Abstract
Objective
Stress testing is commonly performed in emergency department (ED) patients with suspected acute coronary syndrome (ACS). We hypothesised that changes in N-terminal pro-B type natriuretic peptide (NT-proBNP) concentrations from baseline to post-stress testing (stress-delta values) differentiate patients with ischaemic stress tests from controls.
Methods
We prospectively enrolled 320 adult patients with suspected ACS in an ED-based observation unit who were undergoing exercise stress echocardiography. We measured plasma NT-proBNP concentrations at baseline and at 2 and 4 hours post-stress and compared stress-delta NT-proBNP between patients with abnormal stress tests versus controls using non-parametric statistics (Wilcoxon test) due to skew. We calculated the diagnostic test characteristics of stress-delta NT-proBNP for myocardial ischaemia on imaging.
Results
Among 320 participants, the median age was 51 (IQR 44–59) years, 147 (45.9%) were men, and 122 (38.1%) were African–American. Twenty-six (8.1%) had myocardial ischaemia. Static and stress-deltas NT-proBNP differed at all time points between groups. The median stress-deltas at 2 hours were 10.4 (IQR 6.0–51.7) ng/L vs 1.7 (IQR −0.4 to 8.7) ng/L, and at 4 hours were 14.8 (IQR 5.0–22.3) ng/L vs 1.0 (−2.0 to 10.3) ng/L for patients with ischaemia versus those without. Areas under the receiver operating curves were 0.716 and 0.719 for 2-hour and 4-hour stress-deltas, respectively. After adjusting for baseline NT-proBNP levels, the 4-hour stress-delta NT-proBNP remained significantly different between the groups (p=0.009).
Conclusion
Among patients with ischaemic stress tests, static and 4-hour stress-delta NT-proBNP values were significantly higher. Further study is needed to determine if stress-delta NT-proBNP is a useful adjunct to stress testing.
Keywords: echocardiography, emergency medicine, coronary artery disease, acute coronary syndrome, myocardial ischaemia and infarction (IHD), biomarkers
Key questions.
What is already known about this subject?
Patients in the emergency department with potential acute coronary syndrome are often admitted to the hospital to receive provocative cardiac stress testing.
In the case of exercise echocardiography testing, imaging is used to identify abnormal wall motion abnormalities after stress.
It is known that N-terminal pro-B type natriuretic peptide (NT-proBNP) is also released with acute ventricular wall stress.
What does this study add?
We examined whether NT-proBNP elevates acutely when patients have an abnormal exercise echocardiography test by comparing the change in plasma levels of NT-proBNP in patients with abnormal stress tests with patients with non-ischaemic stress test results.
We found that stress-delta NT-proBNP had modest areas under the receiver operating curves for predicting ischaemia on stress test imaging.
How might this impact on clinical practice?
If further validated, our work suggests a potential role for stress-delta NT-proBNP for identifying inducible myocardial ischaemia in patients presenting with acute coronary syndrome symptoms.
Introduction
Background
Accurate diagnosis and management of patients with suspected acute coronary syndromes (ACS) has been a vexing problem for decades.1–5 Patients can be rapidly assessed for myocardial infarction (MI) by cardiac troponin assays.6–9 However, even in the era of high-sensitivity troponin assays, patients frequently require inpatient admission or observation for further risk stratification, including stress testing.10–14 However, stress tests require specialty expertise and equipment15 16 that are not routinely available in the emergency department (ED). Multiple factors, including medicolegal risk aversion17 18 and supply-induced demand,19 20 have led to widespread, low-yield use of stress testing.21 A new paradigm is needed for risk stratification of patients with suspected ACS in whom MI has been excluded.
Importance
Prior research suggests that myocardial ischaemia induced by stress testing is associated with dynamic increases (stress-deltas) in N-terminal pro-B type natriuretic peptide (NT-proBNP) concentrations.22–29 These studies only included outpatients with symptoms of stable angina. In this investigation, we test a novel means for assessing ED patients with potential ACS: a biomarker-based stress test. A stress test that uses biomarkers (as opposed to imaging) to assess ischaemia could have several possible benefits for ED patients with ACS symptoms and could improve the current paradigm.
Goals of this investigation
We hypothesised that ED patients undergoing stress testing with myocardial ischaemia would demonstrate higher increases in NT-proBNP (stress-delta NT-proBNP) than patients without myocardial ischaemia. We calculated the diagnostic test characteristics for the index test of stress-delta NT-proBNP compared with a reference standard of standard stress test imaging. For exploratory analyses, we evaluated whether patients who subsequently suffered adverse cardiac events, such as MI, urgent percutaneous coronary intervention (PCI) or coronary artery bypass grafting, would similarly demonstrate significantly higher stress-delta NT-proBNP.
Methods
Study design and setting
We conducted an a priori planned observational cohort substudy of a prospectively collected biorepository compiled at an urban academic medical centre ED with an approximate yearly census of 75 000 visits. All patients provided informed consent prior to participating. We have previously described the methods for this biorepository,30 but we briefly review it here and follow previously suggested reporting guidelines.31
Selection of participants
From November 2012 to September 2014, we recruited patients from our ED-based observation unit daily on weekdays and intermittently on weekends when staffing was available. During this time, we saw an estimated 5000 patients with undifferentiated chest pain per year in our ED and patients are placed in the ED-based observation unit based on treating physician gestalt with some exclusion criteria as previously described.3 32 33 In short, patients cannot be placed in the observation unit (and were therefore excluded from our study) if they have significant arrhythmias, unstable vital signs, aortic aneurysm or dissection, active myocarditis or pericarditis, acute or decompensated heart failure, or severe aortic stenosis. Likewise, patients had to have non-diagnostic results from serial ECGs and three cardiac troponin assays (Roche Elecsys fourth-generation troponin T) over 8 hours below our institutional cut-off (<0.1 ng/mL, 10% coefficient of variation (CV) level ≤0.03 ng/mL). Advanced practice providers provided care in our observation unit, but final decisions about eligibility for the unit rested with the attending emergency physician caring for the patient in the ED. Approximately 65% of patients in our observation unit for ACS have stress echocardiograms as part of their work-up as opposed to other modalities (myocardial perfusion imaging or cardiac MRI).
