Abstract
Natural products with antispasmodic activity have been used in traditional medicine to alleviate different illnesses since the remote past. We searched the literature and compiled the antispasmodic activity of 248 natural compounds isolated from terrestrial plants. In this review, we summarized all the natural products reported with antispasmodic activity until the end of 2017. We also provided chemical information about their extraction as well as the model used to test their activities. Results showed that members of the Lamiaceae and Asteraceae families had the highest number of isolated compounds with antispasmodic activity. Moreover, monoterpenoids, flavonoids, triterpenes, and alkaloids were the chemical groups with the highest number of antispasmodic compounds. Lastly, a structural comparison of natural versus synthetic compounds was discussed.
1. Introduction
Antispasmodic compounds are currently used to reduce anxiety, emotional and musculoskeletal tension, and irritability. Although most of the available antispasmodic compounds are synthetic or semisynthetic, traditional uses of this group of compounds are still popular.
We collected information about natural compounds with antispasmodic activity isolated from terrestrial plants. We searched the databases of Google Scholar, PubMed, and SciFinder and compiled the information about 248 compounds published until December 2017. This review focuses on the antispasmodic activity of isolated compounds and activities from extracts without further purification are not discussed.
2. The Neurons
Nerve cells or neurons are responsible for receiving, conducting, and transmitting signals. A neuron consists of a nucleated body, a long thin extension called an axon, and several dendrites or prolongations extended from the cell body. Axons conduct signals from the nucleated body towards distant targets, while dendrites provide an enlarged surface area to receive signals from the axons of other neurons.
Signal transmission through axons is driven by a change in the electrical potential across the plasma membrane of neurons. This plasma membrane contains voltage-gated cation channels, which are responsible for generation of action potentials. An action potential is triggered by a depolarization of the plasma membrane or a shift to a less negative value.
In nerve and skeletal muscle cells, a stimulus can cause sufficient depolarization to open voltage-gated Na+ channels allowing the entrance of Na+ into the cell. This influx of Na+ depolarizes the membrane further causing the opening of more Na+ channels. To avoid a permanent influx, Na+ channels are able to reclose rapidly even when the membrane is still depolarized. This function is based on the presence of voltage-gated K+ channels, which are responsible for K+ efflux equilibrating the membrane potential even before the total inactivation of Na+ channels. In some cases, the action potential in some muscles depends on voltage-gated Ca2+ channels.
2.1. Transmission of Signals
The transmission of signals occurs mainly between neurons or from neurons to skeletal muscles, which are the final acceptors of electrical signals, causing a muscular contraction.
2.1.1. Signal Transmission between Neurons
Neuronal signals are transmitted between neurons at specialized sites of contact known as synapses. Neurons are separated by a synaptic cleft where a release of a neurotransmitter occurs. This neurotransmitter is stored in vesicles and is released by exocytosis. Upon triggering, the neurotransmitter is released into the cleft provoking an electrical change in the postsynaptic cell by binding to the transmitter-gated ion channels. To avoid a continuous electrical change and to ensure both spatial and temporal precision of signal transmission, the neurotransmitter is rapidly removed from the cleft either by specific enzymes in the synaptic cleft or by reuptake mediated by neurotransmitter carrier proteins [1].
Neurotransmitters can also open cation channels causing an influx of Na+ and then called excitatory neurotransmitters (e.g., acetylcholine, glutamate, and serotonin) or produce an opening of Cl− channels and then inhibiting the signal transmission by maintaining the postsynaptic membrane polarization [e.g., γ-aminobutyric acid (GABA) and glycine].
2.1.2. Neuromuscular Signal Transmission
The transmission of electrical signals to muscles involves five sequential and orchestrated steps: (i) nerve electric signal reaches the nerve terminal, (ii) it depolarizes the plasma membrane of the terminal, (iii) voltage-gated Ca2+ channels opens causing an increase in Ca2+ concentration in the neuron cytosol, and (iv) release of acetylcholine into the synaptic cleft is triggered. Acetylcholine binds to acetylcholine receptors in the muscle plasma membrane opening Na+ channels and provoking a membrane depolarization. This depolarization enhances the opening of more Na+ channels causing a self-propagating depolarization. The generalized depolarization of the muscle plasma membrane activates Ca2+ channels in specialized regions on the membrane causing Ca2+ release from the sarcoplasmic reticulum (Ca2+ storage) into the cytosol.
As a consequence of an increase in the Ca2+ concentration, myofibrils in the muscle cell contract. The increase of Ca2+ in the cytosol is transient because Ca2+ is rapidly pumped back into the sarcoplasmic reticulum causing a relaxation of the myofibrils. This process is very fast and Ca2+ concentration at resting levels is restored within 30 milliseconds [2].
3. Receptors
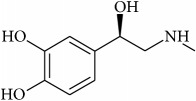
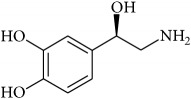
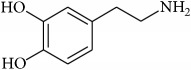
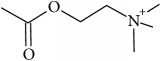
The autonomic nerve system controls and monitors the internal environment of the body. The input of its activity is provided by neurons that are associated with specific sensory receptors located in the blood vessels, muscles, and visceral organs (Table 1). According to the neurotransmitter secreted, these neurons are classified as adrenergic or cholinergic. The adrenergic neurons secrete the neurotransmitter noradrenalin termed also norepinephrine. Adrenergic receptors include the types α and β, which are further categorized as α1, α2, β1, β2, and β3. On the other hand, cholinergic neurons secrete acetylcholine, which induces a postsynaptic event. There are two types of cholinergic receptors, the nicotinic receptor (abundant at the neuromuscular junction) and the muscarinic receptor (abundant on smooth and cardiac muscles and glands).
Table 1.
Receptors targeted by neurotransmitters in the body.
| Receptor | Targeted by | |
|---|---|---|
| Adrenergic | Epinephrine (adrenaline) |
|
| Norepinephrine (noradrenaline) |
|
|
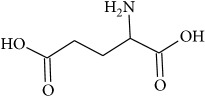
| Dopaminergic | Dopamine |
|
| Cholinergic | Acetylcholine |
|
| GABAergic | GABA |
|
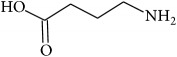
| Glutaminergic | Glutamate |
|
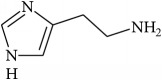
| Histaminergic | Histamine |
|
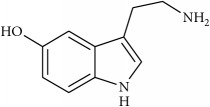
| Serotonergic | Serotonin |
|
| Glycinergic | Glycine |
|
| Opioid | Dynorphin |
|
| Enkephalin |
|
|
| Endorphin |
|
|
| Endomorphin |
|
|
| Nociceptin |
|
There are several agonists (neurotransmitters, hormones, and others) able to bind to specific receptors and activate the contraction of smooth muscle. Upon binding the agonist to the receptor, the mechanism of contraction is based on an increase of phospholipase C. This enzyme hydrolyzes phosphatidylinositol 4,5-bisphosphate located on the membrane, producing two powerful secondary messengers termed diacylglycerol (DG) and inositol 1,4,5 triphosphate (IP3). IP3 binds to specific receptors in the sarcoplasmic reticulum, causing release of Ca2+ within the muscle. DG together with Ca2+ activates the protein kinase C (PKC), which phosphorylates specific proteins. In most smooth muscles, the contraction process commences when PKC phosphorylates Ca2+ channels or other proteins that regulate the cyclic process. For instance, Ca2+ binds to calmodulin (a multifunctional intermediate calcium-binding messenger protein), triggering the activation of the myosin light chain (MLC) kinase, which phosphorylates the light chain of myosin and together with actin carries out the process of initiating the shortening of the smooth muscle cell [147]. However, the elevation of the intracellular concentration of Ca2+ is transient, and the contractile response is maintained by a mechanism sensitized by Ca2+ modulated by the inhibition of myosin phosphatase activity by Rho kinase. This mechanism sensitized to Ca2+ is initiated at the same time that phospholipase C is activated and involves the activation of the small RhoA protein bound to guanosine triphosphate (GTP). Above activation, RhoA increases the activity of Rho kinase, leading to the inhibition of myosin phosphatase. This promotes the contractile state, since the myosin light chain cannot be dephosphorylated [147].
Relaxation of smooth muscle occurs as a result of either removing the contractile stimuli or by the direct action of a substance that stimulates the inhibition of the contractile mechanism. In any circumstance, the relaxation process requires a decrease in the intracellular Ca2+ concentration and an increase in the activity of the MLC phosphatase. The sarcoplasmic reticulum and plasma membrane remove Ca2+ from the cytosol. Na+/Ca2+ channels are located on the plasma membrane and help to reduce the intracellular concentration of Ca2+. During relaxation, other contributors that restrict the Ca2+ entry into the cell are the voltage-operated channels and Ca2+ receptors in the plasma membrane, which remain closed [147].
4. Spasmodic Compounds
The historical antecedents date from the year 1504 when South American natives inhabiting the basins of the high Amazon and the Orinoco prepared a mixture of alkaloids termed curare. This substance was placed in the tips of arrows in order to hunt (prey paralyzing) and fight in wars. Curare produces muscle weakness, paralysis, respiratory failure, and death [148]. In 1800, Alexander von Humboldt, identified that curare was made from the extracts of the species Chondrodendron tomentosum and Strychnos toxifera.
In 1935, the French physiologist Claude Bernard managed to isolate the alkaloid d-tubocurarine from the curare [149]; and one year later, it was elucidated that this compound had the ability to inhibit acetylcholine, blocking the transmission of nerve impulses to the muscles [150]. Lastly, new benzylisoquinoline alkaloids were isolated from curare by Galeffi et al. in 1977 [151, 152].
In 1822, the pharmacist Rudolph Brandes obtained an impure alkaloid from Atropa belladonna (Solanaceae), which after purification was named atropine. Interestingly, atropine was not produced as a natural compound from the plant and it was a derivative generated from the alkaloid hyoscyamine during the process of purification [153]. It is important to note that atropine has been naturally found in small quantities in other members of the Solanaceae family such as Datura stramonium, Duboisia myoporoides, and Scopolia japonica [154–156].
The use of the plant Papaver somniferum (opium poppy) (Papaveraceae) dates back to about 4000 BC. At present the plant is only used to extract a base material for the manufacture of other alkaloids, such as noscapine and codeine, both discovered by the French pharmacist Pierre-Jean Robiquet in 1831 and 1832, respectively [157]. In 1848, papaverine was another substance extracted from the same plant by the German chemist Georg Merck [158], which is rarely used today because of the high doses needed (approximately 6 to 12 mg). However, it is still used as a control in experimental models with the purpose of studying antispasmodic activity of plant extracts.
In the 20th century, extracts and powders derived from A. belladonna were widely used as antispasmodics, but from the 1950s these preparations were displaced by synthetic and semisynthetic anticholinergic compounds in order to obtain a better response [159], such as the case of methocarbamol and guaifenesin. On the other hand, a series of compounds such as dantrolene, glutethimide, methaqualone, chlormezanone, metiprilone, and ethchlorvynol were introduced to replace the meprobamate, which had to be withdrawn from the market in 1960 due to problems resulting from use such as abstinence, addictions, and overdoses.
In 1962, the Swiss chemist Heinrich Keberle synthesized baclofen, which can be obtained by reacting glutarimide with an alkaline solution [160]. Glutarimide can also be found in plants such as Croton cuneatus and C. membranaceus (Euphorbiaceae) [161, 162].
The arrival of the quaternary compounds of nitrogen reinforce their peripheral anticholinergic activity offering also the advantages of being poorly absorbed in the gastrointestinal tract, producing a more powerful and longer lasting sedative effect unlike atropine [1]. For example, ipratropium bromide was developed by the German company Boehringer Ingelheim in 1976 and used to treat asthma. This compound was obtained by reacting atropine with isopropyl bromide [163]. Another quaternary compound was the n-butylhyoscine bromide, which is possible to obtain by the organic synthesis of scopolamine and the cimetropium bromide found in the A. belladonna [164]. Although at present the preparations of plant mixtures are no longer used for therapeutic purposes, these compounds formed a part of and served as the basis for modern pharmacology for their applicability as antispasmodics and anesthetics.
Spasms are involuntary contractions of the muscles, which are normally accompanied by pain and interfere with the free and effective muscular voluntary activity. Muscle spasm can originate from multiple medical conditions and is often associated with spinal injury, multiple sclerosis, and stroke.
Spasticity and rigidity are caused by a disinhibition of spinal motor mechanisms. There are several scenarios where a muscle can produce a spasm: (i) unstable depolarization of motor axons; (ii) muscular contractions persist even if the innervation of muscle is normal and despite attempts of relaxation (myotonia); (iii) after one or a series of contractions, the muscle can decontract slowly, as occurring in hypothyroidism; and (iv) muscles lack the energy to relax.
4.1. Distribution of Spasmodic Compound in Nature
Spasmodic compounds are widely distributed in nature (Table 2). Frequently, these compounds are found in animals that paralyze their preys or used for defense. Some examples include the venom of the black widow and tarantula spiders [11, 165] and the venom of snakes [166]. Plants also produce spasmodic metabolites, such as strychnine, an alkaloid obtained from the tree Strychnos nux-vomica (Loganiaceae). Furthermore, microorganisms synthesize spasmodic compounds such as the neurotoxins tetanospasmin and botulinum toxin from the Gram-positive bacteria Clostridium tetani and C. botulinum, respectively. These toxins produce a toxic disorder, which is characterized by persistent spasms of skeletal muscles on spinal neurons similar to strychnine.
Table 2.
Representative organisms producing spasmodic compounds.
| Compound | Organism | Symptoms | Mechanism | Reference |
|---|---|---|---|---|
| Bacterial | ||||
| Botulinum toxin | Clostridium botulinum | Muscular relaxation | Secretion of acetylcholine into synapses is blocked | [3] |
| Tetanospasmin | Clostridium tetani | Muscular spasm | Inhibits the binding of GABA and glycine | [4] |
|
| ||||
| Marine | ||||
| Nematocyst venom extract | Sea anemones | Nausea, vomiting, muscle cramp, severe pain, paralysis | Delay in the voltage-dependent Na+ channels inactivation | [5] |
| Nematocyst venom extract | Chironex fleckeri (Cnidaria) | Contraction of arterial smooth muscle | Increase of cytosolic Ca2+ concentration | [6] |
| Ciguatoxin | Gambierdiscus toxicus (Dinoflagellate) | Nausea, vomiting, abdominal pain, intestinal spasm | Interact with voltage-gated increasing the Na+ permeability and Ca2+ homeostasis | [7] |
| Chordata | Plotosus lineatus (Catfish) | Violent pain, shock, spasm | Increase of the vascular permeability in peritoneum | [8] |
|
| ||||
| Terrestrial | ||||
| Ergotamine | Claviceps purpurea (fungus) | Seizure, spasms psychosis, nausea, vomiting | Agonist of several neurotransmitter receptors | [9] |
| α-Latrotoxin | Latrodectus tredecimguttatus (black widow spider) | Facial flushing, hypertension, muscle spasm, tachycardia | Causes Ca2+-dependent and -independent release of neurotransmitters | [10] |
| Vanillo-toxin, hanatoxin, huwentoxin | Tarantula species | Severe pain, cramps, erythema, swelling, tachycardia | Unrevealed | [11–14] |
| β-Neurotoxin | Mesobuthus martensii (scorpion) | Increases muscular contraction, spasm, convulsion | Modulates Ca2+ channels | [15] |
| Crotoxin | Crotalus durissus terrificus (rattlesnake) | Severe pain, drooping eyelids, low blood pressure, muscle weakness | Blocks the cholinergic post-synaptic response | [16] |
4.2. Mechanisms of Antispasmodic Activity of Natural Products
Antispasmodic compounds exert their activity in different ways, such as antispasmodic activity through inhibition of the response to the neurotransmitters 5-hydroxytryptamine (5-HT) or serotonin and acetylcholine. However, other authors attribute the antispasmodic effect to (i) capsaicin-sensitive neurons, (ii) the participation of vanilloid receptors [167], (iii) the activation of K+ ATP channels, (iv) the blockade of Na+ channels and muscarinic receptors, (v) the reduction of extracellular Ca2+, or (vi) the blockade of Ca2+ channels [22, 168, 169]. The above is merely a reflection of the ambiguity of the studies showing the mechanisms of action of the antispasmodic compounds [36]. For example, the hydroalcoholic extract of Marrubium vulgare showed antispasmodic effect, having the ability to inhibit the neurotransmitters acetylcholine, bradykinin, prostaglandin E2, histamine, and oxytocin [170], whereas a dual effect of antidiarrheal and laxative activities was reported in Fumaria parviflora [171].
5. Methods Used to Evaluate Antispasmodic Compounds
5.1. Gastrointestinal Model
The small intestine is characterized by its large surface area as a result of its circular folds, villi, and microvilli. It is the longest part of the GI system (approximately 5 meters) and comprises about 5% of its initial length, which corresponds to the duodenum (characterized by the absence of the mesentery) and then the jejunum (around 40% of the intestinal length), ending with the ileum. It is the organ of absorption of nutrients and digestion in organisms. These functions are carried out mainly in the duodenum and jejunum.
The main types of bowel movement are the segmentation and peristaltism. The segmentation is most frequent in the small intestine and consists of contractions of the circular muscle layer in very close areas. Contractions last for 11-12 and 8-9 contractions per min in the duodenum and ileum, respectively. When this segmentation is rhythmic, the contractions are alternated with relaxation. This type of movement results in a mixed effect of the chyme (acidic fluid that passes from the stomach to the small intestine) with the digestive secretions, allowing an optimal contact with the intestinal mucosa. In the case of peristalsis, contractions of successive sections of the circular smooth muscle cause the movement of the intestinal contents in anterograde form. The short peristaltic movement also takes place in the small intestine, but less frequently than the segmentation movements. Peristaltic waves rarely cross more than 10 cm of intestine and, due to the low frequency of propulsion of the chyme, it is in this zone where digestion and absorption are preferably carried out. Peristalsis is regulated mainly by the nervous action of the myenteric plexus (major nerve supply to the gastrointestinal tract that controls GI tract motility) in the intestinal wall.
