Abstract
OBJECTIVES
The choice of treatment according to the inflammation type in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) has been of recent interest. This study investigated the role of novel biomarkers, hospital outcomes, and readmission rates in the first month in patients with eosinophilic or neutrophilic AECOPD.
MATERIALS AND METHODS
We conducted a retrospective observational cohort study in a Chest Teaching Hospital with hospitalized AECOPD patients. Subjects’ characteristics, hemogram results, C-reactive protein (CRP), neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), platelet/mean platelet volume (PLT/MPV), length of hospital stay, mortality, and steroid use were recorded. Eosinophilic AECOPD defined as peripheral blood eosinophilia (PBE) was >2% and neutrophilic AECOPD as PBE ≤2%. Readmission within 28 days of discharge was recorded.
RESULTS
Of 2727(31.5% females) patients, eosinophilic AECOPD was found in 510 (18.7%) patients. Leucocytes, CRP, NLR, and PLR were significantly higher in neutrophilic AECOPD than in eosinophilic AECOPD (p<0.001). Steroid use and mortality rate were 45% and 0.6% in eosinophilic AECOPD and 71%, and 1.4% in neutrophilic AECOPD, respectively (p=0.001, p=0.19). Age >75 years, albumin <2.5 g/dL, CRP >50 mg/dL, and PLT/MPV <20×103 were found to be risks factors for hospital mortality (p<0.05 each). Readmission rates within 28 days of discharge were 5% (n=136), and this rate was higher in eosinophilic AECOPD patients not taking steroids (p<0.001).
CONCLUSION
NLR, PLR, and CRP levels were higher in neutrophilic AECOPD compared with eosinophilic AECOPD. These markers decreased with treatment in neutrophilic AECOPD. A PLT/MPV ratio of <20×103 resulted in an increased mortality rate. Thus, appropriate steroid therapy may reduce readmission rates in the first 28 days after discharge in eosinophilic AECOPD.
Keywords: Chronic obstructive pulmonary diseases, exacerbation, peripheral eosinophilia, neutrophil to lymphocyte ratio, steroid treatment
INTRODUCTION
Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) requiring hospitalization is a significant cause of morbidity and mortality [1]. Different cumulative environmental exposures (air pollution, cigarette smoking, feeding habits, allergens, and infections) lead to pathobiological changes in the airway, and these changes can be addressed as endotypes in patients with chronic obstructive pulmonary disease (COPD) [2]. During AECOPD, these multi pathobiological changes are determined by some biomarkers, which are easily obtained (peripheral blood eosinophil) or require high-end technology (exhaled nitric oxide) [3,4]. The clinical presentations of COPD, such as no symptom or with very severe symptoms and having muscle wasting or obesity, are defined as COPD phenotypes [2]. The awareness of endotypes can lead to a mechanistic approach to COPD stratification and treatment. Determining the nature of AECOPD according to the endotype of inflammation may be important for treatment options in the future. Predominantly neutrophilic and to a lesser extent, eosinophilic inflammation, is observed with COPD, although recent studies have shown that the eosinophilic inflammation rate may reach up to 45% [5–8]. Some studies have shown that corticosteroid treatment of AECOPD may be less effective if it is not the eosinophilic endotype [3,7,9]. A number of studies have investigated sputum and bronchial biopsy eosinophilia, steroid response, and frequency of attacks [7,10,11]. Very recently studies have focused on peripheral blood eosinophilia (PBE) as a biomarker, which reflects sputum eosinophilia, increasing in patients with AECOPD [3,12]. The eosinophilic and neutrophilic endotype of AECOPD can be easily identified using peripheral blood analysis.
Making a decision regarding corticosteroid and/or antibiotic treatment is important for the length of stay (LOS) in the hospital, morbidity, and mortality in patients with hospitalized AECOPD. The results of sputum culture C-reactive protein (CRP), which is a well-known inflammatory biomarker, or any other biomarker are not helpful to physicians when deciding the avenue of treatment with antibiotics in patients with AECOPD [13,14]. The AECOPD endotypes can however provide clues for accurate treatment, thus shortening the LOS in the hospital [15]. In addition to peripheral blood eosinophil percentage, other novel biomarkers have recently been investigated to define the endotype of AECOPD, namely neutrophil/lymphocyte ratio (NLR), platelet/mean platelet volume (PLT/MPV), and platelet/lymphocyte ratio (PLR) [16–18]. The studies have assessed these biomarkers in light of defining the attack severity and managing the treatment approach to shorten hospital stay and decrease hospital mortality, together with reducing readmission rates to hospital. In previous studies, we evaluated outcomes with respect to eosinophilic and noneosinophilic COPD exacerbation and identified a new biomarker (NLR) for predicting the long-term survival (6 months). However, this previous study did not analyze the patient data with respect to hospital stay and readmission rates within the first 28-days post discharge [19].
In the current study, we retrospectively assessed the real-life treatment approach and outcomes of hospitalized eosinophilic and neutrophilic AECOPD patients. We also investigated the LOS in the hospital, mortality risk factors, and the effect of steroid treatment in these AECOPD patients hospitalized with eosinophilic and neutrophilic endotypes.
METHODS
Study Design
A retrospective observational cohort study was performed in a chest disease training and research hospital between January 2014 and December 2014. This study was approved by the Süreyyapaşa Chest Disease and Thoracic Surgery Training and Research Hospital local ethics committee (2015/06/22). Ethical approval was in accordance with the Declaration of Helsinki. All data were collected retrospectively from the hospital database. As informed consent was not obtained due to the retrospective nature of the study, the patient data were de-identified.