Eligible patients for the biorepository had the following characteristics: (1) over the age of 30 years, (2) placed in our observation unit for symptoms suggestive of ACS and (3) scheduled to have exercise stress echocardiography as part of usual care.
Interventions
As part of usual care, patients underwent stress echocardiogram that included symptom-limited treadmill exercise using the Bruce Protocol followed by standard echocardiogram. Stress echocardiograms were interpreted by board-certified cardiologists who were blinded to the results of study-specific NT-proBNP results and overall study hypothesis. The reports generated by these usual-care studies were used for our outcome. Two reviewers (including ATL) further reviewed these reports to confirm abnormal studies and to adjudicate indeterminate results. A third reviewer (LKN), a board-certified cardiologist, adjudicated the remaining indeterminate reports. All reviews were conducted blinded to NT-proBNP results.
We obtained serum samples from a peripheral vein before stress testing and 2 hours after stress testing using existing intravenous catheters when possible. Whenever possible, we obtained a 4-hour post-stress blood sample but did not exclude patients if this sample could not be obtained.
We centrifuged blood within 1 hour of collection for plasma, which was aliquoted into 0.4 mL samples and frozen at −80°C within 8 hours of collection. A core laboratory at the University of Maryland determined plasma concentrations of NT-proBNP using an assay from Roche Diagnostics (Indianapolis, Indiana). The limit of detection for this assay is 5.0 ng/L, the 97.5th percentile cut-off has been established as 115 ng/L, and the manufacturer-recommended cut-off for clinical use is 125 ng/L. At a level of 125 ng/L, the CV for the system is 2.7%. We blinded the core laboratory to all clinical data, including the outcomes of stress testing and subsequent cardiac events. Likewise, all clinical staff caring for patients were blinded to the research NT-proBNP results.
Measurements
In our repository, we collected demographics, patient history including cardiac risk factors and comorbidities, and usual care laboratory and radiography testing results. Clinical data were collected from patients via standardised data collection form and confirmed with medical providers. We prospectively contacted participants at 90 days from enrolment to ascertain the occurrence of any adverse events. We also recorded the results of any relevant tests and other events occurring during the patient’s index visit and for 1 year afterwards via medical records review. We specifically recorded the occurrence of any subsequent MI (International Classification of Diseases-9 code or medical record evidence), abnormal stress testing, significant coronary disease by angiography (lesions >50% in a major epicardial coronary artery), PCI or coronary artery bypass graft surgery (CABG), or death (cardiovascular and all-cause) within 90 days and 1 year from enrolment. At enrolment, we obtained consent to review outside hospital records to confirm any events that occurred outside of our health system. Any discrepancies or ambiguity of follow-up outcome were adjudicated by the lead author. We entered study data into a REDCap (Research Electronic Data Capture)34 data repository hosted at Duke University.
Outcomes
For this a priori planned substudy of the parent biorepository, we compared stress-delta NT-proBNP concentrations between patients with inducible myocardial ischaemia (wall motion abnormality in at least one segment with stress that was not present at rest) on their stress tests versus those without. We calculated the diagnostic test characteristics—sensitivity, specificity and area under the receiver operating curve (AUC)—for the index test of 2-hour and 4-hour stress-delta NT-proBNP values compared with a reference standard of stress echocardiography imaging results.
In an exploratory analysis, we also compared stress-delta NT-proBNP results between patients who had a composite adverse cardiac event outcome at 90 days from index visit and those who did not. We included any patient who had any revascularisation procedure (PCI or CABG), MI or death within 90 days of the index visit. We also extended the follow-up period to 1 year.
To assess the sensitivity of stress-delta NT-proBNP for myocardial ischaemia on stress testing, our targeted sample size of 333 patients would provide a 95% CI range of approximately ±15%, assuming a true sensitivity of 85%.35 For 85% power to detect a difference between groups with alpha <0.05, we needed 25 patients with myocardial ischaemia. Based on prior studies in this observation unit, we anticipated being able to find at least an 8% prevalence of ischaemia on stress tests being performed in our unit.32
Analysis
Absolute and relative intraindividual changes in NT-proBNP concentrations across time points (stress-delta values) were calculated for each patient. In order to calculate these values, we used a value of 0 ng/L for any NT-proBNP assay that was below the limit of detection for this assay (5 ng/L). We used the Wilcoxon rank-sum test to compare the distribution of individual stress-delta values between patients with ischaemia on stress testing versus those without. Additionally, 2-hour and 4-hour stress-delta NT-proBNP values were analysed using analysis of variance models to adjust for differences in baseline values between groups; in these models, all NT-proBNP values were log-transformed due to skew. The receiver operating characteristic (ROC) curves were plotted and the AUC calculated, with calculation of optimal cut-off for maximal sensitivity and specificity. We did not adjust for multiple comparisons. A two-sided p value of <0.05 was considered statistically significant. All analyses were conducted using SAS V.9.4 statistical software.
Results
Characteristics of study subjects
We approached 413 eligible patients and ultimately enrolled 320 patients. Patients who were approached but did not enrol fell into one of the following non-exclusive categories: 30 patients declined or otherwise could not provide informed consent, 43 patients were unable to provide adequate blood samples or accurate lab assays, and 35 patients had their stress test changed to a non-exercise modality after consent. Four-hour post-stress samples were available for 173 patients.
Table 1 shows the demographic and clinical characteristics of enrolled patients. The prevalence of known coronary artery disease and of coronary artery disease risk factors was low.
Table 1.
