Abstract
Objective
Patients with univentricular hearts (UVH) have high mortality despite modern treatment, and better methods to identify patients at highest risk are needed. We wanted to improve risk stratification in patients with UVH by focusing on the prognostic significance of single right versus single left ventricular morphology (SRV vs SLV).
Methods
All 395 patients with UVH operated at our centre were prospectively included from 1972 to 2016 (195 SRV, 166 SLV, 34 mixed or indeterminate ventricular morphology). Diagnoses, UVH morphology, types of all operations and time and causes of death or heart transplantation (HTX) were recorded. The primary endpoint was death or HTX.
Results
Among the 111 non-Fontan patients, 88 died (SRV 62 vs SLV 20; p<0.0001), 32 due to heart failure (SRV 23 vs SLV 5; p=0.0012). Twenty-five years of cumulative SRV versus SLV survival among the 284 Fontan patients (41 deaths/HTX) was 66.9% vs 87.9% (p=0.0027), partly explained by more deaths/HTX due to heart failure among patients with SRV (p=0.0006). Survival in patients with SRV with and without hypoplastic left heart syndrome (HLHS) was similar. SRV versus SLV was a strong predictor of death/HTX in multivariable proportional hazards analyses (RR 3.3, 95% CI 1.6 to 6.6).
Conclusion
SRV versus SLV is a strong short-term and long-term predictor of survival among patients with UVH, mainly explained by higher rates of death/HTX due to heart failure in the SRV group. Our findings apply to patients with SRV both with and without HLHS.
Keywords: congenital heart defects, heart surgery, univentricular hearts, ventricular morphology, survival
Summary.
What is already known about this subject?
One previous study showed that single right ventricular morphology (SRV) versus single left ventricular morphology (SLV) was a predictor of death or heart transplantation (HTX) following initial surgical palliation in patients with univentricular heart (UVH), but conflicting evidence exists on the prognostic significance of SRV versus SLV after establishment of Fontan circulation.
What does this study add?
SRV versus SLV was a strong predictor of death or HTX in patients with UVH both prior to and after Fontan surgery, apparently mainly driven by increased risk of death or HTX due to heart failure among patients with SRV.
The increased risk includes patients with SRV with hypoplastic left heart syndrome, and a broader group of patients with UVH with SRV.
How might this impact on clinical practice?
Ventricular morphology should be included as an important risk factor during the initial evaluation for surgical treatment of patients with UVH.
After Fontan surgery, there should be particular emphasis in patients with SRV on treatment of modifiable risk factors for development of heart failure, like volume or pressure overload.
Introduction
Patients with univentricular hearts (UVH) selected for surgical intervention typically undergo a sequence of operations usually culminating in the establishment of Fontan circulation. Substantial advances in surgical techniques and other treatment modalities1 2 have improved survival considerably.3 Still, patients with UVH—currently accounting for about 20% of patients with complex heart defects (CHD)—have the highest mortality,4 and mortality is high both around initial treatment and during long-term follow-up. Thus, there is a need for preoperative risk stratification algorithms that may discriminate high and low-risk patients. Age at operation, gender, cardiac anatomy and cardiac function have been associated with long-term mortality.5 6 Further, in a recent Editorial,1 7 Gersony hypothesised higher long-term mortality due to heart failure among patients with single right ventricular morphology (SRV) compared with single left ventricular morphology (SLV). However, conflicting evidence exists on the prognostic significance of SRV versus SLV.2 3 6–9 The present study has two aims: to study the long and short-term prognostic significance of SRV versus SLV based on prospective data from 395 patients with UVH operated at Oslo University Hospital (OUS), and further to study potential differences in the patterns of fatal complications.
Methods
Materials
The study was conducted at the Departments of Thoracic Surgery and Pediatric Cardiology, and at the Unit of Adult Congenital Heart Disease, Department of Cardiology, OUS, Rikshospitalet. All operations on patients with UVH, including heart transplantations (HTX), were registered prospectively in the DATACOR database from 5 May 1972 to 31 December 2016, along with dates and causes of death as described previously.4 No patients were lost to follow-up.
Data handling was facilitated by using each patient’s unique 11-digit social security number, which includes individual birth date. CHD diagnoses were based on International Classification of Diseases (ICD)-9 until 1998, and ICD-10 thereafter. The dates and types of all operations were recorded, and a maximum of three diagnoses were given. In cases where the ICD was inadequate, diagnoses were adjusted according to van Mierop.10
All patients with UVH who underwent one or more heart operations and had their first operation at Rikshospitalet were included. Between 1971 and 2003, eighty per cent of all CHD surgical procedures in Norway were performed at this institution. Since 2003, all CHD surgery in Norway has been performed at Rikshospitalet. Between 1987 and 1998, fifty-one patients with hypoplastic left heart syndrome (HLHS) from Norway had their Fontan surgery in the USA or Switzerland (25 survivors) due to government regulations.11 These patients have not been included.
