ABSTRACT
Introduction: Idiopathic pulmonary fibrosis (IPF) is a chronic disease with high morbidity and mortality. A reliable and manageable questionnaire that assesses patients’ quality of life is needed to help to improve the content of clinical consultations. The chronic obstructive pulmonary disease (COPD) Assessment Test (CAT) is used in clinical practice; therefore, the aim of this study was to investigate correlations among the CAT, St. George’s Respiratory Questionnaire (SGRQ) and clinical parameters among patients diagnosed with IPF.
Methods: A retrospective cohort design was employed in which 87 patients diagnosed with IPF who were receiving treatment at an outpatient clinic were included.
Results: The CAT was found to be significantly correlated with SGRQ (p < 0.001). Furthermore, the CAT was significantly correlated with all of the included physiological variables.
Conclusions: This add to support the validity of the COPD Assessment test (CAT) as a measure of symptoms in patients with IPF which in clinical practice in combination with dialogue between healthcare professionals and the patient, can help to improve the content of a clinical consultation.
KEYWORDS: CAT, idiopathic pulmonary fibrosis, quality of life
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive disease that is associated with increased morbidity and mortality [1], and is the most common type of idiopathic interstitial pneumonia (IIP). It accounts for approximately 55% of all new cases [2,3]. It is characterized by fibrotic scarring in the lung tissue subsequent to lung injury that remains poorly understood [1]. The incidence of IPF has been shown to be between 6.8 and 7.4 pr. 100.000 person-years [4,5], and seems to increase due to the ageing population and increased awareness of the disease among both patients and healthcare professionals [6,7]. IPF predominantly occurs in older adults, as it is uncommon under the age of 50 years, with a peak among people in their 80s [8].
Pulmonary fibrosis results in reduced lung volume and impaired diffusion capacity. The cardinal symptoms are increased exertional dyspnoea and dry cough. Progression of fibrosis leads to respiratory insufficiency and death [1,7,9–11]. Disease progress is often slow and occurs gradually over a few years. However, some patients experience an accelerated decline, with acute respiratory worsening and exacerbations [12,13]. It is the type of lung fibrosis with the highest mortality rate, as the median length of survival is 3–5 years [8–11,14], which means that IPF has a poorer prognosis than a number of malignant diseases [7,15]. In 2006, Gribbin and colleagues reported a 43% 5-year survival rate among patients with IPF, and Navaratnam later estimated a 5-year survival rate of 37% in 2011 [4,16].
It is known from patients with other life-threatening diseases that a relationship exists between the patients’ experience of symptoms and quality of life [17] and that many patients experience symptoms that are under-treated [18]. A validated test that measures the impact of IPF on health as it is experienced in the patients’ everyday life, therefore, is needed and may potentially contribute to improving future treatment and palliation for the single patient by more precisely targeting treatment and care.
The St. George’s Respiratory Questionnaire (SGRQ) was originally developed to measure health-related quality of life among patients diagnosed with chronic obstructive pulmonary disease (COPD) and later was validated for use among patients with IPF [19]. However, this questionnaire is a research tool and too complex to use in daily clinical practice. Several validated tests are available for use with patients with COPD, a disease with symptoms that are similar to IPF. One of those is the COPD Assessment Test (CAT), which is a short instrument to quantify COPD’s impact in practice [20]. The CAT has previously been tested and shown good psychometric properties among patients with a mixed group of ILD [21] but has not been tested among patients diagnosed with IPF. Given the overlap in symptoms between IPF and COPD and due to the lack of a short IPF-specific tool for symptom screening, we applied the CAT questionnaire in our daily clinical practice as an aid to improve systematically the content of clinical consultations of IPF patients.
The aim of this study was to investigate whether CAT score correlates with the SGRQ score and clinical parameters in patients with IPF.
Materials and methods
Study design
The study was designed as a retrospective cohort study.