Patient demographics and clinical characteristics
| Characteristics | All patients (N=320) (%) |
Positive for ischaemia (n=26*) (%) |
Negative for ischaemia (n=292) (%) |
| Age (years), median (25th, 75th) | 51.0 (44.0, 59.5) | 56.0 (49.0, 63.0) | 50.0 (43.0, 59.0) |
| Sex | |||
| Male | 147 (45.9) | 12 (46.2) | 133 (45.5) |
| Female | 173 (54.1) | 14 (53.8) | 159 (54.5) |
| Race/Ethnicity | |||
| Hispanic or Latino | 15 (4.7) | 1 (3.8) | 14 (4.8) |
| Asian | 4 (1.3) | 0 (0) | 4 (1.4) |
| Black or African–American | 122 (38.1) | 10 (38.5) | 111 (38.0) |
| White/Caucasian | 188 (58.8) | 16 (61.5) | 171 (58.6) |
| Other | 6 (1.9) | 0 | 6 (2.1) |
| Hypertension | 155 (48.4) | 18 (69.2) | 136 (46.6) |
| Diabetes | 59 (18.4) | 5 (19.2) | 54 (18.5) |
| History of tobacco use | 118 (36.9) | 11 (44.0) | 106 (37.2) |
| Hyperlipidaemia | 105 (32.8) | 16 (61.5) | 89 (30.5) |
| Cocaine use | 21 (6.6) | 2 (8.0) | 19 (6.6) |
| Renal disease/insufficiency | 5 (1.5) | 2 (7.7) | 3 (1.0) |
| Past myocardial infarction | 7 (2.2) | 5 (19.2) | 2 (0.7) |
| Coronary artery disease | 16 (5.0) | 7 (26.9) | 9 (3.1) |
| History of coronary intervention | 10 (3.2) | 3 (11.5) | 7 (2.4) |
| Congestive heart failure | 5 (1.6) | 1 (3.8) | 4 (1.4) |
| Chief complaint: chest pain | 290 (90.6) | 24 (92.3) | 264 (90.4) |
| Peak pain score (median) | 7 | 7 | 6.9 |
| Duration of symptoms (median, min) | 2 | 1 | 2 |
| Pain description | |||
| Was your pain worse if you walk quickly, climb stairs or exert yourself? | 66 (20.6) | 3 (11.5) | 63 (21.6) |
| Was your pain worse if you take a deep breath or cough? | 75 (23.4) | 6 (23.1) | 69 (23.6) |
| Was your pain worse if you pressed on the chest wall? | 48 (15) | 3 (11.5) | 45 (15.4) |
| Associated symptoms? | |||
| Nausea or vomiting | 107 (33.4) | 10 (38.5) | 97 (33.2) |
| Dyspnoea | 136 (42.5) | 12 (46.2) | 123 (42.1) |
| Diaphoresis | 89 (27.8) | 11 (42.3) | 77 (26.4) |
| Syncope | 55 (17.2) | 1 (3.8) | 54 (18.5) |
*2 patients with indeterminate stress tests excluded.
For the primary comparison, we further excluded two patients who had indeterminate stress test results in the opinion of our three reviewers’ adjudication. Our review of stress test reports resulted in only one disagreement between two reviewers out of 292 stress test reports reviewed—a case initially reported as ischaemia that the second reviewer found to be artefact. In total, 26 patients had myocardial ischaemia on stress echocardiographic testing. In follow-up, we were able to contact 241 (75.3%) by phone at 90 days and conduct medical record review on all patients at 1 year. Within 1 year, 48 patients had had some form of coronary artery imaging, with 16 patients demonstrating at least one artery with >50% stenosis. Ultimately, nine patients had any composite outcome within 90 days and 11 patients had any within 1 year. Each individual patient’s downstream outcomes are shown in online supplementary table 1.
openhrt-2018-000847supp001.pdf (35.8KB, pdf)
Main results
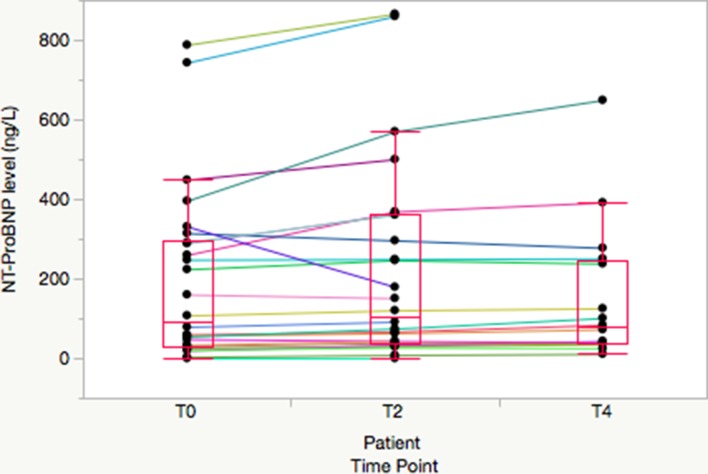
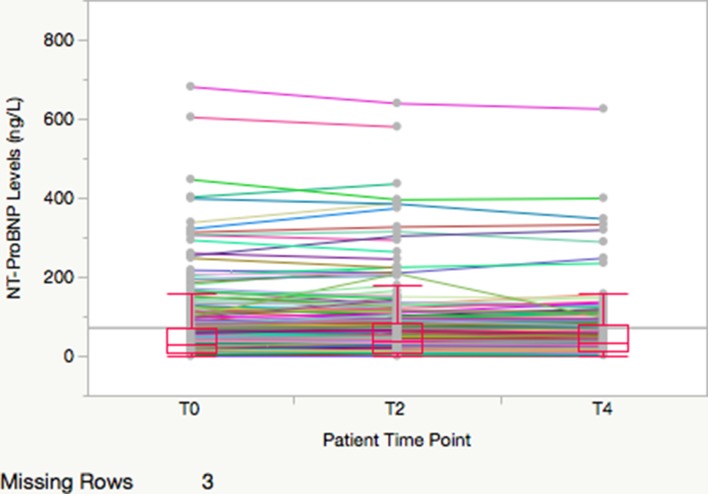
Table 2 shows the median (25th and 75th percentiles) NT-proBNP concentrations at each time point, along with the median patient-level absolute and relative changes in NT-proBNP concentrations (stress-delta). The median absolute stress-deltas at 2 hours were 10.4 (IQR 6.0–51.7) vs 1.7 (IQR −0.4 to 8.7) ng/L and at 4 hours were 14.8 (IQR 5.0–22.3) ng/L vs 1.0 (−2.0 to 10.3) ng/L for patients with ischaemia on stress testing versus those without. Patients with abnormal stress tests had significantly higher NT-proBNP concentrations at baseline and at all subsequent time points (all p<0.05). Figures 1 and 2 show spaghetti plots with boxplots of NT-proBNP concentrations at baseline and at 2-hour and 4-hour post-stress comparing patients who had ischaemia on stress testing versus those without.
