Abstract
Objective
China has undertaken several initiatives to improve the accessibility of safe and effective medicines for children. The aim was to determine the availability, price and affordability of essential medicines for children.
Design
Cross-sectional survey.
Setting
Six cities of Jiangsu Province, China.
Participants
30 public hospitals and 30 retail pharmacies.
Primary and secondary outcome measures
The WHO/Health Action International standardised methodology was used to collect the availability and price data for 40 essential medicines for children. Availability was measured as the percentage of drug outlets per sector where the individual medicine was found on the day of data collection, and prices were measured as median price ratios (MPRs). Affordability was measured as the number of days’ wages required for the lowest paid unskilled government worker to purchase standard treatments for common conditions.
Results
The mean availabilities of originator brands (OBs) and lowest priced generics (LPGs) were 7.5% and 34.2% in the public sector and 8.9% and 29.4% in the private sector. The median MPRs of LPGs in both sectors ranged from 1.41 to 2.12 and 1.10 to 2.24, respectively. However, the patient prices of OBs far exceeded the critical level in both sectors, with median MPRs ranging from 2.47 to 8.22. More than half of these LPGs were priced at 1.5 times their international reference prices in the public sector. Most LPGs were affordable for treatment of common conditions in both public and private sectors, as they each cost less than the daily wage for the lowest paid unskilled government worker.
Conclusions
Access to essential medicines for children is hampered by low availability. Further measures to enhance access to paediatric essential medicines should be taken, such as developing a national essential medicine list for children and mobilising the enthusiasm of pharmaceutical firms to develop and manufacture paediatric medicines.
Keywords: availability, affordability, prices, essential medicines, children, china
Strengths and limitations of this study.
The use of a previously validated WHO/Health Action International methodology allows for the measurement of medicine prices and availability in a reliable and standardised way.
Utilisation of international reference prices in this study can allow for valid international comparisons between China and other countries.
The data refer to the availability of a given medicine in a particular dosage form and strength on the day of data collection at each outlet in six cities of Jiangsu Province. This cross-sectional study is unable to reflect the average monthly, quarterly or yearly availability of medicines at individual outlets.
Due to the limitation of suitable dosage forms on the National Essential Medicines List, only medicines having international reference prices were selected as survey objects and compared with those in other countries, which gave rise to a lack of oral liquid dosage forms in the survey list.
The treatment affordability of three common paediatric conditions was calculated by using the cost of tablets or capsules, which may have caused bias in estimating the affordability of standard treatment regiments.
Introduction
Access to healthcare, including essential medicines, is a fundamental human right.1 Specifically, essential medicines are the backbone of healthcare, which can satisfy the priority healthcare needs of the population.2–4 Equitable access to essential medicines is one of the millennium development goals of the United Nations.1 However, data from some surveys in developing countries have shown that the availability of essential medicines, particularly for children, is generally low and that the medicines are unaffordable.5–8 Less access to essential medicines has been a significant global public health issue. The WHO has estimated that at least one-third of the world’s population does not have regular access to essential medicines,9 and the challenges of poor access are also common for children.6 8 10 The reasons for the lack of access to paediatric essential medicines can include the absence of sustainable financing and efficient supply systems, no regulated medicines, irrational selection and use of medicines or out-of-pocket payments that make the prices unaffordable.2 11 12 Importantly, one of the reasons is that the essential medicines are rarely found in public hospitals and retail pharmacies in the recommended dosages and formulations for children.10 13
It is estimated that 5.9 million children under 5 years of age died in 2015, with a global under-five mortality rate of 42.5 per 1000 live births. Levels of child mortality are higher in developing countries. Moreover, leading causes of child death in the postneonatal period were pneumonia, diarrhoea, injuries and malaria.14 Essential medicines for children can save lives and improve child health when they are available, affordable, of assured quality and properly used. To escalate the accessibility of essential medicines for children, the WHO published the first WHO Model List of Essential Medicines for Children (WHO EMLc) and launched the ‘Make Medicines Child Size’ effort in December of 2007 (WHO EMLc). The WHO EMLc highlights the most critical medicines for paediatric patients, which are intended for use by children up to 12 years of age.15 The WHO EMLc has been updated every 2 years since 2007. Six editions of the WHO EMLc have been published between 2007 and 2017.
As in many developing countries, lack of access to paediatric essential medicines has caused growing concern in China. Since 2009, the central government officially has taken a series of measures to establish the National Essential Medicine System (NEMS) to meet the public’s basic healthcare needs. Based on the WHO model list of essential medicines, the National Health Commission of China (NHC) launched the first Chinese National Essential Medicines List (NEML) in August 2009,16 which included 307 Western and Chinese medications. In 2012, the second NEML was released by the NHC, which included approximately 130 medicines for children and 70 formulations and specifications indicated for paediatric use.6 The NEMS requires that only essential medicines should be stocked and dispensed in the public primary healthcare institutions. The secondary and tertiary hospitals and private hospitals should provide essential medicines as priority drugs for patients.
Despite this NHC initiative, China is still confronted with low access to paediatric essential medicines. Most medicines on the NEML are suitable for adults, which do not sufficiently satisfy paediatric patients’ basic medical needs,6 and the formulations, strengths and dosage forms suitable for children are still in short supply in the healthcare facilities. The medicines for children account for only 2% of the total medicines available. Moreover, there is still no list of essential medicines for children. In 2016, the Chinese central government announced the relaxation of its one-child policy to encourage births. It is estimated that the fertility rate of childbearing-age women will start to increase. The public demand for safe, effective and quality paediatric essential medicines is growing. Therefore, the government should take targeted measures to improve medicine access to children to solve the problems.
A vital first step to improving essential medicine access for children is measuring the availability, prices and affordability of essential medicines in all sectors. Data on the accessibility of essential medicines for children will help health policy makers develop national or regional policy, regulations and strategies to enhance access to them. The WHO and Health Action International (HAI) developed a standardised method for investigating medicine prices, availability and affordability in selected sectors in May 2003.17 However, most surveys focused on the medicines for adults according to the WHO/HAI methodology.18–22 Only a few studies provided these types of data on the accessibility of the paediatric essential medicines for health policy makers.6 8 10 23 The study conducted by Balasubramaniam et al assessed the availability of essential medicines for children and demonstrated that essential medicines for children were less available in public hospitals than in private pharmacies.23 Similar findings have been reported in other developing countries. For instance, Anson et al revealed that the public sector had a lower average availability (25%) compared with the private sector (35%) for paediatric essential medicines in Guatemala. These findings also showed the essential medicines were generally unaffordable.10 Sado et al showed that the availability of paediatric essential medicines was low and that these medicines were sold at higher prices, making them unaffordable for people with low incomes, in Ethiopia.8
Some surveys on the availability, prices and affordability of essential medicines for adults have been conducted in China using the WHO/HAI standardised methodology.13 22 24–26 However, only one study has been conducted, in Shaanxi Province in 2014, using the WHO/HAI methodology to evaluate the prices, availability, price components and affordability of paediatric medicines.6 The study demonstrated that the lowest priced generic (LPG) equivalents of paediatric medicines had better availability than originator brands (OBs) across the sectors. Hence, to our knowledge, this is the second study of this type since the NEMS was established in China and the first conducted in Jiangsu Province.