Patients
Hospitalized patients recorded as J44.0-J44.9 according to the International Classification of Diseases (ICD) 10 coding system and previously diagnosed with COPD by a pulmonology specialist using spirometry test results according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 American Thoracic Society, European Respiratory Society (ATS/ERS) criteria, were included in the study [1]. The diagnosis of COPD was also controlled by a chest training center, and the diagnosis of COPD was checked at least four times in our center. Each patient was to be followed only in the study center and not in other center and city.
Study patients were divided into two groups according to the level of PBE. PBE >2% defined the eosinophilic COPD exacerbation group and PBE ≤2% defined the noneosinophilic COPD exacerbation group (Figure 1).
Figure 1.
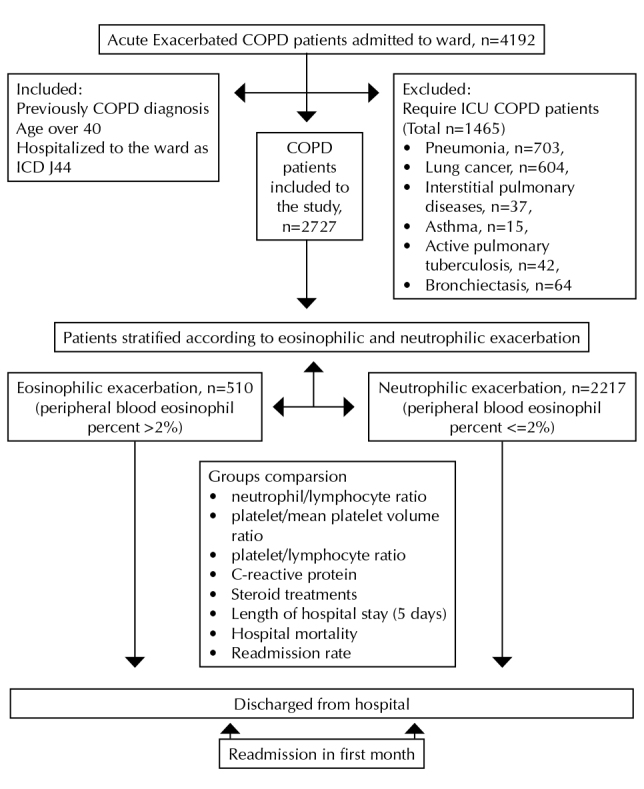
Flow chart showing study enrollment of patients with AECOPD
Patients were further divided into subgroups according to LOS in the hospital. An LOS of <7 days defined the “short stay” group and an LOS ≥7 days defined the “long stay” group. These subgroups were evaluated according to the presence eosinophil predominance, steroid use, and hospital mortality.
Definitions
Chronic obstructive pulmonary disease diagnosis was established by a pulmonologist who evaluated airflow obstruction on spirometry, forced expiratory volume in 1 second (FEV1) of 70% predicted or less, and FEV1 to forced vital capacity ratio of 70% or less [1].
AECOPD was defined as an acute change in a patient’s respiratory symptoms, such as dyspnea, sputum production, volume, and alteration in color, resulting in a change in current therapy [1]. The reasons for COPD exacerbations according to the ICD 10 coding system were as follows: infections, arrhythmia, heart failure, pleurisy, pneumothorax, and pulmonary embolism.
Exclusion criteria were patients diagnosed with pneumonia, lung cancer, interstitial pulmonary diseases, asthma, bronchiectasis, or active pulmonary tuberculosis. We accepted peripheral blood eosinophil percent as a biomarker of airway inflammation, which reflects sputum eosinophil in patients with AECOPD [3].
Neutrophilic endotype was defined as an inflammation of noneosinophilic airway in patients with AECOPD, and a PBE of <2% was the biomarker [2,3]. PBE, which reflects sputum eosinophilia, increases in patients with AECOPD [3,12].
Eosinophilic endotype was defined as an inflammation of eosinophilic airway in patients with AECOPD, and a PBE of >2% was the biomarker [2,3].
Neutrophil/lymphocyte ratio as a marker of systemic inflammation was defined as the absolute neutrophil count divided by the absolute lymphocyte count [20]. An NLR of 2–20 was assessed for an effect on LOS. PLT/MPV ratio as a marker of systemic inflammation was calculated as the ratio of platelet count to the MPV.
Platelet/lymphocyte ratio as a marker of systemic inflammation was defined as the absolute platelet count divided by the absolute lymphocyte count [18]. Hospital readmission was defined as rehospitalization within the first 28 days after discharge from the hospital.
Comorbidities were recorded as diabetes mellitus, hypertension, congestive heart failure, coronary artery diseases, arrhythmia, renal failure, anemia, and anxiety/depression.
Recorded Data
The following patient information from the hospital database was recorded: age, gender, hemogram values, blood biochemistry and laboratory results on admission and discharge from the hospital, and mortality in the hospital. As inflammatory markers, peripheral blood eosinophil count, neutrophil count, and CRP were recorded, and NLR, PLR, and PLT/MPV ratio were calculated. Pulmonary function tests results could not be obtained due to the absence of an electronic database of spirometry values. The LOS at hospital and cases of rehospitalization within 28 days of hospital discharge were also recorded.
The total leukocyte, neutrophil, eosinophil, lymphocyte, and platelet counts and MPV were determined using a Coulter LH 780 Hematology Analyzer (Beckman Coulter, USA). The CRP was checked by the nephelometry method using a BN II System (Siemens, Germany). The normal range of CRP is 0–5 mg/L.
Management of COPD exacerbation
COPD exacerbation treatment was managed by an academic pulmonology specialist using protocol-based treatment in accordance with national and international guidelines [1,21].