Participants
Included in the study were patients diagnosed with IPF who were referred to the outpatient clinic at the Department of Respiratory Medicine at Gentofte University Hospital. The diagnosis was based on predetermined clinical, radiological, and histological criteria based on current international guidelines [1]. Consecutive patients with a definitive diagnosis of IPF were included if they had a consultation from October 2015 to April 2016 and had completed CAT questionnaire at the time of consultation.
Ethics
Approval to perform the study was obtained from the Danish Data Protection Agency (journal no. HGH-2016–049) and the Danish Health Authority (journal no. 3-3013-1586/1/).
Data
The demographic data that were collected from the patients’ medical records were gender, age, and treatment with antifibrotic drugs. Furthermore, completed CAT, SGRQ, and the Medical Research Councils (MRC) scales were collected.
CAT
The CAT was developed in 2009. It is a self-administered QOL questionnaire that measures the impact of COPD on health status. It is relatively easy to complete and has been shown to be suitable for clinical use. It consists of eight items, each of which is scored on a scale from 1 to 5. The total score ranges from 0 to 40. Higher scores indicate a greater impact on the patient’s life. The score is divided into four impact levels, with associated examples of how the patient is affected by the disease and proposals of management considerations [20]. The CAT questionnaire is provided in a Danish translation by the Danish Society of Respiratory Medicine and can be downloaded from https://www.lungemedicin.dk/fagligt/skemaer/143-danishcatest.html. The English version is available from http://www.catestonline.org/images/pdfs/CATest.pdf. The CAT was validated for use with patients with COPD. The Cronbach’s alpha value is excellent, and the instrument has good test-retest reliability and a high correlation with the SGRQ in American patients [20]. Positive correlations among the CAT, SGRQ, and MRC were found in Korean and Asian COPD patients [22,23] and in patients with ILD in a Japanese study with 55 patients, 15 of whom had IPF [21].
SGRQ
The SGRQ is a self-administered instrument. It consists of 50 items that can be divided into three categories: symptoms (8 items), activity (16 items), and impact (26 items). Scores are weighted; therefore, every domain score and the total score range from 0 to 100. Higher scores indicate poorer health-related quality of life. The scores are calculated after filling in an Excel file developed by the creators of the questionnaire [24,25]. The SGRQ is a validated tool for patients with airflow limitations [24]. Swigris and colleagues reviewed the literature and found that the SGRQ was useful for measuring HRQL in patients with IPF [25].
MRC
The MRC dyspnoea scale is a short scale with item scores ranging from 1 to 5, where the degree of dyspnoea is reported by the patient. A higher score indicates a larger impact of breathlessness on daily activities. In an English study with COPD patients, there were significant associations between the MRC and SGRQ [26]. Correlations between the MRC dyspnoea scale and lung function parameters have been found in patients with IPF [27].
Physiological variables
Phsychological variables were forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1), and diffusion capacity of the lung for carbon monoxide (DLCO). The 6 Minutes’ Walking Distance (6 MWD) test was performed indoors in a long, straight corridor according to international guidelines [28] without oxygen or with the patient’s current oxygen flow if he or she was receiving home oxygen therapy. SpO2 was measured before and immediately after walking for 6 min, and the difference was calculated.
Gender, age, and physiology GAP index
The GAP index is a staging system for IPF based on a point score that is calculated based on the patient’s gender, age and physiological variables (FVC and DLCO). Ley and colleagues found that GAP index correlated well with 1-year mortality among patients with IPF [29,30].
Statistical analysis
The data were analysed using SPSS (version 19) (SPSS Inc., Chicago, IL, USA). The characteristics of participants are presented as numbers or means and using descriptive statistics to include percentages or standard deviations. The correlation between the CAT and SGRQ was initially inspected using a scatterplot. This was followed by an analysis of the correlation between the scores on the CAT and SGRQ and the other outcome measures using Spearman’s correlations coefficients.