The purpose of this study was to investigate prices and availability of OBs and generic essential medicines across the public sector (primary healthcare facilities, secondary hospitals, tertiary hospitals) and private sector (retail pharmacies) in six of its cities to assess the availability, prices and affordability of essential medicines for children to determine their accessibility.
Methods
We conducted a survey of the availability, prices and affordability of children’s essential medicines in Jiangsu Province, China, using a standardised methodology developed by WHO and HAI.18 All data on the availability and patient prices of medicines in the public and private sectors was collected from 10 July 2017 to 5 September 2017. Convenience sampling was used in this study, which is a non-probability sampling technique where subjects are selected because of their convenient accessibility and proximity to the researchers.
Survey area
Jiangsu Province is located in eastern China, having 13 cities and a population of 79.73 million. Nanjing, the capital city of Jiangsu Province, was chosen as the major urban centre. As recommended by the WHO/HAI methodology, considering geographical position and level of economic development, six representative cities of this province were selected as survey areas for data collection by convenience sampling: Suzhou, Changzhou, Nanjing, Zhenjiang, Xuzhou and Huaian. The selected cities can be reached within 1 day of travel from the capital.
Selections of medicines outlets
Based on the government records, the sampling frame for the public sector facilities was designed, and the facility type was consistently defined and recorded. In each survey area, we first selected the main public tertiary hospital, which was a children’s hospital or women and children’s health hospital. An additional four public medicine outlets, two secondary hospitals and two primary healthcare facilities per survey area were then chosen within 3 hours’ travel of the main hospital by convenience sampling. Therefore, five public medicine outlets in each of the six cities were included in the public sector, yielding a sample of 30 public outlets.
Private sector facilities were identified by selecting five retail pharmacy outlets in each city that were in geographic proximity to the nearest public facility by convenience sampling. In each city, two retail chain pharmacies and three retail pharmacies were included. Thus, 30 private facilities in all were surveyed. In this study, a retail pharmacy was defined as a single outlet that provided prescription drugs, among other products. A retail pharmacy was not directly affiliated with any chain of pharmacies and was not owned (or operated) by a publicly traded company. However, retail chain pharmacies were retail outlets that shared a brand and central management and usually had standardised business methods and practices. In contrast to the retail pharmacies, retail chain pharmacies had multiple store locations and a larger business scale. Thus, in total, this study was undertaken in 30 public outlets and 30 retail pharmacies.
In each survey city, we selected one county-level secondary hospital or township health centre and one rural retail pharmacy outlet by convenience sampling. Therefore, one rural public medicine outlet in each of the six areas was included, and one rural retail pharmacy outlet per survey area was sampled. Thus, 12 rural facilities in all were surveyed, which accounted for 20% of the sampled public and private facilities.
Selection of medicines to be surveyed
According to the requirements of the WHO/HAI methodology, the systematic survey should identify core and supplementary lists of medicines selected by each country based on local disease burden and needs.27 A total of 40 medicines were surveyed, all of which had international reference prices (IRPs) and were registered in China. Twenty-nine of these medicines were identified as core medicines, which were on the WHO’s EMLc.28 However, only three of the 29 core medicines were not on the 2012 NEML. Apart from these core medicines, a supplementary list of medicines was added. The other 11 were identified as supplementary medicines, which were selected based on the local children’s disease needs, the 2012 NEML, feedback from several paediatric experts and literature reviews. Five of these were selected from the core medicines list but in different dose forms. Table 1 lists all the surveyed medicines.
Table 1.
List of essential medicines for children surveyed in Jiangsu Province
| No. | Name | Strength | Dosage form | NEM |
| Core list | ||||
| 1 | Aciclovir | 200 mg | Cap/tab | Yes |
| 2 | Amoxicillin | 250 mg | Cap/tab | Yes |
| 3 | Amoxicillin/clavulanic acid | 125/31.25 mg/5 mL | Suspen | No |
| 4 | Azithromycin | 250 mg | Cap/tab | Yes |
| 5 | Calamine | 100 mL | Lotion | Yes |
| 6 | Calcium gluconate | 100 mg/mL | Ampoule | Yes |
| 7 | Carbamazepine | 200 mg | Cap/tab | Yes |
| 8 | Ceftriaxone | 1 g | Phial | Yes |
| 9 | Ceftazidime | 1 g | Phial | Yes |
| 10 | Clarithromycin (sustained-release) | 500 mg | Cap/tab | No |
| 11 | Clindamycin | 150 mg | Cap/tab | Yes |
| 12 | Diazepam | 5 mg/mL | Ampoule | Yes |
| 13 | Fluconazole | 50 mg | Cap/tab | Yes |
| 14 | Folic Acid | 5 mg | Cap/tab | Yes |
| 15 | Furosemide | 10 mg/mL | Ampoule | Yes |
| 16 | Hydrochlorothiazide | 25 mg | Cap/tab | Yes |
| 17 | Hydrocortisone | 100 mg | Phial | Yes |
| 18 | Ibuprofen | 200 mg | Cap/tab | Yes |
| 19 | Loratadine | 10 mg | Cap/tab | Yes |
| 20 | Miconazole nitrate | 2% | Cream | Yes |
| 21 | Mupirocin | 2% | Cream | No |
| 22 | Omeprazole (enteric-coated) | 20 mg | Cap/tab | Yes |
| 23 | Paracetamol | 500 mg | Cap/tab | Yes |
| 24 | Phenobarbital | 30 mg | Cap/tab | Yes |
| 25 | Phenytoin | 100 mg | Cap/tab | Yes |
| 26 | Propylthiouracil | 50 mg | Cap/tab | Yes |
| 27 | Ranitidine | 150 mg | Cap/tab | Yes |
| 28 | Salbutamol | 100 mcg/dose | Inhaler | Yes |
| 29 | Sodium valproate | 200 mg | Cap/tab | Yes |
| Supplementary list | ||||
| 1 | Aminophylline | 100 mg | Cap/tab | Yes |
| 2 | Amoxicillin/clavulanic acid | 1000/200 mg | Phial | Yes |
| 3 | Cefuroxime | 250 mg | Cap/tab | Yes |
| 4 | Chlorphenamine maleate | 4 mg | Cap/tab | Yes |
| 5 | Dexamethasone | 5 mg/mL | Ampoule | Yes |
| 6 | Clarithromycin | 250 mg | Cap/tab | Yes |
| 7 | Ibuprofen | 100 mg/5 mL | Suspen | Yes |
| 8 | Phenobarbital | 100 mg/mL | Ampoule | Yes |
| 9 | Vitamin B6 | 50 mg/mL | Ampoule | Yes |
| 10 | Vitamin C | 100 mg | Cap/tab | Yes |
| 11 | Sodium valproate (sustained-release) | 500 mg | Cap/tab | No |
Cap, capsule; NEM, National Essential Medicines; suspen, suspension; tab, tablet.