Table 2.
Patients with ischaemic cardiac stress tests had higher NT-proBNP levels at all time points, as well as higher absolute and relative stress-delta values
| Characteristics | Positive for ischaemia (n=26) |
Negative for ischaemia (n=292) |
All patients (N=318*) |
P values |
| Baseline NT-proBNP | <0.001 | |||
| n | 26 | 292 | 318 | |
| Median (25th, 75th) | 93.3 (31.1, 291.2) | 31.7 (9.8, 71.0) | 34.3 (10.7, 78.4) | |
| Min, max | 0.0, 788.0 | 0.0, 2343 | 0.0, 2343 | |
| 2-Hour post-stress NT-proBNP† | <0.001 | |||
| n | 25 | 289 | 314 | |
| Median (25th, 75th) | 120.5 (38.8, 360.8) | 38.9 (10.3, 81.6) | 40.0 (11.3, 88.3) | |
| Min, max | 0.0, 865.8 | 0.0, 1800 | 0.0, 1800 | |
| Absolute 2-hour stress-delta NT-proBNP | <0.001 | |||
| n | 25 | 289 | 314 | |
| Median (25th, 75th) | 10.4 (6.0, 51.7) | 1.7 (−0.4, 8.7) | 2.1 (−0.3, 9.5) | |
| Min, max | −152, 174.3 | −543, 108.0 | −543, 174.3 | |
| Percentage 2-hour stress-delta NT-proBNP‡ | 0.05 | |||
| n | 23 | 240 | 263 | |
| Median (25th, 75th) | 15.7 (10.1, 31.1) | 7.9 (−4.6, 25.1) | 9.3 (−4.1, 25.5) | |
| Min, max | −45.9, 75.8 | −100, 189.0 | −100, 189.0 | |
| 4-Hour post-stress NT-proBNP | 0.009 | |||
| n | 16 | 155 | 171 | |
| Median (25th, 75th) | 78.2 (38.2, 244.5) | 35.5 (13.3, 81.4) | 39.3 (14.8, 87.3) | |
| Min, max | 10.9, 649.2 | 0.0, 1757 | 0.0, 1757 | |
| Absolute 4-hour stress-delta NT-proBNP | 0.004 | |||
| n | 16 | 155 | 171 | |
| Median (25th, 75th) | 14.8 (5.0, 22.3) | 1.0 (−2.0, 10.3) | 1.8 (−1.3, 12.4) | |
| Min, max | −36.9, 253.2 | −586, 64.5 | −586, 253.2 | |
| Percentage 4-hour stress-delta NT-proBNP | 0.05 | |||
| n | 15 | 135 | 150 | |
| Median (25th, 75th) | 26.9 (6.4, 63.9) | 6.3 (−8.2, 30.5) | 8.2 (−7.3, 39.1) | |
| Min, max | −17.1, 109.5 | −100, 285.5 | −100, 285.5 |
*2 patients with indeterminate stress tests excluded.
†Four patients had baseline and 4-hour samples drawn, but no 2-hour samples.
‡Unable to calculate percentage delta in patients with undetectable baseline values.
NT-proBNP, N-terminal pro-B type natriuretic peptide.
Figure 1.
Spaghetti plot of individual patient N-terminal pro-B type natriuretic peptide (NT-proBNP) concentrations for patients with ischaemic stress test results. Median, 25th and 75th percentiles are noted by boxes and whiskers extend to 1.5x interquartile range..
Figure 2.
Spaghetti plot of individual patient N-terminal pro-B type natriuretic peptide (NT-proBNP) concentrations for patients with normal or indeterminate stress test results. Median, 25th and 75th percentiles are noted by boxes and whiskers extend to 1.5x interquartile range. Scale is truncated in order to match figure 1 and due to two extreme outlier patients who are excluded from the graph but not from our analysis.
In unadjusted analyses, relative and absolute stress-delta NT-proBNP significantly differed according to the presence or absence of ischaemia on stress testing at both 2-hour and 4-hour time points. After log transformation of NT-proBNP values and adjusting for baseline concentrations, 2-hour stress-delta NT-proBNP concentrations were not significantly different between patients with ischaemic versus normal stress tests (p=0.07), but the 4-hour stress-delta NT-proBNP concentrations were significantly different (p=0.0085).
Figure 3 shows the ROC curves of 2-hour and 4-hour stress-delta NT-proBNP for predicting myocardial ischaemia on stress testing. It demonstrates that 2-hour and 4-hour stress-delta NT-proBNP had modest predictive value of ischaemia with an AUC of 0.716 and 0.719, respectively. The diagnostic test characteristics of 2-hour and 4-hour stress-delta NT-proBNP for predicting myocardial ischaemia on stress test imaging are shown in table 3. Stress-delta NT-proBNP has modest sensitivity and specificity, high negative predictive value but poor positive predictive value.
Figure 3.
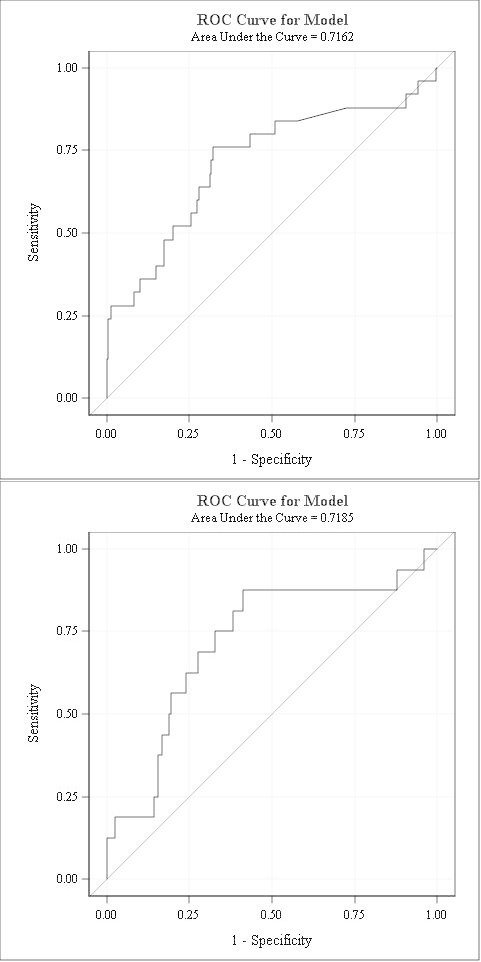
ROC curve of 2-hour (A) and 4-hour (B) stress-delta NT-proBNP. Two-hour stress-delta NT-proBNP shows modest predictive ability of ischaemia on stress test imaging with an AUC of 0.7162. Four-hour stress-delta shows an AUC of 0.7185. AUC, area under the curve; NT-proBNP, N-terminal pro-B type natriuretic peptide; ROC, receiver operating characteristic.
