curative treatment if informed appropriately: proof-of-concept observational study. Ann Rheum Dis. 2013;72:826–830. doi: 10.1136/annrheumdis-2012-201676. [DOI] [PubMed] [Google Scholar]
- 16.Kiltz U, Smolen J, Bardin T. Treat-to-target (T2T) recommendations for gout. Ann Rheum Dis. 2017;76:632–638. doi: 10.1136/annrheumdis-2016-209467. [DOI] [PubMed] [Google Scholar]
- 17.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Hirsch JD, Terkeltaub R, Khanna D. Gout disease-specific quality of life and the association with gout characteristics. Patient Relat Outcome Meas. 2010;1:1–8. doi: 10.2147/PROM.S8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roddy E, Zhang W, Doherty M. Concordance of the management of chronic gout in a UK primary-care population with the EULAR gout recommendations. Ann Rheum Dis. 2007;66:1311–1315. doi: 10.1136/ard.2007.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roddy E, Zhang W, Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatology. 2007;46:1441–1444. doi: 10.1093/rheumatology/kem150. [DOI] [PubMed] [Google Scholar]
- 22.National Insitute for Health and Care Excellence Pegloticase for treating severe debilitating chronic tophaceous gout: technology appraisal guidance [TA291] June 26, 2013. https://www.nice.org.uk/Guidance/TA291
- 23.Harrold LR, Mazor KM, Velten S, Ockene IS, Yood RA. Patients and providers view gout differently: a qualitative study. Chronic Illn. 2010;6:263–271. doi: 10.1177/1742395310378761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldfien RD, Ng MS, Yip G. Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study. BMJ Open. 2014;4:e003627. doi: 10.1136/bmjopen-2013-003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fields TR, Rifaat A, Yee AMF. Pilot study of a multidisciplinary gout patient education and monitoring program. Semin Arthritis Rheum. 2017;46:601–608. doi: 10.1016/j.semarthrit.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldfien R, Pressman A, Jacobson A, Ng M, Avins A. A pharmacist-staffed, virtual gout management clinic for achieving target serum uric acid levels: a randomized clinical trial. Perm J. 2016;20:18–23. doi: 10.7812/TPP/15-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipworth W, Kerridge I, Brett J, Day R. How clinical and research failures lead to suboptimal prescribing: the example of chronic gout. BMJ. 2011;343:d7459. doi: 10.1136/bmj.d7459. [DOI] [PubMed] [Google Scholar]
- 28.Harrold LR, Mazor KM, Negron A, Ogarek J, Firneno C, Yood RA. Primary care providers' knowledge, beliefs and treatment practices for gout: results of a physician questionnaire. Rheumatology. 2013;52:1623–1629. doi: 10.1093/rheumatology/ket158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Eligibility for and prescription of urate-lowering treatment in patients with incident gout in England. JAMA. 2014;312:2684–2686. doi: 10.1001/jama.2014.14484. [DOI] [PubMed] [Google Scholar]
- 30.Qaseem A, Harris RP, Forciea MA. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58–68. doi: 10.7326/M16-0570. [DOI] [PubMed] [Google Scholar]
- 31.Dalbeth N, Bardin T, Doherty M. Discordant American College of Physicians and international rheumatology guidelines for gout management: consensus statement of the Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN) Nat Rev Rheumatol. 2017;13:561–568. doi: 10.1038/nrrheum.2017.126. [DOI] [PubMed] [Google Scholar]
- 32.Khanna D, FitzGerald JD, Khanna PP. 2012 American College of Rheumatology guidelines for management of gout part i: systematic non-pharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.