For each medicine, two forms, OB and LPG, were surveyed. The OB product had a unique originator pharmaceutical company, and LPG equivalents were defined as the same product sold under the generic name with the lowest unit price at each medicine outlet at the time of data collection.22
Data collection
To verify the feasibility and effectiveness of the survey, a pilot study was conducted in Nanjing prior to the data collection. In addition, a standardised data collection form was designed and used to ensure data accuracy and reliability. Six well-trained research assistants (RAs) visited the enrolled public and private outlets to finish collecting data on the availability and patient prices of paediatric essential medicines. At the end of each day, the RAs checked the completed data collection forms and ensured that the data were integral, consistent and legible at the end of each day. The data collection was completed within 2 months.
The trained RAs entered survey data into the preprogrammed MS Excel Workbook provided by the WHO/HAI. Data were double-entered, and the data checker function on the spreadsheet was used to avoid data entry errors.
Data analysis
This study focused on three key endpoints: medicine availability, patient prices and affordability. The availability of individual medicines is calculated as the percentage (%) of the surveyed outlets where the medicine was found on the day of data collection. Mean availability was calculated for OBs and LPGs for the overall basket of all 40 medications surveyed within the public and private sectors.
To facilitate national and international comparisons, patient prices were presented as median price ratios (MPRs). The MPR is the ratio of the local median unit price of a medicine divided by the median IRP. The MPRs were calculated to express how much greater or less the median local medicine price was than the IRP. For instance, an MPR of 1.5 would mean that the local medicine price was 1.5 times the IRP. MPRs were only calculated when the medicine was available at a minimum of four medicine outlets. In this study, medicine prices from the Drug Prices Guide in 2015 issued by Management Science for Health (MSH) were adopted as the IRPs for surveyed medicines. MSH IRPs represent actual procurement prices for medicines offered to developing countries by non-profit suppliers, which are generally recommended as the most useful standard. In general, an MPR of one or less is taken as an efficient procurement system in the public sector, while below 2.5 is considered efficient for the private sector. For the purposes of discussion in this study, an MPR of 1.5 and 2.0 was the cut-off point for patient price in the public sector and private sector, respectively.6 Meanwhile, less than 30% is regarded as very low availability, and greater than 80% is regarded as high availability.29
The exchange rate used to calculate MPRs was 1 US$=$C6.7964; this was the commercial ‘buy’ rate taken from State Administration of Foreign Exchange.com on the first day of data collection (10 July 2017).30
According to the results of the fifth national health service survey on child healthcare in China and paediatric experts’ opinions, eight common conditions in childhood were chosen to assess the affordability by comparing the total cost of medicines at a standard dose to the daily wage of the lowest paid unskilled government worker, which was RMB 53.0/day (US$7.7982 per day) at the time of the survey.31 Treatment affordability was calculated by using the cost of medicine for a full course of therapy for acute diseases or the cost of a 30-day supply of medicines for chronic diseases. The duration of a full course of treatment for acute diseases was determined by the seventh edition of Paediatrics published by People’s Health Publishing House.32 If the treatment cost was less than a daily wage, we categorised it as an affordable medicine, while it was unaffordable if its cost was over a day’s wage. The 5- year-old boys were taken as objects, whose average weight was approximately 20 kg in China.33
Patient and public involvement
The patients and public were not involved in this study.
Results
Availability
Table 2 shows the availability of individual medicines in the public sector and the private sector. The availability of the selected medicines in both sectors was low. Among LPGs, dexamethasone injection and loratadine tablet had the highest availability in the public sector and the private sector, respectively.
Table 2.
Availability of individual medicines in the public sector and the private sector
| Name of medicine | Public sector | Private sector | ||
| OBs availability (%) | LPGs availability (%) | OBs availability (%) | LPGs availability (%) | |
| Aciclovir Ttab 200 mg | 0 | 26.7 | 0 | 6.7 |
| Amoxicillin tab/cap 250 mg | 0 | 56.7 | 0 | 60.0 |
| Amoxicillin/clavulanic acid suspension 125/31.25 mg/5 mL | 0 | 3.3 | 0 | 3.3 |
| Azithromycin tab/cap 250 mg | 10.0 | 30.0 | 16.7 | 53.3 |
| Calamine lotion 100 mL | 0 | 50.0 | 3.3 | 56.7 |
| Calcium gluconate injection 100 mg/mL | 0 | 63.3 | 0 | 13.3 |
| Carbamazepine tablet 200 mg | 33.3 | 0 | 46.7 | 0 |
| Ceftriaxone injection 1 g/phial | 10.0 | 16.7 | 3.3 | 26.7 |
| Ceftazidime injection 1 g/phial | 6.7 | 40.0 | 0 | 3.3 |
| Clarithromycin tablet 500 mg (sustained-release) | 0 | 23.3 | 0 | 43.3 |