Results
Patient characteristics
The study included 87 patients with IPF who had a mean age of 70.1 years (SD = 10.1). Table 1 shows the characteristics of the included patients. There were slightly more men (62%) than women. When the data were collected, 42 patients were treated with pirfenidone, 5 patients were treated with nintedanib, and 40 patients were not on treatment, either because they were assessed before treatment start or they have declined antifibrotic therapy. The severity of IPF was graded according to the GAP score, 37 patients had mild IPF (GAP 1), 38 had moderate IPF (GAP 2) and only 7 had severe IPF (GAP 3). Figures 1 and 2 show the distribution of the SGRQ and CAT scores in relation to the GAP index. Assessing both figures visually, patients who belong to GAP index 2 had scorings covering almost all possible values in both the CAT score and the SGRQ score. Univariate analysis showed a significant increase in disease impact in the different GAP grades, whether assessed by SGRQ (p = 0.013) or CAT score (p = 0.03).
Table 1.
Characteristics of the study population, 87 patients with idiopathic pulmonary fibrosis.
| N (%) or Mean (SD) | |
|---|---|
| Male | 54 (62 %) |
| Age (years) | 70.1 (10.1) |
| Treatment | |
| Pirfenidone | 42 (48.3 %) |
| Nintedanib | 5 (5.7 %) |
| None | 40 (46.0 %) |
| FVC (litre)1 | 2.88 (0.85) |
| FEV1 (%)2 | 84.21 (19.76) |
| CAT | 16.76 (6.83) |
| DLCO (%)3 | 45.01 (13.16) |
| 6 MWD (m)4 | 424.6 (108.1) |
| Saturation before 6 MWD4 | 95.23 (2.37) |
| Saturation after 6 MWD4 | 84.39 (7.99) |
| GAP Index5 | 1.63 (0.64) |
| MRC6 | 2.69 (1.08) |
| SGRQ Symptoms7 | 54.05 (21.18) |
| SGRQ Activity8 | 57.45 (24.12) |
| SGRQ Impact9 | 33.88 (19.57) |
| SGRQ Total score10 | 45.09 (19.76) |
16 values missing; 27 values missing; 37 values missing; 431 values missing; 55 values missing; 632 values missing; 741 values missing; 840 missing; 943 missing; 1046 values missing.
Figure 1.
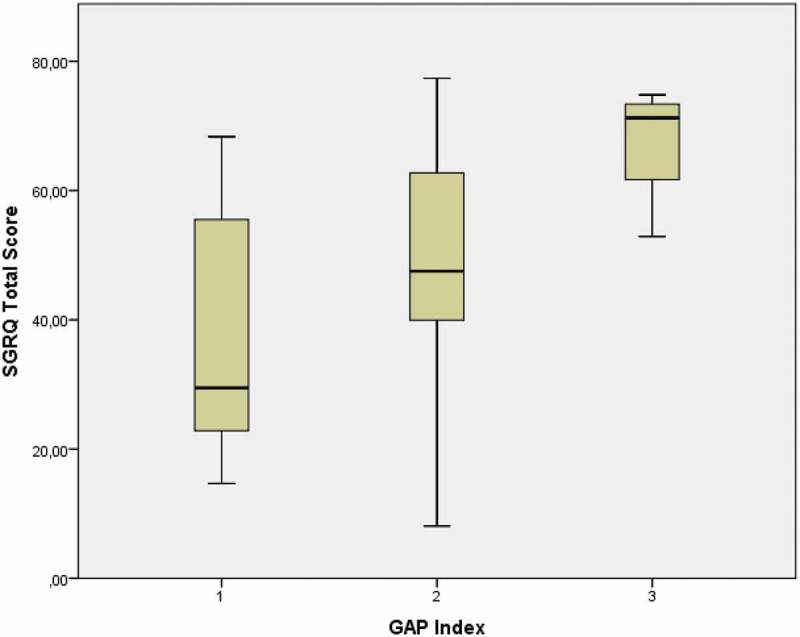
Distribution of the SGRQ total score according to the GAP index in patients with IPF
(GAP index 1 n = 37, GAP index 2 n = 38, GAP index 3 n = 7).
