Abstract
Several drugs used in the treatment of multidrug-resistant tuberculosis (MDR-TB) have been reported as teratogenic. Treatment of such cases during gestation is disputable. Some experts favor the termination of pregnancy, whereas others suggest reducing the dose of teratogenic drugs or even suspending the regimen during pregnancy. There have been no clinical trials on the subject, but case reports and case series show excellent outcomes for children exposed during pregnancy to second-line agents, indicating that aggressive management of gestational MDR-TB may benefit not only the mother but also the fetus. We present a case of pregnancy in a teenager while she was under treatment for MDR-TB and continued with full treatment and nevertheless delivered a healthy child.
Keywords: Multidrug-resistant, tuberculosis, treatment, teratogenicity, pregnancy
INTRODUCTION
Multidrug-resistant tuberculosis (MDR-TB) mainly affects young adults, including women of childbearing age. Several drugs used in the treatment of MDR-TB (ethionamide, fluoroquinolones, and aminoglycosides) have been reported as teratogenic. The management of MDR-TB during pregnancy is disputable [1]. Although some experts favor the termination of pregnancy, others suggest reducing the dose of teratogenic drugs or even suspending the regimen during pregnancy [2]. We present a case of pregnancy in a teenager who was under treatment for drug-resistant tuberculosis.
CASE PRESENTATION
During contact investigation, the 15-year-old daughter of an MDR-TB case (her mother) was diagnosed with active pulmonary tuberculosis resistant to isoniazid, rifampin, and ethambutol and classified as a new MDR-TB case; HIV rapid testing was negative. A chest radiograph at the outset showed airspace infiltrates in the lingula region (Figure 1).
Figure 1.
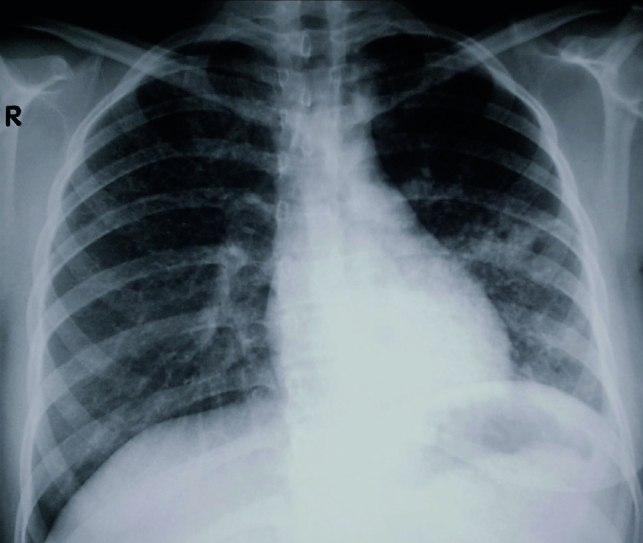
Chest radiograph at the time of diagnosis showing an airspace infiltrate in the lingula
Treatment was initiated in November 2015, and her regimen included levofloxacin, prothionamide, amikacin, cycloserine, and pyrazinamide. She responded rapidly and had converted her culture by mid-January 2016. After 8 months of treatment and 6 months with consecutive negative sputum cultures, she reported the start of sexual relations. When referred for contraceptive measures, the pregnancy test was already positive, and an obstetric ultrasound revealed an 8-week pregnancy. Although amikacin had been stopped before pregnancy started, the patient was still receiving levofloxacin and prothionamide, two potentially teratogenic drugs. The case was evaluated by the hospital bioethics committee, and the patient was informed of the risk of congenital malformations and the option of undergoing a therapeutic abortion owing to the risk of relapse if treatment was stopped; besides, the fetus had been already exposed to the drugs for at least 2months. Nonetheless, she decided to continue with the pregnancy. In March 2017, she delivered a healthy male child (weighing 2.9 kg) with no congenital defects. The patient was discharged as asymptomatic and with a normal chest radiograph, and declared as cured of MDR-TB in June 2017.
DISCUSSION
Ethionamide and fluoroquinolones are classified as pregnancy category C drugs and amikacin as category D (category C drugs: animal reproduction studies have shown adverse effects to the fetus and there are no adequate and well-controlled human clinical trials; category D drugs: there is evidence in clinical trials in humans of teratogenic risk). The loss of auditory acuity associated with the use of amikacin is usually severe and irreversible and may occur even after its use for just a few days; its effect is usually associated with the cumulative dose of the drug. Other aminoglycosides (streptomycin, kanamycin, tobramycin, and gentamicin) and capreomycin (a polyene) are also associated with auditory toxicity [3]. The use of ethionamide during the first trimester of pregnancy has been associated with defects of the central nervous system [4]. Animal studies with fluoroquinolones have suggested that there are risks of damage to the articular cartilages of the fetus and, consequently, of joint lesions [5].