The diversity of experimental models used for the testing of antispasmodic compounds is large. These models mainly use isolated organs or live animals. Once the organ is extracted from the animal, the intestinal motility is assessed with the administration of a substance. The use of extracted organs can be sustained for hours when placed in a physiological solution, such as Ringer, Jalon, Tyrode, and Krebs [172].
The most used organs to perform the studies are guinea pig ileum, duodenum, heart, trachea, and jejunum. The same organs can be also extracted from rabbit, mouse, rat, and hamster (Table 3). The preparation of ileum is preferred because it evaluates the spasmolytic activity. However, although the jejunum contracts spontaneously, it allows evaluating the spasmolytic activity directly and without the use of an agonist [173].
Table 3.
Natural products with antispasmodic activity isolated from terrestrial plants.
| Compound name | Species (Family) | Preparation (Solvent) | Model tested | Source | Reference |
|---|---|---|---|---|---|
| Monoterpenoids | |||||
| 1 Myrcene, β-myrcene | Plectranthus barbatus (Lamiaceae) | Leaf (MeOH) | ACh, BaCl2, KCl in guinea pig ileum | EO | [17] |
| 2 Citral B, β-citral, Neral | Aloysia triphylla (Verbenaceae) | Leaf (Hexane) | Carbachol, KCl, O, PGF (2α) in rat uterus | IC | [18] |
| Cymbopogon citratus (Poaceae) | Leaf (MeOH 70%) | ACh, KCl in rabbit ileum | IC | [19] | |
| Melissa officinalis (Lamiaceae) | Aerial part (EtOH 70%) | ACh, KCl in rat ileum | EO | [20] | |
| 3 Geranyl formate | Anthemis mauritiana (Compositae) | Flower (Distillation) | Ca2+, carbachol, KCl in rabbit and rat jejunum | EO | [21] |
| 4 Geranyl acetate | Nepeta cataria (Lamiaceae) | Leaf (Aqueous) | Carbachol, KCl in guinea pig trachea and rabbit jejunum | EO | [22] |
| 5 Geraniol | Rosa damascene (Rosaceae) | Flower (hydrodistillation) | ACh, KCl, electrical field stimulation in rat ileum | IC | [23] |
| 6 Citronellol | Rosa damascene (Rosaceae) | Flower (hydrodistillation) | ACh, KCl, electrical field stimulation in rat ileum | IC | [23] |
| 7 (±)-α-Phellandrene | Zingiber officinale (Zingiberaceae) | Rhizome (MeOH) | Serotonin in rat ileum | EO | [24] |
| 8 (±)-β-Phellandrene | Croton sonderianus (Euphorbiaceae) | Leaf (Distillation) | ACh, KCl in rat tracheal smooth muscle | EO | [25] |
| 9 Terpinolene | Zingiber officinale (Zingiberaceae) | Rhizome (MeOH) | Serotonin in rat ileum | EO | [24] |
| 10 D-(+)-Limonene | Zingiber roseum (Zingiberaceae) | Fresh seeds (Hydrodistilled with diethyl ether) | Carbachol, KCl in rat duodenal smooth muscle | EO | [26] |
| Mentha x villosa (Lamiaceae) | Leaf infusion (MeOH) | KCl in guinea pig ileum | IC | [27] | |
| Dracocephalum kotschyi (Lamiaceae) | Aerial part (Hydrodistillation) | ACh, electrical field stimulation, KCl in rat ileum | EO | [28] | |
| 11 γ-Terpinene | Acalypha phleoides (Euphorbiaceae) | Aerial part infusion MeOH-CHCl3 (1:1) | ACh, BaCl2, H, S in guinea pig ileum and rabbit jejunum | IC | [29] |
| 12 Thymoquinone | Nigella sativa (Ranunculaceae) | Seed infusion (Aqueous) | BaCl2, carbachol, leukotriene in rat trachea | IC | [30] |
| 13 (R)-(+)-Pulegone | Calamintha glandulosa (Lamiaceae) | Aerial parts infusion (Diethyl ether) | KCl in rat ileum | IC | [31] |
| Mentha x villosa (Lamiaceae) | Leaf infusion (MeOH) | KCl in guinea pig ileum | IC | [27] | |
| 14 (-)-Menthol | Mentha piperita (Lamiaceae) | Leaf and flower infusion (EtOH) | S in rat ileum | IC | [32] |
| 15 dl-α-Terpineol | Casimiroa pringlei (Rutaceae) | Aerial part infusion (Ethylic ether) | KCl in rat uterine smooth muscle | IC | [33] |
| Zingiber roseum (Zingiberaceae) | Fresh seeds (Hydrodistilled with diethyl ether) | Carbachol, KCl in rat duodenal smooth muscle | EO | [26] | |
| Dracocephalum kotschyi (Lamiaceae) | Aerial part (Hydrodistillation) | ACh, electrical field stimulation, KCl in rat ileum | EO | [28] | |
| 16 (-)-Piperitone | Casimiroa pringlei (Rutaceae) | Aerial part infusion (Ethylic ether) | KCl in rat uterine smooth muscle | IC | [33] |
| 17 (+)-Rotundifolone | Mentha x villosa (Lamiaceae) | Leaf infusion (MeOH) | KCl in guinea pig ileum | IC | [27] |
| 18 (R)-(-)-Carvone | Mentha x villosa (Lamiaceae) | Leaf infusion (MeOH) | KCl in guinea pig ileum | IC | [27] |
| 19 (R,R,R)-Carvone-1,2-oxide) | Mentha x villosa (Lamiaceae) | Leaf infusion (MeOH) | KCl in guinea pig ileum | IC | [27] |
| 20 (S)-(+)-Carvone | Mentha x villosa (Lamiaceae) | Leaf infusion (MeOH) | KCl in guinea pig ileum | IC | [27] |
| 21 1,8-Cineole | Ocimum gratissimum (Lamiaceae) | Leaf infusion (MeOH) | ACh, KCl in guinea pig ileum | IC | [34] |
| Nepeta cataria (Lamiaceae) | Leaf infusion (Aqueous) | Carbachol, KCl in guinea pig trachea and rabbit jejunum | EO | [22] | |
| Casimiroa pringlei (Rutaceae) | Aerial part infusion (Ethylic ether) | KCl in rat uterine smooth muscle | IC | [33] | |
| 22 p-Cymene | Lippia graveolens (Verbenaceae) | Leaf infusion (Distillation) | Carbachol, H in guinea pig ileum | IC | [35] |
| Zingiber roseum (Zingiberaceae) | Fresh seeds (Hydrodistilled with diethyl ether) | Carbachol, KCl in rat duodenal smooth muscle | EO | [26] | |
| Poliomintha longiflora (Lamiaceae) | Leaves stem infusion (Distillation) | Carbachol, H in guinea pig ileum | IC | [35] | |
| 23 Carvacrol | Origanum acutidens (Lamiaceae) | Leaf, stem and flower infusion (MeOH) | Spontaneous contraction in rat ileum | EO | [36] |
| Thymus vulgaris (Lamiaceae) | Whole plants (Ethanol) | ACh, BaCl2, KCl in rat trachea and ileum | IC | [37] | |
| 24 Thymol | Acalypha phleoides (Euphorbiaceae) | Aerial part infusion [MeOH-CHCl3 (1:1)] | ACh, BaCl2, H, KCl, S in guinea pig ileum and rabbit jejunum | IC | [29] |
| Thymus vulgaris (Lamiaceae) | Whole plants (Ethanol) | ACh, BaCl2, KCl in rat trachea and ileum | IC | [37] | |
| 25 Thujane or Sabinane | Anthemis mauritiana (Asteraceae) | Flower infusion (Aqueous) | Carbachol, KCl in rabbit jejunal smooth muscle | EO | [21] |
| 26 (±)-Camphor | Acalypha phleoides (Euphorbiaceae) | Aerial part infusion [MeOH-CHCl3 (1:1)] | ACh, BaCl2, H, KCl, S in guinea pig ileum and rabbit jejunum | IC | [29] |
| Lippia dulcis (Verbenaceae) | Leaf infusion (Steam distillation) | Carbachol, H in porcine bronchi | EO | [38] | |
| 27 (+)-α-Pinene | Anthemis mauritiana (Asteraceae) | Flower infusion (Aqueous) | Carbachol, KCl in rabbit jejunal smooth muscle | EO | [21] |
| Nepeta cataria (Lamiaceae) | Leaf infusion (Aqueous) | Carbachol, KCl in guinea pig trachea and rabbit jejunum | EO | [22] | |
| Plectranthus barbatus (Lamiaceae) | Leaf infusion (MeOH) | ACh, BaCl2, H, KCl in guinea pig ileum | EO | [17] | |
| 28 (-)-α-Pinene | Dissotis rotundifolia (Melastomataceae) | Leaf infusion (EtOH) | Carbachol in mouse intestinal motility | E | [39] |
| Eucalyptus tereticornis (Myrtaceae) | Commercial | ACh, KCl in rat trachea | EO | [40] | |
| Zingiber roseum (Zingiberaceae) | Fresh seeds (Hydrodistilled with diethyl ether) | Carbachol, KCl in rat duodenal smooth muscle | EO | [26] | |
| 29 (+)-β-Pinene | Ferula gummosa (Apiaceae) | Resin infusion (Hydroalcoholic, ether, MeOH) | ACh, KCl in rat ileum | IC | [41] |
| Zingiber officinale (Zingiberaceae) | Rhizome infusion (MeOH) | S in rat ileum | EO | [24] | |
| Zingiber roseum (Zingiberaceae) | Fresh seeds (Hydrodistilled with diethyl ether) | Carbachol, KCl in rat duodenal smooth muscle | EO | [26] | |
| 30 Cantleyine | Strychnos trinervis (Loganiaceae) | Root bark (EtOAc) | Carbachol, H, KCl in guinea pig trachea | IC | [42] |
| 31 Penstemonoside | Parentucellia latifolia (Scrophulariaceae) | Whole plant infusion (Butanol) | ACh, CaCl2, KCl in rat uterus | IC | [43] |
| 32 Aucubine or aucuboside | Parentucellia latifolia (Scrophulariaceae) | Whole plant infusion (Butanol) | ACh, CaCl2, KCl in rat uterus | IC | [43] |
| 33 2′-O-Acetyldihydropenstemide | Viburnum prunifolium (Caprifoliaceae) | Root and stem bark infusion (MeOH) | Carbachol in rabbit jejunum and guinea pig trachea | E | [44] |
| 34 2′-O-trans-p-Coumaroyl-dihydropenstemide | Viburnum prunifolium (Caprifoliaceae) | Root and stem bark infusion (MeOH) | Carbachol in rabbit jejunum and guinea pig trachea | E | [44] |
| 35 2′-O-Acetylpatrinoside | Viburnum prunifolium (Caprifoliaceae) | Root and stem bark infusion (MeOH) | Carbachol in rabbit jejunum and guinea pig trachea | E | [44] |
| 36 Patrinoside | Viburnum prunifolium (Caprifoliaceae) | Root and stem bark infusion (MeOH) | Carbachol in rabbit jejunum and guinea pig trachea | E | [44] |
| 37 Valtriate or Valepotriate | Valeriana procera (Valerianeaceae) | Root infusion (EtOH) | BaCl2, carbachol, KCl in guinea pig ileum and stomach | IC | [45] |
| 38 Isovaltrate or Isovaltratum | Valeriana procera (Valerianeaceae) | Root infusion (EtOH) | BaCl2, carbachol, KCl in guinea pig ileum and stomach | IC | [45] |
| 39 Epoxygaertneroside | Morinda morindoides (Rubiaceae) | Leaf infusion (Aqueous) | ACh, KCl in guinea pig ileum | IC | [46] |
| 40 Gaertneroside | Morinda morindoides (Rubiaceae) | Leaf infusion (Aqueous) | ACh, KCl in guinea pig ileum | IC | [46] |
| 41 Catalpinoside or Catapol | Parentucellia latifolia (Scrophulariaceae) | Whole plant infusion (Butanol) | ACh, CaCl2, KCl in rat uterus | IC | [43] |
|
| |||||
| Sesquiterpenes | |||||
| 43 (±)-Hernandulcin | Lippia dulcis (Verbenaceae) | Leaf infusion (Steam distillation) | Carbachol, H in porcine bronchi | EO | [38] |
| 43 Humulene or α-Caryophyllene | Nepeta cataria (Lamiaceae) | Leaf infusion (Aqueous) | Carbachol, KCl, in guinea pig trachea and rabbit jejunum | EO | [22] |
| 44 β-Caryophyllene epoxide | Conyza filaginoides (Asteraceae) | Leaf infusion [CHCl3:MeOH (1:1)] | Spontaneous contraction in rat ileum | IC | [47] |
| Croton sonderianus (Euphorbiaceae) | Leaf infusion (Steam distillation) | ACh, KCl in rat tracheal smooth muscle | EO | [25] | |
| 45 β-Caryophyllene | Croton sonderianus (Euphorbiaceae) | Leaf infusion (Steam distillation) | ACh, KCl in rat tracheal smooth muscle | EO | [25] |
| Conyza filaginoides (Asteraceae) | Leaf infusion [CHCl3:MeOH (1:1)] | Spontaneous contraction in rat ileum | IC | [47] | |
| Plectranthus barbatus (Lamiaceae) | Leaf infusion (MeOH) | ACh, BaCl2, H, KCl in guinea pig ileum | EO | [17] | |
| Pterodon polygalaeflorus (Fabaceae) | Seed (Steam distillation) | ACh, KCl in rat ileum smooth muscle | IC | [48] | |
| 46 Bicyclogermacrene or Lepidozene | Croton sonderianus (Euphorbiaceae) | Leaf infusion (Steam distillation) | ACh, KCl in rat tracheal smooth muscle | EO | [25] |
| 47 (+)-Capsidiol | Nicotiana silvestri (Solanaceae) | Leaf infusion (EtOAc) | ACh, BaCl2, bradykinin, carbachol in guinea pig ileum and trachea | IC | [49] |
| 48 S-Petasin | Petasites formosanus (Compositae) | Aerial parts (EtOH) | CaCl2, carbachol, H, KCl in guinea pig trachea | IC | [50] |
| 49 (+)-Isopetasin | Petasites formosanus (Compositae) | Aerial parts (EtOH) | CaCl2, carbachol, H, KCl in guinea pig trachea | IC | [50] |
| 50 Valeranone o Jatamansone | Valeriana procera (Valerianeaceae) | Root infusion (EtOH) | BaCl2, carbachol, KCl in guinea pig ileum and stomach | IC | [45] |
| 51 Chamazulene | Matricaria recutita (Asteraceae) | Plant infusion (Aqueous) | Human platelet | E | [51] |
| 52 Spathulenol | Croton sonderianus (Euphorbiaceae) | Leaf infusion (Steam distillation) | ACh, KCl in rat tracheal smooth muscle | EO | [25] |
| Lepechinia caulescens (Lamiaceae) | Leaf infusion (Hexane) | KCl in rat uterus | IC | [52] | |
| 53 Cynaropicrin | Cynara scolymus (Asteraceae) | Leaf and flower infusion (MeOH 70%) | ACh in guinea pig ileum | IC | [53] |
| 54 Cedrenol | Anthemis mauritiana (Asteraceae) | Flower infusion (Aqueous) | Carbachol, KCl in rabbit jejunal smooth muscle | EO | [21] |
| 55 (+)-Bakkenolide A | Hertia cheirifolia (Asteraceae) | Aerial parts (MeOH) | ACh, BaCl2 in rat duodenum | IC | [54] |
| 56 Himachalol | Cedrus deodara (Pinaceae) | Wood infusion | ACh, BaCl2, H, nicotine, S in guinea pig ileum and seminal vesicle, rabbit jejunum and rat uterus | IC | [55] |
| 57 (E)-Damascenone | Ipomoea pes-caprae (Convolvulaceae) | Leaf infusion (Aqueous) | H in guinea pig ileal smooth muscle | IC | [56] |
| 58 (-)-Isogermacrene D | Artemisia vulgaris (Compositae) | Stem and leaf infusion (Aqueous) | guinea pig ileum | [57] | |
| 59 Ezoalantonin | Artemisia vulgaris (Compositae) | Leaf (CHCl3) | H, PMA, S in guinea pig ileum and trachea | IC | [57] |
| 60 Costunolide | Radix aucklandiae (Asteraceae) | Rhizome (MeOH) | ACh, KCl, S in rat jejunum | IC | [58] |
| 61 Dehydrocostuslactone | Radix aucklandiae (Asteraceae) | Rhizome (MeOH) | ACh, KCl, S in rat jejunum | IC | [58] |
|
| |||||
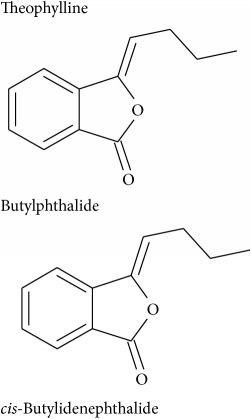
| Diterpenes | |||||
| 62 E-Phytol | Ipomoea pes-caprae (Convolvulaceae) | Leaf infusion (Aqueous) | H in guinea pig ileal smooth muscle | IC | [56] |
| 63 3α-Angeloyloxy-2α-hydroxy-13,14Z-dehydrocativic acid | Brickellia paniculata (Compositae) | Leaf infusion (MeOH) | KCl in rat myometrial tissue | IC | [59] |
| 64 15-Epicyllenin A | Marrubium globosum ssp. libanoticum (Lamiaceae) | Aerial part infusion (MeOH) | ACh in mouse ileum | IC | [60] |
| 65 Cyllenin A | Marrubium globosum ssp. libanoticum (Lamiaceae) | Aerial part infusion (MeOH) | ACh in mouse ileum | IC | [60] |
| 66 Marrulibacetal | Marrubium globosum ssp. libanoticum (Lamiaceae) | Aerial part infusion (MeOH) | ACh in mouse ileum | IC | [60] |
| 67 (13R)-9α,13α-epoxylabda-6β(19),16(15)-diol dilactone | Marrubium globosum ssp. libanoticum (Lamiaceae) | Aerial part infusion (MeOH) | ACh in mouse ileum | IC | [60] |
| 68 Marrubin | Marrubium vulgare (Lamiaceae) | Aerial parts (Aqueous) | KCl in rat aorta | IC | [61] |
| 69 Marrubenol or Marrubiol | Marrubium vulgare (Lamiaceae) | Aerial parts (Aqueous) | KCl in rat aorta | IC | [61] |
| 70 Marrulanic acid | Marrubium globosum ssp. libanoticum (Lamiaceae) | Aerial part infusion (MeOH) | ACh in mouse ileum | IC | [60] |
| 71 Marrulactone | Marrubium globosum ssp. libanoticum (Lamiaceae) | Aerial part infusion (MeOH) | ACh in mouse ileum | IC | [60] |
| 72 (+)-Dehydroabietic acid | Lepechinia caulescens (Lamiaceae) | Leaf infusion (Hexane) | KCl in rat uterus | IC | [52] |