SGRQ – The St. George’s Respiratory Questionnaire, IPF- Idiopathic Pulmonary Fibrosis, GAP Index – Gender, age and physiology.
Figure 2.
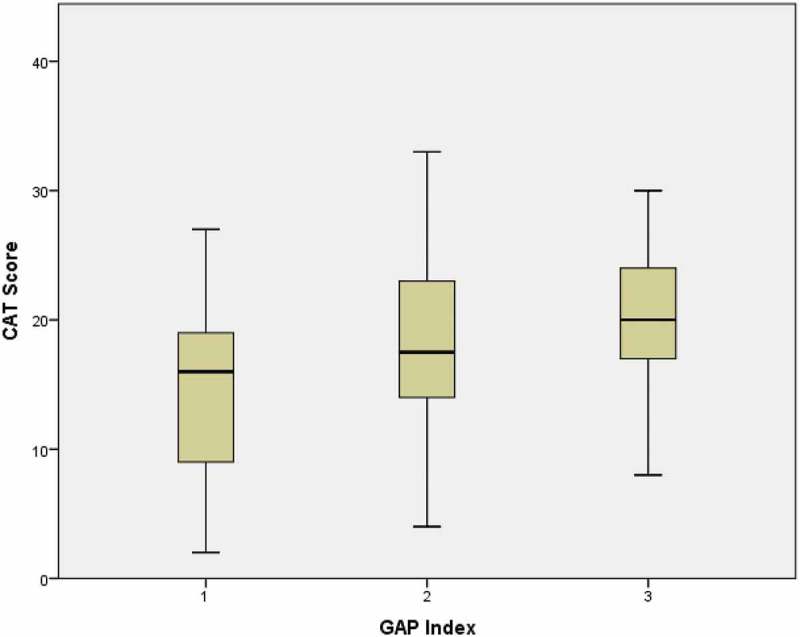
Distribution of the CAT score according to the GAP index in patients with IPF.
(GAP index 1 n = 37, GAP index 2 n = 38, GAP index 3 n = 7)
SGRQ – The St. George’s Respiratory Questionnaire, IPF- Idiopathic Pulmonary Fibrosis, GAP Index – Gender, age and physiology.
Correlations
The correlation between the CAT score and SGRQ score was initially inspected visually in a scatterplot to look for a possible relationship between the two variables (Figure 3). The Spearman’s correlation coefficient was r = 0.8 (p < 0.001), indicating that the two scores shared approximately 64% of the variance. Table 2 shows the Spearman’s correlation coefficients between the CAT and SGRQ scores in combination with the physiological variables. The CAT score was significantly correlated with all physiological variables. The SGRQ total score was significantly correlated with the FVC, FEV1 (%), 6 MWT distance and MRC. The SGRQ did not show significant correlation with FEV1, DLCO and Desaturation at 6 MWD, as did the CAT score. Neither the CAT nor the SGRQ was significantly correlated with age.
Figure 3.
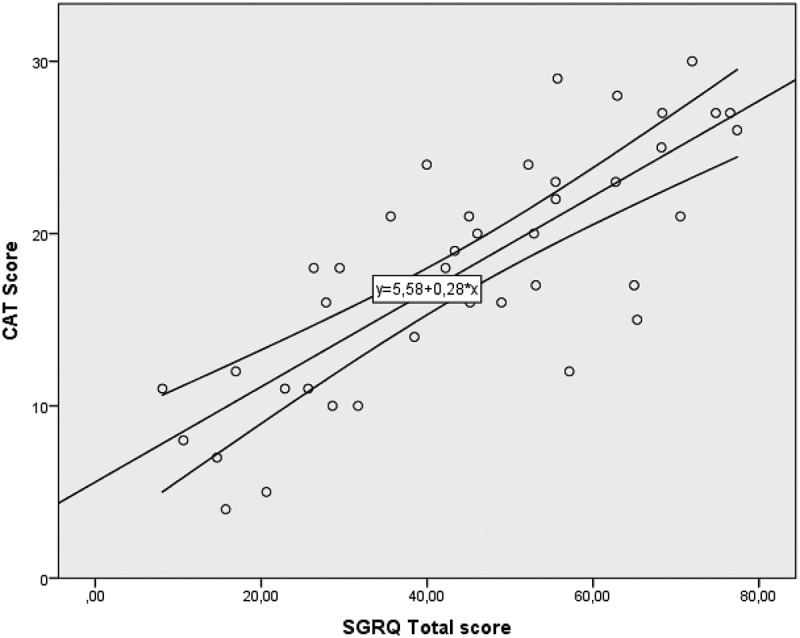
Correlation between the CAT and SGRQ total scores in patients with IPF.