| Clindamycin capsule 150 mg | 0 | 0 | 0 | 20.0 |
| Diazepam injection 5 mg/mL | 0 | 60.0 | 0 | 3.3 |
| Fluconazole tab/cap 50 mg | 10.0 | 33.3 | 10.0 | 43.3 |
| Folic acid tablet 5 mg | 0 | 36.7 | 0 | 43.3 |
| Furosemide injection 10 mg/mL | 3.3 | 60.0 | 0 | 13.3 |
| Hydrochlorothiazide tablet 25 mg | 0 | 73.3 | 0 | 46.7 |
| Hydrocortisone injection 100 mg/phial | 0 | 30.0 | 0 | 10.0 |
| Ibuprofen tablet 200 mg | 0 | 0 | 16.7 | 10.0 |
| Loratadine tablet 10 mg | 23.3 | 46.7 | 16.7 | 80.0 |
| Miconazole nitrate cream 2% | 36.7 | 6.7 | 23.3 | 26.7 |
| Mupirocin cream 2% | 36.7 | 3.3 | 63.3 | 13.3 |
| Omeprazole tab/cap 20 mg (enteric-coated) | 26.7 | 66.7 | 40.0 | 70.0 |
| Paracetamol tablet 500 mg | 3.3 | 13.3 | 0 | 16.7 |
| Phenobarbital tablet 30 mg | 0 | 50.0 | 0 | 0 |
| Phenytoin tablet 100 mg | 0 | 30.0 | 0 | 40.0 |
| Propylthiouracil tablet 50 mg | 0 | 30.0 | 3.3 | 23.3 |
| Ranitidine tablet 150 mg | 0 | 26.7 | 3.3 | 63.3 |
| Salbutamol inhaler 100 µg/dose | 30.0 | 13.3 | 30.0 | 40.0 |
| Sodium valproate tablet 200 mg | 0 | 53.3 | 0 | 33.3 |
| Aminophylline tablet 100 mg | 0 | 33.3 | 0 | 56.7 |
| Amoxicillin/clavulanic acid injection 1000/200 mg/phial | 6.7 | 10.0 | 0 | 0 |
| Cefuroxime tablet 250 mg | 3.3 | 13.3 | 13.3 | 33.3 |
| Chlorphenamine maleate tablet 4 mg | 0 | 23.3 | 0 | 50.0 |
| Dexamethasone injection 5 mg/mL | 0 | 86.7 | 0 | 13.3 |
| Clarithromycin tablet 250 mg | 3.3 | 46.7 | 6.7 | 50.0 |
| Ibuprofen suspension 100 mg/5 mL | 13.3 | 30.0 | 33.3 | 23.3 |
| Phenobarbital injection 100 mg/mL | 0 | 53.3 | 0 | 0 |
| Vitamin B6 injection 50 mg/mL | 0 | 66.7 | 0 | 20.0 |
| Vitamin C tablet 100 mg | 0 | 56.7 | 0 | 56.7 |
| Sodium valproate tablet 500 mg (sustained-release) | 43.3 | 3.3 | 26.7 | 10.0 |
LPGs, lowest priced generics; OBs, originator brands.
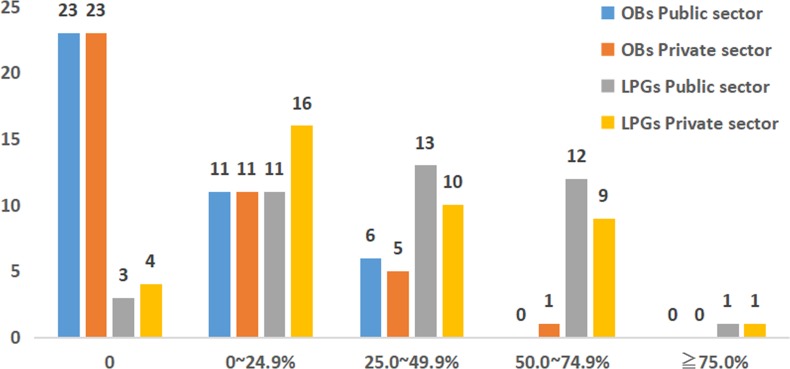
The availability of OBs and LPGs in both the public and private sectors is shown in figure 1. In both sectors, 23 OBs were not found. In the public sector, 11 OBs had availabilities of less than 25.0%, 6 OBs were between 25.0% and 49.9%, and no OBs were found in 50.0% or more of outlets. Meanwhile, in the private sector, 11 OBs were less than 25.0%, 5 OBs were 25.0%–49.9% and only 1 medicine was found in 50.0% or more of retail pharmacies. Three LPGs were not found, 11 LPGs had availabilities of less than 25.0%, 13 LPGs were 25.0% to 49.9% and 12 LPGs were found in 50.0%–74.9% of outlets in the public sector. The situation in the private sector was different: 4 LPGs were not found, 16 LPGs were less than 25.0%, 10 LPGs were 25.0%–49.9% and 9 LPGs were found in 50.0%–74.9% of outlets. Only one medicine was found in 75.0% or more of both sectors.
Figure 1.
Availability of OBs and LPGs in the public sector and the private sector. LPGs, lowest priced generics; OBs, originator brands.
As shown in table 3, the mean availability of medicines varied by medicine list and sector. The mean availability of OBs and LPGs was 7.5% and 34.2% in the public sector and 8.9% and 29.4% in the private sector. For the medicines listed on the EMLc, the mean availability of LPGs was 32.8% in the public sector and 29.7% in the private sector. For the medicines listed on the NEML, the mean availability in the public sector was 6.0% for OBs and 36.9% for LPGs, compared with 7.4% for OBs and 30.8% for LPGs in the private sector.
Table 3.
The mean availability of medicines in the public sector and the private sector
| Type | Primary healthcare facilities (n=12) | Public hospitals (n=18) | Public sector (n=30) | Private sector (n=30) |
| OBs | ||||
| All | 7.7 | 9.4 | 7.5 | 8.9 |
| Core | 8.4 | 10.0 | 8.2 | 9.5 |
| Supplementary | 5.9 | 7.9 | 5.8 | 7.3 |
| NEM | 6.4 | 7.4 | 6.0 | 7.4 |
| LPGs | ||||
| All | 30.1 | 35.6 | 34.2 | 29.4 |
| Core | 30.0 | 34.1 | 32.8 | 29.7 |
| Supplementary | 30.5 | 39.4 | 37.9 | 28.8 |
| NEM | 32.0 | 38.0 | 36.9 | 30.8 |
LPGs, lowest priced generics; NEM, National Essential Medicines; OBs, originator brands.
The public sector in this study was divided into two categories: primary healthcare facilities and secondary and tertiary hospitals. The mean availability of OBs and LPGs was 7.7% and 30.1% in primary healthcare facilities, respectively. The mean availability of OBs and LPGs was 9.4% and 35.6% in secondary and tertiary hospitals, respectively. Overall, for the medicines listed on the supplementary list, the higher availability of LPGs (30.5%) and the lowest of OBs (5.9%) were observed at primary healthcare facilities, whereas the LPGs were most available (39.4%) and OBs had lower availability (7.9%) at secondary and tertiary hospitals.
Overall, OBs were less available than LPGs in both the public and private sectors.
Medicine prices
In the public sector, as shown in table 4, the median MPRs of all LPGs ranged from 1.41 to 2.12, which indicated that the patient prices of LPGs appeared to be close to the IRPs and were acceptable. However, the patient prices of OBs exceeded the cut-off point, with median MPRs ranging from 2.47 to 7.70. Coincidentally, in the private sector, the patient prices of LPGs were similar to the IRPs, with median MPRs ranging from 1.10 to 2.24. However, the patient prices of OBs were all above the threshold level and higher than those in public sector, with median MPRs ranging from 5.07 to 8.22.