Definitions
UVHs were retrospectively classified as having either a single dominant morphologically right ventricle (SRV) and a rudimentary left ventricle, a single dominant morphologically left ventricle (SLV) and a rudimentary right ventricle, or a functionally single ventricle with mixed or indeterminate ventricular morphology (MIX) according to a recognised nomenclature.12 The diagnoses were based on echocardiographic studies, MRI and invasive studies.
A bidirectional cavopulmonary connection (BCPC) involves rerouting the superior vena cava into the pulmonary artery. During a Fontan procedure, systemic venous return from the upper and lower body is diverted to the pulmonary artery without passing through the ventricle(s). We divided the Fontan procedures among our patients in three main groups: (1) extracardiac total cavopulmonary connection13; (2) intracardiac total cavopulmonary connection (lateral tunnel)14; and (3) others, including variants of the classical atriopulmonary Fontan.15 The first palliative shunt operation in our material was performed on 22 May 1972, and the first Fontan procedure on 5 May 1979.
The term ‘failing Fontan’ represents a variety of pathophysiologies that may develop secondary to the heart defect and mode of correction, especially low cardiac output and elevated central venous pressure.16 17 End-stage Fontan failure is refractory to optimised medical therapy.
The primary endpoint in the survival analyses was death or HTX. Depending on the analysis, the starting point was the date of birth or the date of the Fontan operation. ‘Early mortality’ includes all deaths occurring 30 days or less after surgery.
Statistical analyses
We used Kaplan-Meier analyses to study survival after birth and after the Fontan procedure. The log-rank test (Mantel-Cox) was used to test for differences in survival.
Proportional hazards (Cox) analyses were used to explore the prognostic significance of right versus left ventricular morphology after correction for possible confounders (gender, Fontan age, Fontan era and Fontan method). Variables that were found to be significantly associated with outcome in univariable analyses were included in the multivariable analysis. The proportional hazards assumption was adequately fulfilled for all covariables.
Two-sample t-tests were used when comparing clinical variables among survivors.
P values (two sided) <0.05 were considered significant. The statistical package StatView V.5.0 was used for analyses.
Results
Surgical treatment
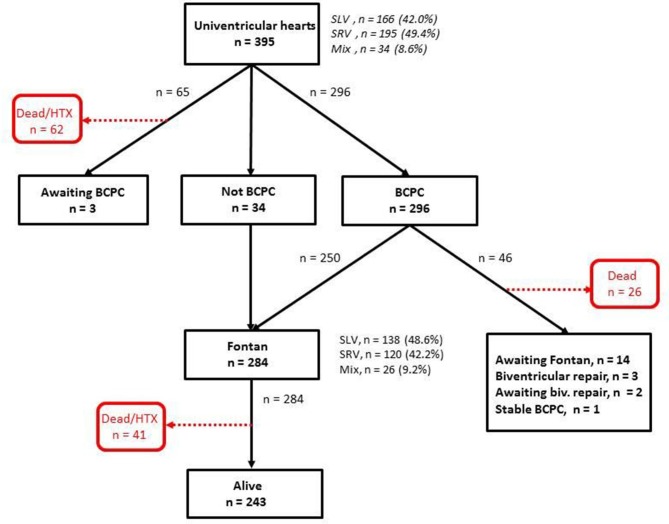
In total, 395 patients with UVH were included (figure 1). All had at least one operation, and total number of operations was 997. The number of patients with SLV was 166 (42.0%), SRV 195 (49.4%) and MIX 34 (8.6%). There were substantial SLV versus SRV differences in types of initial surgical procedures, reflecting differences in UVH diagnostic profiles (table 1). Numbers of women versus men were 177 vs 218 (p=0.022). Median age at initial surgery was 15 vs 6 days in patients with SLV versus SRV (p<0.0001).
Figure 1.
Treatment history of 395 study patients with univentricular hearts. BCPC, bidirectional cavopulmonary connection; HTX, heart transplantation; Mix, mixed or indeterminate ventricular morphology; SLV, single left ventricular morphology; SRV, single right ventricular morphology.
Table 1.
Demographic data, initial surgical procedures and primary UVH diagnoses
| SLV | SRV | MIX | Total | |
| Female, n (%) | 84 (50.6) | 82 (42.1) | 11 (32.4) | 177 (44.8) |
| Initial surgical procedures | ||||
| BAP | 38 | 19 | 3 | 60 |
| BCPC | 28 | 9 | 10 | 47 |
| Shunt | 83 | 41 | 16 | 140 |
| Norwood I | 6 | 123 | 4 | 133 |
| Fontan | 5 | 2 | 1 | 8 |
| Other operations | 6 | 1 | 0 | 7 |
| Total | 166 | 195 | 34 | 395 |
| Primary UVH diagnoses | ||||
| TA | 72 | 0 | 0 | 72 |
| HLHS | 0 | 121 | 0 | 121 |
| DILV | 40 | 0 | 3 | 43 |
| DORV | 0 | 28 | 4 | 32 |
| AVSD | 6 | 12 | 13 | 31 |
| PA-IVS | 32 | 0 | 1 | 33 |
| Misc | 16 | 34 | 13 | 63 |
| Total | 166 | 195 | 34 | 395 |
AVSD, atrioventricular septal defect; BAP, banding of the pulmonary artery; BCPC, bidirectional cavopulmonary connection; DILV, double inlet left ventricle; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; MIX, mixed or indeterminate ventricular morphology; Misc, miscellaneous heart defects; PA-IVS, pulmonary atresia with intact ventricular septum; SLV, single left ventricular morphology; SRV, single right ventricular morphology; Shunt, central aorticopulmonary shunt (Waterston, Blalock-Taussig, etc.); TA, tricuspid atresia; UVH, univentricular heart.