Anti-inflammatory and bronchodilator treatment
Steroid treatment was used if the COPD exacerbation was believed to have a noninfectious origin, and the steroid protocol was 40–60 mg/day of oral methylprednisolone, if there were no gastrointestinal (GI) symptoms. If GI symptoms were present, intravenous steroid was administered. The duration of steroid use was 5–7 days. Discontinuation of steroid treatment was done abruptly.
Theophylline was administered orally (100/200/300 mg) or intravenously (200 mg/100 mL or 400 mg/500 mL) every 12 hours.
Bronchodilator
A short-acting β2 agonist (salbutamol, 100 μg per puff) and ipratropium bromide (100 μg/20 μg per puff) were given every 4–6 hours (one puff per use) via a metered dose inhaler chamber (Aerovent, Altech®, Altera Firm, İzmir, Turkey). A nebular form of salbutamol (2.5 mg/2.5 mL per nebule) was given every 4–6 hours, or ipratropium bromide/salbutamol (0.5 mg/3.01 mg/2.5 mL per nebule) was given every 4–6 hours.
A combined form of long-acting β2 agonists and inhaler steroid as formoterol plus budesonide (4.5/160 μg, 9/320 μg, 12/200 μg, 12/400 μg) or salmeterol plus fluticasone (50/250 μg, 50/500 μg) were used in COPD patients.
Statistical Analysis
A descriptive analysis was used to investigate the subject demographics and hospital data. Groups were compared using the Mann-Whitney U-test for nonparametric continuous variables or Student’s t-test for parametric continuous variables. The chi-square test was employed for dichotomous variables. If n was <5, the Fisher’s exact test was used. The median with interquartile range was employed for nonparametric continuous variables, and mean ± standard deviation was used for parametric continuous variables. Count and percentage were used when applicable. A logistic regression analysis of hospital mortality was performed. In the hospital mortality model, we included NLR >15, LOS >7 days, age >75 years, serum albumin <2.5 g/dL, CRP >50 mg/dL, PLT/MPV <20, and steroid use. A p<0.05 was accepted as statistically significant. Hospital readmission within 28 days relative to the use of steroids in the two the groups were compared using the chi-square test.
RESULTS
During the study period, 4192 patients were hospitalized with AECOPD. In total, 2727 eligible patients with AECOPD were included into the study. There were 510 (18.7%) in the eosinophilic AECOPD group. Patient enrollment is summarized in Figure 1.
Table 1 shows a comparison of the eosinophilic and neutrophilic AECOPD study groups. The study groups were compared according to the patients’ demographic characteristics, comorbidities, steroid use, hospital stay, mortality rates, and biochemistry on admission. The male/female ratio, average age, rate of steroid use, and serum biochemistry values (except blood glucose) were very similar between the two groups. The eosinophilic group had a significantly shorter LOS in hospital (p<0.001), a significantly lower leucocyte and neutrophil count, and a significantly higher percentage of monocytes, basophils, and lymphocytes (p<0.001).
Table 1.
Demographics and laboratory findings of COPD patients at admission for eosinophilic and neutrophilic acute exacerbation
| Eosinophilic Exacerbation | Neutrophilic Exacerbation | p | |
|---|---|---|---|
| Number of patients, n | 510 | 2217 | |
| Male, % | 68.8 | 68.4 | 0.85 |
| Age, year, mean± SD | 69 ±11 | 70 ±10 | 0.67 |
| Steroid use, n (%) | 340 (67) | 1452 (66) | 0.62 |
| LOS, days, | 6.6 (4.6–8.0) | 7.0 (5.0–9.0) | 0.001 |
| Hospital mortality, n (%) | 3 (0.6) | 32 (1.5) | 0.19 |
| LTOT, n (%) | 176 (34.5) | 845 (38.1) | 0.13 |
| Comorbidities n (%) | |||
| Diabetes mellitus | 54 (10.5) | 182 (8.2) | 0.9 |
| Hypertension | 77 (15.0) | 328 (14.7) | 0.86 |
| Congestive heart failure | 71 (13.9) | 355 (16.0) | 0.24 |
| Coronary artery disease | 17 (3.3) | 72 (3.2) | 0.92 |
| Arrhythmias | 13 (2.5) | 45 (2.0) | 0.46 |
| Chronic renal failure | 8 (1.5) | 35 (1.5) | 0.99 |
| Anemia | 3 (0.5) | 10 (0.4) | 0.66 |
| Anxiety/Depression | 10 (1.9) | 30 (1.3) | 0.48 |