Table 4.
Median MPRs of surveyed medicines in public sector and retail pharmacies
| Sector | Median MPRs of core medicines | Median MPRs of supplementary medicines | ||
| OBs | LPGs | OBs | LPGs | |
| Primary healthcare facilities (n=12) | 7.22 | 1.41 | 2.47 | 1.85 |
| Secondary and tertiary healthcare facilities (n=18) | 7.70 | 2.12 | 2.47 | 1.44 |
| Retail pharmacies (n=30) | 8.22 | 2.24 | 5.07 | 1.10 |
MPRs were calculated only for medicines with price data from at least four medicine outlets.
LPGs, lowest priced generics; MPRs, median price ratios; OBs, originator brands.
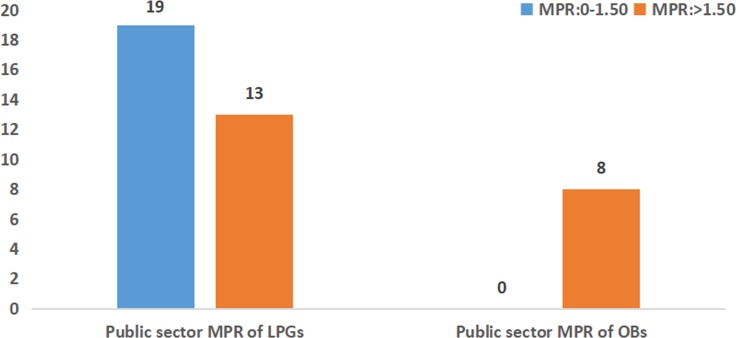
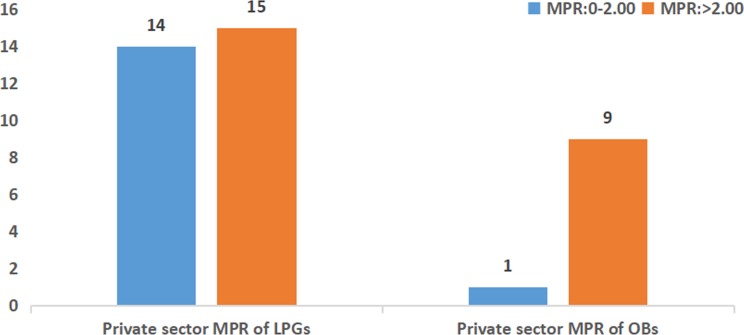
As shown in figures 2 and 3, in the public sector, the patient prices were more than 1.5 times the IRPs for 13 LPGs and less than 1.5 times the IRPs for 19 LPGs. However, in the private sector, 15 LPGs were sold at more than 2.0 times their IRPs, and nearly half of the LPGs (n=14) were priced at less than 2.0 times their IRPs. However, all OBs were priced at more than 1.5 times their IRPs in the public sector, and the MPRs were more than 2.0 times their IRPs for nine OBs in the private sector. Only one OB was sold at less than 2.0 times the reference price in retail pharmacies: salbutamol inhaler (1.90 times the IRP).
Figure 2.
The frequency distribution of median price ratios (MPRs) of medicines in the public sector. LPGs, lowest priced generics; OBs, originator brands.
Figure 3.
The frequency distribution of median price ratios (MPRs) of medicines in the private sector. LPGs, lowest priced generics; OBs, originator brands.
Affordability of standard treatment regiments
The affordability of standard treatments for eight different health conditions was calculated. Due to the low availability of OBs, we finally included eight LPGs and five OBs from the public sector and seven LPGs and five OBs from the private sector.
Table 5 shows the affordability of LPGs in the surveyed sectors. As LPGs were prescribed and dispensed in the public hospitals and retail pharmacies, only two treatments were costly. Treatments of paediatric urinary tract infection with the LPG of ceftriaxone injection from retail pharmacies and otitis media with the LPG of ceftazidime injection from public sector would cost 2.18 and 6.86 days’ wages, respectively. In addition, the most affordable LPGs were ibuprofen for treating pain and inflammation, which cost 0.03 days’ wages in the public sector and 0.07 days’ wages in the private sector. The cost of purchasing other LPGs in both public and private sectors was between 0.12 and 0.90 days’ wages, which demonstrated that generic paediatric essential medicines in Jiangsu Province were fairly affordable. Similarly, for OB medicines found in the surveyed sectors, three medicines cost over a day’s wage and were less affordable. The OBs of cefuroxime tablet for acute bronchitis from retail pharmacies, ceftazidime injection for otitis media from public sector and ceftriaxone injection for urinary tract infection from both sectors would cost 19.05, 22.63 and 26.03 days’ wages, respectively. It was noteworthy that ibuprofen for treating pain and inflammation was fairly affordable and highly available, costing between 0.06 and 0.09 days’ wages. Furthermore, the LPGs and OBs of ibuprofen were present in both public and private sectors.
Table 5.
Affordability: number of days’ wages of lowest paid unskilled government worker needed to purchase standard treatments
| Condition | Drug name, strength, dosage form | Treatment schedule | Days’ wages to pay for treatment | |
| LPGs: public sector | LPGs: private sector | |||
| Upper respiratory tract infection | Amoxicillin Ttb/cap 250 mg | Child 5–12 years: 250 mg*3*7 days, 5250 mg | 0.14 | 0.12 |
| Otitis media | Ceftazidime injection 1 g/phial | 5-year-old child: maximum 50 mg/kg*20 kg*3*7 days, 21 phials | 6.86 | n/a |
| Acute bronchitis | Cefuroxime tablet 250 mg | Child 2–12 years: maximum 250 mg*2*7 days 3500 mg | 0.68 | 0.71 |
| Urinary tract infection | Ceftriaxone Injection 1 g/phial | Child over 1 year: 75 mg/kg*20*14 days, 21 vials | 0.90 | 2.18 |
| Seizure disorder | Sodium valproate tablet 200 mg | 5-year-old child: 40 mg/kg *20*42 days, 33 600 mg | 0.32 | 0.29 |
| Asthma | Salbutamol inhaler 100 µg/dose | One inhaler of 200 doses, as needed | 0.38 | 0.34 |
| Acute eczema | Calamine lotion 100 mL | 100 mL as need | 0.15 | 0.19 |
| Pain/inflammation | Ibuprofen suspension 100 mg/5 mL | Child 3 months–12 years old: maximum 40 mg*20 kg*3 days. 2400 mg | 0.03 | 0.07 |
LPGs, lowest priced generics; NA, not available; OBs, originator brands.
As a whole, the standard treatments cost less than 1 day’s wage for LPGs (except for ceftriaxone and ceftazidime injection) in both sectors.