| 73 9β-Hydroxydehydroabietyl alcohol | Lepechinia caulescens (Lamiaceae) | Leaf infusion (Hexane) | KCl in rat uterus | IC | [52] |
| 74 9α,13α-Epidioxyabiet-8(14)-en-18-oic acid methyl ester | Lepechinia caulescens (Lamiaceae) | Leaf infusion (Hexane) | KCl in rat uterus | IC | [52] |
| 75 4-epi-Hyalic acid | Croton argyrophylloides (Euphorbiaceae) | Bark infusion (MeOH) | ACh, KCl in rat tracheal smooth muscle | IC | [62] |
| 76 Pimaradienoic acid or Continentalic acid | Viguiera arenaria (Asteraceae) | Root infusion (CH2Cl2) | ACh, KCl in rat carotid artery | IC | [63] |
| 77 8(14),15-Sandaracopimaradiene-7α,18-diol | Tetradenia riparia (Lamiaceae) | Leaf infusion (CHCl3) | BaCl2, H, methacholine in guinea pig ileum | IC | [64] |
| 78 3,4-Secoisopimara-4(18),7,15-triene-3-oic acid | Salvia cinnabarina (Lamiaceae) | Aerial parts (EtOH) | ACh, BaCl2, H in guinea pig ileum | IC | [65] |
| 79 ent-Kaurenoic acid | Viguiera arenaria (Asteraceae) | Root infusion (CH2Cl2) | ACh, KCl in rat carotid artery | IC | [63] |
| Viguiera hypargyrea (Asteraceae) | Root infusion (Hexane) | Spontaneous contraction in guinea pig ileum | IC | [66] | |
| 80 Beyerenic acid or Monogynoic acid | Viguiera hypargyrea (Asteraceae) | Root infusion (Hexane) | Spontaneous contraction in guinea pig ileum | IC | [66] |
| 81 ent-7α-Acetoxytrachyloban-18-oic acid | Xylopia langsdorfiana (Annonaceae) | Stem infusion (EtOH 95%) | BaCl2, H, KCl in guinea pig ileum | IC | [67] |
| 82 ent-7α -hydroxytrachyloban-18-oic acid | Xylopia langsdorfiana (Annonaceae) | Stem infusion (EtOH 95%) | BaCl2, H, KCl in guinea pig ileum | IC | [67] |
| 83 Phorbol 12-acetate-13-tiglate | Crotonis tiglium (Euphorbiaceae) | Fruit (MeOH) | Spontaneous contraction in rabbit jejunum | E | [68] |
| 84 3,7,10,14,15-pentaacetyl-5-butanoyl-13,17-epoxy-8-myrsinene | Pycnocycla spinosa (Umbelliferae) | Aerial parts (MeOH) | KCl in rat illeum | IC | [69] |
|
| |||||
| Triterpenoids | |||||
| 85 Agapanthagenin 3-O-β-D-glucopyranoside | Allium elburzense (Alliaceae) | Flower and bulb infusion (Hexane) | H in guinea pig ileum | IC | [70] |
| 86 Agapanthagenin | Allium elburzense (Alliaceae) | Flower and bulb infusion (Hexane) | H in guinea pig ileum | IC | [70] |
| 87 β-sitosterol | Eucalyptus camaldulensis (Myrtaceae) | Leaf infusion (EtOAc) | KCl, spontaneous contraction in rabbit jejunum | IC | [71] |
| 88 β-sitosterol 3-O-β-D-glucopyranoside | Eucalyptus camaldulensis (Myrtaceae) | Leaf infusion (EtOAc) | KCl, spontaneous contraction in rabbit jejunum | IC | [71] |
| 89 α-Spinasteryl β-D-glucoside | Conyza filaginoides (Asteraceae) | Leaf infusion [CHCl3:MeOH (1:1)] | Spontaneous contraction in rat ileum | IC | [47] |
| 90 Tropeoside B1 and B2 | Allium cepa(Alliaceae) | Bulbs [CHCl3:MeOH (9:1)] | ACh, H in guinea pig ileum | IC | [72] |
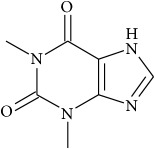
| 91 Tropeoside A1 and A2 | Allium cepa(Alliaceae) | Bulbs [CHCl3:MeOH (9:1)] | ACh, H in guinea pig ileum | IC | [72] |
| 92 Elburzensoside A1 and A2 | Allium elburzense (Alliaceae) | Flower and bulb infusion (Hexane) | H in guinea pig ileum | IC | [70] |
| 93 Elburzensoside C1 and C2 | Allium elburzense (Alliaceae) | Flower and bulb infusion (Hexane) | H in guinea pig ileum | IC | [70] |
| 94 Galphimin A | Galphimia glauca (Malpighiaceae) | Leaf infusion (MeOH) | Electrical-induced contraction in guinea pig ileum | IC | [73] |
| 95 Galphimin B | Galphimia glauca (Malpighiaceae) | Leaf infusion (MeOH) | Electrical-induced contraction in guinea pig ileum | IC | [73] |
| 96 Galphimin C | Galphimia glauca (Malpighiaceae) | Leaf infusion (MeOH) | Electrical-induced contraction in guinea pig ileum | IC | [73] |
| 97 Galphimin E | Galphimia glauca (Malpighiaceae) | Leaf infusion (MeOH) | Electrical-induced contraction in guinea pig ileum | IC | [73] |
| 98 Galphimin F | Galphimia glauca (Malpighiaceae) | Leaf infusion (MeOH) | Electrical-induced contraction in guinea pig ileum | IC | [73] |
| 99 Handianol | Herissanthia tiubae (Malvaceae) | Leaf infusion (EtOH) | Carbachol, H, KCl in guinea pig ileum and trachea, and rat aorta | IC | [74] |
| 100 Cycloartanol | Herissanthia tiubae (Malvaceae) | Leaf infusion (EtOH) | Carbachol, H, KCl in guinea-pig ileum, trachea and rat aorta | IC | [74] |
| 101 Taraxasteryl acetate | Brickellia veronicifolia (Asteraceae) | Aerial parts [CH2Cl2:MeOH (1:1)] | Gastrointestinal motility test in mouse | E | [75] |
| 102 Pomolic acid or Benthamic acid or Randialic acid A | Licania pittieri (Rosaceae) | Leaf infusion (EtOH) | Carbachol, KCl in rat aorta | IC | [76] |
| 103 Ursolic acid | Agastache mexicana (Lamiaceae) | Aerial part (MeOH) | ACh, KCl in guinea pig ileum | IC | [77] |
| 104 Ehretiolide | Eucalyptus camaldulensis (Myrtaceae) | Leaf infusion (EtOAc) | KCl, spontaneous contraction in rabbit jejunum | IC | [78] |
| 105 Ehretiolide acetate | Eucalyptus camaldulensis (Myrtaceae) | Leaf infusion (EtOAc) | KCl, spontaneous contraction in rabbit jejunum | IC | [78] |
| 106 Camaldulin | Eucalyptus camaldulensis (Myrtaceae) | Leaf infusion (EtOAc) | KCl, spontaneous contraction in rabbit jejunum | IC | [71] |
| 107 Zygophyloside N | Zygophyllum gaetulum (Zygophyllaceae) | Root infusion (MeOH) | Electrically-induced contractions of isolated guinea pig ileum | E | [79] |
| 108 Erythrodiol | Conyza filaginoides (Asteraceae) | Leaf infusion [CHCl3:MeOH (1:1)] | Spontaneous contraction in rat ileum | IC | [47] |
| 109 3-β-tridecanoyloxy-28-hydroxyolean-12-ene | Conyza filaginoides (Asteraceae) | Leaf infusion [CHCl3:MeOH (1:1)] | Spontaneous contraction in rat ileum | IC | [47] |
| 110 3-β-Hydroxyolean-9(11),12-dien-28-oic acid | Eucalyptus camaldulensis (Myrtaceae) | Leaf infusion (EtOAc) | KCl, spontaneous contraction in rabbit jejunum | IC | [78] |
| 111 4-epi-Hederagenin | Hedera helix (Araliaceae) | Leaf infusion (EtOH) | ACh in guinea pig ileum | IC | [80] |
| 112 Hederacoside C | Hedera helix (Araliaceae) | Leaf infusion (EtOH) | ACh in guinea pig ileum | IC | [80] |
| 113 Betulinic acid | Eucalyptus camaldulensis (Myrtaceae) | Leaf infusion (EtOAc) | KCl, spontaneous contraction in rabbit jejunum | IC | [78] |
| 114 α-Amyrin acetate | Tylophora hirsuta (Asclepiadaceae) | Aerial parts (MeOH) | KCl in rabbit jejunum | IC | [81] |
|
| |||||
| Phloroglucinol derivatives | |||||
| 115 Hyperforin | Hypericum perforatum (Hypericaceae) | Aerial parts (EtOH 70%) | KCl in rabbit jejunum | IC | [82] |
| 116 Hypericin | Hypericum perforatum (Hypericaceae) | Aerial parts (EtOH 70%) | KCl in rabbit jejunum | IC | [82] |
|
| |||||
| Coumarins | |||||
| 117 Scopoletin | Brunfelsia hopeana (Solanaceae) | Root infusion (EtOH) | Phenylephrine, KCl, PGF2, serotonin in rat aorta | IC | [83] |
| 118 Todannone | Toddalia asiatica var. floribunda (Rutaceae) | Aerial parts (EtOH 95%) | ACh, BaCl2, H, nicotine in guinea pig ileum | IC | [84] |
| 119 (2S∗,3R∗)-2-[(3E)-4,8-dimethylnona-3,7-dien-1-yl]-2,3-dihydro-7-hydroxy-2,3-dimethylfuro[3,2c] coumarin | Ferula heuffelii (Apiaceae) | Underground part (CHCl3) | ACh, KCl in rat ileum | IC | [85] |
| 120 Osthole | Prangos ferulacea (Apiaceae) | Root (Acetone) | ACh, KCl, electric field stimulation in rat ileum | IC | [86] |
| 121 Angelicin | Heracleum thomsoni (Apiaceae) | Aerial part infusion (EtOH) | ACh, BaCl2, H, S in cat ureter, guinea pig bile duct and trachea, monkey gall bladder, rabbit jejunum, and rat uterus | IC | [87] |
| 122 Glycycoumarin | Glycyrrhizae radix (Leguminosae) | Root infusion (Aqueous) | A23187, BaCl2, carbachol, KCl in mouse jejunum | IC | [88] |
| Glycyrrhiza ularensis (Leguminosae) | Root infusion (Aqueous) | Carbachol in mouse jejunum | E | [89] | |
|
| |||||
| Chalcones | |||||
| 123 Davidigenin | Mascarenhasia arborescens (Apocynaceae) | Leaf and stem infusion (MeOH) | ACh, H in guinea pig and rat duodenum | IC | [90] |
| 124 Isoliquiritigenin | Glycyrrhiza glabra (Leguminosae) | Root infusion (Aqueous) | ACh, KCl, O, spontaneous contraction in rat uterus | IC | [91] |
| Glycyrrhiza ularensis (Leguminosae) | Root infusion (Aqueous) (Aqueous) | BaCl2, carbachol, KCl in mouse jejunum,ileum and rectum | IC | [92] | |
| 125 Licochalcone A | Glycyrrhiza inflata (Leguminosae) | Root infusion (Aqueous) | A23187, BaCl2, carbachol, KCl in mouse jejunum | IC | [93] |
|
| |||||
| Flavonoids | |||||
| 126 (-)-Pinostrobin | Conyza filaginoides (Asteraceae) | Leaf infusion [CHCl3:MeOH (1:1)] | Spontaneous contraction in rat ileum | IC | [47] |
| 127 (-)-(S)-Sakuranetin | Dodonaea viscosa (Sapindaceae) | Leaf infusion [CHCl3:MeOH (1:1)] | ACh, BaCl2, H in rat uterus | IC | [94] |
| 128 (±)-Sternbin | Artemisia monosperma (Compositae) | Aerial part (EtOH) | ACh, O in rat ileum, pulmonary artery, urinary bladder, trachea, and uterus | IC | [95] |
| 129 Ouratea catechin | Maytenus rigida (Celastraceae) | Stem bark (EtOH) | BaCl2, carbachol, KCl, H in guinea pig ileum | IC | [96] |
| 130 Apegenin | Achillea millefolium (Asteraceae) | Whole plant infusion (MeOH 40%) | ACh, CaCl2, H, PE, S in rat ileum | IC | [97] |
| 131 Buddleoflavonol or Linarigenin | Agastache mexicana (Lamiaceae) | Aerial part (MeOH) | ACh, KCl in guinea pig ileum | IC | [77] |
| 132 Luteolin | Achillea millefolium (Asteraceae) | Whole plant infusion (MeOH 40%) | ACh, CaCl2, H, PE, S in rat ileum | IC | [97] |
| Artemisia copa (Compositae) | Aerial parts (Aqueous) | KCl, PE, S in rat aorta | E | [98] | |
| Plantago lanceolata (Plantaginaceae) | Aerial part (EtOH) | ACh, BaCl2, H, KCl in guinea pig ileum and trachea | IC | [99] | |
| Thymus vulgaris (Lamiaceae) | Leaf and flower (EtOH) | ACh, BaCl2, carbachol, H in guinea pig ileum and trachea, and rat vas deferens | IC | [100] | |
| 133 Scutellarein 6-β-D-glucoside (isovitexin) | Aloysia citridora (Verbenaceae) | Leaf infusion (Aqueous) | ACh, CaCl2, KCl in rat duodenum | IC | [101] |
| 134 Vitexin | Aloysia citridora (Verbenaceae) | Leaf infusion (Aqueous) | ACh, CaCl2, KCl in rat duodenum | IC | [101] |
| Aspalathus linearis (Fabaceae) | Commercial (Aqueous) | KCl in rabbit jejunum | IC | [102] | |
| 135 Xanthomycrol | Brickellia paniculata (Compositae) | Leaf infusion (MeOH) | KCl, O in rat uterus | IC | [59] |
| 136 Demethoxycentaureidin | Piptadenia stipulacea (Leguminosae) | Aerial parts, (CHCl3) | Carbachol, H, O, in guinea pig ileum and trachea, rat aorta and uterus | IC | [103] |
| 137 Gnaphaliin B | Gnaphalium liebmannii (Asteraceae) | Aerial parts (Hexane) | ACh, carbachol in guinea pig trachea | IC | [104] |
| 138 Kaempferol or Kaempherol | Hedera helix (Araliaceae) | Aerial parts (EtOH 30%) | ACh in guinea pig ileum | IC | [80] |
| 139 Gnaphaliin A | Gnaphalium liebmannii (Asteraceae) | Aerial parts (Hexane) | ACh, carbachol in guinea pig trachea | IC | [104] |
| 140 Quercetin | Achillea millefolium (Asteraceae) | Whole plant infusion (MeOH 40%) | ACh, CaCl2, H, PE, serotonin in rat ileum | IC | [97] |
| Psidium guajava (Myrtaceae) | Leaf extract (MeOH) | Peristalsis in guinea pig ileum | IC | [105] | |
| Drosera madascariensis (Droseraceae) | Leaf extract (EtOH 70%) | Carbachol, H, PGF2 in guinea pig ileum and trachea | IC | [106] | |
| Drosera rotundifolia (Droseraceae) | Aerial parts (EtOH 70%) | Carbachol in guine pig ileum | IC | [107] | |
| Morinda morindoides (Rubiaceae) | Leaf extract (Aqueous) | Ac, KCl in guinea pig ileum | IC | [46] | |
| 141 3-O-Methylquercetin | Rhamnus nakaharai (Rhamnaceae) | Stem bark (not reported) | Carbachol, H, KCl in guinea pig trachea | IC | [108] |
| 142 3,4′-Dimethylquercetin | Artemisia abrotanum (Asteraceae) | Aerial part (MeOH 67%) | Carbachol in guinea pig trachea | IC | [109] |
| 143 3,7-Dimethylquercetin | Artemisia abrotanum (Asteraceae) | Aerial part (MeOH 67%) | Carbachol in guinea pig trachea | IC | [109] |
| 144 Isoquercetin | Conyza filaginoides (Asteraceae) | Leaf infusion [CHCl3:MeOH (1:1)] | Spontaneous contraction in rat ileum | IC | [47] |
| Hedera helix (Araliaceae) | Aerial parts (EtOH 30%) | ACh in guinea pig ileum | IC | [80] | |
| Drosera rotundifolia (Droseraceae) | Aerial parts (EtOH 70%) | Carbachol in guinea pig ileum | IC | [107] | |
| Drosera madascariensis (Droseraceae) | Leaf extract (EtOH 70%) | Carbachol, H, PGF2 in guinea pig ileum and trachea | IC | [106] | |
| Psidium guajava (Myrtaceae) | Leaf extract (MeOH) | Peristalsis in guinea pig ileum | IC | [105] | |
| 145 Quercetin 3-α-rhamnoside or Quercitroside | Psidium guajava (Myrtaceae) | Leaf extract (MeOH) | Peristalsis in guinea pig ileum | IC | [105] |
| Morinda morindoides (Rubiaceae) | Leaf extract (Aqueous) | ACh, KCl in guinea pig ileum | IC | [46] | |
| 146 Quercetin 3-O-β-L-arabinoside | Psidium guajava (Myrtaceae) | Leaf extract (MeOH) | Peristalsis in guinea pig ileum | IC | [105] |
| 147 Quercetin 3-O-β-D-galactoside | Psidium guajava (Myrtaceae) | Leaf extract (MeOH) | Peristalsis in guinea pig ileum | IC | [105] |
| Drosera madascariensis (Droseraceae) | Leaf extract (EtOH 70%) | Carbachol, H, PGF2 in guinea pig ileum and trachea | IC | [106] | |
| 148 Quercetin 3-O-β-gentiobioside 3-O-β-D- | Morinda morindoides (Rubiaceae) | Leaf extract (Aqueous) | ACh, KCl in guinea pig ileum | IC | [46] |
| Glucopyranosylquercetin | Drosera rotundifolia (Droseraceae) | Aerial parts (EtOH 70%) | Carbachol in guinea pig ileum | EO | [107] |
| 149 Centaureidin | Artemisia abrotanum (Asteraceae) | Aerial part (MeOH 67%) | Carbachol in guinea pig trachea | IC | [109] |
| 150 Casticin or Vitexicarpin | Artemisia abrotanum (Asteraceae) | Aerial part (MeOH 67%) | Carbachol in guinea pig trachea | IC | [109] |
| 151 Prunetol or Sophoricol | Genista tridentata (Papilionaceae) | Not reported | AC, electric field stimulation, 6-oxo PGE1 in guinea pig ileum | IC | [110] |
| 152 Boeravinone E | Boerhaavia diffusa (Nyctaginaceae) | Root infusion (MeOH) | ACh in guinea pig ileum | IC | [111] |
| 153 4,6,11-trihydroxy-9-methoxy-10-methyl-6,12-dihydro-5,7-dioxatetraphen-12-one | Boerhaavia diffusa (Nyctaginaceae) | Root infusion (MeOH) | ACh in guinea pig ileum | IC | [111] |
| 154 Boeravinone G | Boerhaavia diffusa (Nyctaginaceae) | Root infusion (MeOH) | ACh in guinea pig ileum | IC | [111] |