Table 3.
Diagnostic test characteristics of stress-delta NT-proBNP for myocardial ischaemia on stress echocardiogram imaging
| Sensitivity (%) | Specificity (%) | LR+ | LR− | |
| 2-Hour stress-delta NT-proBNP (cut-off 6 ng/L) | 76 | 68 | 2.38 | 0.35 |
| 4-Hour stress-delta NT-proBNP (cut-off 5.9 ng/L) | 75 | 67 | 2.27 | 0.37 |
LR+, positive likelihood ratio;LR−, negative likelihood ratio;NT-proBNP, N-terminal pro-B type natriuretic peptide.
In exploratory analyses we found nine patients with a 90-day composite outcome, and the median NT-proBNP values were non-significantly higher at all static time points, and only the absolute 2-hour stress-delta comparison was significantly different (online supplementary table 2). Similarly, none of the NT-proBNP concentrations were significantly different for the 11 patients with a 1-year composite outcome compared with those without such outcome (online supplementary table 3).
openhrt-2018-000847supp002.pdf (59KB, pdf)
openhrt-2018-000847supp003.pdf (63.8KB, pdf)
Discussion
The optimal assessment of suspected ACS remains controversial despite decades of research. The current standard of care uses stress testing,8 36–38 in which the patient who has ruled out for MI undergoes a protocolised stressor and is assessed via imaging modalities such as echocardiography. The current paradigm remains inefficient, and many question the utility of routine stress testing in ED patients with symptoms of ACS.21 39–42 Based on the event rate in the reported literature (eg, <1% in Sandhu et al 42), it appears that stress testing is currently overused. However, there remains concern for adverse cardiac outcomes following serial cardiac troponin testing, even with high-sensitivity assays.7 30
In this context, we sought to determine whether a biomarker-based stress test could improve the current paradigm. Currently, biomarkers are only serially measured in resting patients as a test for MI, heart failure or as a predictor of cardiac complications following major non-cardiac surgery.43 44 Measuring dynamic changes in biomarker levels during stress testing is a novel application of cardiac biomarkers in ED patients. If a biomarker-based stress test could be developed, it would have several advantages. Since laboratory testing is routinely available 24 hours a day to ED patients, a biomarker stress test could serve as a triage test in the ED to determine which patients need further work-up. If it could provide equivalent predictive value, it might represent a cost-effective and time-effective alternative to standard stress testing that does not require special equipment nor specialists to obtain or interpret the images. Based on prior literature,45 46 it would appear that using two serial NT-proBNP tests compared with an echocardiogram would be approximately 30% the cost of using echocardiography with stress testing. However, for biomarkers to replace the use of stress echocardiography, considerably higher sensitivities than our current results would likely be needed. Alternatively, it could be used as an adjunct to aid in disposition of patients with indeterminate results on standard stress tests.
In this study, we assessed the feasibility of a biomarker-based stress test using NT-proBNP. We found that NT-proBNP concentrations differed significantly according to patients’ stress test imaging results. Our study is one of the largest to examine this biomarker-based stress test concept with NT-proBNP. Prior authors found that patients with ischaemia had a differential stress-delta response,22 25–29 while others did not.23 24 Our patient sample of recently symptomatic ED patients makes our study unique. Obtaining a baseline measurement of NT-proBNP just prior to stress testing limits any potential impact that the patients’ recent symptoms may have had on results.
NT-proBNP is the prohormone of BNP released first in the bloodstream and has a shorter half-life, which could potentially lead to differences in results between these two markers. However, others have studied BNP in this paradigm and found a similar range of response in patients with inducible ischaemia.47–54
Prior studies used the stress test imaging component as their gold standard for outcome. We built on these by considering a broader range of downstream outcomes, although these occurred infrequently in our population. Our study did not find significantly different stress-delta results in this comparison, a finding that we attribute to underpowered analyses.
We also found that baseline NT-proBNP levels were significantly higher among patients who ultimately had myocardial ischaemia. This was not our primary intent in this study so these findings should be considered preliminary. However, NT-proBNP has long been noted to have high independent prognostic capability as a traditional ACS biomarker.55–57 Although professional society guidelines acknowledge their potential use for ED risk stratification of ACS,58 they have not entered into common use for this purpose. These findings suggest a potential role for NT-proBNP in ED ACS risk stratification that should be further explored.
Study limitations
Our study has some important limitations. It was performed at a single institution and in a population that had been selected for relatively low risk for ACS. During this time, our unit did not have objective criteria for entry in the ACS pathway. However, it has been shown that clinician gestalt can effectively risk-stratify patients to lower risk groups,59 and our unit’s prevalence of ischaemia is similar to that described in other settings.60 Furthermore, during the time of this study, high-sensitivity troponin assays were not available in our setting. The introduction of these assays will likely alter the prevalence of disease in the population being referred for observation unit care in the future. Although we attempted to approach consecutive patients, we did not have the ability to reach all patients on weekends.