| Hemogram values | |||
| Leucocyte count, 109 L | 8.02 (6.40–9.81) | 10.110.001 (7.67–13.11) | |
| Eosinophil count above 0.34×109 L | 147 (28.8) | 9 (0.4) | 0.001 |
| Eosinophil count 109 L | 0.26 (0.20–0.36) | 0.05 (0.01–0.10) | 0.001 |
| Neutrophil, % | 67.9 (61.6–73.6) | 82.4 (74.4–89) | 0.001 |
| Monocyte, % | 7.5 (5.8–9.3) | 5.4 (3.1–7.7) | 0.001 |
| Lymphocyte, % | 19.2 (14.9–24.4) | 10.2 (6.3–16.5) | 0.001 |
| Basophil, % | 0.50 (0.30–0.90) | 0.30 (0.10–0.61) | 0.001 |
| Erythrocyte count, 1012L | 4.34 (3.90–4.77) | 4.35 (3.92–7.79) | 0.39 |
| Hemoglobin, g/dL | 12.1 (10.8–13.6) | 12.3 (10.9–13.6) | 0.16 |
| Hematocrit, % | 36.7 (32.8–41.4) | 37.2 (33.4–41.3) | 0.31 |
| MCV, fL | 86 (82–90) | 86 (82–90) | 0.87 |
| Platelet count, 109 L | 249 (197–313) | 250 (198–315) | 0.97 |
| Mean Platelet Volume, fL | 8.43 (7.80–9.20) | 8.54 (7.85–9.22) | 0.35 |
| Biochemistry values | |||
| Blood glucose mg/dL | 111 (93–144) | 135 (102–182) | 0.001 |
| Blood urea nitrogen, mg/dL | 25 (16–39) | 30 (20–48) | 0.001 |
| Serum creatinine, mg/dL | 0.80 (0.68–1.05) | 0.82 (0.68–1.06) | 0.82 |
| Sodium, mmol/L | 139(137–141) | 139 (137–141) | 0.005 |
| Potassium, mmol/L | 4.2(3.9–4.7) | 4.3 (3.9–4.7) | 0.43 |
| SGOT, U/L | 20 (14–27) | 18 (14–26) | 0.12 |
| SGPT, U/L | 16(10–25) | 17 (11–26) | 0.07 |
| Albumin, g/dL | 3.1 (2.7–3.5) | 3.2 (2.8–3.6) | 0.50 |
IQR: interquartile range (25%-75%), Values median (IQR). Mann Whitney U Test used; LOS: length of hospital stay; LTOT: long-term oxygen therapy; MCV: mean corpuscular volume; SGOT: serum glutamic-oxalacetic transaminase; SGPT: serum glutamic-pyruvic transaminase
Table 2 shows a comparison of the novel inflammatory biomarkers NLR, PLR, PLT/MPV, and CRP and the sedimentation rate in the eosinophilic and neutrophilic AECOPD groups on the day of admission to the hospital (baseline) and the day of discharge from the hospital. The neutrophilic AECOPD group had a significantly higher level of NLR and PLR on the first and last day of hospitalization. CRP was significantly higher in the neutrophilic group on admission, but not at the time of discharge. Both groups had similar PLT/MPV ratios and erythrocyte sedimentation rate on the first and last days of hospitalization.
Table 2.
The inflammatory biomarkers on admission and discharge from the hospital of eosinophilic and neutrophilic COPD exacerbation groups
| Eosinophilic Exacerbation | Neutrophilic Exacerbation | ||||
|---|---|---|---|---|---|
|
|
|
||||
| N | Variables | N | Variables | p | |
| NLR (baseline) | 510 | 3.60 (2.56–4.91) | 2217 | 8.09 (4.50–13.94) | 0.001 |
| NLR (on discharge) | 489 | 3.67 (2.60–5.17) | 2117 | 6.19 (3.75–11.00) | 0.001 |
| PLR (baseline) | 510 | 166.69 (121.51–228.47) | 2217 | 247.62 (156.43–388.30) | 0.001 |
| PLR (on discharge) | 489 | 166.19 (122.55–231.34) | 2121 | 213.59 (139.79–341.05) | 0.001 |
| PLT/MPVx103(baseline) | 510 | 30 (22–39) | 2217 | 29 (22–38) | 0.58 |
| PLT/MPVx103(on discharge) | 489 | 31 (23–40) | 2116 | 30 (23–41) | 0.97 |
| CRP, mg/dL (baseline) | 484 | 22.4 (7.7–62.4) | 2120 | 34.9 (11.5–96.7) | 0.001 |
| CRP, mg/dL (on discharge) | 478 | 12.8 (5.5–30.5) | 2093 | 12.5 (4.6–32.0) | 0.95 |
| ESR mm/h (baseline) | 223 | 42 (26–65) | 1060 | 48 (26–70) | 0.23 |
| ESR mm/h (on discharge) | 226 | 40 (23–65) | 1064 | 44 (22–66) | 0.64 |
Baseline: Day of admission to the hospital; Discharge: the day of discharge from hospital; NLR: neutrophil to lymphocyte ratio, PLR: platelet to lymphocyte ratio; PLT/MPV: platelet to mean platelet volume; CRP: C reactive protein; ESR: erythrocyte sedimentation rate
Table 3 shows the patients’ demographics, comorbid diseases, eosinophilic endotype, steroid use, and biomarkers in the survival and nonsurvival groups. Nonsurvival patients with AECOPD had very similar demographics to the survival group. All the inflammatory biomarkers besides PLR were significantly different in the nonsurvival group compared with the survival group. The nonsurvival group had a shorter LOS than the survival group.
Table 3.