| 155 (2R,3S,2”R,3”R)-Manniflavonone | Garcinia buchananii (Clusiaceae) | Stem bark (EtOH 70%) | Bay K 8644 in mouse ileum | IC | [112] |
| 156 Hyperoside | Hypericum perforatum (Hypericaceae) | Aerial parts (EtOH 70%) | KCl in rabbit jejunum | IC | [82] |
| 157 Chrysoeriol | Artemisia copa (Compositae) | Aerial parts (Aqueous) | KCl, PE, S in rat aorta | E | [98] |
| Aspalathus linearis (Fabaceae) | Commercial (Aqueous) | KCl in rabbit jejunum | IC | [102] | |
| 158 Spinacetin | Artemisia copa (Compositae) | Aerial parts (Aqueous) | KCl, PE, S in rat aorta | E | [98] |
| 159 Vicenin 2 | Perilla frutescens (Lamiaceae) | Commercial (Aqueous) | ACh, BaCl2 i rat ileum | IC | [113] |
| 160 Orientin | Aspalathus linearis (Fabaceae) | Commercial (Aqueous) | KCl in rabbit jejunum | IC | [102] |
|
| |||||
| Phenylmetanoids | |||||
| 161 Salicylic acid methyl ether | Brickellia veronicifolia (Asteraceae) | Aerial parts [CH2Cl2:MeOH (1:1)] | Gastrointestinal motility test in mouse | E | [75] |
| 162 O-Anisic acid or 6-Methoxysalicylic acid | Brickellia veronicifolia (Asteraceae) | Aerial parts [CH2Cl2:MeOH (1:1)] | Gastrointestinal motility test in mouse | E | [75] |
| 163 Protocatechuic acid | Hedera helix (Araliaceae) | Aerial parts (EtOH 30%) | ACh in guinea pig ileum | IC | [80] |
| 164 Benzyl 2,5-dimethoxybenzoate | Brickellia veronicifolia (Asteraceae) | Aerial parts [CH2Cl2-MeOH (1:1)] | Gastrointestinal motility test in mouse | E | [75] |
|
| |||||
| Phenylethanoids | |||||
| 165 O-Methylbalsamide | Zanthoxylum hyemale (Rutaceae) | Stem bark infusion (EtOH) | ACh, BaCl2 in rat ileum | IC | [114] |
| 166 (-)-Tembamide | Zanthoxylum hyemale (Rutaceae) | Stem bark infusion (EtOH) | ACh, BaCl2 in rat ileum | IC | [114] |
| 167 O-Methyltembamide | Zanthoxylum hyemale (Rutaceae) | Steam bark infusion (EtOH) | ACh, BaCl2 in rat ileum | IC | [114] |
|
| |||||
| Phenylpropanoids | |||||
| 168 Eugenol | Ocimum gratissimum (Lamiaceae) | Not reported | ACh, KCl in guinea pig ileum | EO | [34] |
| 169 Rosemaric acid or Rosemary acid or trans-Rosmarinic acid | Thymus vulgaris (Lamiaceae) | Commercial | KCl in rat trachea | IC | [100] |
| 170 trans-Chlorogenic acid | Hedera helix (Araliaceae) | Aerial parts (EtOH 30%) | ACh in guinea pig ileum | IC | [80] |
| 171 cis-Chlorogenic acid | Hedera helix (Araliaceae) | Aerial parts (EtOH 30%) | ACh in guinea pig ileum | IC | [80] |
| 172 3,5-Dicaffeoylquininic acid | Hedera helix (Araliaceae) | Aerial parts (EtOH 30%) | ACh in guinea pig ileum | IC | [80] |
| 173 Verbascoside | Plantago lanceolata (Plantaginaceae) | Aerial part infusion (EtOH 20%) | ACh, BaCl2, H, KCl in guinea pig ileum and trachea | E | [99] |
| 174 Isoacteoside or Isoverbascoside | Plantago lanceolata (Plantaginaceae) | Aerial part infusion (EtOH 20%) | ACh, BaCl2, H, KCl in guinea pig ileum and trachea | E | [99] |
| 175 Plantamajoside or Plantamoside or Purpureaside A | Plantago lanceolata (Plantaginaceae) | Aerial part infusion (EtOH 20%) | ACh, BaCl2, H, KCl in guinea pig ileum and trachea | E | [99] |
| 176 Lavandulifolioside | Plantago lanceolata (Plantaginaceae) | Aerial part infusion (EtOH 20%) | ACh, BaCl2, H, KCl in guinea pig ileum and trachea | E | [99] |
| 177 Echinacoside | Cistanche tubulosa (Orobanchaceae) | No reported (EtOH) | KCl, PE in rat aorta | IC | [115] |
| 178 Schisandrin A or Wuweizisu A | Schisandra chinensis (Schisandraceae) | Academic | Spontaneous contractions in rat colon | IC | [116] |
| 179 Schisandrin B or Wuweizisu B | Schisandra chinensis (Schisandraceae) | Fruit decoction (Aqueous) | ACh, KCl, S in guinea pig ileum | IC | [117] |
| 180 Schisandrol B | Schisandra chinensis (Schisandraceae) | Fruit decoction (Aqueous) | ACh, KCl, S in guinea pig ileum | IC | [117] |
|
| |||||
| Stilbenoids | |||||
| 181 Aloifol II or Dendrophenol or Moscatilin | Nidema boothii (Orchidaceae) | Whole plant infusion [CH2Cl2-MeOH 1:1)] | Spontaneous contraction in guinea pig ileum | IC | [118] |
| 182 Batatasin III | Nidema boothii (Orchidaceae) | Whole plant infusion [CH2Cl2-MeOH 1:1)] | Spontaneous contraction in guinea pig ileum | IC | [118] |
| Scaphyglottis livida (Orchidaceae) | Whole plant infusion [CH2Cl2-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [119] | |
| 183 4-[2-(3-hydroxy-5-methoxyphenyl)ethyl]-2-methoxyphenol | Scaphyglottis livida (Orchidaceae) | Whole plant infusion [CH2Cl2-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [119] |
| 184 Gigantol | Nidema boothii (Orchidaceae) | Whole plant infusion [CH2Cl2-MeOH (1:1)] | Spontaneous contraction in guinea pig ileum | IC | [118] |
| 185 Coelonin | Scaphyglottis livida (Orchidaceae) | Whole plant infusion [CH2Cl2-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [119] |
| 186 Erianthridin | Maxillaria densa (Orchidaceae) | Whole plant infusion [CHCl3-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [120] |
| 187 Ephemeranthoquinone | Nidema boothii (Orchidaceae) | Whole plant infusion [CH2Cl2-MeOH (1:1)] | Spontaneous contraction in guinea pig ileum | IC | [118] |
| 188 Nudol | Maxillaria densa (Orchidaceae) | Whole plant infusion [CHCl3-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [120] |
| 189 3,4- dimethoxyphenanthrene-2,5-diol | Maxillaria densa (Orchidaceae) | Whole plant infusion [CHCl3-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [120] |
| 190 Denthyrsinin | Scaphyglottis livida (Orchidaceae) | Whole plant infusion [CH2Cl2-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [119] |
| 191 Gymnopusin | Maxillaria densa (Orchidaceae) | Whole plant infusion [CHCl3-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [120] |
| 192 Fimbriol A | Maxillaria densa (Orchidaceae) | Whole plant infusion [CHCl3-MeOH (1:1)] | ACh, BaCl2, H in rat ileum | IC | [120] |
|
| |||||
| Curcuminoid | |||||
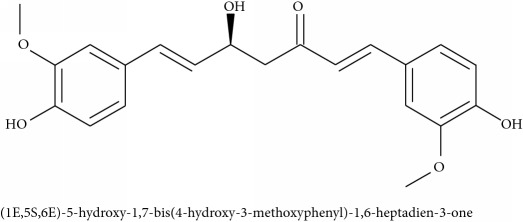
| 193 (1E,5S,6E)-5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3-one | Curcuma longa (Zingiberaceae) | Macerated rhizome (EtOH 70%) | ACh, BaCl2, CaCl2, H, KCl, O in guinea pig Ileum and rat uterus | IC | [121] |
|
| |||||
| Benzofurans and Related | |||||
| 194 (+)-Vitisin C | Vitis spp. (Vitaceae) | Stem infusion (MeOH) | PE in rabbit aorta | IC | [122] |
| 195 Butylphthalide | Ligusticum wallichii (Umbelliferae) | Rhizome (hydrodistillation) | CaCl2, KCl in rat aorta | EO | [123] |
| 196 cis-Butylidenephthalide | Ligusticum wallichii (Umbelliferae) | Rhizome (hydrodistillation) | CaCl2, KCl in rat aorta | EO | [123] |
| 197 Ligustilide A or cis-Ligustilide | Ligusticum wallichii (Umbelliferae) | Rhizome (hydrodistillation) | CaCl2, KCl in rat aorta | EO | [123] |
| 198 12-acetoxytremetone | Helichrysum italicum ssp. italicum (Asteraceae) | Flowers (EtOH) | ACh, BaCl2 in mouse ileum | IC | [124] |
| 199 1-[(2R)-2-(3-hydroxyprop-1-en-2-yl)-2,3-dihydro-1-benzofuran-5-yl]ethan-1-one | Helichrysum italicum ssp. italicum (Asteraceae) | Flowers (EtOH) | ACh, BaCl2 in mouse ileum | IC | [124] |
|
| |||||
| Alkaloids | |||||
| 200 Indicaxanthin | Opuntia ficus indica (Cactaceae) | Fruit pulp infusion (Aqueous) | Carbachol, KCl in mouse ileum | IC | [125] |
| 201 Papaverine | Daucus carota (Apiaceae) | Seed infusion (MeOH 90%) | ACh, BaCl2, H, KCl, S, O in dog trachea, guinea pig, rabbit, rat ilea, rat uterus | IC | [126] |
| 202 Higenamine | Nandina domestica (Berberidaceae) | Fruit (Aqueous) | ACh, H, KCl in guinea pig trachea | IC | [127] |
| 203 Atherosperminine | Fissistigma glaucescens (Annonaceae) | Bark (MeOH) | Carbachol, KCl, LTC4, PGF2α, U46619 in guinea pig trachea | IC | [128] |
| 204 (+)-Domestine or (+)-Nantenine | Platycapnos spicata (Fumariaceae) | Academic supplier | BaCl2, CaCl2, KCl, PE, S in rat aorta and atria | IC | [129] |
| 205 10-Methylacridone | Citrus deliciosa (Rutaceae) | Root juice (MeOH) | Rabbit ileum | IC | [130] |
| 206 Spermatheridine or liriodenin | Fissistigma glaucescens (Annonaceae) | Leaf infusion (MeOH) | Carbachol in canine trachea | IC | [131] |
| 207 Citpressine I | Citrus deliciosa (Rutaceae) | Root juice (MeOH) | Rabbit ileum | IC | [130] |
| 208 Jatrorhizine or Neprotine | Berberis aristata (Berberidaceae) | Institutional supplier | ACh, S, spontaneous contractions in rat ileum | IC | [132] |
| Coptis chinensis (Ranunculaceae) | Rhizoma (EtOH 70%) | ACh in guinea pig ileum | IC | [133] | |
| 209 Coptisine | Coptis chinensis (Ranunculaceae) | Rhizoma (EtOH 70%) | ACh in guinea pig ileum | IC | [133] |
| 210 Escholine or Thalictrine | Mahonia aquifolium (Berberidaceas) | Cortex and fruit infusion | KCl, PE in rat aorta | IC | [134] |
| 211 (+)-Isothebaine | Mahonia aquifolium (Berberidaceas) | Cortex and fruit infusion | KCl, PE in rat aorta | IC | [134] |
| 212 (+)-Corytuberine | Mahonia aquifolium (Berberidaceas) | Cortex and fruit infusion | KCl, PE in rat aorta | IC | [134] |
| 213 (+)-Isocorydine or Luteanine | Mahonia aquifolium (Berberidaceas) | Cortex and fruit infusion | KCl, PE in rat aorta | IC | [134] |
| 214 (+)-Chelidonine or Stylophorine | Chelidonium majus (Papaveraceae) | Commercial supplier | BaCl2, carbachol in guinea pig ileum | IC | [135] |
| 215 (-)-8 beta-(4′-hydroxybenzyl)-2,3-dimethoxyberbin-10-ol | Aristolochia constricta (Aristolochiaceae) | Aerial part infusion (MeOH) | ACh, electrical contraction, H in guinea pig ileum | IC | [136] |
| 216 3-O-methylconstrictosine | Aristolochia constricta (Aristolochiaceae) | Aerial part infusion (MeOH) | ACh, electrical contraction, H in guinea pig ileum | IC | [136] |
| 217 3,5-di-O-methylconstrictosine | Aristolochia constricta (Aristolochiaceae) | Aerial part infusion (MeOH) | ACh, electrical contraction, H in guinea pig ileum | IC | [136] |
| 218 5,6-dihydro-3,5-di-O-methylconstrictosine | Aristolochia constricta (Aristolochiaceae) | Aerial part infusion (MeOH) | ACh, electrical contraction, H in guinea pig ileum | IC | [136] |
| 219 5,6-dihydroconstrictosine | Aristolochia constricta (Aristolochiaceae) | Aerial part infusion (MeOH) | ACh, electrical contraction, H in guinea pig ileum | IC | [136] |
| 220 Constrictosine | Aristolochia constricta (Aristolochiaceae) | Aerial part infusion (MeOH) | ACh, electrical contraction, H in guinea pig ileum | IC | [136] |
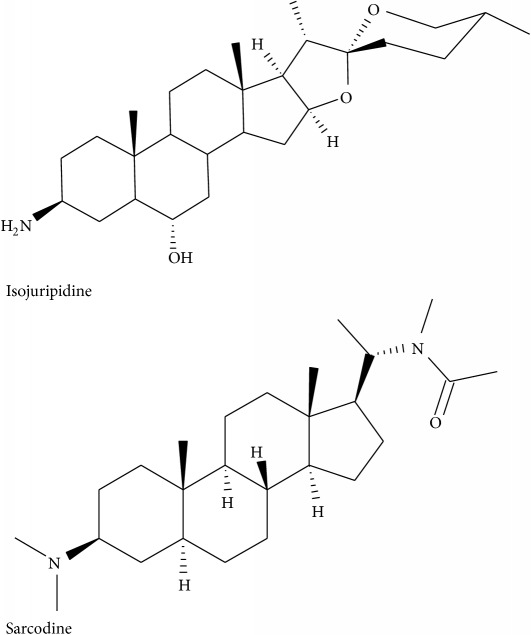
| 221 Isojuripidine | Solanum asterophorum (Solanaceae) | Leaf infusion (MeOH) | ACh, CaCl2, H in guinea pig ileum | IC | [137] |
| 222 Sarcodine | Sarcocca saligna (Buxaceae) | Whole plant (MeOH) | ACh, KCl in guinea pig ileum, rat stomach fundus, rabbit jejunum | IC | [138] |
| 223 Saracorine or Sarcorine | Sarcococca saligna (Buxaceae) | Whole plant infusion (MeOH) | ACh, KCl in rabbit jejunum | IC | [139] |
| 224 Saracocine | Sarcocca saligna (Buxaceae) | Whole plant (MeOH) | ACh, KCl in guinea pig ileum, rat stomach fundus, rabbit jejunum | IC | [138] |
| 225 Alkaloid C | Sarcocca saligna (Buxaceae) | Whole plant (MeOH) | ACh, KCl in guinea pig ileum, rat stomach fundus, rabbit jejunum | IC | [138] |
| 226 (-)-Pachyaximine A | Sarcococca saligna (Buxaceae) | Whole plant infusion (MeOH) | ACh, KCl in rabbit jejunum, KCl | IC | [139] |
| 227 (-)-(R)-Geibalansine or (-)-R-Geilbalansine | Zanthoxylum hyemale (Rutaceae) | Stem bark infusion (EtOH) | ACh, BaCl2 in rat ileum | IC | [114] |
| 228 Hyemaline | Zanthoxylum hyemale (Rutaceae) | Stem bark infusion (EtOH) | ACh, BaCl2 in rat ileum | IC | [114] |
| 229 Theophylline | Fissistigma glaucescens (Annonaceae) | Leaf infusion (MeOH) | Carbachol in canine trachea | IC | [131] |
| 230 Carboxyscotangamine A | Scopolia tangutica (Solanaceae) | Root (95% EtOH) | Carbachol in Chinese hamster ovarian cell | IC | [140] |
| 231 Scotanamine A | Scopolia tangutica (Solanaceae) | Root (95% EtOH) | Carbachol in Chinese hamster ovarian cell | IC | [140] |
| 232 Piperine | Piper nigrum (Piperaceae) | Fruit (EtOH) | Ileum loop in mice | IC | [141] |
|
| |||||
| Amines | |||||
| 233 Scotanamine B | Scopolia tangutica (Solanaceae) | Root (95% EtOH) | Carbachol in Chinese hamster ovarian cell | IC | [123] |
| 234 Scotanamine C | Scopolia tangutica (Solanaceae) | Root (95% EtOH) | Carbachol in Chinese hamster ovarian cell | IC | [140] |
| 235 Scotanamine D | Scopolia tangutica (Solanaceae) | Root (95% EtOH) | Carbachol in Chinese hamster ovarian cell | IC | [140] |
| 236 N1-Caffeoyl-N3-dihydrocaffeoylspermidine | Scopolia tangutica (Solanaceae) | Root (95% EtOH) | Carbachol in Chinese hamster ovarian cell | IC | [140] |
| 237 N1, N10-Bis(dihydrocaffeoyl)spermidine | Scopolia tangutica (Solanaceae) | Root (95% EtOH) | Carbachol in Chinese hamster ovarian cell | IC | [140] |
| 238 Caffeoylputrescine | Scopolia tangutica (Solanaceae) | Root (95% EtOH) | Carbachol in Chinese hamster ovarian cell | IC | [140] |
|
| |||||
| Isothiocyanates | |||||
| 239 Redskin or Senfoel | Cruciferous vegetables (Brassicaceae) | Commercial source | ACh, electrical contraction in mouse ileum | IC | [142] |
|
| |||||
| Alcohols | |||||
| 240 (3E)-4-(3,4-dimethoxyphenyl)but-3-en-1-ol | Zingiber cassumunar (Zingiberaceae) | Chemically synthesized | O in rat uterus | IC | [143] |
|
| |||||
| Ketones | |||||
| 241 2-Decanone | Ruta chalepensis (Rutaceae) | Leaf (EtOH 70%) | KCl in rat ileum | E | [144] |
| 242 2-Undecanone | Ruta chalepensis (Rutaceae) | Leaf (EtOH 70%) | KCl in rat ileum | E | [144] |
| 243 2-Tridecanone | Ruta chalepensis (Rutaceae) | Leaf (EtOH 70%) | KCl in rat ileum | E | [144] |
| 244 Latifolone | Ferula heuffelii (Apiaceae) | Underground part (CHCl3) | ACh, KCl in rat ileum | IC | [85] |
| 245 Dshamirone | Ferula heuffelii (Apiaceae) | Underground part (CHCl3) | ACh, KCl in rat ileum | IC | [85] |
|
| |||||
| Phenolic compounds | |||||
| 246 6-(4-hydroxy-3-methoxyphenyl)-hexanonic acid (HMPHA) | Pycnocycla spinosa (Umbelliferae) | Aerial parts (MeOH) | KCl in rat ileum | IC | [145] |
| 247 Isovanillin | Pycnocycla spinosa (Umbelliferae) | Aerial parts (MeOH) | KCl in rat ileum | IC | [146] |
| 248 Iso-acetovanillon | Pycnocycla spinosa (Umbelliferae) | Aerial parts (MeOH) | KCl in rat ileum | IC | [146] |
IC = isolated compound, E = extract, EO = essential oil, ACh = acetylcholine, O = oxytocin, PMA = β-Phenylethyl amsine, PGF = Prostaglandin F2α, H = histamine, S = serotonin.