This was an observational study that is subject to ascertainment bias of downstream outcomes, although all patients had the reference standard test as an inclusion criterion. Furthermore, a small sample size with a low event rate led to underpowered analyses of downstream composite outcomes. We did not control for multiple other baseline risk factors other than NT-proBNP to avoid overfitting our small number of patients with ischaemia. We did use an admittedly surrogate outcome for patient-oriented adverse events; however, we felt that for this low-risk cohort, the stress test result is the key data point that determines further testing and thus a valid outcome. In the future, cardiac MRI or angiography could be considered as diagnostic standard, but this would require significant resources to conduct in a study and has less practical relevance for most ED practices. Nonetheless, our findings warrant further study in larger samples with higher risk of ACS. Although we conducted multiple comparisons between groups, the consistency of results at multiple time points and between static and stress-delta comparisons is compelling.
We were not able to obtain 4-hour post-stress samples on all patients in our biorepository. In almost all cases, this was because the patient had been discharged from the ED within 4 hours of completion of their stress test. Despite the smaller sample size for this comparison, we still noted results that were directionally the same as our 2-hour analyses and that were statistically significant in our adjusted analyses. It remains to be seen whether our proposed paradigm of biomarker-based stress testing would be clinically acceptable to clinicians and patients. Furthermore, a complete financial analysis of a biomarker-based stress test strategy is beyond the scope of this paper.
In conclusion, patients with ischaemia on stress testing demonstrated higher concentrations of NT-proBNP at baseline and all subsequent post-stress time points.
Relative and absolute stress-delta NT-proBNP differed significantly based on stress test imaging results at the 2-hour and 4-hour time points, although when adjusted for baseline NT-proBNP only the 4-hour time point remained significant. These findings warrant further study of this biomarker stress test paradigm in a larger number of patients.
Acknowledgments
We would like to acknowledge Ms Ashley Morgan for proof-reading the manuscript. We would like to acknowledge Roche Diagnostics International for their financial support of this project via an investigator-initiated grant.
Footnotes
Twitter: alimkakeng
Contributors: ATL conceived the study. ATL, LKN and YL designed the analysis. ATL and LKN obtained funding for the project. ATL, EJ, JCL and MG oversaw enrolment, data collection, blood specimen collection and storage. RC oversaw the analysis of blood samples and provided expertise on laboratory-related content. ATL, EJ, YL and JCL managed the data. YL provided the statistical analysis plan and analysed the data. ATL drafted the manuscript, and all authors contributed substantially to its revision. ATL takes responsibility as guarantor for the content of the paper as a whole.
Funding: Financial support was not dependent on the results of the study. Roche Diagnostics International provided salary support for investigators and materials for testing samples. The investigators retained control of the data throughout the study and the decision of whether to publish the results. The Duke Office of Clinical Research’s support of this project was made possible by Grant Number 1 UL1 RR024128-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research. Its content is solely the responsibility of the authors and does not necessarily represent the official view of the NCRR or the NIH.
Competing interests: ATL would like to disclose that he has received research support from Roche Diagnostics, Siemens Healthcare Diagnostics and Abbott Laboratories, all of which are manufacturers of troponin and B-type natriuretic peptide assays. RHC has served as a consultant and member of scientific advisory groups for Roche Diagnostics, Quidel Diagnostics, Beckman Coulter Diagnostics, and Siemens Healthcare Diagnostics. LKN reports receiving consulting honoraria from Roche Diagnostics and Philips Healthcare, which produce products related to the current research, and from AstraZeneca HCF, Metanomics and Medscape. She has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, Google Life Sciences (Verily), NHLBI and the MURDOCK Study.
Patient consent: Not required.
Ethics approval: The Duke University Medical Center Institutional Review Board approved this protocol.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Limkakeng A, Gibler WB, Pollack C, et al. . Combination of goldman risk and initial cardiac troponin I for emergency department chest pain patient risk stratification. Acad Emerg Med 2001;8:696–702. 10.1111/j.1553-2712.2001.tb00187.x [DOI] [PubMed] [Google Scholar]
- 2. Mark DB, Hlatky MA, Harrell FE, et al. . Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987;106:793 10.7326/0003-4819-106-6-793 [DOI] [PubMed] [Google Scholar]
- 3. Ely S, Chandra A, Mani G, et al. . Utility of observation units for young emergency department chest pain patients. J Emerg Med 2013;44:306–12. 10.1016/j.jemermed.2012.07.048 [DOI] [PubMed] [Google Scholar]
- 4. Amsterdam EA, Kirk JD, Bluemke DA, et al. . Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American heart association. Circulation 2010;122:1756–76. 10.1161/CIR.0b013e3181ec61df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Go AS, Mozaffarian D, Roger VL, et al. . Executive summary: heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation 2014;129:399–410. 10.1161/01.cir.0000442015.53336.12 [DOI] [PubMed] [Google Scholar]
- 6. Mitka M. New definition of myocardial infarction puts biomarkers front and center. JAMA 2012;308:1511–2. 10.1001/jama.2012.12794 [DOI] [PubMed] [Google Scholar]
- 7. Reichlin T, Schindler C, Drexler B, et al. . One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012;172:1211–8. 10.1001/archinternmed.2012.3698 [DOI] [PubMed] [Google Scholar]