Pearson correlation 0.8
SGRQ – The St. George’s Respiratory Questionnaire, IPF- Idiopathic Pulmonary Fibrosis, GAP Index – Gender, age and physiology.
Table 2.
Spearman correlations between the CAT and SGRQ scores and physiological variables in patients with IPF.
| CAT | SGRQ total score | |
|---|---|---|
| Age | −.053 | −.008 |
| FVC | −.338** | −.339* |
| FVC (%) | −.425** | −.433** |
| FEV1 | −.252* | −.127 |
| FEV1 (%) | −.323** | −.367* |
| FEV1/FVC | .0162 | .229 |
| DLCO | −.346** | −.299 |
| DLCO (%) | −.348** | −.280 |
| 6 MWD | −.582** | −.796** |
| MRC | .646** | . 805** |
| CAT | .8** | |
| Desaturation at 6 MWD | .679** | .06 |
**Correlation is significant at the 0.01 level (2-tailed).
*Correlation is significant at the 0.05 level (2-tailed).
Discussion
In this study, we found a strong correlation between the CAT score and SGRQ score, indicating that it is feasible to use the test to measure health-related quality of life among patients with IPF. The CAT score generally showed higher correlations with physiological parameters than the SGRQ score, supporting its validity as a test to assess the impact of IPF on health status. Interestingly, desaturation at 6 MWT, which is a prognostic parameter in IPF, only showed significance in relation to CAT.
The results are supported in a recent study from Japan, where Matsudo and colleagues also found that the CAT was significantly correlated with SGRQ score in a group of patients with IPF [31].
Symptoms such as dyspnoea [32] and physiological parameters such as DLCO [33] and 6 MWD [34] have been shown to correlate with quality of life among patients with IPF. In our study, the CAT score correlated to more physiological parameters than the SGRQ score did, indicating that it may provide a better picture of patients’ health-related quality of life, even though it is short and simple.
Patients with IPF are highly affected by the chronic, progressive disease, given its high morbidity and mortality. The lung tissue changes irreversibly, which results in symptoms such as dyspnoea and dry cough, which again affect patients’ everyday life. It has been shown that patients have a clear understanding of their prognosis but lack assistance with understanding how their disease will progress [35]. Exploring the patients’ perspective, Overgaard and colleagues found that the fatality of the disease was overwhelming for patients and, therefore, the dose and timing of information are tricky [36]. Sampson and colleagues showed that the patients’ initial relief associated with not being diagnosed with cancer was replaced by shock associated with the prognosis of IPF [35]. Clinical encounters, timely identification of changes in health status and functional activity and understanding of symptoms influence patients’ experiences of care. Therefore, the use of a standardized questionnaire such as the CAT score cannot stand alone. Instead, it must be accompanied with dialogue among the patient, patient’s family and health care professionals.
Given the lack of a disease-specific questionnaire for IPF, other health-related quality of life tools have been tested among patients with IPF. The Patient-Reported Outcome Measurement Information System (PROMIS), with the exception of sleep disturbance, has shown the ability to generate scores with a high level of sensitively in relation to the MRC [37]. Other available questionnaires are the Tool to Assess Quality of Life in Idiopathic Fibrosis (ATAQ-IPF) [38]. The SGRQ-I, which is an IPF-specific version of the SGRQ [19], and King’s Brief Interstitial Lung Disease (K-BILD) questionnaire [39] also exist. However, they have not been independently validated and are not widely used, and the K-BILD does not include questions about cough, a cardinal symptom in IPF patients. Currently, studies are needed to evaluate and validate all four questionnaires, although the reliability and validity of the SGRQ-I were found to be acceptable and comparable to the original SGRQ.