Some advantages of performing ex vivo experiments are as follows: (i) different substances can be evaluated in fresh tissues without absorption factors, metabolic excretion or interference due to nerve reflexes; (ii) it is possible to quantify the effect produced by a precisely determined drug; and (iii) it is easier to obtain dose-effect curves, such as the smooth muscle where the contraction obtained under the influence of a spasm or in tissue homogenates is measured by determination of the enzyme activities [172, 174].
5.2. Guinea Pig Ileum and Rat Stomach
The ileum is removed and cut in strips of approximately 2 cm long and then placed in a bath filled with an isotonic solution as mentioned earlier. Electrophysiological studies are performed by graphically recording the contractions with the aid of a transducer, which is calibrated 30 min before the treatment begins. A range of 0.01 to 0.03 μM is generally used to determine dose response curves of the antispasmodic substance [175].
In rats, the stomach is removed and the corpus and fundus are cut in strips of approximately 5 mm x 15 mm and placed on a prewarmed warm solution as mentioned before.
5.3. Compounds Used to Elicit a Spasmodic Activity
The main compounds used are acetylcholine, atropine, BaCl2, carbachol, histamine, KCl, and serotonin.
Acetylcholine is a postganglionic neurotransmitter in the parasympathetic neurons that innervate the intestine. The response to acetylcholine is regulated by activation of the two types of muscarinic receptors: M2 and M3 [176]. The activation of these receptors causes contractions by increasing the intracellular concentration of Ca2+ via IP3 [176]. Atropine is a competitive reversible antagonist of muscarinic acetylcholine receptors M1, M2, M3, M4, and M5.
Different substances are used to produce contractions. For example, BaCl2 induces contractions by mobilizing membrane-bound Ca2+ [177], carbachol is a cholinomimetic drug (cholinergic agonist) that binds and activates acetylcholine receptors [178], histamine acts by either accelerating the release of acetylcholine or interacting supra-additively with the acetylcholine at the smooth muscle [179], whereas KCl increases the voltage-operated Ca2+ channel activity by increasing intracellular free Ca2+ in smooth muscle [180]. Serotonin is also an important neurotransmitter mainly stored in the digestive tract, affecting the secretory and motor activities. At high concentrations, it acts as a vasoconstrictor by contracting endothelial smooth muscle directly or by potentiating the effects of other vasoconstrictors [181, 182].
6. Antispasmodic Activity of Natural Compounds
Compounds isolated from terrestrial plants have shown the ability to function as antispasmodic compounds. The chemical group with the highest number of members of antispasmodic compounds is the monoterpenoid group (41 compounds) followed by flavonoids (35 compounds), alkaloids (with 33 compounds), and triterpenes with 31 (Figure 1). Although we summarize in Table 3 248 compounds, in most of the cases the mechanism behind their activity has not been elucidated.
Figure 1.
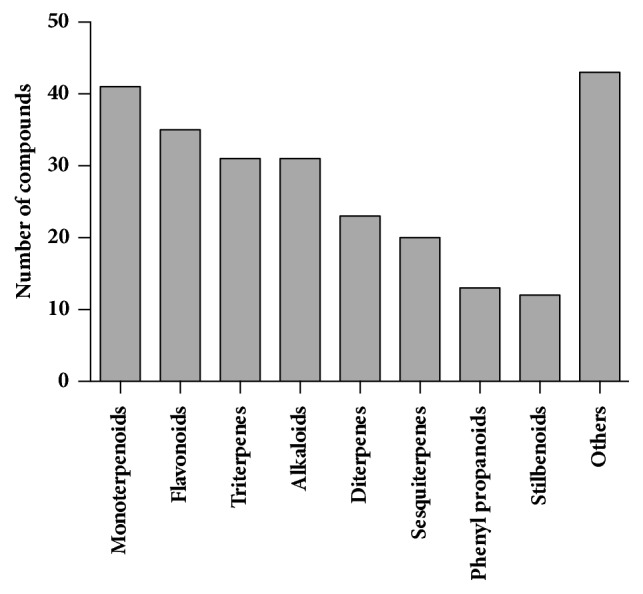
Number of isolated compounds with antispasmodic activity. The total number was obtained from Table 3. “Others” is the sum of the compounds belonging to alcohols, amines, benzofurans, chalcones, coumarins, curcuminoids, isothiocyanates, ketones, phenolic, phenylmethanoids, phenylethanoids, glucinols, and phloroglucinols.
7. Mutagenicity
Studies related to the mutagenicity of antispasmodics are very scarce. This topic has been underestimated when testing the bioactivities of ethnomedicinal plants. Probably the most useful method to determine the mutagenicity of natural products or plant extracts is the Ames method [183]. This test is based on the rate of mutations detected in genetically modified strains of Salmonella typhimurium. Moreover, this test has also been developed to detect mutagenicity of metabolized compounds in the liver. In this situation, a mixture of liver enzymes (S9 microsomal fraction) is used to mimic the metabolites that will be produced in the liver [184].
Few studies have been performed to determine the mutagenicity of natural products with antispasmodic activity. For example, the flavonoids quercetin and luteolin were tested using the Ames method and the appearance of point mutations in four of the tested bacterial strains was shown [185]. In another study, the extracts of the plants Brickellia veronicaefolia, Gnaphalium sp., Poliomintha longiflora, and Valeriana procera were studied. Compounds isolated from these plants are listed as antispasmodic compounds (Table 3). Results of the mutagenicity test indicated that Gnaphalium sp., Poliomintha longiflora (used in the Mexican cuisine and as a traditional medicine), and Valeriana procera induced mutagenesis in the tested bacterial strain [186].
8. Chemical Similarities between Natural and Synthetic Antispasmodic Compounds
To determine whether or not there is an analogy between synthetic (Table 4) and natural antispasmodic compounds, the structures of both groups were compared. Results showed that no similarities were found except for alkaloids, amines, and amino acids.
Table 4.
Synthetic antispasmodic compounds used in medicine.
| Synthetic compound | Receptor targeted | Main use |
|---|---|---|
| Alkaloids | ||
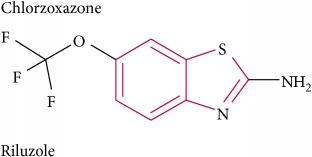
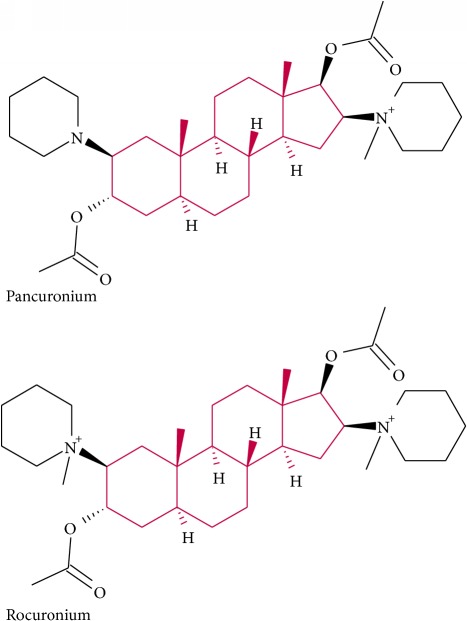
| Chlorzoxazone | Prevents release of histamine | Muscular spasm |
| Pancuronium | Nicotinic acetylcholine | Muscle relaxant |
| Riluzole | Sodium channels | Amyotrophic lateral sclerosis |
| Rocuronium | Antagonist of neuromuscular junction | Muscle relaxant and anaesthesia |
| Tizanidine | α 2 adrenergic agonist | Muscle relaxant |
| Vecuronium | Nicotinic acetylcholine | Muscle relaxant and anaesthesia |
|
| ||
| Curcuminoids | ||
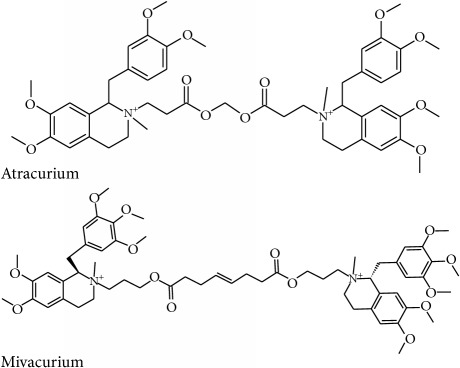
| Atracurium | Nicotinic acetylcholine | Muscle relaxant and anaesthesia |
| Cisatracurium | Nicotinic acetylcholine | Muscle relaxant and anaesthesia |
| Mivacurium | Nicotinic acetylcholine | Muscle relaxant and anaesthesia |
|
| ||
| Methylpropanoid | ||
| Diazepam | GABAA | Anxiety, alcohol withdrawal syndrome, muscle spasms, seizures, and restless legs syndrome |
| Prograbide | GABAA+B | Epilepsy |
| Orphenadrine | Skeletal muscle relaxant that is used for the treatment of acute muscle aches, pain, or spasms. | |
|
| ||
| Phenylpropanoids | ||
| Baclofen | GABAB | Spinal cord injury, cerebral palsy, and multiple sclerosis |
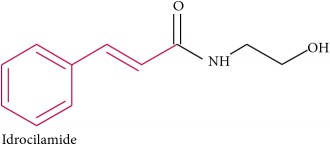
| Idrocilamide | Prevents release of intracellular Ca2+ | Skeletal muscle relaxant and muscular pain |
One of the main differences is that commercial alkaloids are methylated in their nitrogen to make them positive, increasing their solubilities because of salt formation. In contrast, natural products have no positive nitrogen, rendering the molecule neutral and pH dependent. Thus, the compound may or may not be protonated, resulting in a change in its solubility and consequently a change on the targeting tissues.
The comparison can perhaps be focused on the distribution of charges rather than by functional groups or families of compounds, emphasizing the electron distribution. For example, a physical characterization such as the heat of formation, the surface electrostatic potential, the molecular weight, the surface tension, the refractive index, the lipophilicity, and others has been used to characterize the structure-activity relationship of alkaloids extracted from the Amaryllidaceae family [187]. These alkaloids were selected because of their ability to inhibit the effect of the acetylcholinesterase enzyme.
Of special interest is the natural compound salvinorin A isolated from the Mexican hallucinogenic Salvia divinorum (Lamiaceae) used in the traditional medicine as an antidiarrheal. It has been reported that this compound inhibited the intestinal motility through the activation of other receptors such as κ-opioid receptors (KORs). Upon inflammation of the gut, the cannabinoid C, B1, and KOR receptors are upregulated. It appears that salvinorin A interacts in the cross-talk between these receptors with a reduction of the inflammation as demonstrated in murine and guinea pig models [188, 189].
Analysis of the similarities between synthetic and natural antispasmodic structures is depicted in Table 5.
Table 5.
Similarities between natural and synthetic compounds.
| Synthetic | Natural |
|---|---|
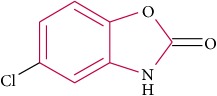
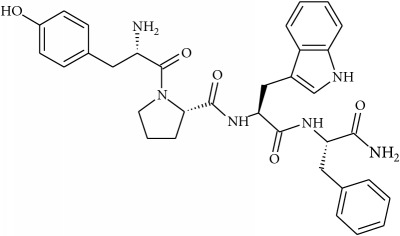
|
|
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
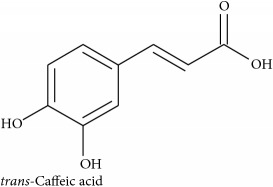
|
9. Conclusions
A large number of natural products with antispasmodic activities have been reported. Although the use of plants in traditional medicine is still relevant, it is necessary to perform new studies to elucidate the mechanism of action of antispasmodics. Moreover, more information about cytotoxicity and mutagenesis should be explored to ensure that these compounds are safe for consumption. The findings of this study corroborated the need for safety studies on plants extensively used for primary health care in countries such as Mexico. Such studies must be carried out before continuing with the widespread use of some species, which may provoke long-term and irreversible damage.
Acknowledgments
The authors thank Marilyn Robertson for helpful discussion.
Contributor Information
Luis R. Hernández, Email: luisr.hernandez@udlap.mx.
Horacio Bach, Email: hbach@mail.ubc.ca.
Conflicts of Interest
The authors declare no conflicts of interest.
Supplementary Materials
This file contains the structures of the compounds described in the main text.