- 8. Wright RS, Anderson JL, Adams CD, et al. . 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2011;57:e215–367. 10.1016/j.jacc.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 9. Newby LK, Goldmann BU, Ohman EM. Troponin: an important prognostic marker and risk-stratification tool in non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 2003;41:S31–S36. 10.1016/S0735-1097(02)02832-2 [DOI] [PubMed] [Google Scholar]
- 10. Chandra A, Rudraiah L, Zalenski RJ. Stress testing for risk stratification of patients with low to moderate probability of acute cardiac ischemia. Emerg Med Clin North Am 2001;19:87–103. 10.1016/S0733-8627(05)70169-3 [DOI] [PubMed] [Google Scholar]
- 11. Christenson J, Innes G, McKnight D, et al. . A clinical prediction rule for early discharge of patients with chest pain. Ann Emerg Med 2006;47:1–10. 10.1016/j.annemergmed.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 12. Committee O. Single photon emission computed tomography for the diagnosis of coronary artery disease: an Evidence-Based Analysis. Ontario Health Technology Assessment Series 2010;10:1–64. [PMC free article] [PubMed] [Google Scholar]
- 13. Mikhail MG, Smith FA, Gray M, et al. . Cost-effectiveness of mandatory stress testing in chest pain center patients. Ann Emerg Med 1997;29:88–98. 10.1016/S0196-0644(97)70314-7 [DOI] [PubMed] [Google Scholar]
- 14. Mowatt G, Vale L, Brazzelli M, et al. . Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Health Technol Assess 2004;8:1–207. 10.3310/hta8300 [DOI] [PubMed] [Google Scholar]
- 15. Miyamoto MI, Vernotico SL, Majmundar H, et al. . Pharmacologic stress myocardial perfusion imaging: a practical approach. J Nucl Cardiol 2007;14:250–5. 10.1016/j.nuclcard.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 16. Peteiro J, Bouzas-Mosquera A. Exercise echocardiography. World J Cardiol 2010;2:223–32. 10.4330/wjc.v2.i8.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Newman DH, Ackerman B, Kraushar ML, et al. . Quantifying patient-physician communication and perceptions of risk during admissions for possible acute coronary syndromes. Ann Emerg Med 2015;66:13–18. 10.1016/j.annemergmed.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 18. Pines JM, Isserman JA, Szyld D, et al. . The effect of physician risk tolerance and the presence of an observation unit on decision making for ED patients with chest pain. Am J Emerg Med 2010;28:771–9. 10.1016/j.ajem.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 19. Blecker S, Gavin NP, Park H, et al. . Observation units as substitutes for hospitalization or home discharge. Ann Emerg Med 2016;67:706–13. 10.1016/j.annemergmed.2015.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin GP, Wright B, Ahmed A, et al. . Use or abuse? A qualitative study of emergency physicians' views on use of observation stays at three hospitals in the united states and england. Ann Emerg Med 2017;69:284–92. 10.1016/j.annemergmed.2016.08.458 [DOI] [PubMed] [Google Scholar]
- 21. Foy AJ, Liu G, Davidson WR, et al. . Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med 2015;175:428–36. 10.1001/jamainternmed.2014.7657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Başkurt M, Aktürk F, Keskin K, et al. . Serum high-sensitivity C-reactive protein, amyloid associated protein and N-terminal proBNP levels do not predict reversible myocardial ischaemia. Cardiovasc J Afr 2011;22:85–9. 10.5830/CVJA-2010-041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karabinos I, Karvouni E, Chiotinis N, et al. . Acute changes in N-terminal pro-brain natriuretic peptide induced by dobutamine stress echocardiography. Eur J Echocardiogr 2007;8:265–74. 10.1016/j.euje.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 24. Kurz K, Voelker R, Zdunek D, et al. . Effect of stress-induced reversible ischemia on serum concentrations of ischemia-modified albumin, natriuretic peptides and placental growth factor. Clin Res Cardiol 2007;96:152–9. 10.1007/s00392-007-0469-5 [DOI] [PubMed] [Google Scholar]
- 25. Staub D, Jonas N, Zellweger MJ, et al. . Use of N-terminal pro-B-type natriuretic peptide to detect myocardial ischemia. Am J Med 2005;118:1287–1287. 10.1016/j.amjmed.2005.05.020 [DOI] [PubMed] [Google Scholar]
- 26. Szardien S, Nef HM, Möllmann H, et al. . Transient elevation of NT-pro-BNP as a predictor for myocardial ischemia. Clin Res Cardiol 2010;99:857–9. 10.1007/s00392-010-0211-1 [DOI] [PubMed] [Google Scholar]
- 27. van der Zee PM, Verberne HJ, van Spijker RC, et al. . Relation of N-terminal pro B-type natriuretic peptide levels after symptom-limited exercise to baseline and ischemia levels. Am J Cardiol 2009;103:604–10. 10.1016/j.amjcard.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 28. Vanzetto G, Jacon P, Calizzano A, et al. . N-terminal pro-brain natriuretic peptide predicts myocardial ischemia and is related to postischemic left-ventricular dysfunction in patients with stable coronary artery disease. J Nucl Cardiol 2007;14:835–42. 10.1016/j.nuclcard.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 29. Weber M, Bazzino O, Navarro Estrada JL, et al. . N-terminal B-type natriuretic peptide assessment provides incremental prognostic information in patients with acute coronary syndromes and normal troponin T values upon admission. J Am Coll Cardiol 2008;51:1188–95. 10.1016/j.jacc.2007.11.054 [DOI] [PubMed] [Google Scholar]
- 30. Limkakeng A, Drake W, Lokhnygina Y. Myocardial ischemia on cardiac stress testing is not associated with changes in troponin t Levels. J Appl Lab Med 2017;1:532–43. [DOI] [PubMed] [Google Scholar]
- 31. Hollander JE, Blomkalns AL, Brogan GX, et al. . Standardized reporting guidelines for studies evaluating risk stratification of emergency department patients with potential acute coronary syndromes. Ann Emerg Med 2004;44:589–98. 10.1016/S0196064404012806 [DOI] [PubMed] [Google Scholar]
- 32. Chavez J, Srinivasan A, Ely S, et al. . Thrombolysis in myocardial infarction risk score in an observation unit setting. Crit Pathw Cardiol 2013;12:137–40. 10.1097/HPC.0b013e3182998bc1 [DOI] [PubMed] [Google Scholar]
- 33. Limkakeng AT, Chandra A. Impact of renal dysfunction on acute coronary syndrome evaluation in observation unit patients. Am J Emerg Med 2010;28:658–62. 10.1016/j.ajem.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 34. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Committee O. Stress echocardiography for the diagnosis of coronary artery disease: an evidence-based analysis. Ont Health Technol Assess Ser 2010;10:1–61. [PMC free article] [PubMed] [Google Scholar]