Discussion
Until now, only one study on access to paediatric essential medicines has been carried out in China using the standardised WHO/HAI methodology.6 As the first paediatric medicine survey to apply the methodology to the eastern region of China, the findings of this study, together with the previously conducted survey in western China, provide a comprehensive report on availability, prices and affordability of essential children’s medicines in China. The main findings of the present study concern the availability and prices of 40 paediatric essential medicines in public and private sectors of six cities in Jiangsu Province. The results revealed that the availability of essential medicines for children was low in both sectors. The mean availability of existing generic medicines and their original products was less than 40% in both the public and private sectors. Specifically, the mean availability of LPGs was 34.2% in the public sector and 29.4% in the private sector. Compared with the study conducted by Wang et al 6 in Shaanxi Province, China, our findings showed higher availability of paediatric essential medicines in both sectors. In Shaanxi Province, their analyses revealed that the mean availabilities of OBs and LPGs were 10.8% and 27.3% in the public hospitals versus 11.9% and 20.6% in the private pharmacies.6 Their findings are consistent with studies in some undeveloped countries, such as Ethiopia, Guatemala and Sri Lanka,8 10 23 which also showed low availability of paediatric essential medicines.
China is the largest developing country in the world. Nevertheless, what is not fitting is that access to children’s essential medicines is hampered by poor availability. In China, the list of essential medicines for children is still unavailable, and lack of access to paediatric essential medicines has caused increasing concern. Strengths and dosage forms suitable for children, such as oral solutions, are in short supply in the market.6 In this study, the dosage forms of survey medicines mainly include oral solid dosage forms (eg, tablets, capsules, granules and dry suspensions) and injections. However, few surveyed medicines have oral liquid dosage forms on the 2012 NEML. NEML lacks oral liquid dosage forms such as suspensions, which is a problem, especially for children. In routine clinical treatment, doctors have become used to reducing the doses of adult medicines and have to divide the tablets for adults into pieces to deal with paediatric diseases.6
Three reasons might explain these findings. First, due to low profit margins, the Chinese pharmaceutical manufacturers lack the motivation to produce the children’s essential medicines. Although there are more than 4000 pharmaceutical manufacturers in China, only approximately 5% of the pharmaceutical manufacturers are willing to produce children’s essential medicines. According to the statistical results, just 0.17% of pharmaceutical enterprises are specialised in the production of paediatric medicines.34 Second, the physicians’ willingness to procure and prescribe children’s essential medicines is limited because of inappropriate prescription behaviour. Admittedly, the physicians can obtain sizeable commissions from pharmaceutical firms on prescriptions of medicines in China. Thus, the physicians have a direct financial incentive to prescribe more expensive medications. However, the patient prices of LPGs for children’s essential medicines are relatively low, which leads to the removal of sales commissions. Therefore, the physicians might be reluctant to prescribe children’s essential medicines. Third, the bidding and distribution systems for paediatric medicines are inefficient. Since 2009, the centralised bidding procurement and distribution system for drugs was established at the provincial level to support the implementation of essential medicine policy.35 The new centralised purchase policy has been implemented to shift purchasing power from medical institutions to the provincial committees.36 Henceforth, all public facilities must be enrolled in the province-level centralised bidding and purchasing system, and 100% of medicines in public hospitals should be procured through this centralised tender. The pharmaceutical firms and suppliers were selected through a competitive bidding process by the provincial committee’s jurisdiction, and then the products were distributed to all public facilities. Specifically, the pharmaceutical firms and suppliers who can win the tenders are limited. For a specific essential medicine with same strength and dosage form, only 3–5 firms can win the tender. Based on the present procurement policies in Jiangsu Province, only the firms that offer the lowest prices can win bids, which probably leads to shrinking revenues from drug sales and drives some firms to pull medicines from the market. Therefore, once the firms who win bids choose to abandon the tenders, some essential medicines are out of supply and stock.22
In addition, our findings reveal that the mean availability of LPGs was higher in the public sector than that in the private sector. Conversely, the mean availability of OBs was higher in the private sector than that in the public sector. This may have been due to the 15% drug markups in the private retail pharmacies. Since 2014, to eliminate drug markups and to encourage appropriate use of medicines, the public secondary and tertiary hospitals and primary healthcare facilities began to implement a zero-markup policy for drug sales in Jiangsu Province. Nonetheless, the drug policy permits private retail pharmacies to add a 15% markup to the wholesale prices of drugs. The lower the procurement price, the lower the revenue from the markup. In fact, the retail pharmacies usually obtain their profit from the fixed wholesale and retail margins. Unlike in Western countries, not all of the retail pharmacists could get additional dispensing or professional fees, so their incomes depend mainly on wages and bonuses. To compensate the pharmacists for their dispensing services, the retail pharmacies tend to sell OBs because the patient prices of OBs are higher. It is difficult for the retail pharmacies to stay in business by dispensing large amounts of LPGs. Therefore, the retail pharmacies prefer to sell more expensive OBs and might be reluctant to procure cheap LPGs.
Similar to the findings of the availability survey22 that has been conducted for adult medicines in Jiangsu Province, this study demonstrated that the availability of child-specific generic medicines far exceeded that of originator products in both sectors. Even so, most outlets only carried 7.7 %-35.6 % of paediatric essential medications. However, the results are different from the previous survey22 of adult medicines, which showed that the mean availability of LPGs was 100% in public primary healthcare facilities and 42.9% in the private sector. Access to paediatric essential medicines could be hindered by the poor availability of medicines in the dosages and formulations preferable for use in children. However, the availability of adult medicines was relatively high. To deal with this shortage, physicians, pharmacists and nurses have to calculate the children’s dose from the adult dosage based on the child’s age, weight and body surface area. This calculation may lead to incorrect dose use, which might cause adverse drug reactions.
For two medicines, clindamycin capsule and ibuprofen tablet, neither their originator brands nor generic equivalents were found in the public sector. One possible reason is that there exist therapeutic alternatives or alternate dosage forms in the public hospitals. Amoxicillin/clavulanic acid injection, phenobarbital injection and phenobarbital tablet were not available in the private sector. All of them were prescription drugs, and the former two were injections. Unlike in Western countries, the outpatients usually fill their prescriptions in the same hospital or community health centre where they go for medical care in China. Thus, some prescription medicines, especially for injections, are not available in retail pharmacies. Furthermore, phenobarbital, which is listed as a psychotropic substance by regulatory authorities, is subject to strong control and stringent regulations for retail pharmacies in China. Like most developing countries, the fear that inadequate prescription records or discrepancies in record keeping between hospital and pharmacy could lead to punitive consequences is a major barrier to access to phenobarbital in retail pharmacies.37
Compared with the IRPs, the OBs were expensive in public hospitals and private retail pharmacies. In contrast, according to the IRPs, the patient prices were acceptable for generics in both sectors. In China, LPGs of the same medication are manufactured and marketed by more than 4000 pharmaceutical enterprises, which leads to fierce market competition. Most importantly, these generic products do not vary obviously in quality or efficacy, and firms have to depend on price advantages to survive. Therefore, the prices of LPGs are low.