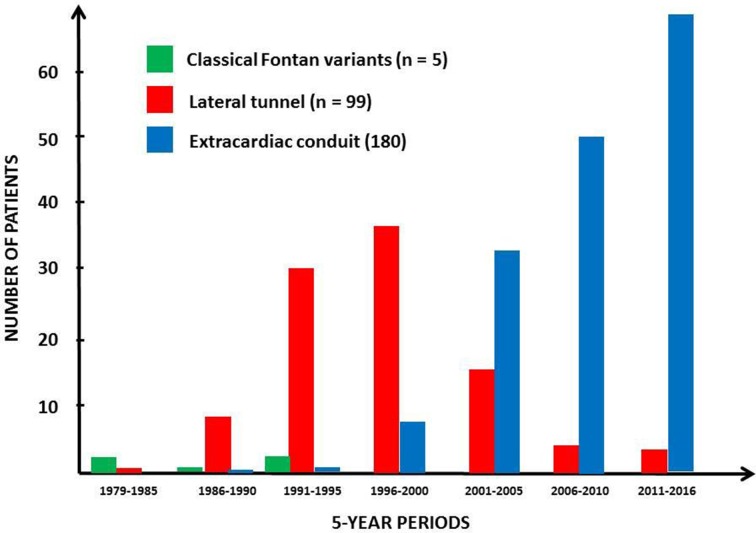
Fontan circulation was established in 284/395 patients (71.9%); 250 after BCPC and 34 without previous BCPC, and in a larger proportion of patients with SLV than SRV (138/166 (83.1%) vs 120/195 (61.5%), p<0.0001). A total of 219/284 patients (77.4%) had their Fontan surgery before 5 years of age. Figure 2 shows the distribution of various Fontan methods in different eras. Fenestrations were created in 99 patients (34.1%).
Figure 2.
Numbers and proportions of different Fontan procedures in different eras.
Age at Fontan surgery among patients with SLV and SRV was similar, as were proportions of classical, lateral tunnel and extracardiac conduit Fontan, patients with fenestration and patients who had BCPC before Fontan. However, the percentage of patients who had palliative surgery before BCPC was larger in the SRV group than in the SLV group (p=0.0002) (table 2). Proportions of female versus male patients having Fontan surgery were similar to the female versus male ratio in the entire UVH population.
Table 2.
Demographic data and surgical procedures in Fontan patients
| SLV (n=138) |
SRV (n=120) |
MIX (n=26) |
Total (n=284) |
|
| Female (%) | 71 (51.4) | 45 (37.5) | 9 (34.6) | 125 (44.0) |
| BCPC⁴ before Fontan (%) | 118 (85.5) | 108 (90.0) | 24 (92.3) | 250 (88.0) |
| Palliation before BCPC (%) | 88 (63.8) | 101 (84.2) | 13 (50.0) | 202 (71.1) |
| Fontan age (median, years) | 3.3 | 3.2 | 4.7 | 3.4 |
| Classical Fontan (%) | 3 (2.1) | 2 (1.7) | 0 (0.0) | 5 (1.8) |
| Lateral tunnel (%) | 53 (38.4) | 36 (30.0) | 10 (38.5) | 99 (34.9) |
| Extracardiac conduit (%) | 82 (59.4) | 82 (68.3) | 16 (61.5) | 180 (63.4) |
| Fenestration (%) | 46 (33.3) | 45 (37.5) | 8 (30.8) | 99 (34.9) |
BCPC, bidirectional cavopulmonary connection; MIX, mixed or indeterminate ventricular morphology; SLV, single left ventricular morphology; SRV, single right ventricular morphology.
Reoperations and transcatheter interventions
Among the 284 Fontan patients, eight underwent reoperation within 30 days after the Fontan procedure. Forty (14.1%) patients had different types of late surgical revisions: 18 had conversion to extracardiac conduit or lateral tunnel (1 patient), 9 had opening/closure of fenestrations, 6 had valve surgery, 3 had intraventricular resections and 4 had other types of surgical procedures. There was no difference between SRV and SLV with respect to proportions of patients undergoing Fontan conversion (p=0.82; details not shown).