References
- 1.Warburton D. M. Behavioral effects of central and peripheral changes in acetylcholine systems. Journal of Comparative and Physiological Psychology. 1969;68(1):56–64. doi: 10.1037/h0027662. [DOI] [PubMed] [Google Scholar]
- 2.Anthony Lai F., Erickson H. P., Rousseau E., Liu Q.-Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- 3.Apostolidis A., Haferkamp A., Aoki K. R. Understanding the Role of Botulinum Toxin A in the Treatment of the Overactive Bladder-More than Just Muscle Relaxation. European Urology, Supplements. 2006;5(11):670–678. doi: 10.1016/j.eursup.2006.05.006. [DOI] [Google Scholar]
- 4.Rossetto O., Scorzeto M., Megighian A., Montecucco C. Tetanus neurotoxin. Toxicon. 2013;66:59–63. doi: 10.1016/j.toxicon.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Marino A., Valveri V., Muià C., et al. Cytotoxicity of the nematocyst venom from the sea anemone Aiptasia mutabilis. Comparative Biochemistry and Physiology - C Toxicology and Pharmacology. 2004;139(4):295–301. doi: 10.1016/j.cca.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Hughes R. J. A., Angus J. A., Winkel K. D., Wright C. E. A pharmacological investigation of the venom extract of the Australian box jellyfish, Chironex fleckeri, in cardiac and vascular tissues. Toxicology Letters. 2012;209(1):11–20. doi: 10.1016/j.toxlet.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen-Huu T. D., Mattei C., Wen P. J., et al. Ciguatoxin-induced catecholamine secretion in bovine chromaffin cells: Mechanism of action and reversible inhibition by brevenal. Toxicon. 2010;56(5):792–796. doi: 10.1016/j.toxicon.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Junqueira M. E. P., Grund L. Z., Orii N. M., et al. Analysis of the inflammatory reaction induced by the catfish (Cathorops spixii) venoms. Toxicon. 2007;49(7):909–919. doi: 10.1016/j.toxicon.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Sawynok J. GABAergic mechanisms of analgesia: an update. Pharmacology Biochemistry & Behavior. 1987;26(2):463–474. doi: 10.1016/0091-3057(87)90148-1. [DOI] [PubMed] [Google Scholar]
- 10.Quan D., Ruha A.-M. Priapism associated with Latrodectus mactans envenomation. The American Journal of Emergency Medicine. 2009;27(6):759–e2. doi: 10.1016/j.ajem.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed N., Pinkham M., Warrell D. A. Symptom in search of a toxin: Muscle spasms following bites by Old World tarantula spiders (Lampropelma nigerrimum, Pterinochilus murinus, Poecilotheria regalis) with review. QJM: An International Journal of Medicine. 2009;102(12):851–857. doi: 10.1093/qjmed/hcp128. [DOI] [PubMed] [Google Scholar]
- 12.Liang S. An overview of peptide toxins from the venom of the Chinese bird spider Selenocosmia huwena Wang [=Ornithoctonus huwena (Wang)] Toxicon. 2004;43(5):575–585. doi: 10.1016/j.toxicon.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Swartz K. J. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon. 2007;49(2):213–230. doi: 10.1016/j.toxicon.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cromer B. A., McIntyre P. Painful toxins acting at TRPV1. Toxicon. 2008;51(2):163–173. doi: 10.1016/j.toxicon.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Chai Z.-F., Zhu M.-M., Bai Z.-T., et al. Chinese-scorpion (Buthus martensi Karsch) toxin BmK αIV, a novel modulator of sodium channels: From genomic organization to functional analysis. Biochemical Journal. 2006;399(3):445–453. doi: 10.1042/BJ20060035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bon C. Synergism of the two subunits of crotoxin. Toxicon. 1982;20(1):105–109. doi: 10.1016/0041-0101(82)90173-8. [DOI] [PubMed] [Google Scholar]
- 17.Câmara C. C., Nascimento N. R. F., Macêdo-Filho C. L., Almeida F. B. S., Fonteles M. C. Antispasmodic Effect of the Essential Oil of Plectranthus barbatus and some Major Constituents on the Guinea-Pig Ileum. Planta Medica. 2003;69(12):1080–1085. doi: 10.1055/s-2003-45186. [DOI] [PubMed] [Google Scholar]
- 18.Ponce-Monter H., Fernández-Martínez E., Ortiz M. I., et al. Spasmolytic and anti-inflammatory effects of Aloysia triphylla and citral, in vitro and in vivo studies. Journal of Smooth Muscle Research. 2010;46(6):309–319. doi: 10.1540/jsmr.46.309. [DOI] [PubMed] [Google Scholar]
- 19.Devi R. C., Sim S. M., Ismail R. Spasmolytic effect of citral and extracts of Cymbopogon citratus on isolated rabbit ileum. Journal of Smooth Muscle Research. 2011;47(5):143–156. doi: 10.1540/jsmr.47.143. [DOI] [PubMed] [Google Scholar]
- 20.Sadraei H., Ghannadi A., Malekshahi K. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contractions. Fitoterapia. 2003;74(5):445–452. doi: 10.1016/S0367-326X(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 21.Karim A., Berrabah M., Mekhfi H., et al. Effect of essential oil of Anthemis mauritiana Maire & Sennen flowers on intestinal smooth muscle contractility. Journal of Smooth Muscle Research. 2010;46(1):65–75. doi: 10.1540/jsmr.46.65. [DOI] [PubMed] [Google Scholar]
- 22.Gilani A. H., Shah A. J., Zubair A., et al. Chemical composition and mechanisms underlying the spasmolytic and bronchodilatory properties of the essential oil of Nepeta cataria L. Journal of Ethnopharmacology. 2009;121(3):405–411. doi: 10.1016/j.jep.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Sadraei H., Asghari G., Emami S. Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction. Research in Pharmaceutical Sciences. 2013;8(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Riyazi A., Hensel A., Bauer K., Geißler N., Schaaf S., Verspohl E. J. The effect of the volatile oil from ginger rhizomes (Zingiber officinale), its fractions and isolated compounds on the 5-HT3 receptor complex and the serotoninergic system of the rat ileum. Planta Medica. 2007;73(4):355–362. doi: 10.1055/s-2007-967171. [DOI] [PubMed] [Google Scholar]
- 25.Pinho-Da-Silva L., Mendes-Maia P. V., Do Nascimento Garcia T. M., et al. Croton sonderianus essential oil samples distinctly affect rat airway smooth muscle. Phytomedicine. 2010;17(10):721–725. doi: 10.1016/j.phymed.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Prakash O., Kasana V. K., Pant A. K., Zafar A., Hore S. K., Mathela C. S. Phytochemical composition of essential oil from seeds of Zingiber Roseum Rosc. and its antispasmodic activity in rat duodenum. Journal of Ethnopharmacology. 2006;106(3):344–347. doi: 10.1016/j.jep.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 27.De Sousa D. P., Júnior G. A. S., Andrade L. N., et al. Structure and spasmolytic activity relationships of monoterpene analogues found in many aromatic plants. Section C Journal of Biosciences. 2008;63(11-12):808–812. doi: 10.1515/znc-2008-11-1205. [DOI] [PubMed] [Google Scholar]
- 28.Sadraei H., Asghari G., Kasiri F. Comparison of antispasmodic effects of Dracocephalum kotschyi essential oil, limonene and α-terpineol. Research in Pharmaceutical Sciences. 2015;10(2):109–116. [PMC free article] [PubMed] [Google Scholar]
- 29.Astudillo A., Hong E., Bye R., Navarrete A. Antispasmodic activity of extracts and compounds of Acalypha phleoides Cav. Phytotherapy Research. 2004;18(2):102–106. doi: 10.1002/ptr.1414. [DOI] [PubMed] [Google Scholar]
- 30.Wienkötter N., Höpner D., Schütte U., et al. The effect of nigellone and thymoquinone on inhibiting trachea contraction and mucociliary clearance. Planta Medica. 2008;74(2):105–108. doi: 10.1055/s-2008-1034280. [DOI] [PubMed] [Google Scholar]
- 31.Brankovic S. V., Kitic D. V., Radenkovic M. M., Veljkovic S. M., Golubovic T. D. Calcium blocking activity as a mechanism of the spasmolytic effect of the essential oil of Calamintha glandulosa Silic on the isolated rat ileum. General Physiology and Biophysics. 2009;28:174–178. doi: 10.4149/gpb_2009_02_174. [DOI] [PubMed] [Google Scholar]
- 32.Heimes K., Hauk F., Verspohl E. J. Mode of action of peppermint oil and (-)-menthol with respect to 5-HT3 receptor subtypes: Binding studies, cation uptake by receptor channels and contraction of isolated rat ileum. Phytotherapy Research. 2011;25(5):702–708. doi: 10.1002/ptr.3316. [DOI] [PubMed] [Google Scholar]
- 33.Ponce-Monter H., Campos M. G., Pérez S., et al. Chemical composition and antispasmodic effect of Casimiroa pringlei essential oil on rat uterus. Fitoterapia. 2008;79(6):446–450. doi: 10.1016/j.fitote.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Madeira S. V. F., Rabelo M., Soares P. M. G., et al. Temporal variation of chemical composition and relaxant action of the essential oil of Ocimum gratissimum L. (Labiatae) on guinea-pig ileum. Phytomedicine. 2005;12(6-7):506–509. doi: 10.1016/j.phymed.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Rivero-Cruz I., Duarte G., Navarrete A., Bye R., Linares E., Mata R. Chemical composition and antimicrobial and spasmolytic properties of poliomintha longiflora and lippia graveolens essential oils. Journal of Food Science. 2011;76(2):C309–C317. doi: 10.1111/j.1750-3841.2010.02022.x. [DOI] [PubMed] [Google Scholar]
- 36.Taqvi S. I. H., Shah A. J., Gilani A. H. Insight into the possible mechanism of antidiarrheal and antispasmodic activities of piperine. Pharmaceutical Biology. 2009;47(8):660–664. doi: 10.1080/13880200902918352. [DOI] [Google Scholar]
- 37.Begrow F., Engelbertz J., Feistel B., Lehnfeld R., Bauer K., Verspohl E. J. Impact of Thymol in thyme extracts on their antispasmodic action and ciliary clearance. Planta Medica. 2010;76(4):311–318. doi: 10.1055/s-0029-1186179. [DOI] [PubMed] [Google Scholar]
- 38.Görnemann T., Nayal R., Pertz H. H., Melzig M. F. Antispasmodic activity of essential oil from Lippia dulcis Trev. Journal of Ethnopharmacology. 2008;117(1):166–169. doi: 10.1016/j.jep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Abere T. A., Okoto P. E., Agoreyo F. O. Antidiarrhoea and toxicological evaluation of the leaf extract of Dissotis rotundifolia triana (Melastomataceae) BMC Complementary and Alternative Medicine. 2010;10, article 71 doi: 10.1186/1472-6882-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima F. J. B., Brito T. S., Freire W. B. S., et al. The essential oil of Eucalyptus tereticornis, and its constituents α- And β-pinene, potentiate acetylcholine-induced contractions in isolated rat trachea. Fitoterapia. 2010;81(6):649–655. doi: 10.1016/j.fitote.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Sadraei H., Asghari G. R., Hajhashemi V., Kolagar A., Ebrahimi M. Spasmolytic activity of essential oil and various extracts of Ferula gummosa Boiss. on ileum contractions. Phytomedicine. 2001;8(5):370–376. doi: 10.1078/0944-7113-00052. [DOI] [PubMed] [Google Scholar]
- 42.Da Silva T. M. S., Da Silva B. A., Mukherjee R. The monoterpene alkaloid cantleyine from Strychnos trinervis root and its spasmolytic properties. Phytomedicine. 1999;6(3):169–176. doi: 10.1016/S0944-7113(99)80005-1. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz De Urbina A. V., Martin M. L., Fernandez B., San Roman L., Cubillo L. In vitro antispasmodic activity of peracetylated penstemonoside, aucubin and catalpol. Planta Medica. 1994;60(6):512–515. doi: 10.1055/s-2006-959561. [DOI] [PubMed] [Google Scholar]
- 44.Cometa M. F., Parisi L., Palmery M., Meneguz A., Tomassini L. In vitro relaxant and spasmolytic effects of constituents from Viburnum prunifolium and HPLC quantification of the bioactive isolated iridoids. Journal of Ethnopharmacology. 2009;123(2):201–207. doi: 10.1016/j.jep.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Hazelhoff B., Malingre T. M., Meijer D. K. F. Antispasmodic effects of valeriana compounds: An in-vivo and in-vitro study on the guinea-pig ileum. Archives Internationales de Pharmacodynamie et de Thérapie. 1982;257(2):274–287. [PubMed] [Google Scholar]
- 46.Cimanga R. K., Mukenyi P. N. K., Kambu O. K., et al. The spasmolytic activity of extracts and some isolated compounds from the leaves of Morinda morindoides (Baker) Milne-Redh. (Rubiaceae) Journal of Ethnopharmacology. 2010;127(2):215–220. doi: 10.1016/j.jep.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Mata R., Rojas A., Acevedo L., et al. Smooth muscle relaxing flavonoids and terpenoids from Conyza filaginoides. Planta Medica. 1997;63(1):31–35. doi: 10.1055/s-2006-957598. [DOI] [PubMed] [Google Scholar]
- 48.Leonhardt V., Leal-Cardoso J. H., Lahlou S., et al. Antispasmodic effects of essential oil of Pterodon polygalaeflorus and its main constituent β-caryophyllene on rat isolated ileum. Fundamental & Clinical Pharmacology. 2010;24(6):749–758. doi: 10.1111/j.1472-8206.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 49.Nasiri A., Holth A., Bjork L. Effects of the sesquiterpene capsidiol on isolated guinea-pig ileum and trachea, and on prostaglandin synthesis in vitro. Planta Medica. 1993;59(3):203–206. doi: 10.1055/s-2006-959652. [DOI] [PubMed] [Google Scholar]
- 50.Ko W.-C., Lei C.-B., Lin Y.-L., Chen C.-F. Mechanisms of relaxant action of S-petasin and S-isopetasin, sesquiterpenes of Petasites formosanus, in isolated guinea pig trachea. Planta Medica. 2001;67(3):224–229. doi: 10.1055/s-2001-11991. [DOI] [PubMed] [Google Scholar]
- 51.Maschi O., Dal Cero E., Galli G. V., Caruso D., Bosisio E., Dell'Agli M. Inhibition of human cAMP-phosphodiesterase as a mechanism of the spasmolytic effect of Matricaria recutita L. Journal of Agricultural and Food Chemistry. 2008;56(13):5015–5020. doi: 10.1021/jf800051n. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Hernandez N., Ponce-Monter H., Medina J. A., Joseph-Nathan P. Spasmolytic effect of constituents from Lepechinia caulescens on rat uterus. Journal of Ethnopharmacology. 2008;115(1):30–35. doi: 10.1016/j.jep.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 53.Emendörfer F., Bellato F., Noldin V. F., et al. Antispasmodic activity of fractions and cynaropicrin from Cynara scolymus on guinea-pig ileum. Biological & Pharmaceutical Bulletin. 2005;28(5):902–904. doi: 10.1248/bpb.28.902. [DOI] [PubMed] [Google Scholar]
- 54.Ammar S., Edziri H., Mahjoub M. A., Chatter R., Bouraoui A., Mighri Z. Spasmolytic and anti-inflammatory effects of constituents from Hertia cheirifolia. Phytomedicine. 2009;16(12):1156–1161. doi: 10.1016/j.phymed.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Kar K., Puri V. N., Patnaik G. K., et al. Spasmolytic constituents of Cedrus deodara (Roxb.) Loud: Pharmacological evaluation of himachalol. Journal of Pharmaceutical Sciences. 1975;64(2):258–262. doi: 10.1002/jps.2600640213. [DOI] [PubMed] [Google Scholar]
- 56.Pongprayoon U., Baeckstrom P., Jacobsson U., Lindstrom M., Bohlin L. Antispasmodic activity of β-damascenone and E-phytol isolated from Ipomoea pes-caprae. Planta Medica. 1992;58(1):19–21. doi: 10.1055/s-2006-961381. [DOI] [PubMed] [Google Scholar]
- 57.Natividad G. M., Broadley K. J., Kariuki B., Kidd E. J., Ford W. R., Simons C. Actions of Artemisia vulgaris extracts and isolated sesquiterpene lactones against receptors mediating contraction of guinea pig ileum and trachea. Journal of Ethnopharmacology. 2011;137(1):808–816. doi: 10.1016/j.jep.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 58.Guo H., Zhang J., Gao W., Qu Z., Liu C. Gastrointestinal effect of methanol extract of Radix Aucklandiae and selected active substances on the transit activity of rat isolated intestinal strips. Pharmaceutical Biology. 2014;52(9):1141–1149. doi: 10.3109/13880209.2013.879601. [DOI] [PubMed] [Google Scholar]
- 59.Ponce-Monter H., Perez S., Zavala M. A., et al. Relaxant effect of xanthomicrol and 3α-angeloyloxy-2α-hydroxy- 13,14Z-dehydrocativic acid from Brickellia paniculata on rat uterus. Biological & Pharmaceutical Bulletin. 2006;29(7):1501–1503. doi: 10.1248/bpb.29.1501. [DOI] [PubMed] [Google Scholar]
- 60.Rigano D., Aviello G., Bruno M., et al. Antispasmodic effects and structure-activity relationships of labdane diterpenoids from Marrubium globosum ssp. libanoticum. Journal of Natural Products. 2009;72(8):1477–1481. doi: 10.1021/np9002756. [DOI] [PubMed] [Google Scholar]
- 61.El Bardai S., Morel N., Wibo M., et al. The vasorelaxant activity of marrubenol and marrubiin from Marrubium vulgare. Planta Medica. 2003;69(1):75–77. doi: 10.1055/s-2003-37042. [DOI] [PubMed] [Google Scholar]
- 62.Aguiar L. A., Porto R. S., Lahlou S., et al. Antispasmodic effects of a new kaurene diterpene isolated from Croton argyrophylloides on rat airway smooth muscle. Journal of Pharmacy and Pharmacology. 2012;64(8):1155–1164. doi: 10.1111/j.2042-7158.2012.01494.x. [DOI] [PubMed] [Google Scholar]