In addition to assessing health status and quality of life in IPF research, there is an urgent need for a reliable and valid tool for use in routine clinical practice. We needed a short and simple questionnaire that is available in Danish to facilitate our clinical evaluation of IPF patients. CAT is translated into many different languages, and therefor available in many cultural contexts. The focused information on daily symptoms, activity limitations, and disease impact guides consultations on patient-related outcomes and the optimal management plan in the time available for consultation. Nevertheless, whereas it was originally a COPD questionnaire, we chose the CAT for the aforementioned reasons. In fact, the CAT includes items that are valuable for assessing IPF, including fatigue and sleep quality, which are not available in disease-specific questionnaires [39].
There are some limitations in the present study. The CAT score was assessed using a Danish translation and even though it seems as though the scores across other European countries showed no significant difference in reporting [40], a published study of the validity and reliability of the Danish translation was not found. A relatively high number of values related, to SGRQ, to 6 MWD (and saturation before and after the test), and the MRC were missing. Although this was anticipated due to the poor physical condition of some of the included patients, it obviously influenced the strength and quality of our findings. The results from this study must be further validated before they can be considered generalizable due to limited number of patients with severe symptoms.
Conclusions
In conclusion, results support the validity of the CAT as a measure of symptoms in patients with IPF and correlates both to SGRQ and important physiological parameters in this group of patients.
Geolocation
The study was performed in the capital region in Denmark.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Du Bois RM.An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;21:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim DS, Collard HR, King TE Jr.. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Navaratnam V, Fleming KM, West J, et al. The rising incidence of idiopathic pulmonary fibrosis in the UK. Thorax. 2011;66:462–467. [DOI] [PubMed] [Google Scholar]
- [5].Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur Respir J. 2016;48:179–186. [DOI] [PubMed] [Google Scholar]
- [6].Olson AL, Swigris JJ, Lezotte DC, et al. Mortality from pulmonary fibrosis increased in the USA from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. [DOI] [PubMed] [Google Scholar]
- [7].Lee AS, Mira-Avendano I, Ryu JH, et al. The burden of idiopathic pulmonary fibrosis: an unmet public health need. Respir Med. 2014;108:955–967. [DOI] [PubMed] [Google Scholar]
- [8].Loveman E, Copley VR, Colquitt J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015;19:i–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Raimundo K, Chang E, Broder MS, et al. Clinical and economic burden of idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med. 2016;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hyldgaard C. A cohort study of Danish patients with interstitial lung diseases: burden, severity, treatment and survival. Dan Med J. 2015;62:B5069. [PubMed] [Google Scholar]
- [11].Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis: An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265–275. [DOI] [PubMed] [Google Scholar]
- [12].Kistler KD, Nalysnyk L, Rotella P, et al. Lung transplantation in idiopathic pulmonary fibrosis: a systematic review of the literature. BMC Pulm Med. 2014;14:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ley B, Hr C, Te K Jr.. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. [DOI] [PubMed] [Google Scholar]
- [14].Hyldgaard C, Hilberg O, Muller A, et al. A cohort study of interstitial lung diseases in central Denmark. Respir Med. 2014;108:793–799. [DOI] [PubMed] [Google Scholar]
- [15].Vancheri C, Failla M, Crimi N, et al. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. [DOI] [PubMed] [Google Scholar]