Forty-three (15.1%) Fontan patients had transcatheter fenestration closures, 66 (23.2%) had balloon dilations with or without stents in the pulmonary artery or in the Fontan conduits, and 53 (18.7%) had closures of veno-venous or arteriovenous collaterals and 40 (14.1%) had balloon dilations with or without stents in the descending aorta. Among these 40 patients, 36 had SRV (p<0.0001); all patients with HLHS. Otherwise, no SRV versus SLV differences in proportions of patients having transcatheter procedures were found. In addition, 38 patients (13.4%) had permanent pacemakers, 1 patient (0.3%) had a defibrillator and 15 (5.3%) had radiofrequency ablations due to arrhythmias.
Survival
All patients with UVH
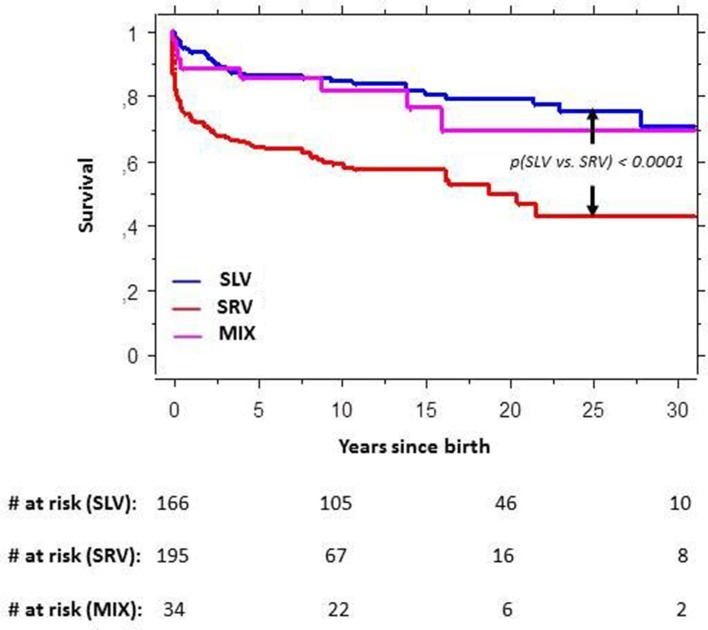
A total of 118 patients (29.9%) died during follow-up, and 13 patients (3.3%) underwent HTX (2 died after HTX). Accordingly, 129 patients died or had HTX. Figure 3 shows a markedly better survival among patients with SLV than among SRV. Twenty-five years of cumulative SLV versus SRV survival was 74.9% vs 42.5% (p<0.0001), and 69.1% in the group with MIX (figure 3). Among patients with atrioventricular septal defect, long-term survival was similar regardless of right or left ventricular dominance. Otherwise, survival within all SLV diagnostic subgroups (table 1) was significantly higher than mean SRV survival, and survival within all SRV subgroups was significantly lower than mean SLV survival (online supplementary figure 1).
Figure 3.
Cumulative survival in patients with univentricular hearts (UVH). MIX, mixed or indeterminate ventricular morphology; SLV, single left ventricular morphology; SRV, single right ventricular morphology; #, number of patients.
openhrt-2018-000902supp001.jpg (65.4KB, jpg)
Non-Fontan patients
Among the 111/395 patients (28.1%) who did not undergo or were awaiting Fontan operation, the majority had SRV (table 3). This difference was mainly due to higher mortality in the SRV group (SRV 62/195 (31.8%) vs SLV 21/166 (12.7%), p<0.0001).
Table 3.
Causes of death or HTX among the 111 non-Fontan patients
| SLV(n= 28) | SRV(n=75) | MIX(n=8) | Total(n=111) | |
| Heart failure* | 5 | 23 | 4 | 32 |
| Postoperative complications† | 3 | 13 | 2 | 18 |
| Thrombosis‡ | 2 | 9 | 0 | 11 |
| Pulmonary vascular disease§ | 4 | 6 | 0 | 10 |
| Sudden death¶ | 2 | 8 | 0 | 10 |
| Infection** | 3 | 2 | 0 | 5 |
| Bleeding†† | 1 | 1 | 0 | 2 |
| Total | 20 | 62 | 6 | 88 |
*Myocardial and/or valvar failure.
†Various complications within 30 days of last surgery.
‡Shunt thrombosis or thrombosis in Fontan circuit, myocardial infarction, stroke.
§Underdeveloped pulmonary vessels and/or high pulmonary vascular resistance.
¶Death occurring within minutes from any cause, including malignant tachyarrhythmias.
**Pneumonia, sepsis, gastroenteritis.
††Cerebral hemorrhage, pulmonary hemorrhage.
HTX, heart transplantation; MIX, mixed or indeterminate ventricular morphology; SLV, single left ventricular morphology; SRV, single right ventricular morphology.
SRV mortality was higher than SLV mortality both among patients with HLHS (40/121 (33.1%); p<0.0001) and among non-HLHS patients (22/74 (29.7%); p=0.001). The dominant cause of death among non-Fontan patients was heart failure (SLV 5, SRV 23; p=0.0012).