It was noted that the MPRs of OBs on the core and supplementary list were generally higher in the private sector than in the public sector. This finding is inconsistent with a previous study on adult medicines that was conducted in 2013 in Jiangsu, which found that the core and supplementary list MPRs were 4.13 and 4.01 for the private sector and 6.78 and 16.72 for the public sector, respectively.22 To some extent, the high prices of OBs in the private sector can be attributed to the 15% markup policy that is still implemented in retail pharmacies. Since 2015, the policy of removal of drug markup has been implemented in public secondary and tertiary hospitals in Jiangsu, which has resulted in price reductions for originator medicines in the public sector. As a result, the price differences of OBs have increase. However, since 2015, the Chinese government has liberated drug price regulation to the maximum extent possible. The wholesale or factory gate prices of all medicines (except for narcotic drugs, psychoactive drugs, radioactive drugs and toxic drugs for medical use) are not regulated by the government. However, due to the bidding system, the pharmaceutical companies that win bids offer the tender prices. As a result, there is relatively little difference between the prices of the same medicine with same strength and dosage form in the public sector. Conversely, due to a lack of regulations on prices, there were outrageous price differences in the private facilities. Furthermore, some OBs had high patient prices in retail pharmacies. Private retail pharmacies adopt the strategy of maintaining low prices of generic medicines to attract consumers and gain market competitiveness. Hence, little difference is observed among the prices of LPGs across all sectors.
In this study, we studied the affordability of medicines for eight common paediatric conditions, mostly focusing on acute diseases. Most LPGs for common conditions were affordable in both sectors. Due to the shorter treatment duration for acute conditions, the standard treatments cost less than 1 day’s wage. This finding was similar to the study of the affordability of paediatric medicines conducted in Shaanxi by Wang et al,6 which showed that most acute medicines for children were affordable. This finding was inconsistent with other studies done on the availability, prices and affordability of the essential medicines for children in low-income countries such as Guatemala and Ethiopia.8 10 In these countries, many of lowest priced treatments in both sectors cost more than the daily wage of a lowest paid government employee. Due to differences in economic development between China and these countries, there may be regional differences in the affordability of essential medicines. Given that only 2% of the population in Jiangsu is living below the national poverty line of less than $700/year, the urban and rural residents in Jiangsu could afford high medical expenses. Moreover, universal health insurance coverage in Jiangsu also reduces out-of-pocket payments for the residents.
A major strength of this study is the use of the WHO/HAI medicine survey, which allowed us to measure availability, prices and affordability in a reliable and standardised way. An additional strength is the utilisation of IRPs to make valid international comparisons. A further strength of the methodology was to take multiple measures to ensure quality data collection. There are several limitations as well. First, 80% of sampled facilities in this study were located in urban areas. Thus, the urban-biased sample might misrepresent the situation for the whole population. Second, the data refer to the availability of a given medicine in a particular dosage form and strength on the day of data collection at each outlet in six cities of Jiangsu Province. As a result, data on medication availability at a single point in time may not reflect the average monthly, quarterly or yearly availability of medicines at individual outlets. Third, for the convenience of calculations, the average weight of 5-year-old children was estimated to be approximately 20 kg, according to relevant reports. Thus, the medicine affordability in this survey may be underestimated. Fourth, therapeutic alternatives or alternate dosage forms, such as traditional Chinese medicines, were not assessed. Due to the limitation of suitable dosage forms on the NEML, only medicines having IRPs were selected as surveyed objects and compared with those in other countries, which gave rise to a lack of oral liquid dosage forms in the survey list. In addition, some survey medicines were selected repeatedly, such as ranitidine, omeprazole, aminophylline and salbutamol, which could have led to sample bias. However, some survey medicines, such as propylthiouracil, could not be expected to be used only for common ambulatory care conditions. Fifth, the treatment affordability of three common paediatric conditions was calculated by using the cost of tablets or capsules. However, it is difficult for 5-year-old children to take oral solid dosage forms, which may have caused bias in estimating the affordability of standard treatment regiments. Finally, this study did not assess the medicine procurement prices.
Conclusions
This study was conducted to assess access to essential medicines for children based on their availability, price and affordability. In Jiangsu Province, the paediatric LPGs had higher availability than OBs, and the availability of paediatric essential medicines was very low in both public and private sectors. Medicines were sold at prices higher than their IRPs, but their affordability was reasonable. Relevant measures should be taken to improve access to medicines for children. First, analysis of the procurement, supply and distribution of paediatric essential medicines is needed to discover the reasons for the low availability. Second, the government should develop a list of national essential medicines for children and mobilise the enthusiasm of pharmaceutical firms to develop and manufacture paediatric medicines, particularly in the dosages and formulations preferable for use in children.
Supplementary Material
Acknowledgments
The authors would like to thank Mr Cheng Ji, who provided invaluable comments and suggestions for this paper. The authors also appreciate all the research assistants for data collection.
Footnotes
Contributors: XS, JW and DY were involved in data collection, data analysis and writing the manuscript. XL coordinated the study design as well as data analysis and interpretation and was the primary investigator involved in writing the manuscript. YuY, QC, XX, JD, YaY and JS were involved in data collection. All the authors have read and approved the entire manuscript.
Funding: This study was supported by the General Project of Philosophy and Social Science of University of Jiangsu Province (Grant No: 2017SJB0277) and the innovation training projects for Nanjing Medical University students (Grant No: 2017YXDC04).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Ethical approval to conduct this study was obtained from the Nanjing Medical University Ethics Committee (grant number: ethical review 201236).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1. Force M D GGT. Delivering on the Global Partnership for Achieving the Millennium Development Goals. United Nations, New York 2008. [Google Scholar]
- 2. World Health Organization. Equitable access to essential medicines: a framework for collective action, 2004. [Google Scholar]
- 3. Mendis S, Fukino K, Cameron A, et al. . The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ 2007;85:279–88. 10.2471/BLT.06.033647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leisinger KM, Garabedian LF, Wagner AK. Improving access to medicines in low and middle income countries: corporate responsibilities in context. South Med Rev 2012;5:3–8. [PMC free article] [PubMed] [Google Scholar]
- 5. Cham M, Sundby J, Vangen S. Availability and quality of emergency obstetric care in Gambia’s main referral hospital: women-users’ testimonies. Reprod Health 2009;6:5 10.1186/1742-4755-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Fang Y, Yang S, et al. . Access to paediatric essential medicines: a survey of prices, availability, affordability and price components in Shaanxi Province, China. PLoS One 2014;9:e90365 10.1371/journal.pone.0090365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gitanjali B, Manikandan S. Availability of five essential medicines for children in public health facilities in India: A snapshot survey. J Pharmacol Pharmacother 2011;2:95–9. 10.4103/0976-500X.81900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sado E, Sufa A. Availability and affordability of essential medicines for children in the Western part of Ethiopia: implication for access. BMC Pediatr 2016;16:40 10.1186/s12887-016-0572-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. The World Medicines Situation 2011-Access to Essential Medicines as Part of the Right to Health. 2011. http://apps.who.int/medicinedocs/en/m/abstract/Js18772en/ (accessed 19 May 2018).