- [16].Gribbin J, Hubbard RB, Le J, et al. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer. 2013;81:288–293. [DOI] [PubMed] [Google Scholar]
- [18].Chan A, Lim E, Ng T, et al. Symptom burden and medication use in adult sarcoma patients. Support Care Cancer. 2015;23:1709–1717. [DOI] [PubMed] [Google Scholar]
- [19].Yorke J, Jones PW, Swigris JJ. Development and validity testing of an IPF-specific version of the St. George’s Respiratory Questionnaire. Thorax. 2010;65:921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. [DOI] [PubMed] [Google Scholar]
- [21].Nagata K, Tomii K, Otsuka K, et al. Evaluation of the chronic obstructive pulmonary disease assessment test for measurement of health-related quality of life in patients with interstitial lung disease. Respirology. 2012;17:506–512. [DOI] [PubMed] [Google Scholar]
- [22].Hwang YI, Jung KS, Lim SY, et al. A validation study for the Korean version of Chronic Obstructive Pulmonary Disease Assessment Test (CAT). Tuberc Respir Dis (Seoul). 2013;74:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kwon N, Amin M, Hui DS, et al. Validity of the COPD assessment test translated into local languages for Asian patients. Chest. 2013;143:703–710. [DOI] [PubMed] [Google Scholar]
- [24].Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. [DOI] [PubMed] [Google Scholar]
- [25].Swigris JJ, Esser D, Conoscenti CS, et al. The psychometric properties of the St George’s Respiratory Questionnaire (SGRQ) in patients with idiopathic pulmonary fibrosis: a literature review. Health Qual Life Outcomes. 2014;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Papiris SA, Daniil ZD, Malagari K, et al. The Medical Research Council dyspnea scale in the estimation of disease severity in idiopathic pulmonary fibrosis. Respir Med. 2005;99:755–761. [DOI] [PubMed] [Google Scholar]
- [28].ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- [29].Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. [DOI] [PubMed] [Google Scholar]
- [30].Kim ES, Choi SM, Lee J, et al. Validation of the GAP score in Korean patients with idiopathic pulmonary fibrosis. Chest. 2015;147:430–437. [DOI] [PubMed] [Google Scholar]
- [31].Matsuda T, Taniguchi H, Ando M, et al. COPD assessment test for measurement of health status in patients with idiopathic pulmonary fibrosis: a cross-sectional study. Respirology. 2017;22:721–727. [DOI] [PubMed] [Google Scholar]
- [32].Baddini Martinez JA, Martinez TY, Lovetro Galhardo FP, et al. Dyspnea scales as a measure of health-related quality of life in patients with idiopathic pulmonary fibrosis. Med Sci Monit. 2002;8:CR405–CR410. [PubMed] [Google Scholar]
- [33].Nishiyama O, Taniguchi H, Kondoh Y, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor? Respir Med. 2005;99:408–414. [DOI] [PubMed] [Google Scholar]
- [34].Verma G, Marras T, Chowdhury N, et al. Health-related quality of life and 6 min walk distance in patients with idiopathic pulmonary fibrosis. Can Respir J. 2011;18:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sampson C, Gill BH, Harrison NK, et al. The care needs of patients with idiopathic pulmonary fibrosis and their carers (CaNoPy): results of a qualitative study. BMC Pulm Med. 2015;15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Overgaard D, Kaldan G, Marsaa K, et al. The lived experience with idiopathic pulmonary fibrosis: a qualitative study. Eur Respir J. 2016;47:1472–1480. [DOI] [PubMed] [Google Scholar]
- [37].Yount SE, Beaumont JL, Chen SY, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. Lung. 2016;194:227–234. [DOI] [PubMed] [Google Scholar]
- [38].Yorke J, Spencer LG, Duck A, et al. Cross-Atlantic modification and validation of the A Tool to Assess Quality of Life in Idiopathic Pulmonary Fibrosis (ATAQ-IPF-cA). BMJ Open Respir Res. 2014;1:e000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Patel AS, Siegert RJ, Brignall K, et al. The development and validation of the King’s Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax. 2012;67:804–810. [DOI] [PubMed] [Google Scholar]
- [40].Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38:29–35. [DOI] [PubMed] [Google Scholar]