- 36. American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association . ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr 2011;24:229–67. 10.1016/j.echo.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 37. Bruce RA, Blackmon JR, Jones JW, et al. . Exercising testing in adult normal subjects and cardiac patients. Pediatrics 1963;32: :742–56. SUPPL. [PubMed] [Google Scholar]
- 38. Tennant R, Wiggers C. The effects of coronary occlusion on myocardial contraction. Am J Physiol 1935;112:351–61. [Google Scholar]
- 39. Hermann LK, Newman DH, Pleasant WA, et al. . Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern Med 2013;173:1128–33. 10.1001/jamainternmed.2013.850 [DOI] [PubMed] [Google Scholar]
- 40. Mudrick DW, Cowper PA, Shah BR, et al. . Downstream procedures and outcomes after stress testing for chest pain without known coronary artery disease in the United States. Am Heart J 2012;163:454–61. 10.1016/j.ahj.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prasad V, Cheung M, Cifu A. Chest pain in the emergency department: the case against our current practice of routine noninvasive testing. Arch Intern Med 2012;172:1506–9. 10.1001/archinternmed.2012.4037 [DOI] [PubMed] [Google Scholar]
- 42. Sandhu AT, Heidenreich PA, Bhattacharya J, et al. . Cardiovascular testing and clinical outcomes in emergency department patients with chest pain. JAMA Intern Med 2017;177:1175 10.1001/jamainternmed.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodseth RN, Biccard BM, Le Manach Y, et al. . The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol 2014;63:170–80. 10.1016/j.jacc.2013.08.1630 [DOI] [PubMed] [Google Scholar]
- 44. Cagini L, Andolfi M, Leli C, et al. . B-type natriuretic peptide following thoracic surgery: a predictor of postoperative cardiopulmonary complications. Eur J Cardiothorac Surg 2014;46:e74–e80. 10.1093/ejcts/ezu348 [DOI] [PubMed] [Google Scholar]
- 45. Goode KM, Clark AL, Cleland JG. Ruling out heart failure in primary-care: the cost-benefit of pre-screening using NT-proBNP and QRS width. Int J Cardiol 2008;130:426–37. 10.1016/j.ijcard.2007.08.131 [DOI] [PubMed] [Google Scholar]
- 46. Ferrandis MJ, Ryden I, Lindahl TL, et al. . Ruling out cardiac failure: cost-benefit analysis of a sequential testing strategy with NT-proBNP before echocardiography. Ups J Med Sci 2013;118:75–9. 10.3109/03009734.2012.751471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zaid G, Tanchilevitch A, Rivlin E, et al. . Diagnostic accuracy of serum B-type natriuretic peptide for myocardial ischemia detection during exercise testing with spect perfusion imaging. Int J Cardiol 2007;117:157–64. 10.1016/j.ijcard.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 48. Salinas G, Daher IN, Okorodudu AO, et al. . B-type natriuretic peptide is not a marker of ischemia during dobutamine stress echocardiography. J Am Soc Echocardiogr 2007;20:23–6. 10.1016/j.echo.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 49. Asada J, Tsuji H, Iwasaka T, et al. . Usefulness of plasma brain natriuretic peptide levels in predicting dobutamine-induced myocardial ischemia. Am J Cardiol 2004;93:702–4. 10.1016/j.amjcard.2003.11.051 [DOI] [PubMed] [Google Scholar]
- 50. Bergeron S, Møller JE, Bailey KR, et al. . Exertional changes in circulating cardiac natriuretic peptides in patients with suggested coronary artery disease. J Am Soc Echocardiogr 2006;19:772–6. 10.1016/j.echo.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 51. Foote RS, Pearlman JD, Siegel AH, et al. . Detection of exercise-induced ischemia by changes in B-type natriuretic peptides. J Am Coll Cardiol 2004;44:1980–7. 10.1016/j.jacc.2004.08.045 [DOI] [PubMed] [Google Scholar]
- 52. Røsjø H, Kravdal G, Høiseth AD, et al. . Troponin I measured by a high-sensitivity assay in patients with suspected reversible myocardial ischemia: data from the Akershus Cardiac Examination (ACE) 1 study. Clin Chem 2012;58:1565–73. 10.1373/clinchem.2012.190868 [DOI] [PubMed] [Google Scholar]
- 53. Staub D, Nusbaumer C, Zellweger MJ, et al. . Use of B-type natriuretic peptide in the detection of myocardial ischemia. Am Heart J 2006;151:1223–30. 10.1016/j.ahj.2005.06.045 [DOI] [PubMed] [Google Scholar]
- 54. Win HK, Chang SM, Raizner M, et al. . Percent change in B-type natriuretic peptide levels during treadmill exercise as a screening test for exercise-induced myocardial ischemia. Am Heart J 2005;150:695–700. 10.1016/j.ahj.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 55. Galvani M, Ottani F, Oltrona L, et al. . N-terminal pro-brain natriuretic peptide on admission has prognostic value across the whole spectrum of acute coronary syndromes. Circulation 2004;110:128–34. 10.1161/01.CIR.0000134480.06723.D8 [DOI] [PubMed] [Google Scholar]
- 56. Heeschen C, Hamm CW, Mitrovic V, et al. . N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation 2004;110:3206–12. 10.1161/01.CIR.0000147611.92021.2B [DOI] [PubMed] [Google Scholar]
- 57. Timóteo AT, Toste A, Ramos R, et al. . Does admission NT-proBNP increase the prognostic accuracy of GRACE risk score in the prediction of short-term mortality after acute coronary syndromes? Acute Card Care 2009;11:236–42. 10.1080/17482940903177036 [DOI] [PubMed] [Google Scholar]
- 58. Amsterdam EA, Kirk JD, Bluemke DA, et al. . Testing of low-risk patients presenting to the emergency department with chest pain. A Scientific Statement From the American Heart Association 2010;122:1756–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chandra A, Lindsell CJ, Limkakeng A, et al. . Emergency physician high pretest probability for acute coronary syndrome correlates with adverse cardiovascular outcomes. Acad Emerg Med 2009;16:740–8. 10.1111/j.1553-2712.2009.00470.x [DOI] [PubMed] [Google Scholar]
- 60. Holly J, Fuller M, Hamilton D, et al. . Prospective evaluation of the use of the thrombolysis in myocardial infarction score as a risk stratification tool for chest pain patients admitted to an ED observation unit. Am J Emerg Med 2012. (published Online First: 2012/09/05). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2018-000847supp001.pdf (35.8KB, pdf)
openhrt-2018-000847supp002.pdf (59KB, pdf)
openhrt-2018-000847supp003.pdf (63.8KB, pdf)