A comparison of acute exacerbation COPD patients’ characteristics and inflammatory biomarkers on admission to hospital, and length of hospital stay relative to mortality
| Survival, n=2692 | Non-survival, n=35 | p | |
|---|---|---|---|
| Age, above 75 year, n (%) | 899 (33) | 22 (63) | 0.001 |
| Gender, Male % | 1144 (69) | 23 (66) | 0.72 |
| Co-morbidities, n (%) | |||
| • Diabetes mellitus | 232 (9) | 4 (11) | 0.56 |
| • Hypertension | 401 (15) | 4 (11) | 0.57 |
| • Congestive heart failure | 417 (16) | 9 (26) | 0.10 |
| • Coronary artery disease | 88 (3) | 1 (3) | 0.89 |
| LTOT, n (%) | 1005 (37) | 16 (46) | 0.31 |
| Steroid use in hospital, n (%) | 1777 (66) | 13 (41) | 0.001 |
| Eosinophil > 2%, n (%) | 507 (19) | 3 (9) | 0.001 |
| NLR, median (IQR) | 6.60 (3.82–12.28) | 9.59 (4.11–18.85) | 0.07 |
| NLR >7, n (%) | 1287 (48) | 23 (66) | 0.035 |
| NLR >15, n (%) | 487 (18) | 11 (31) | 0.042 |
| PLR, median (IQR) | 224.64 (146.75–353.66) | 224.14 (122.54–391.17) | 0.75 |
| PLR >182, n (%) | 1682 (63) | 19 (54) | 0.32 |
| PLT/MPV <20×103, n (%) | 496 (18) | 16 (46) | 0.001 |
| CRP, mg/dL, median (IQR) | 31.7 (10.6–89.5) | 91.9 (18.1–149.0) | 0.045 |
| CRP > 50 mg/dL, n (%) | 411 (16) | 15 (43) | 0.001 |
| Albumin <2.5mg/dL, n (%) | 279 (12) | 13 (41) | 0.001 |
| Length of stay, days, median (IQR) | 7 (5–9) | 4 (2–7) | 0.001 |
LTOT: long term oxygen therapy; NLR: neutrophil to lymphocyte ratio; PLR: platelet to lymphocyte ratio; PLT/MPV: platelet to mean platelet volume; CRP: C reactive protein; IQR: inter quartile range
Figure 2 summarizes the outcomes of hospital stay, which was stratified over 7 days according to eosinophilic and neutrophilic endotype and whether steroid therapy was received in the hospital. There was no mortality in eosinophilic AECOPD patients who received steroid in the hospital independent from LOS at the hospital. Mortality in the shorter (3.8%) and longer (2%) stay groups was higher in the neutrophilic AECOPD patients who did not receive steroid therapy in hospital.
Figure 2.
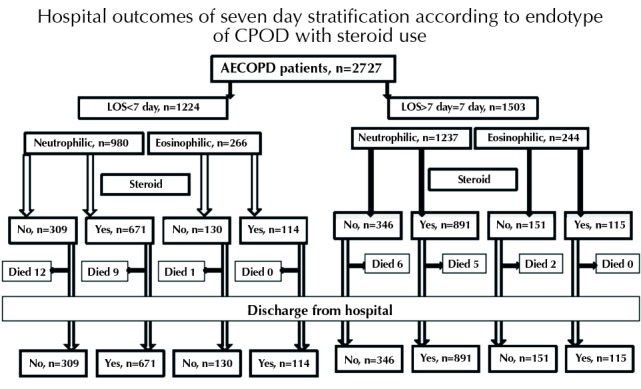
Hospital outcomes of study groups according to eosinophilia and steroid use
Among the 2217 neutrophilic AECOP patients, 32 died in hospital and 18 (56.3%) of these did not receive steroid therapy.
Table 4 shows the multivariate logistic regression analysis results. NLR>15, LOS>7 days, age>75 years, serum albumin <2.5 g/dL, CRP >50 mg/dL, PLT/MPV <20, and steroid use were included in the model. Mortality was observed in 35 patients. Age >75 years, serum albumin <2.5 g/dL, CRP >50 mg/dL, and PLT/MPV <20 were all found to be risks factors for hospital mortality in AECOPD patients.
Table 4.
Logistic regression analysis of mortality risk factors in acute exacerbation of COPD requiring hospitalization
| Variables | Odds ratio | 95% CI, lower-upper | p |
|---|---|---|---|
| C-reactive protein >50 mg/dL on admission | 3.82 | 1.69–8.62 | 0.001 |
| Serum albumin <2.5 mg on admission | 2.60 | 1.12–6.04 | 0.026 |
| PLT to MPV <20×103 | 3.52 | 1.62–7.63 | 0.001 |
| Age >75 years | 2.51 | 1.15–5.49 | 0.021 |
| NLR >15 | 1.13 | 0.46–2.78 | 0.79 |
| Steroid use | 0.50 | 0.22–1.10 | 0.09 |
| Hospital days longer than 7 days | 0.72 | 0.33–1.55 | 0.37 |
| Eosinophilic AECOPD | 0.49 | 0.14–1.72 | 0.27 |
CI: Confidence interval; NLR: Neutrophil to lymphocyte ratio; PLT to MPV: platelet to mean platelet volume ratio
Readmission within 28 days of discharge from hospital was observed in 130 patients (5%). Of these, 20 (15%) were eosinophilic AECOPD patients and 116 (85%) were neutrophilic AECOPD patients. In the eosinophilic group, six patients had received steroid therapy, while in the neutrophilic group, 94 patients had received steroids. Readmission rates were significantly higher in those patients who had not received steroids in the eosinophilic group, while readmission rates were significantly higher in those patients who had received steroids in the neutrophilic group (p<0.001; Figure 3).
Figure 3.
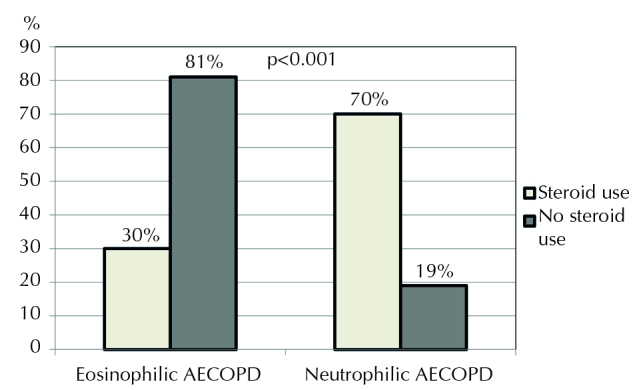
Readmitted COPD patients according to steroid use
DISCUSSION
This study revealed that in AECOPD patients requiring hospitalization, the eosinophilic (peripheral blood eosinophils >2%) and neutrophilic (peripheral blood eosinophils ≤2%) endotypes demonstrated significantly different inflammatory biomarkers. We also found that eosinophilic to neutrophilic endotype rate was 1:4. The level of inflammatory biomarkers in the neutrophilic endotype was much greater than that in the eosinophilic AECOPD group. During the hospital stay, NLR, PLR, and CRP values decreased and PLT/MPV values increased with treatment. A CRP level >50 mg/dL, age >75 years, serum albumin <2.5 g/dL, and a PLT/MPV ratio <20×103 were found to be mortality risk factors for AECOPD. Eosinophilic AECOPD patients had a shorter LOS and better outcome with steroid therapy in the hospital and decreased readmission rates compared with the neutrophilic group.