Fontan patients
Among the 284 Fontan patients, 41 died (29 patients) or had HTX (12 patients). Sixteen patients (5.6%) died within 30 days after the operation. Median follow-up time among Fontan survivors was 11.0 years (mean 11.4 years, SD 7.3 years). Twenty-five years of cumulative survival was significantly higher among patients with SLV compared with patients with SRV (87.9% vs 66.9%, p=0.0027), even when disregarding early mortality (92.5% vs 70.8, p=0.0005) (online supplementary figure 2). Twenty-five years of cumulative survival among patients with MIX was 91.6% (details not shown).
openhrt-2018-000902supp002.jpg (55.3KB, jpg)
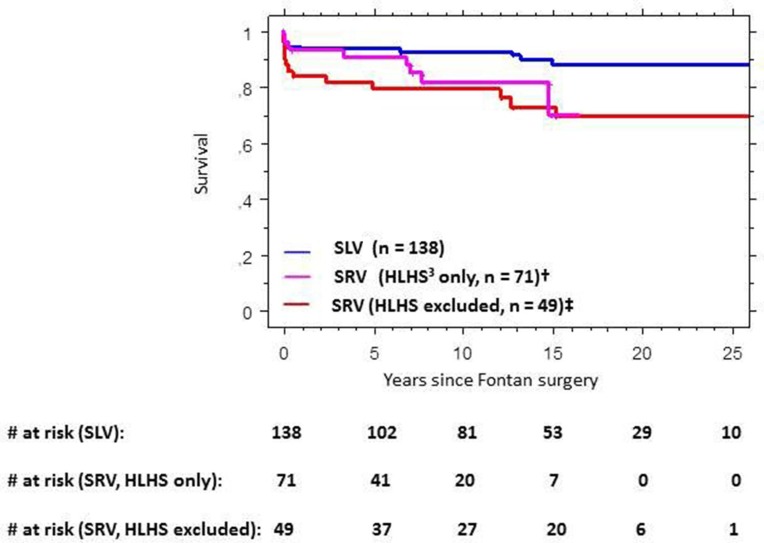
Survival among Fontan patients with SLV was significantly higher than survival among patients with SRV both with and without HLHS (figure 4).
Figure 4.
Cumulative survival in Fontan patients with single left ventricular morphology versus single right ventricular morphology with and without hypoplastic left heart syndrome. HLHS, hypoplastic left heart syndrome; SLV, single left ventricular morphology; SRV, single right ventricular morphology; #, number of patients. †p(SLV vs SRV; HLHS only)=0.034. ‡p(SLV vs SRV; HLHS excluded)=0.0018.
Details on causes of death and HTX among Fontan patients are shown in table 4. In total, one patient with SLV versus 11 patients with SRV died or had HTX due to heart failure (p=0.0006), and one had mixed or indeterminate morphology. Nine of these patients had systolic heart failure, three combined systolic heart failure and significant valvar disease, and one systolic heart failure and intractable arrhythmias. Otherwise, there were no SRV versus SLV differences in causes of death or HTX.
Table 4.
Causes of death or HTX among the 284 Fontan patients
| SLV(n=138) | SRV(n=120) | MIX(n=26) | Total(n=284) | |
| Postoperative complications* | 7 | 7 | 1 | 15 |
| Heart failure† | 1 | 11 | 1 | 13 |
| Sudden death‡ | 1 | 3 | 0 | 4 |
| Failed Fontan§ | 3 | 1 | 0 | 4 |
| Infection¶ | 2 | 1 | 0 | 3 |
| Thrombosis** | 1 | 1 | 0 | 2 |
| Total | 15 | 24 | 2 | 41 |
*Various complications within 30 days of last surgery.
†Myocardial and/or valvar failure.
‡Death occurring within minutes from any cause, including malignant tachyarrhythmias.
§See Definitions.
¶Pneumonia, sepsis, gastroenteritis.
**Shunt thrombosis or thrombosis in Fontan circuit, myocardial infarction, stroke.
HTX, heart transplantation; MIX, mixed or indeterminate ventricular morphology; SLV, single left ventricular morphology; SRV, single right ventricular morphology.
Sequential changes in survival
Among the Fontan patients, there was a gradual improvement in survival from the beginning to the end of the study period (online supplementary figure 3, panel A). However, most of the differences in survival between eras were attributable to differences in early mortality (online supplementary figure 3, panel B).
openhrt-2018-000902supp003.jpg (53.2KB, jpg)
Multivariable analysis
Since survival among patients with SLV and MIX was almost identical, these groups were merged in the proportional hazards analyses. Fontan era was treated as a dichotomous variable (1979–2000 vs 2001–2016). In univariable analyses including ventricular morphology (SRV vs SLV+MIX), Fontan era, Fontan age, gender and Fontan type, only SRV versus SLV+MIX and Fontan era were significantly associated with outcome. In the multivariable analysis both these variables were strong predictors of death or HTX (table 5). In a similar multivariable analysis excluding patients with MIX (n=26), SRV versus SLV was a strong predictor of death or HTX (RR=3.3; 95% CI 1.6 to 6.6)
Table 5.