- 63.Ambrosio S. R., Tirapelli C. R., Bonaventura D., De Oliveira A. M., Da Costa F. B. Pimarane diterpene from Viguiera arenaria (Asteraceae) inhibit rat carotid contraction. Fitoterapia. 2002;73(6):484–489. doi: 10.1016/S0367-326X(02)00170-3. [DOI] [PubMed] [Google Scholar]
- 64.van Puyvelde L., Lefebvre R., Mugabo P., De Kimpe N., Schamp N. Active principles of Tetradenia riparia; II. Antispasmodic activity of 8 (14),15-sandaracopimaradiene-7α,18-diol. Planta Medica. 1987;53(2):156–158. doi: 10.1055/s-2006-962660. [DOI] [PubMed] [Google Scholar]
- 65.Romussi G., Ciarallo G., Bisio A., et al. A new diterpenoid with antispasmodic activity from Salvia cinnabarina. Planta Medica. 2001;67(2):153–155. doi: 10.1055/s-2001-11511. [DOI] [PubMed] [Google Scholar]
- 66.Zamilpa A., Tortoriello J., Navarro V., Delgado G., Alvarez L. Antispasmodic and antimicrobial diterpenic acids from Viguiera hypargyrea roots. Planta Medica. 2002;68(3):281–283. doi: 10.1055/s-2002-23146. [DOI] [PubMed] [Google Scholar]
- 67.Santos R. F., Martins I. R. R., Travassos R. A., et al. Ent-7α-acetoxytrachyloban-18-oic acid and ent-7α- hydroxytrachyloban-18-oic acid from Xylopia langsdorfiana A. St-Hil. & Tul. modulate K + and Ca 2+ channels to reduce cytosolic calcium concentration on guinea pig ileum. European Journal of Pharmacology. 2012;678(1-3):39–47. doi: 10.1016/j.ejphar.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 68.Hu J., Gao W.-Y., Ma L., Man S.-L., Huang L.-Q., Liu C.-X. Activation of M3 muscarinic receptor and Ca2+ influx by crude fraction from Crotonis Fructus in isolated rabbit jejunum. Journal of Ethnopharmacology. 2012;139(1):136–141. doi: 10.1016/j.jep.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 69.Ghanadian M., Sadraei H., Yousuf S., Asghari G., Choudhary M. I., Jahed M. New diterpene polyester and phenolic compounds from Pycnocycla spinosa Decne. Ex Boiss with relaxant effects on KCl-induced contraction in rat ileum. Phytochemistry Letters. 2014;7(1):57–61. doi: 10.1016/j.phytol.2013.09.016. [DOI] [Google Scholar]
- 70.Barile E., Capasso R., Izzo A. A., Lanzotti V., Sajjadi S. E., Zolfaghari B. Structure-activity relationships for saponins from Allium hirtifolium and Allium elburzense and their antispasmodic activity. Planta Medica. 2005;71(11):1010–1018. doi: 10.1055/s-2005-873134. [DOI] [PubMed] [Google Scholar]
- 71.Begum S., Sultana I., Siddiqui B. S., Shaheen F., Gilani A. H. Structure and spasmolytic activity of eucalyptanoic acid from Eucalyptus camaldulensis var. obtusa and synthesis of its active derivative from oleanolic acid. Journal of Natural Products. 2002;65(12):1939–1941. doi: 10.1021/np020127x. [DOI] [PubMed] [Google Scholar]
- 72.Corea G., Fattorusso E., Lanzotti V., Capasso R., Izzo A. A. Antispasmodic saponins from bulbs of red onion, Allium cepa L. var. Tropea. Journal of Agricultural and Food Chemistry. 2005;53(4):935–940. doi: 10.1021/jf048404o. [DOI] [PubMed] [Google Scholar]
- 73.González-Cortazar M., Tortoriello J., Alvarez L. Norsecofriedelanes as spasmolytics, advances of structure-activity relationships. Planta Medica. 2005;71(8):711–716. doi: 10.1055/s-2005-871224. [DOI] [PubMed] [Google Scholar]
- 74.Gomes A. Y. S., Souza M. D. F. V., Cortes S. F., Lemos V. S. Mechanism involved in the spasmolytic effect of a mixture of two triterpenes, cycloartenol and cycloeucalenol, isolated from Herissanthia tiubae in the guinea-pig ileum. Planta Medica. 2005;71(11):1025–1029. doi: 10.1055/s-2005-871291. [DOI] [PubMed] [Google Scholar]
- 75.Palacios-Espinosa F., Déciga-Campos M., Mata R. Antinociceptive, hypoglycemic and spasmolytic effects of Brickellia veronicifolia. Journal of Ethnopharmacology. 2008;118(3):448–454. doi: 10.1016/j.jep.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 76.Estrada O., González-Guzmán J. M., Salazar-Bookaman M., Fernández A. Z., Cardozo A., Alvarado-Castillo C. Pomolic acid of Licania pittieri elicits endothelium-dependent relaxation in rat aortic rings. Phytomedicine. 2011;18(6):464–469. doi: 10.1016/j.phymed.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 77.González-Trujano M. E., Ventura-Martínez R., Chávez M., Díaz-Reval I., Pellicer F. Spasmolytic and antinociceptive activities of ursolic acid and acacetin identified in Agastache mexicana. Planta Medica. 2012;78(8):793–799. doi: 10.1055/s-0031-1298416. [DOI] [PubMed] [Google Scholar]
- 78.Begum S., Farhat, Sultana I., Siddiqui B. S., Shaheen F., Gilani A. H. Spasmolytic constituents from Eucalyptus camaldulensis var. obtusa leaves. Journal of Natural Products. 2000;63(9):1265–1268. doi: 10.1021/np9902340. [DOI] [PubMed] [Google Scholar]
- 79.Aquino R., Tortora S., Fkih-Tetouani S., Capasso A. Saponins from the roots of Zygophyllum gaetulum and their effects on electrically-stimulated guinea-pig ileum. Phytochemistry. 2001;56(4):393–398. doi: 10.1016/S0031-9422(00)00415-5. [DOI] [PubMed] [Google Scholar]
- 80.Trute A., Gross J., Mutschler E., Nahrstedt A. In vitro antispasmodic compounds of the dry extract obtained from Hedera helix. Planta Medica. 1997;63(2):125–129. doi: 10.1055/s-2006-957627. [DOI] [PubMed] [Google Scholar]
- 81.Ali N. Brine shrimp cytotoxicity of crude methanol extract and antispasmodic activity of α-amyrin acetate from Tylophora hirsuta Wall. BMC Complementary and Alternative Medicine. 2013;13, article 135 doi: 10.1186/1472-6882-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan A.-U., Gilani A.-H., Najeeb-Ur-Rehman Pharmacological studies on Hypericum perforatum fractions and constituents. Pharmaceutical Biology. 2011;49(1):46–56. doi: 10.3109/13880209.2010.494307. [DOI] [PubMed] [Google Scholar]
- 83.Oliveira E. J., Romero M. A., Silva M. S., Silva B. A., Medeiros I. A. Intracellular calcium mobilization as a target for the spasmolytic action of scopoletin. Planta Medica. 2001;67(7):605–608. doi: 10.1055/s-2001-17355. [DOI] [PubMed] [Google Scholar]
- 84.Lakshmi V., Kapoor S., Pandey K., Patnaik G. K. Spasmolytic activity of Toddalia asiatica var. floribunda. Phytotherapy Research. 2002;16(3):281–282. doi: 10.1002/ptr.844. [DOI] [PubMed] [Google Scholar]
- 85.Pavlović I., Krunić A., Nikolić D., et al. Chloroform extract of underground parts of ferula heuffelii: Secondary metabolites and spasmolytic activity. Chemistry & Biodiversity. 2014;11(9):1417–1427. doi: 10.1002/cbdv.201400094. [DOI] [PubMed] [Google Scholar]
- 86.Sadraei H., Shokoohinia Y., Sajjadi S. E., Mozafari M. Antispasmodic effects of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Research in Pharmaceutical Sciences. 2013;8(2):137–144. [PMC free article] [PubMed] [Google Scholar]
- 87.Patnaik G. K., Banaudha K. K., Khan K. A., Shoeb A., Dhawan B. N. Spasmolytic activity of angelicin: A coumarin from Heracleum thomsoni. Planta Medica. 1987;53(6):517–520. doi: 10.1055/s-2006-962799. [DOI] [PubMed] [Google Scholar]
- 88.Sato Y., Akao T., He J.-X., et al. Glycycoumarin from Glycyrrhizae Radix acts as a potent antispasmodic through inhibition of phosphodiesterase 3. Journal of Ethnopharmacology. 2006;105(3):409–414. doi: 10.1016/j.jep.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 89.Nagai H., Yamamoto Y., Sato Y., Akao T., Tani T. Pharmaceutical evaluation of cultivated Glycyrrhiza uralensis roots in comparison of their antispasmodic activity and glycycoumarin contents with those of licorice. Biological & Pharmaceutical Bulletin. 2006;29(12):2442–2445. doi: 10.1248/bpb.29.2442. [DOI] [PubMed] [Google Scholar]
- 90.Desire O., Rivière C., Razafindrazaka R., et al. Antispasmodic and antioxidant activities of fractions and bioactive constituent davidigenin isolated from Mascarenhasia arborescens. Journal of Ethnopharmacology. 2010;130(2):320–328. doi: 10.1016/j.jep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 91.Shi Y., Wu D., Sun Z., et al. Analgesic and uterine relaxant effects of isoliquiritigenin, a flavone from Glycyrrhiza glabra. Phytotherapy Research. 2012;26(9):1410–1417. doi: 10.1002/ptr.3715. [DOI] [PubMed] [Google Scholar]
- 92.Sato Y., He J.-X., Nagai H., Tani T., Akao T. Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biological & Pharmaceutical Bulletin. 2007;30(1):145–149. doi: 10.1248/bpb.30.145. [DOI] [PubMed] [Google Scholar]
- 93.Nagai H., He J.-X., Tani T., Akao T. Antispasmodic activity of licochalcone A, a species-specific ingredient of Glycyrrhiza inflata roots. Journal of Pharmacy and Pharmacology. 2007;59(10):1421–1426. doi: 10.1211/jpp.59.10.0013. [DOI] [PubMed] [Google Scholar]
- 94.Rojas A., Cruz S., Ponce-Monter H., Mata R. Smooth muscle relaxing compounds from Dodonaea viscosa. Planta Medica. 1996;62(2):154–159. doi: 10.1055/s-2006-957840. [DOI] [PubMed] [Google Scholar]
- 95.Abu-Niaaj L., Abu-Zarga M., Sabri S., Abdalla S. Isolation and biological effects of 7-O-methyleriodictyol, a flavanone isolated from Artemisia monosperma, on rat isolated smooth muscles. Planta Medica. 1993;59(1):42–45. doi: 10.1055/s-2006-959601. [DOI] [PubMed] [Google Scholar]
- 96.Da Rocha M. B., Souza F. V. M., Estevam C. D. S., Pizza C., Sant'Ana A. E. G., Marçal R. M. Antispasmodic effect of 4′-methylepigallocatechin on guinea pig ileum. Fitoterapia. 2012;83(7):1286–1290. doi: 10.1016/j.fitote.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Lemmens-Gruber R., Marchart E., Rawnduzi P., Engel N., Benedek B., Kopp B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium s.l. on isolated guinea-pig ilea. Arzneimittel-Forschung/Drug Research. 2006;56(8):582–586. doi: 10.1055/s-0031-1296755. [DOI] [PubMed] [Google Scholar]
- 98.Gorzalczany S., Moscatelli V., Ferraro G. Artemisia copa aqueous extract as vasorelaxant and hypotensive agent. Journal of Ethnopharmacology. 2013;148(1):56–61. doi: 10.1016/j.jep.2013.03.061. [DOI] [PubMed] [Google Scholar]
- 99.Fleer H., Verspohl E. J. Antispasmodic activity of an extract from Plantago lanceolata L. and some isolated compounds. Phytomedicine. 2007;14(6):409–415. doi: 10.1016/j.phymed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Engelbertz J., Lechtenberg M., Studt L., Hensel A., Verspohl E. J. Bioassay-guided fractionation of a thymol-deprived hydrophilic thyme extract and its antispasmodic effect. Journal of Ethnopharmacology. 2012;141(3):848–853. doi: 10.1016/j.jep.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 101.Ragone M. I., Sella M., Conforti P., Volonté M. G., Consolini A. E. The spasmolytic effect of Aloysia citriodora, Palau (South American cedrón) is partially due to its vitexin but not isovitexin on rat duodenums. Journal of Ethnopharmacology. 2007;113(2):258–266. doi: 10.1016/j.jep.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Gilani A. H., Khan A.-U., Ghayur M. N., Ali S. F., Herzig J. W. Antispasmodic effects of Rooibos tea (Aspalathus linearis) is mediated predominantly through K+-channel activation. Basic & Clinical Pharmacology & Toxicology. 2006;99(5):365–373. doi: 10.1111/j.1742-7843.2006.pto_507.x. [DOI] [PubMed] [Google Scholar]
- 103.Macêdo C. L., Vasconcelos L. H. C., Correia A. C. D. C., et al. Spasmolytic effect of galetin 3,6-dimethyl ether, a flavonoid obtained from Piptadenia stipulacea (Benth) Ducke. Journal of Smooth Muscle Research. 2011;47(5):123–134. doi: 10.1540/jsmr.47.123. [DOI] [PubMed] [Google Scholar]
- 104.Rodríguez-Ramos F., Navarrete A. Solving the confusion of gnaphaliin structure: Gnaphaliin A and gnaphaliin B identified as active principles of Gnaphalium liebmannii with tracheal smooth muscle relaxant properties. Journal of Natural Products. 2009;72(6):1061–1064. doi: 10.1021/np800746j. [DOI] [PubMed] [Google Scholar]
- 105.Lozoya X., Meckes M., Abou-Zaid M., Tortoriello J., Nozzolillo C., Arnason J. T. Quercetin glycosides in Psidium guajava L. leaves and determination of a spasmolytic principle. Archives of Medical Research. 1994;25(1):11–15. [PubMed] [Google Scholar]
- 106.Melzig M. F., Pertz H. H., Krenn L. Anti-inflammatory and spasmolytic activity of extracts from Droserae Herba. Phytomedicine. 2001;8(3):225–229. doi: 10.1078/0944-7113-00031. [DOI] [PubMed] [Google Scholar]
- 107.Krenn L., Beyer G., Pertz H. H., et al. In vitro antispasmodic and anti-inflammatory effects of Drosera rotundifolia. Arzneimittel-Forschung/Drug Research. 2004;54(7):402–405. doi: 10.1055/s-0031-1296991. [DOI] [PubMed] [Google Scholar]
- 108.Ko W. C., Wang H. L., Lei C. B., Shih C. H., Chung M. I., Lin C. N. Mechanisms of relaxant action of 3-O-methylquercetin in isolated guinea pig trachea. Planta Medica. 2002;68(1):30–35. doi: 10.1055/s-2002-20059. [DOI] [PubMed] [Google Scholar]
- 109.Bergendorff O., Sterner O. Spasmolytic flavonols from Artemisia abrotanum. Planta Medica. 1995;61(4):370–371. doi: 10.1055/s-2006-958106. [DOI] [PubMed] [Google Scholar]
- 110.Herrera M. D., Marhuenda E., Gibson A. Effects of genistein, an isoflavone isolated from Genista tridentata, on isolated guinea-pig ileum and guinea-pig ileal myenteric plexus. Planta Medica. 1992;58(4):314–316. doi: 10.1055/s-2006-961474. [DOI] [PubMed] [Google Scholar]
- 111.Borrelli F., Milic N., Ascione V., et al. Isolation of new rotenoids from Boerhaavia diffusa and evaluation of their effect on intestinal motility. Planta Medica. 2005;71(10):928–932. doi: 10.1055/s-2005-871282. [DOI] [PubMed] [Google Scholar]
- 112.Balemba O. B., Stark T. D., Lösch S., et al. (2R,3S,2''R,3''R)-manniflavanone, a new gastrointestinal smooth muscle L-type calcium channel inhibitor, which underlies the spasmolytic properties of Garcinia buchananii stem bark extract. Journal of Smooth Muscle Research. 2014;50(1):48–65. doi: 10.1540/jsmr.50.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verspohl E. J., Fujii H., Homma K., Buchwald-Werner S. Testing of Perilla frutescens extract and Vicenin 2 for their antispasmodic effect. Phytomedicine. 2013;20(5):427–431. doi: 10.1016/j.phymed.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 114.De Moura N. F., Morel A. F., Dessoy E. C., et al. Alkaloids, amides and antispasmodic activity of Zanthoxylum hyemale. Planta Medica. 2002;68(6):534–538. doi: 10.1055/s-2002-32550. [DOI] [PubMed] [Google Scholar]
- 115.He W.-J., Fang T.-H., Ma X., Zhang. K., Ma Z.-Z., Tu P.-F. Echinacoside elicits endothelium-dependent relaxation in rat aortic rings via an NO-cGMP pathway. Planta Medica. 2009;75(13):1400–1404. doi: 10.1055/s-0029-1185745. [DOI] [PubMed] [Google Scholar]
- 116.Yang J., Ip P. S. P., Yeung J. H. K., Che C.-T. Inhibitory effect of schisandrin on spontaneous contraction of isolated rat colon. Phytomedicine. 2011;18(11):998–1005. doi: 10.1016/j.phymed.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang J.-M., Ip P. S. P., Che C.-T., Yeung J. H. K. Relaxant effects of Schisandra chinensis and its major lignans on agonists-induced contraction in guinea pig ileum. Phytomedicine. 2011;18(13):1153–1160. doi: 10.1016/j.phymed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Hernández-Romero Y., Rojas J.-I., Castillo R., Rojas A., Mata R. Spasmolytic Effects, Mode of Action, and Structure-Activity Relationships of Stilbenoids from Nidema boothii. Journal of Natural Products. 2004;67(2):160–167. doi: 10.1021/np030303h. [DOI] [PubMed] [Google Scholar]
- 119.Estrada S., Rojas A., Mathison Y., Israel A., Mata R. Nitric oxide/cGMP mediates the spasmolytic action of 3,4'-dihydroxy- 5,5'-dimethoxybibenzyl from Scaphyglottis livida. Planta Medica. 1999;65(2):109–114. doi: 10.1055/s-1999-14056. [DOI] [PubMed] [Google Scholar]
- 120.Estrada S., López-Guerrero J. J., Villalobos-Molina R., Mata R. Spasmolytic stilbenoids from Maxillaria densa. Fitoterapia. 2004;75(7-8):690–695. doi: 10.1016/j.fitote.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Itthipanichpong C., Kemsri W., Ruangrungsi N., Sawasdipanich A. Antispasmodic effects of curcuminoids on isolated guinea-pig ileum and rat uterus. Journal of the Medical Association of Thailand. 2003;86(2):S299–S309. [PubMed] [Google Scholar]
- 122.Seya K., Furukawa K.-I., Taniguchi S., et al. Endothelium-dependent vasodilatory effect of vitisin C, a novel plant oligostilbene from Vitis plants (Vitaceae), in rabbit aorta. Clinical Science. 2003;105(1):73–79. doi: 10.1042/CS20020288. [DOI] [PubMed] [Google Scholar]