Endotypes of AECOPD: Eosinophilic versus Neutrophilic
Bafadhel et al. [3] and Pascoe et al. [22] showed that the peripheral blood eosinophil count is a valid biomarker of COPD exacerbation, and they also showed that the 2% threshold value is a sensitive marker for the presence of an eosinophilic attack that can be responsive to corticosteroids. The evaluation of COPD longitudinally to identify predictive surrogate end-points (Eclipse) cohort study accepted the cut-off value of ≥2% eosinophils in peripheral blood and sputum in COPD patients, and persistent eosinophilia was detected in 37% of patients [23]. Vedel-Krogh and colleagues recently published a similar study, but instead of a blood eosinophil percent, they showed that an eosinophil cell count >0.34×109/L can be associated with an increased risk of AECOPD requiring hospitalization in patients with COPD [12]. In the present study, a PBE rate >2% was observed in nearly one-fifth of the hospitalized patients with COPD exacerbations. However, eosinophilic AECOPD (eosinophils >2%) revealed a cell count of eosinophils (0.26×109/L) lower than that defined in the Vedel-Krogh’s study (0.34×109). The classification of patients with eosinophilic inflammation suggests a change in the treatment plan and management of the disease. Identifying this inflammation with peripheral blood samples, which is cheap and easily accessible, will provide more practical solutions for the management of these patients.
The Behavior of CRP and Novel Biomarkers (NLR, PLR, and PLT/MPV) in Eosinophilic and Neutrophilic AECOPD
C-reactive protein levels higher than 8 mg/mL and the Anthonisen’s criteria were reported to support the diagnosis of AECOPD [19,24]. In our previous study that evaluated factors affecting long-term (6month) survival in AECOPD, CRP values greater than 19 indicated a high risk for mortality in a noneosinophilic attack [19]. In another prior study, Salturk and coworkers evaluated AECOPD outcomes, and patients were grouped as eosinophilic and noneosinophilic; CRP values were significantly lower in the eosinophilic group compared to the noneosinophilic group (39.3 and 52.7, respectively), and AECOPD patients with CRP values >50 had a 1.7 times increased risk of intensive care unit (ICU) mortality [25]. The AECOPD patients requiring ICU care in their study had higher CRP values compared to the patients presented in this study. CRP values can also indicate the severity of AECOPD. In the present study, long-term mortality was not investigated; however, CRP values >50 were associated with a nearly four times increased risk of hospital mortality. Gunay et al. [16] reported that NLR values of COPD patients were higher than the control group in stable COPD and AECOPD (AECOPD, 4.28; stable COPD, 2.59; and control group, 1.71; p<0.001). Gunay and coworkers made no distinction between the AECOPD endotypes in their analysis. In the present study, we found almost two-fold greater NLR values in the neutrophilic AECOPD group than the values reported by Gunay and coworkers, while NLR values in the eosinophilic AECOPD subgroup were lower than those reported by Gunay and coworkers. Salturk and coworkers evaluated patients with very severe AECOPD requiring intensive care admission, and they reported NLR values nearly 2.8 times lower in the eosinophilic group compared to the noneosinophilic group (NLR=4.6 versus NLR=13.0, respectively) [25]. However, the NLR values of their patients requiring ICU admission were higher than the NLR values of the patients presented in the current study. The NLR values have been reported to increase as the severity of attack increases [16,25]. Kurtipek and coworkers published a study on 94 COPD patients; 46 of the 94 patients had AECOPD, and 48 of them had stable COPD. They reported that an NLR >3.3 and a PLR >150 could be used for the diagnosis of AECOPD [26]. Our study group had a larger sample size than the Kurtipek study, and all the patients had severe AECOPD. In addition, Kurtipek et al. [26] did not categorize the AECOPD patients into subgroups with respect to endotypes. NLR values in our eosinophilic AECOPD subgroup were similar to those reported by Kurtipek et al. [26]; however, the neutrophilic AECOPD subgroup presented here had NLR values nearly two times higher than those of the eosinophilic group.
The relationship between MPV and COPD is controversial. MPV is reported to be higher in stable COPD patients compared with healthy individuals (stable COPD, 10.6 and smoker control group, 9.9) [27]. In contrast, Wang et al. [17] reported that MPV was lower in the stable period and during the exacerbation of COPD compared with healthy individuals (COPD exacerbation, 9.5; stable COPD, 9.8; and control group, 10.4). They found that a reduced MPV was positively related to white blood cell count and CRP levels in exacerbated COPD patients. In the present study, PLT/MPV rate was found to be significantly higher in the longer stay group, but it is difficult to make a clinical interpretation solely on this result. PLT/MPV values were similar in the eosinophilic and noneosinophilic groups at both hospital admission and discharge. However, a PLT/MPV ratio of <20 was found to indicate a nearly 3.5 times higher risk of hospital mortality in the logistic regression model.