Proportional hazards analysis for death or heart transplantation in Fontan patients
| Covariables | Univariable analyses | Multivariable analysis | ||
| RR (95% CI) | P values | RR (95% CI) | P values | |
| SRV* versus SLV+MIX | 2.6 (1.4 to 5.0) | 0.0041 | 2.9 (1.6 to 5.9) | 0.0010 |
| Intracardiac versus extracardiac Fontan | 1.8 (0.9 to 3.5) | 0.083 | ||
| Fontan era: 1979–2000 vs 2001–2016 | 2.7 (1.3 to 5.3) | 0.0051 | 2.1 (1.3 to 7.5) | 0.010 |
| Fontan age (+1 SD) | 1.1 (0.8 to 1.4) | 0.68 | ||
| Male versus female | 1.2 (0.6 to 2.3) | 0.59 | ||
MIX, mixed or indeterminate ventricular morphology; SLV, single left ventricular morphology; SRV, single right ventricular morphology.
Discussion
In the present prospective study we explore predictors of survival in patients with UVH throughout the surgical treatment process and beyond the establishment of Fontan circulation. Our main findings are that SRV versus SLV is a strong predictor of increased mortality or HTX both before and after Fontan surgery, and that the SRV versus SLV differences are mainly driven by differences in mortality or HTX due to heart failure. Moreover, our findings apply to patients with SRV both with and without HLHS.
Previous studies
The prognostic significance of SRV versus SLV following initial surgical palliation in patients with UVH has been explored in one large previous study.8 In that study, including 499 patients born in Australia between 1990 and 2008, SRV was found to be a strong predictor of mortality during the first years of life before BCPC and Fontan surgery—similar to our findings. However, that study did not show that SRV was a predictor of mortality after establishment of BCPC.
In a study from the Mayo Clinic including 1052 Fontan patients undergoing operation between 1973 and 2012, differences in ventricular morphology did not predict differences in long-term survival.6 In two subsequent studies from Australia and New Zealand, HLHS was a significant predictor of long-term Fontan failure,3 9 but not of death. In a recent study from Spain including 91 Fontan patients operated in 1995–2013,2 SRV was a significant predictor of all-cause mortality.
In summary, previous studies have not unambiguously clarified the relevance of SRV versus SLV as a predictor of mortality in UVH prior to and after Fontan operations. However, according to our data, SRV versus SLV was a strong predictor of mortality through all stages of surgical correction in patients with UVH.
Selection issues
Data on the true birth incidence of different CHDs compared with the proportions of various types of CHD given in different studies suggest differences in selection of patients. However, the use of different diagnostic classification systems makes comparisons among studies difficult. Nevertheless, according to a meta-analysis based on 44 published studies on the birth incidence of CHD (median CHD incidence 1.0%), the ratio between the median birth incidence of HLHS (all morphologically SRV) and the median birth incidence of tricuspid atresia (TA; virtually all morphologically SLV) was about 2.5.18 In our study, the initial ‘HLHS/TA ratio’ was 1.6, indicating that some selection in favour of patients with TA had already taken place. At the time of Fontan surgery, this ratio was reduced to 1.1 because of high pre-Fontan mortality in patients with SRV. In the previously mentioned Fontan studies, the HLHS/TA ratios were as low as 0.2 (7), 0.1 (6), 0.3 (3) and 0.6 (2), respectively. Accordingly, a much higher proportion of patients with SRV were included in our study compared with the other studies. This indicates a substantial selection based on ventricular morphology prior to Fontan surgery in the other studies. In the study by d’Udekem and coworkers,8 the HLHS/TA ratio was similar to our data. However, only 209/383 (54.7%) of the BCPC patients underwent Fontan surgery compared with 250/284 (84.4%) in our study.
In summary, differences in patient selection and treatment routines make comparisons among Fontan studies difficult. Our patient population had an HLHS/TA ratio that was close to the birth incidence of these heart defects. Consequently, our study material may provide more representative estimates of the prognostic impact of ventricular morphology in Fontan patients than previous studies.