- 10. Anson A, Ramay B, de Esparza AR, et al. . Availability, prices and affordability of the World Health Organization’s essential medicines for children in Guatemala. Global Health 2012;8:22–10. 10.1186/1744-8603-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hinsch M, Kaddar M, Schmitt S. Enhancing medicine price transparency through price information mechanisms. Global Health 2014;10:34–11. 10.1186/1744-8603-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaidi S, Bigdeli M, Aleem N, et al. . Access to essential medicines in Pakistan: policy and health systems research concerns. PLoS One 2013;8:e63515 10.1371/journal.pone.0063515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang M, Yang S, Yan K, et al. . Measuring access to medicines: a survey of prices, availability and affordability in Shaanxi province of China. PLoS One 2013;8:e70836 10.1371/journal.pone.0070836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. World Health Statistics 2016: Monitoring Health for the Sustainable Development Goals (SDGs) World Health Organization, 2016. [Google Scholar]
- 15. Braine T. WHO to launch first essential medicines list for children. Bull World Health Organ 2007;85:249–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The National Health Commission. The Chinese national EML. 2012. http://www.moh.gov.cn/mohywzc/s3580/201303/f01fcc9623284509953620abc2ab189e/files/961cfc3a86584f8888e9140b1c208438.pdf (accessed 7 Apr 2018).
- 17. World Health Organization, Health Action International. Medicine prices: a new approach to measurement, 2003. [Google Scholar]
- 18. Khuluza F, Heide L. Availability and affordability of antimalarial and antibiotic medicines in Malawi. PLoS One 2017;12:e0175399 10.1371/journal.pone.0175399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ewen M, Zweekhorst M, Regeer B, et al. . Baseline assessment of WHO’s target for both availability and affordability of essential medicines to treat non-communicable diseases. PLoS One 2017;12:e0171284 10.1371/journal.pone.0171284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kotwani A. Where are we now: assessing the price, availability and affordability of essential medicines in Delhi as India plans free medicine for all. BMC Health Serv Res 2013;13:285 10.1186/1472-6963-13-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chahal HS, St Fort N, Bero L. Availability, prices and affordability of essential medicines in Haiti. J Glob Health 2013;3:20405 10.7189/jogh.03.020405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xi X, Li W, Li J, et al. . A survey of the availability, prices and affordability of essential medicines in Jiangsu Province, China. BMC Health Serv Res 2015;15:345 10.1186/s12913-015-1008-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balasubramaniam R, Beneragama BV, Sri Ranganathan S. A national survey of availability of key essential medicines for children in Sri Lanka. Ceylon Med J 2011;56:101–7. 10.4038/cmj.v56i3.3597 [DOI] [PubMed] [Google Scholar]
- 24. Qiang S. A survey of medicine prices, availability, affordability and price components in Shandong province. China, 2005. http://www.haiweb.org/medicineprices/surveys/200410MY/survey_report. [Google Scholar]
- 25. Ye L. A survey of medicine prices, availability and affordability in Shanghai, China using the WHO/HAI methodology. 2010. http://www.haiweb.org/medicineprices/surveys/200609CNS/survey_report.pdf.
- 26. Yang H, Dib HH, Zhu M, et al. . Prices, availability and affordability of essential medicines in rural areas of Hubei Province, China. Health Policy Plan 2010;25 219–29. 10.1093/heapol/czp056 [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization. Measuring medicine prices, availability, affordability and price components. 2nd edition: Geneva World Health Organization, 2008:8;504–7. [Google Scholar]
- 28. World Health Organization. WHO model list of essential medicines for children: 6th list, 2017. [Google Scholar]
- 29. Gelders S, Ewen M, Noguchi N, et al. . Price, availability and affordability: An International comparison of chronic diseases medicines. Comercialização De Medicamentos 2006. [Google Scholar]
- 30. State Administration of Foreign Exchange. http://www.safe.gov.cn/wps/portal/sy/sy (accessed 10 Jul 2017).
- 31. Jiangsu Provincial Department of Human Resources and Social Security. Notice of Jiangsu Provincial Department of human resources and social security on adjusting the minimum wage standard of the whole province. 2015. http://www.jshrss.gov.cn/sy/zcfg/201512/t20151221_193319.html (accessed 21 Otc 2017).
- 32. Xiaoming S. Wang Weiping.[Pediatric. 7th ed] China: People’s Medical Publishing House, 2011:p188–400. [Google Scholar]
- 33. National Health and Family Planning Commission of the People ‘s Republic of China, 2016. The Results of the Fifth Childhood Physical Development Survey in China http://www.scio.gov.cn/xwfbh/gbwxwfbh/xwfbh/wsb/Document/1479692/1479692.html (accessed 13 Dec 2017).
- 34. Zhang Xiaoyu.[Sales of paediatric drugs: professional success, brand first]. Zhongguo Yao Dian Za Zhi. 2017;3:68. [Google Scholar]
- 35. Yin J, Li Q, Sun Q. Antibiotic consumption in Shandong Province, China: an analysis of provincial pharmaceutical centralized bidding procurement data at public healthcare institutions, 2012-16. J Antimicrob Chemother 2017;73:814–20. 10.1093/jac/dkx469 [DOI] [PubMed] [Google Scholar]
- 36. Shi J, Liu R, Jiang H, et al. . Moving towards a better path? A mixed-method examination of China’s reforms to remedy medical corruption from pharmaceutical firms. BMJ Open 2018;8:e018513 10.1136/bmjopen-2017-018513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chomba EN, Haworth A, Mbewe E, et al. . The current availability of antiepileptic drugs in Zambia: implications for the ILAE/WHO "out of the shadows" campaign. Am J Trop Med Hyg 2010;83:571–4. 10.4269/ajtmh.2010.10-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.