Steroid Therapy and Readmission in Eosinophilic and Neutrophilic AECOPD
Studies have shown the presence of sputum eosinophilia with good response to steroids in COPD [28,29]. Bafadhel et al. [3] reported that a steroid regimen determined by the presence of peripheral blood eosinophils greater than 2% did not result in treatment failure or deterioration of symptoms compared with the standard treatment regimen. In another study, treatment failure was 11% for patients receiving steroids and 66% for patients not receiving steroids in the group with peripheral blood eosinophils ≥2% [8]. Steroid use did not affect the success of treatment in the group with peripheral blood eosinophils <2% [8]. In the present study, the shorter- and longer stay group with eosinophilic AECOPD patients who received steroid therapy, had no incidence of hospital mortality (Figure 2). In this study, hospital readmission within the first 28 days after hospital discharge was found to be higher in the eosinophilic patients who did not receive steroid therapy and higher in the noneosinophilic patients who received steroid therapy. These findings may suggest that steroid usage in an eosinophilic attack of COPD is important; however, in noneosinophilic exacerbations, steroids may not be the first choice of treatment. The COPD guidelines suggest the use of steroid as “consider” in patients with AECOPD [1]. However, there are no detail definitions for the criteria of considering steroids and also for antibiotics. The unnecessary use of steroid or not to use antibiotic can lead to undesired complications, such as progress the infections and prolonged hospitalization. However, the logic that “consider steroid if peripheral blood eosinophil >2%” appears relevant. Further well-designed studies will support this approach.
Length of Hospital Stay and Mortality
The LOS in the hospital and mortality is reported to be longer and higher as the severity of the disease increases [30]. In different studies, the length of hospitalization was found to be 8.4–9 days, and hospital mortality was found to be 5.9–7.4% [30–32]. Advanced age, poor performance status, low albumin, and pulse oxygen saturation levels have been identified as independent risk factors for prolonged hospitalization. Age, blood urea, serum albumin, arterial pH, arterial oxygen saturation levels, and performance status were independent factors that increased mortality [32]. In a previous study, the 6-month mortality was found to be similar in the eosinophilic and noneosinophilic groups (14.2% and 15.2%, respectively) [19]. In the present study, CRP values >50 mg/dL, age >75 years, serum albumin <2.5 g/dL, and PLT/MPV <20 were associated with an increased risk of hospital mortality. Some studies have investigated an association between COPD severity and mortality and eosinophilia. In the study of Holland and coworkers comparing COPD exacerbations with eosinopenia and COPD exacerbations with a normal eosinophil count, the days of hospitalization were 8 and 5 days, respectively, and mortality rates were reported to be 17% and 2%, respectively [33]. In the present study, the shorter- and longer stay group had lower mortality rates, both in the eosinophilic and noneosinophilic groups, compared with those in the Holland’s study. Salturk and coworkers showed that the LOS in hospital and mortality in eosinophilic and noneosinophilic patients with COPD exacerbation were 4 days and 6 days and 12.9% and 24.9%, respectively [25]. Recently Yao and colleagues conducted a study on 303 AECOPD patients to evaluate NLR and PLR as potential prognostic biomarkers for hospital mortality (n=37, 12.2%) [18]. They defined an NLR >6.24, PLR >182.68, and CRP >16.45 as high risk for increased mortality. Thus, increased NLR and PLR may be useful prognostic biomarkers in AECOPD for hospital mortality. In their study, both survivors and nonsurvivors had a mean LOS in the hospital of 15 days. In the present study, the mortality rate of patients with AECOPD was nearly one-eighth less than that reported by Yao et al. [18]. Also, LOS was shorter in the nonsurvivors (a fourth less) and survivors (half), respectively, in our study.
In the present study, the PLR (182.68) was evaluated for an association with mortality in a binary logistic regression model; however, there was no significant difference between the groups.
There were some limitations in this study. Firstly, it was a retrospective study; however, we believe that it provides valuable clinical information for hospital-assessed outcomes of patients with eosinophilic and noneosinophilic exacerbations of COPD. Secondly, the COPD severity and spirometry results were not recorded. However, all patients were previously diagnosed using spirometry results by a pulmonologist in a teaching hospital for chest diseases. All study patients were chronic followed-up patients, and patients were not included from other center and city. Lastly, this study was carried out at a single center. The study center is however the biggest chest teaching hospital in the country (503 beds), and patient numbers could be high enough for an acceptable valuable support to future studies.
In conclusion, this study showed the inflammatory indicators of eosinophilic and neutrophilic AECOPD. If AECOPD has an eosinophilic endotype (i.e., peripheral blood eosinophils>2%), the novel inflammatory markers NLR and PLR are not helpful for follow-up treatment response due to nonsignificant changes during the hospital stay. However, NLR and PLR can be used to follow-up treatment and clinical response in the neutrophilic endotype (i.e., peripheral blood eosinophil ≤2%) of AECOPD. In routine clinical assessment, the treatment choice for AECOPD is made without focusing on the endotype of AECOPD. For patients hospitalized with COPD exacerbation and grouped according to their endotype, i.e., noneosinophilic (eosinophils≤2%) and eosinophilic (eosinophils>2%), clinicians can more effectively plan their treatment regimen. Blood count values can guide the clinician when deciding on antibiotics (infectious attack) or steroids (noninfectious attack). We found a longer LOS in the hospital when steroid therapy was received in cases of infectious exacerbation and when antibiotics were used for inflammatory COPD exacerbations. Distinguishing an infectious or inflammatory exacerbation of COPD can be very simply achieved by checking peripheral blood eosinophil levels, NLR, and even PLR. If the appropriate treatment regimen for COPD exacerbations is carried out, LOS in the hospital may be shorter than 7 days and readmission rates may be decreased.