- 123.Liang M.-J., He L.-C., Yang G.-D. Screening, analysis and in vitro vasodilatation of effective components from Ligusticum Chuanxiong. Life Sciences. 2005;78(2):128–133. doi: 10.1016/j.lfs.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 124.Rigano D., Formisano C., Senatore F., et al. Intestinal antispasmodic effects of Helichrysum italicum (Roth) Don ssp. italicum and chemical identification of the active ingredients. Journal of Ethnopharmacology. 2013;150(3):901–906. doi: 10.1016/j.jep.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 125.Baldassano S., Tesoriere L., Rotundo A., Serio R., Livrea M. A., Mulè F. Inhibition of the mechanical activity of mouse ileum by cactus pear (Opuntia ficus Indica, L, Mill.) fruit extract and its pigment indicaxanthin. Journal of Agricultural and Food Chemistry. 2010;58(13):7565–7571. doi: 10.1021/jf100434e. [DOI] [PubMed] [Google Scholar]
- 126.Gambhir S. S., Sen S. P., Sanyal A. K., Das P. K. Antispasmodic activity of the tertiary base of Daucus carota, Linn. seeds. Indian Journal of Physiology and Pharmacology. 1979;23(3):225–228. [PubMed] [Google Scholar]
- 127.Tsukiyama M., Ueki T., Yasuda Y., et al. β2-adrenoceptor-mediated tracheal relaxation induced by higenamine from nandina domestica thunberg. Planta Medica. 2009;75(13):1393–1399. doi: 10.1055/s-0029-1185743. [DOI] [PubMed] [Google Scholar]
- 128.Lin C.-H., Ko F.-N., Wu Y.-C., Lu S.-T., Teng C.-M. The relaxant actions on guinea-pig trachealis of atherosperminine isolated from Fissistigma glaucescens. European Journal of Pharmacology. 1993;237(1):109–116. doi: 10.1016/0014-2999(93)90099-4. [DOI] [PubMed] [Google Scholar]
- 129.Orallo F. Pharmacological effects of (+)-nantenine, an alkaloid isolated from Platycapnos spicata, in several rat isolated tissues. Planta Medica. 2003;69(2):135–142. doi: 10.1055/s-2003-37700. [DOI] [PubMed] [Google Scholar]
- 130.El-Shafae A. M., Soliman A. S. A pyranocoumarin and two alkaloids (one with antispasmodic effect) from Citrus deliciosa. Die Pharmazie. 1998;53(9):640–643. [PubMed] [Google Scholar]
- 131.Lin C., Yang C., Ko F., Wu Y., Teng C. Antimuscarinic action of liriodenine, isolated from Fissistigma glaucescens, in canine tracheal smooth muscle. British Journal of Pharmacology. 1994;113(4):1464–1470. doi: 10.1111/j.1476-5381.1994.tb17161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yuan J., Zhou J., Hu Z., Ji G., Xie J., Wu D. The effects of jatrorrhizine on contractile responses of rat ileum. European Journal of Pharmacology. 2011;663(1-3):74–79. doi: 10.1016/j.ejphar.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 133.Zhao M., Xian Y., Ip S., Fong H. H. S., Che C. A new and weakly antispasmodic protoberberine alkaloid from rhizoma coptidis. Phytotherapy Research. 2010;24(9):1414–1416. doi: 10.1002/ptr.3154. [DOI] [PubMed] [Google Scholar]
- 134.Sotníková R., Kettmann V., Kostálová D., Táborská E. Relaxant properties of some aporphine alkaloids from Mahonia aquifolium. Methods and Findings in Experimental and Clinical Pharmacology. 1997;19(9):589–597. [PubMed] [Google Scholar]
- 135.Hiller K.-O., Ghorbani M., Schlicher H. Antispasmodic and relaxant activity of chelidonine, protopine, coptisine, and Chelidonium majus extracts on isolated guinea-pig ileum. Planta Medica. 1998;64(8):758–760. doi: 10.1055/s-2006-957576. [DOI] [PubMed] [Google Scholar]
- 136.Rastrelli L., Capasso A., Pizza C., De Tommasi N., Sorrentino L. New protopine and benzyltetrahydroprotoberberine alkaloids from Aristolochia constricta and their activity on isolated guinea-pig ileum. Journal of Natural Products. 1997;60(11):1065–1069. doi: 10.1021/np960710b. [DOI] [PubMed] [Google Scholar]
- 137.Oliveira R. C., Lima J. T., Ribeiro L. A. A., et al. Spasmolytic action of the methanol extract and isojuripidine from Solanum asterophorum Mart. (Solanaceae) leaves in guinea-pig ileum. Zeitschrift fur Naturforschung - Section C Journal of Biosciences. 2006;61(11-12):799–805. doi: 10.1515/znc-2006-11-1205. [DOI] [PubMed] [Google Scholar]
- 138.Gilani A.-U. H., Khalid A., Zaheer-ul-Haq, Choudhary M. I., Atta-ur-Rahman Presence of antispasmodic, antidiarrheal, antisecretory, calcium antagonist and acetylcholinesterase inhibitory steroidal alkaloids in Sarcococca saligna. Planta Medica. 2005;71(2):120–125. doi: 10.1055/s-2005-837777. [DOI] [PubMed] [Google Scholar]
- 139.Khalid A., Zaheer-Ul-Haq, Ghayur M. N., et al. Cholinesterase inhibitory and spasmolytic potential of steroidal alkaloids. The Journal of Steroid Biochemistry and Molecular Biology. 2004;92(5):477–484. doi: 10.1016/j.jsbmb.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Y., Long Z., Guo Z., et al. Hydroxycinnamic acid amides from Scopolia tangutica inhibit the activity of M1 muscarinic acetylcholine receptor in vitro. Fitoterapia. 2016;108:9–12. doi: 10.1016/j.fitote.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pongkorpsakol P., Wongkrasant P., Kumpun S., Chatsudthipong V., Muanprasat C. Inhibition of intestinal chloride secretion by piperine as a cellular basis for the anti-secretory effect of black peppers. Pharmacological Research. 2015;100:271–280. doi: 10.1016/j.phrs.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 142.Capasso R., Aviello G., Romano B., et al. Modulation of mouse gastrointestinal motility by allyl isothiocyanate, a constituent of cruciferous vegetables (Brassicaceae): Evidence for TRPA1-independent effects. British Journal of Pharmacology. 2012;165(6):1966–1977. doi: 10.1111/j.1476-5381.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kanjanapothi D., Soparat P., Panthong A., Tuntiwachwuttikul P., Reutrakul V. A uterine relaxant compound from Zingiber cassumunar. Planta Medica. 1987;53(4):329–332. doi: 10.1055/s-2006-962729. [DOI] [PubMed] [Google Scholar]
- 144.Moazedi A. A., Dabir N., Gharib Naseri M. K., Zadkarami M. R. The role of NO and cGMP in antispasmodic activity of Ruta chalepensis leaf extract on rat ileum. Pakistan Journal of Biological Sciences. 2010;13(2):83–87. doi: 10.3923/pjbs.2010.83.87. [DOI] [PubMed] [Google Scholar]
- 145.Sadraei H., Ghanadian M., Asghari G., Madadi E., Azali N. Antispasmodic and antidiarrhoeal activities of 6-(4-hydroxy-3- methoxyphenyl)-hexanonic acid from Pycnocycla spinosa Decne. exBoiss. Research in Pharmaceutical Sciences. 2014;9(4):279–286. [PMC free article] [PubMed] [Google Scholar]
- 146.Sadraei H., Ghanadian M., Asghari G., Azali N. Antidiarrheal activities of isovanillin, iso-acetovanillon and Pycnocycla spinosa Decne ex.Boiss extract in mice. Research in Pharmaceutical Sciences. 2014;9(2):83–89. [PMC free article] [PubMed] [Google Scholar]
- 147.Webb R. C. SMOOTH MUSCLE CONTRACTION AND RELAXATION. Advances in Physiology Education. 2003;27(4):201–206. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- 148.Copyright page. Clinical Pharmacology & Therapeutics. 2003;73(6):p. 578. doi: 10.1016/S0009-9236(03)00161-9. [DOI] [Google Scholar]
- 149.Wintersteiner O., Dutcher J. D. Curare alkaloids from Chondodendron tomentosum. Science. 1943;97(2525):467–470. doi: 10.1126/science.97.2525.467. [DOI] [PubMed] [Google Scholar]
- 150.Dale H. H., Feldberg W., Vogt M. Release of acetylcholine at voluntary motor nerve endings. The Journal of Physiology. 1936;86(4):353–380. doi: 10.1113/jphysiol.1936.sp003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Galeffi C., Scarpetti P., Marini-Bettolo G. B. Peinamine, a new bisbenzylisoquinoline alkaloid from arrow tips (pei-namô) of the Upper Orinoco. Il Farmaco; edizione scientifica. 1977;32(9):665–671. [PubMed] [Google Scholar]
- 152.Galeffi C., Scarpetti P., Marini Bettolo G. B. New curare alkaloids. II. New bisbenzylisoquinoline alkaloids from Abuta grisebachii (Menispermaceae) Farmaco, Edizione Scientifica. 1977;32(12):853–865. [PubMed] [Google Scholar]
- 153.Geiger. Darstellung des Atropins. Annalen der Pharmacie. 1833;5(1):43–81. doi: 10.1002/jlac.18330050108. [DOI] [Google Scholar]
- 154.Coulson J. F., Griffin W. J. The alkaloids of Duboisia myoporoides. I. Aerial parts. Planta Medica. 1967;15(4):459–466. doi: 10.1055/s-0028-1100007. [DOI] [PubMed] [Google Scholar]
- 155.Coulsen J. F., Griffin W. J. The alkaloids of Duboisia myoporoides. II. Roots. Planta Medica. 1968;16(2):174–181. doi: 10.1055/s-0028-1099896. [DOI] [PubMed] [Google Scholar]
- 156.Miraldi E., Masti A., Ferri S., Barni Comparini I. Distribution of hyoscyamine and scopolamine in Datura stramonium. Fitoterapia. 2001;72(6):644–648. doi: 10.1016/S0367-326X(01)00291-X. [DOI] [PubMed] [Google Scholar]
- 157.Wisniak J. Pierre-Jean Robiquet. Educación Química. 2013;24:139–149. doi: 10.1016/S0187-893X(13)72507-2. [DOI] [Google Scholar]
- 158.Hayes A. N., Gilbert S. G. Historical milestones and discoveries that shaped the toxicology sciences. EXS. 2009;99:1–35. doi: 10.1007/978-3-7643-8336-7_1. [DOI] [PubMed] [Google Scholar]
- 159.Grynkiewicz G., Gadzikowska M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacological Reports. 2008;60(4):439–463. [PubMed] [Google Scholar]
- 160.Keberle H., Faigle J. W., Wilhelm M. Beta-(para-halo-phenyl)-glutaric acid imides. https://patents.google.com/patent/US3634428A/en, 1972. [Google Scholar]
- 161.Aboagye F. A., Sam G. H., Massiot G., Lavaud C. Julocrotine, a glutarimide alkaloid from Croton membranaceus. Fitoterapia. 2000;71(4):461–462. doi: 10.1016/s0367-326x(00)00141-6. [DOI] [PubMed] [Google Scholar]
- 162.Suárez A. I., Blanco Z., Delle Monache F., Compagnone R. S., Arvelo F. Three new glutarimide alkaloids from Croton cuneatus. Natural Product Research (Formerly Natural Product Letters) 2004;18(5):421–426. doi: 10.1080/14786410310001622004. [DOI] [PubMed] [Google Scholar]
- 163.Oates J. A., Wood A. J. J., Gross N. J. Ipratropium Bromide. The New England Journal of Medicine. 1988;319(8):486–494. doi: 10.1056/NEJM198808253190806. [DOI] [PubMed] [Google Scholar]
- 164.Litta Modignani R., Mazzolari M., Barantani E., Bertoli D., Vibelli C. Relative potency of the atropine-like effects of a new parasympatholytic drug, scopolamine-n-(Cyclopropy 1 methyl) bromide and those of hyoscine-n-butyl bromide. Current Medical Research and Opinion. 1977;5(4):333–340. doi: 10.1185/03007997709110189. [DOI] [PubMed] [Google Scholar]
- 165.Timms P. K., Gibbons R. B. Latrodectism - Effects of the black widow spider bite. Western Journal of Medicine. 1986;144(3):315–317. [PMC free article] [PubMed] [Google Scholar]
- 166.Toyama D. O., Boschero A. C., Martins M. A., Fonteles M. C., Monteiro H. S., Toyama M. H. Structure-function relationship of new crotamine isoform from the Crotalus durissus cascavella. The Protein Journal. 2005;24(1):9–19. doi: 10.1007/s10930-004-0601-1. [DOI] [PubMed] [Google Scholar]
- 167.McNamara F. N., Randall A., Gunthorpe M. J. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) British Journal of Pharmacology. 2005;144(6):781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gálvez J., Sánchez De Medina F., Jiménez J., Zarzuelo A. Bioactive Natural Products (Part F) Vol. 25. Elsevier; 2001. Effects of flavonoids on gastrointestinal disorders; pp. 607–649. (Studies in Natural Products Chemistry). [DOI] [Google Scholar]
- 169.Mehmood M. H., Siddiqi H. S., Gilani A. H. The antidiarrheal and spasmolytic activities of Phyllanthus emblica are mediated through dual blockade of muscarinic receptors and Ca2+ channels. Journal of Ethnopharmacology. 2011;133(2):856–865. doi: 10.1016/j.jep.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 170.Schlemper V., Ribas A., Nicolau M., Cechinel Filho V. Antispasmodic effects of hydroalcoholic extract of Marrubium vulgare on isolated tissues. Phytomedicine. 1996;3(2):211–216. doi: 10.1016/S0944-7113(96)80038-9. [DOI] [PubMed] [Google Scholar]
- 171.Najeeb-ur-Rehman, Bashir S., Al-Rehaily A. J., Gilani A.-H. Mechanisms underlying the antidiarrheal, antispasmodic and bronchodilator activities of Fumaria parviflora and involvement of tissue and species specificity. Journal of Ethnopharmacology. 2012;144(1):128–137. doi: 10.1016/j.jep.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 172.Peddireddy M. K. R. In vitro evaluation techniques for gastrointestinal motility. Indian Journal of Pharmaceutical Education and Research (IJPER) 2011;45(2):184–191. [Google Scholar]
- 173.Astudillo-Vázquez A., Mata R., Navarrete A. El reino vegetal, fuente de agentes antiespasmódicos gastrointestinales y antidiarreicos. Revista Latinoamericana de Química. 2009;37:7–44. [Google Scholar]
- 174.Ariens E. J., Lehmann P. A., Simonis A. M. Introduccion a la toxicologia general. Diana: MΘxico D.F; 1978. [Google Scholar]
- 175.Khan M., Khan A.-U., Najeeb-Ur-Rehman, Gilani A.-H. Gut and airways relaxant effects of Carum roxburghianum. Journal of Ethnopharmacology. 2012;141(3):938–946. doi: 10.1016/j.jep.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 176.Ehlert F. J. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sciences. 2003;74(2-3):355–366. doi: 10.1016/j.lfs.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 177.Clement J. G. BaCl2-induced contractions in the guinea pig ileum longitudinal muscle: Role of presynaptic release of neurotransmitters and Ca2+ translocation in the postsynaptic membrane. Canadian Journal of Physiology and Pharmacology. 1981;59(6):541–547. doi: 10.1139/y81-081. [DOI] [PubMed] [Google Scholar]
- 178.Blackwood A. M., Bolton T. B. Mechanism of carbachol‐evoked contractions of guinea‐pig ileal smooth muscle close to freezing point. British Journal of Pharmacology. 1993;109(4):1029–1037. doi: 10.1111/j.1476-5381.1993.tb13725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shore S., Irvin C. G., Shenkier T., Martin J. G. Mechanisms of histamine-induced contraction of canine airway smooth muscle. Journal of Applied Physiology. 1983;55(1):22–26. doi: 10.1152/jappl.1983.55.1.22. [DOI] [PubMed] [Google Scholar]
- 180.Ratz P. H., Berg K. M., Urban N. H., Miner A. S. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. American Journal of Physiology-Cell Physiology. 2005;288(4):C769–C783. doi: 10.1152/ajpcell.00529.2004. [DOI] [PubMed] [Google Scholar]
- 181.Ratz P. H., Flaim S. F. Mechanism of 5-HT contraction in isolated bovine ventricular coronary arteries. Evidence for transient receptor-operated calcium influx channels. Circulation Research. 1984;54(2):135–143. doi: 10.1161/01.RES.54.2.135. [DOI] [PubMed] [Google Scholar]
- 182.Sumner M. J., Feniuk W., McCormick J. D., Humphrey P. P. A. Studies on the mechanism of 5‐HT1 receptor‐induced smooth muscle contraction in dog saphenous vein. British Journal of Pharmacology. 1992;105(3):603–608. doi: 10.1111/j.1476-5381.1992.tb09026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test combining liver homogenates for activation and bacteria for detection. Proceedings of the National Acadamy of Sciences of the United States of America. 1973;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutation Research. 1983;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 185.Czeczot H., Tudek B., Kusztelak J., et al. Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutation Research - Genetic Toxicology and Environmental Mutagenesis. 1990;240(3):209–216. doi: 10.1016/0165-1218(90)90060-F. [DOI] [PubMed] [Google Scholar]
- 186.Déciga-Campos M., Rivero-Cruz I., Arriaga-Alba M., et al. Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. Journal of Ethnopharmacology. 2007;110(2):334–342. doi: 10.1016/j.jep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 187.Elgorashi E. E., Malan S. F., Stafford G. I., van Staden J. Quantitative structure-activity relationship studies on acetylcholinesterase enzyme inhibitory effects of Amaryllidaceae alkaloids. South African Journal of Botany. 2006;72(2):224–231. doi: 10.1016/j.sajb.2005.08.001. [DOI] [Google Scholar]
- 188.Capasso R., Borrelli F., Capasso F., et al. The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A inhibit enteric cholinergic transmission in the guinea-pig ileum. Neurogastroenterology & Motility. 2006;18(1):69–75. doi: 10.1111/j.1365-2982.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 189.Capasso R., Borrelli F., Cascio M. G., et al. Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between kappa-opioid and cannabinoid CB1 receptors. British Journal of Pharmacology. 2008;155(5):681–689. doi: 10.1038/bjp.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains the structures of the compounds described in the main text.