Physiological considerations
In a study of Fontan patients operated in 2005–2013, ventricular stress–strain relationship in patients with SRV suggested heart failure, in part explaining unfavourable outcomes in Fontan patients with RV morphology.19 Echocardiographic and MR studies have demonstrated reduced strain rate and abnormal contraction pattern in SRVs of patients with transposition of the great arteries operated with atrial switch when compared with patients with systemic LVs.20 RV morphology in paediatric Fontan patients was associated with worse ventricular and valvar function compared with LV or MIX.21 Inadequate ventricular preload may be a major determinant of reduced diastolic function in Fontan patients,16 22 although it is not clear whether this affects SLV and SRV in the same manner. In summary, pathophysiological data suggest that the performance of hearts with SRVs—including SRVs in Fontan patients, is inferior to the function of hearts with SLV. These data are in agreement with observations that patients with SRVs have a high frequency of heart failure and high mortality.23 24
Similarly, our study demonstrated a significantly better survival in patients with SLV compared with patients with SRV, mainly associated with differences in incidence of death or HTX due to heart failure. This finding was in fact predicted by an Editorial in 2008,1 based on a retrospective study of 261 Fontan patients from Boston operated in 1973–1991 suggesting increased long-term mortality due to heart failure in patients with SRV.7 The present study is the first to confirm this hypothesis. Moreover, our findings do not only apply to excess mortality due to heart failure in patients with SRV with Fontan circulation, but throughout the surgical treatment process of patients with UVH.
According to our data, patients with SLV had favourable survival when compared with patients with SRV both with and without HLHS, and these differences were seen both in Fontan and non-Fontan patients. One of the studies from Australia and New Zealand reported that HLHS was a predictor of adverse events in Fontan patients, whereas data specifically focusing on non-HLHS SRV were not provided.9 Our data indicate that the negative prognostic impact of right ventricular morphology is not limited to HLHS, but encompasses a broader spectrum of UVH defects characterised by right ventricular morphology—a suggestion further supported by the observation that survival in SRV diagnostic subgroups was poorer than SLV survival, and that survival in SLV diagnostic subgroups was better than SRV survival (online supplementary figure 1).
Limitations
Distinguishing between right versus left ventricular morphology in patients with UVH may be difficult. Although we have tried to adhere to an established classification scheme,12 determination of definite ventricular morphology is sometimes impossible. Therefore, we defined a group denoted ‘mixed or indeterminate morphology’.
In later surgical eras, there was a marked sequential improvement in survival in patients undergoing Fontan surgery. However, after exclusion of early deaths, the differences between eras were much smaller. Given the shorter follow-up duration of patients with extracardiac Fontan compared with patients with lateral tunnel Fontan, we were unable to determine whether this impacts survival. There was a sequential increase in the number of patients with UVH having Fontan surgery despite stable birth incidence— suggesting that more patients previously considered inoperable are now operated. The number of Fontan patients doubled from 1991–2000 to 2011–2015, likely as a result of the shift in surgical technique from direct Fontan to a staged procedure,25 26 making more patients suitable for Fontan surgery.
Echocardiographic and haemodynamic studies were done several times prior to all surgical procedures in all of our patients, but standardised data were not available for the present study. Such data might have allowed for a deeper understanding of the significance of SRV versus SLV in relation to survival. As mentioned above, it has, for example, been hypothesised that low ventricular preload may be an important determinant of diastolic function in Fontan patients.16 22 Echocardiographic and invasive data might have helped to elucidate the role of diastolic dysfunction as a cause of poor outcome due to heart failure in our patients. Factors like low birth weight, prematurity and genetic syndromes clearly affect survival, and availability of such data might have been valuable. Further studies are needed to explore these important issues.
Conclusions
Our prospective, long-term follow-up study with low selection bias shows that SRV is a strong predictor of death or HTX in patients with UVH both prior to and following Fontan surgery. This increased risk among patients with SRV compared with patients with SLV may be driven mainly by differences in risk of death or HTX due to heart failure. Importantly, the increased risk is not confined to patients with SRV with HLHS, but includes a broader group of patients with UVH with RV morphology.
According to our data, ventricular morphology should be included as an important risk factor in the initial evaluation for surgical treatment of patients with UVH. After establishment of Fontan circulation, there should be particular emphasis in patients with SRV on early treatment of potentially modifiable risk factors for development of heart failure, like volume or pressure overload. Finally, there is a need for more studies to define subgroups of patients with SRV who are at particularly high risk.
Acknowledgments
We express our deepest gratitude to Susan Tower Gibbs, RN, at the Department of Thoracic Surgery, Rikshospitalet, for initiating the work on the database in 1971.