Acknowledgements
The authors thank Dr Sharon Forsyth for editing the manuscript. The English in this document has been checked by at least two professional editors, and both are native speakers of English. (http://www.biomedicalediting.com). Some of the work data were presented at the ATS congress 2016 in “ATS and TTS sister society session”.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013).
Informed Consent: The study designed as a retrospective study, hospital electronic database was used, patients’ identity information is confidential.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - Z.K., E.A., S.G.; Design - C.S., E.T., M.Y. ; Supervision - M.Ç A., İ.Ö., D.D. ; Resource - N.D.K., Ü.A.A., B.O ; Materials - E.T., S.G., E.A. ; Data Collection and/or Processing - D.D., M.Ç.A., B.O.; Analysis and/or Interpretation - Z.K., E.A., C.S. ; Literature Search - E.A., E.T., İ.Ö. ; Writing - Z.K., E.A., S.G. ; Critical Reviews - Z.K., E.A., S.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD (Revised 2017) http://www.goldcopd.org.
- 2.Agustí A, Celli B, Faner R. What does endotyping mean for treatment in chronic obstructive pulmonary disease? Lancet. 2017;390:980–7. doi: 10.1016/S0140-6736(17)32136-0. [DOI] [PubMed] [Google Scholar]
- 3.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharitonov SA, Yates D, Robbins RA, et al. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–5. doi: 10.1016/S0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 5.Saetta M, Di Stefano A, Maestrelli P, et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150:1646–52. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 6.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:39–47. doi: 10.2147/copd.2006.1.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–71. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 8.Bafadhel M, Davies L, Calverley PM, et al. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J. 2014;44:789–91. doi: 10.1183/09031936.00062614. [DOI] [PubMed] [Google Scholar]
- 9.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibilty to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 10.Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–21. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 11.Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906–13. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 12.Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193:965–74. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 13.Clark TW, Medina MJ, Batham S, et al. C-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation of COPD. Eur Respir J. 2015;45:76–86. doi: 10.1183/09031936.00020015. [DOI] [PubMed] [Google Scholar]
- 14.Peng C, Tian C, Zhang Y, et al. C-reactive protein levels predict bacterial exacerbation in patients with chronic obstructive pulmonary disease. Am J Med Sci. 2013;345:190–4. doi: 10.1097/MAJ.0b013e318253c921. [DOI] [PubMed] [Google Scholar]
- 15.Gao P, Zhang J, He X, et al. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2013;8:e57678. doi: 10.1371/journal.pone.0057678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Günay E, Sarınç Ulaşlı S, Akar O, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation. 2014;37:374–80. doi: 10.1007/s10753-013-9749-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang RT, Li JY, Cao ZG, et al. Mean platelet volume is decreased during an acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2013;18:1244–8. doi: 10.1111/resp.12143. [DOI] [PubMed] [Google Scholar]
- 18.Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–90. doi: 10.2147/COPD.S141760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duman D, Aksoy E, Agca MC, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469–78. doi: 10.2147/COPD.S90330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon NB, Son C, Um SJ. Role of the neutrophil-lymphocyte count ratio in the differential diagnosis between pulmonary tuberculosis and bacterial community-acquired pneumonia. Ann Lab Med. 2013;33:105–10. doi: 10.3343/alm.2013.33.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turkish Thoracic Society. Chronic Obstructive Pulmonary Disease Prevention, Diagnosis and Treatment Report. 2014. [Google Scholar]
- 22.Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–42. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 23.Singh D, Kolsum U, Brightling CE, et al. ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 24.Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:867–74. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 25.Saltürk C, Karakurt Z, Adiguzel N, et al. Does eosinophilic COPD exacerbation have a better patient outcome than non-eosinophilic in the intensive care unit? Int J Chron Obstruct Pulmon Dis. 2015;10:1837–46. doi: 10.2147/COPD.S88058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtipek E, Bekci TT, Kesli R, et al. The role of neutrophil-lymphocyte ratio and platelet lymphocyte ratio in exacerbation of chronic obstructive pulmonary disease. J Pak Med Assoc. 2015;65:1283–7. [PubMed] [Google Scholar]
- 27.Steiropoulos P, Papanas N, Nena E, et al. Mean platelet volume and platelet distribution width in patients with chronic obstructive pulmonary disease: the role of comorbidities. Angiology. 2013;64:535–9. doi: 10.1177/0003319712461436. [DOI] [PubMed] [Google Scholar]
- 28.Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193–8. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitaguchi Y, Komatsu Y, Fujimoto K, et al. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int J Chron Obstruct Pulmon Dis. 2012;7:283–9. doi: 10.2147/COPD.S30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lykkegaard J, Søndergaard J, Kragstrup J, et al. All Danish first-time COPD hospitalisations 2002–2008: incidence, outcome, patients, and care. Respir Med. 2012;106:549–56. doi: 10.1016/j.rmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Fuhrman C, Roche N, Vergnenègre A, et al. Hospital admissions related to acute exacerbations of chronic obstructive pulmonary disease in France, 1998–2007. Respir Med. 2011;105:595–601. doi: 10.1016/j.rmed.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Connolly MJ, Lowe D, Anstey K, et al. British Thoracic Society and the Royal College of Physicians Clinical Effectiveness Evaluation Unit (CEEu) Admissions to hospital with exacerbations of chronic obstructive pulmonary disease: Effect of age related factors and service organisation. Thorax. 2006;61:843–8. doi: 10.1136/thx.2005.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland M, Alkhalil M, Chandromouli S, et al. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology. 2010;15:165–7. doi: 10.1111/j.1440-1843.2009.01651.x. [DOI] [PubMed] [Google Scholar]