There are not many management options for pregnant women with MDR-TB; the available options include interruption of pregnancy, interruption of the mother’s MDR-TB treatment, or continuation of the regimen with potentially teratogenic drugs despite pregnancy [6]. Therapeutic abortion has been proposed in these cases, since discontinuation of treatment puts the mother at grave risk and favors the possible transmission of a resistant strain in the community [7].
Our patient had stopped the aminoglycoside treatment before getting pregnant but was still receiving both prothionamide and levofloxacin. Human studies yield conflicting data regarding the teratogenicity of ethionamide/protionamide. In general, thioamides are considered as potentially teratogenic, and it is recommended to avoid pregnancy while under treatment with this class of drugs [8].
On the contrary, experiences from Peru and India reveal excellent outcomes for children exposed to second-line drugs during pregnancy, suggesting that aggressive management of gestational MDR-TB may benefit both the mother and the fetus [6–8]. Long-term follow-up (>5 years) in 6 children exposed to multiple potentially teratogenic antituberculosis drugs showed a virtually normal psychomotor development [9].
Patients should have the right to choose once they have been thoroughly informed of their options. On the basis of available evidence, it appears that the demonstrated and hypothetical benefits of continued MDR-TB treatment despite pregnancy are greater than the theoretical risks for the mother and fetus [6, 10, 11]. Finally, all women of childbearing age (even the very young, as we have learned from this case) should be screened for pregnancy before initiation of treatment. In addition, family planning that does not require perfect adherence (e.g. birth control implant or an intrauterine birth control device) should be made a strong recommendation for the duration of therapy.
Footnotes
Informed Consent: Informed consent was obtained from the parents of the patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - R.L.L., K.C.L., A.H.P.; Supervision - R.L.L.; Resource - R.L.L.; Materials - R.L.L.; Data Collection and/or Processing - K.C.L., A.H.P.; Analysis and/or Interpretation - R.L.L., K.C.L., A.H.P.; Literature Search - K.C.L., A.H.P.; Writing - R.L.L.; Critical Reviews - K.C.L., A.H.P.
Conflict of Interest: Authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Curry International Tuberculosis Center and California Department of Public Health; Curry International Tuberculosis Center, editor. Drug-Resistant Tuberculosis: A Survival Guide for Clinicians. 3rd Edition. 2016. pp. 187–8. [Google Scholar]
- 2.Craig GM, Booth H, Story A, et al. The impact of social factors on tuberculosis management. J Adv Nurs. 2007;58:418–24. doi: 10.1111/j.1365-2648.2007.04257.x. [DOI] [PubMed] [Google Scholar]
- 3.Sagwa EL, Ruswa N, Mavhunga F, et al. Comparing amikacin and kanamycin induced hearing loss in multidrug-resistant tuberculosis treatment under programmatic conditions in a Namibian retrospective cohort. BMC Pharmacol Toxicol. 2015;16:36. doi: 10.1186/s40360-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loto OM, Awowole I. Tuberculosis in Pregnancy: A Review. J Pregnancy. 2012;2012 doi: 10.1155/2012/379271. 379271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 6.Palacios E, Dallman R, Munoz M, et al. Drug-Resistant Tuberculosis and Pregnancy: Treatment Outcomes of 38 Cases in Lima, Peru. Clin Infect Dis. 2009;48:1413–9. doi: 10.1086/598191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin S, Guerra D, Rich M, et al. Treatment of Multidrug-Resistant Tuberculosis during Pregnancy: a Report of 7 Cases. Clin Infect Dis. 2003;36:996–1003. doi: 10.1086/374225. [DOI] [PubMed] [Google Scholar]
- 8.Rohilla M, Joshi B, Jain V, et al. Multidrug-Resistant Tuberculosis during Pregnancy: Two Case Reports and Review of the Literature. Case Rep Obstet Gynecol. 2016;2016 doi: 10.1155/2016/1536281. 1536281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drobac PC, del Castillo H, Sweetland A, et al. Treatment of multidrug-resistanttuberculosis during pregnancy: long-term follow-up of 6 children with intrauterine exposure to second-line agents. Clin Infect Dis. 2005;40:1689–92. doi: 10.1086/430066. [DOI] [PubMed] [Google Scholar]
- 10.Arbex MA, de Varella MC, Siqueira HR, et al. Antituberculosis drugs: Drug interactions, adverse effects, and use in special situations. Part 2: Second-line drugs. J Bras Pneumol. 2010;36:641–56. doi: 10.1590/S1806-37132010000500017. [DOI] [PubMed] [Google Scholar]
- 11.T Joint. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax. 1988;53:536–48. [PMC free article] [PubMed] [Google Scholar]