Footnotes
Contributors: GE wrote the manuscript with input from all authors. JA, JL and HLL participated in conceiving the project and revised it critically for intellectual content. KL was the main responsible for the statistical analyses. GD, GE and HLL participated in data acquisition. MEE, OG and HS revised the paper critically for intellectual content. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Data Protection Officer at Oslo University Hospital.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1. Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation 2008;117:13–15. 10.1161/CIRCULATIONAHA.107.748566 [DOI] [PubMed] [Google Scholar]
- 2. Franco E, Domingo EJB, Del Val VA, et al. Percutaneous interventions in Fontan circulation. Int J Cardiol Heart Vasc 2015;8:138–46. 10.1016/j.ijcha.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. d'Udekem Y, Iyengar AJ, Galati JC, et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 2014;130(11 Suppl 1):S32–S38. 10.1161/CIRCULATIONAHA.113.007764 [DOI] [PubMed] [Google Scholar]
- 4. Erikssen G, Liestøl K, Seem E, et al. Achievements in congenital heart defect surgery: a prospective, 40-year study of 7038 patients. Circulation 2015;131:337–46. 10.1161/CIRCULATIONAHA.114.012033 [DOI] [PubMed] [Google Scholar]
- 5. Alsaied T, Bokma JP, Engel ME, et al. Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart 2017;103:104–10. 10.1136/heartjnl-2016-310108 [DOI] [PubMed] [Google Scholar]
- 6. Pundi KN, Johnson JN, Dearani JA, et al. 40-Year follow-up after the fontan operation: long-term outcomes of 1,052 patients. J Am Coll Cardiol 2015;66:1700–10. 10.1016/j.jacc.2015.07.065 [DOI] [PubMed] [Google Scholar]
- 7. Khairy P, Fernandes SM, Mayer JE, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85–92. 10.1161/CIRCULATIONAHA.107.738559 [DOI] [PubMed] [Google Scholar]
- 8. d'Udekem Y, Xu MY, Galati JC, et al. Predictors of survival after single-ventricle palliation: the impact of right ventricular dominance. J Am Coll Cardiol 2012;59:1178–85. 10.1016/j.jacc.2011.11.049 [DOI] [PubMed] [Google Scholar]
- 9. Iyengar AJ, Winlaw DS, Galati JC, et al. The extracardiac conduit fontan procedure in australia and new zealand: hypoplastic left heart syndrome predicts worse early and late outcomes. Eur J Cardiothorac Surg 2014;46:465–73. 10.1093/ejcts/ezu015 [DOI] [PubMed] [Google Scholar]
- 10. van Mierop LH. Diagnostic code for congenital heart disease. Pediatr Cardiol 1984;5:331–62. 10.1007/BF02424983 [DOI] [PubMed] [Google Scholar]
- 11. Hagemo PS, Skarbø AB, Rasmussen M, et al. An extensive long term follow-up of a cohort of patients with hypoplasia of the left heart. Cardiol Young 2007;17:51–5. 10.1017/S1047951106001284 [DOI] [PubMed] [Google Scholar]
- 12. Tynan MJ, Becker AE, Macartney FJ, et al. Nomenclature and classification of congenital heart disease. Br Heart J 1979;41:544–53. 10.1136/hrt.41.5.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Backer CL, Deal BJ, Kaushal S, et al. Extracardiac versus intra-atrial lateral tunnel fontan: extracardiac is better. Semin Thorac Cardiovasc Surg 2011;14:4–10. 10.1053/j.pcsu.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 14. de Leval MR, Kilner P, Gewillig M, et al. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg 1988;96:682–95. [PubMed] [Google Scholar]
- 15. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240–8. 10.1136/thx.26.3.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gewillig M, Brown SC, Eyskens B, et al. The fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg 2010;10:428–33. 10.1510/icvts.2009.218594 [DOI] [PubMed] [Google Scholar]
- 17. Deal BJ, Jacobs ML. Management of the failing fontan circulation. Heart 2012;98:1098–104. 10.1136/heartjnl-2011-301133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890–900. 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 19. Ghelani SJ, Colan SD, Harrild DM, et al. The impact of ventricular morphology on wall stress and ventricular strain in Fontan patients. Journal of Cardiovascular Magnetic Resonance 2016;18(Suppl 1):O30 10.1186/1532-429X-18-S1-O30 [DOI] [Google Scholar]
- 20. Pettersen E, Helle-Valle T, Edvardsen T, et al. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol 2007;49:2450–6. 10.1016/j.jacc.2007.02.062 [DOI] [PubMed] [Google Scholar]
- 21. Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol 2008;52:85–98. 10.1016/j.jacc.2008.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu CP, Ting CT, Yang TM, et al. Reduced left ventricular compliance in human mitral stenosis. role of reversible internal constraint. Circulation 1992;85:1447–56. 10.1161/01.CIR.85.4.1447 [DOI] [PubMed] [Google Scholar]
- 23. Piran S, Veldtman G, Siu S, et al. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 2002;105:1189–94. 10.1161/hc1002.105182 [DOI] [PubMed] [Google Scholar]
- 24. Dobson R, Danton M, Nicola W, et al. The natural and unnatural history of the systemic right ventricle in adult survivors. J Thorac Cardiovasc Surg 2013;145:1493–503. 10.1016/j.jtcvs.2013.02.030 [DOI] [PubMed] [Google Scholar]
- 25. Bridges ND, Jonas RA, Mayer JE, et al. Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation 1990;82(5 Suppl):170–6. [PubMed] [Google Scholar]
- 26. Iacona GM, Marianeschi SM, Condoluci C, et al. [The role of a bidirectional cavopulmonary anastomosis in the correction and palliation of complex congenital cardiopathies]. G Ital Cardiol 1998;28:1372–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2018-000902supp001.jpg (65.4KB, jpg)
openhrt-2018-000902supp002.jpg (55.3KB, jpg)
openhrt-2018-000902supp003.jpg (53.2KB, jpg)