ABSTRACT
Several studies support the notion that the kinase inhibitor Imatinib mesylate exerts off-target effects on cells of the immune system. After our first report of continuous daily oral administration in subjects with relapsed/refractory neuroblastoma (NB, EudraCT: 2005–005778-63), here we update the clinical information and report additional information on potential surrogate markers for prediction of efficacy.
Peripheral blood (PB) samples collected at study entry and after the first and second cycle of Imatinib mesylate treatment were tested for IFN-γ, TNF-α, TGF-β, IL-10, CXCL12 and soluble (s) B7-H6 plasma levels. In addition, paired PB and bone marrow (BM) samples collected at study entry and after the second Imatinib cycle were evaluated for CXCL12, CXCR4 and NKp30 isoform mRNA levels. Correlation between each parameter level and response/outcome was then evaluated.
Out of the six subjects still alive at the time of the first report, thee died after additional therapy, two for NB progression and one for a second malignancy. Three are presently alive and cured from NB at 10 years after the first Imatinib cycle. Of these, one achieved complete response (CR) during Imatinib treatment and never relapsed, one had a local relapse removed by surgery and the third received TVD as rescue therapy. Response and outcome were associated with low Imatinib exposure, whereas none of the tested immunological and molecular parameters was predictive of response/outcome. However, after Imatinib treatment NKp30 isoform mRNA levels significantly increase in BM samples, indicating that Imatinib mesylate exerted an off-target effect on NK cells in vivo.
Imatinib mesylate efficacy in relapsed/refractory NB has been confirmed at a longer follow-up, supporting its inclusion in new Phase II trials for these subjects, that should envisage collection of samples to evaluate the predictive power of other potential surrogate markers of efficacy.
Keywords: imatinib mesylate, neuroblastoma, pharmacokinetics, cytokines, NK cells
Introduction
Prognosis for children with relapsed or refractory neuroblastoma (NB) is dismal with cure rate below 5%,1,2 as recently confirmed by a meta-analysis of ITCC/SIOPEN European phase II clinical trials.3 Among the numerous chemotherapeutic trials performed in these subjects, the phase II EudraCT: 2005–005778-63 study with oral daily administration of Imatinib mesylate for up to 12 months in 24 patients showed five complete responses (CR, 21%) and two partial responses (PR, 8%) lasting several months accompanied by low toxicity and high compliance.4 Disease status at study entry, time from diagnosis to enrolment and absent/low Tyrosine hydroxylase (TH) mRNA expression in bone marrow (BM) and peripheral blood (PB) samples at study entry associated with favourable outcome, suggesting that Imatinib mesylate efficacy was achieved in the presence of low tumour burden. Surprisingly, however, response and favourable outcome associated with low Imatinib exposure,4 suggesting that high drug concentration could affect survival of cells involved in anti-NB response.
Based on these data and on observations in adult subjects with gastro-intestinal stromal tumours (GIST),5,6 we investigated the effect of Imatinib mesylate on cells of the innate immunity in vitro. Concentration ≤ 6 μg/ml, comparable to those achieved by subjects with low exposure, did not affect NK cell ability to lyse NB cells nor macrophage secretion of pro-inflammatory and immune-stimulatory cytokines.7 Interestingly, low doses of Imatinib mesylate increased CXCR4 surface expression on lymphocytes and monocytes,7 supporting the hypothesis that homing of immune effectors into the BM, the main site of NB metastases,8 could be favoured by activation of the CXCR4-CXCL12 axis.
In subjects with GIST, response to Imatinib mesylate inversely correlated with NK cell activating receptor NKp30 mRNA expression, ∆BC NKp30 isoform mRNA ratio,9,10 and with plasma level of soluble (s) B7-H6, the ligand of NKp30.10 Interestingly, in three independent cohorts of high-risk NB in CR after induction therapy, ∆BC NKp30 mRNA associated with response to first line chemotherapy.11
Based on these new findings on the off-target effects of Imatinib mesylate and the existence of surrogate biomarkers for its efficacy in adult subjects with GIST, we measured plasma levels of IFN-γ, TNF-α, TGF-β, IL-10, CXCL12 and sB7-H6, and the ratio of NKp30 mRNA isoforms, in samples collected during the Imatinib mesylate trial.4 The objective of this study was to evaluate the association between each of these parameters and clinical response/outcome, and to update the clinical information regarding the subjects enrolled in the trial.
Methods
Subjects and trial
The EudraCT: 2005–005778-63 trial, Phase II study of Imatinib mesylate in 24 patients with relapsed/refractory NB, was a multicentre, single arm, open label, longitudinal, dose-response study, applying a Simon sequential two-stage design12 incorporating response rate and toxicity13 to protect subjects from treatments with toxic or ineffective agents.
Imatinib mesylate was provided by Novartis Pharma, Origgio, Italy, as 100 mg film-coated capsules. Imatinib was administered bis in die (BID) at a dose of 170 mg/sqm for the first 4 weeks (cycle), escalated to 300 mg/sqm for the following cycles, up to 12 cycles or up to progression of the disease.
Complete assessment was carried out every two months and at suspicion of disease progression. Complete information on clinical characteristics of the subjects was given in the first report of the trial.4
Collection of peripheral blood (PB) samples for pharmacokinetics and ELISA assays
PB samples were collected at study entry and after the first and second cycle of Imatinib mesylate treatment from 19 children (age range 2–16 years, median 7 years). Plasma aliquots were stored at −80°C and freshly thawed aliquots were used for the ELISA assays. Plasma at study entry, after the first and second cycle were available for 13 subjects, namely #2, 3, 4, 5, 8, 11, 13, 14, 15, 16, 17, 19 and 22.4 Each subject is indicated by the same symbol in all graphs and figures. All ELISA assays were performed according to manufacturer’ protocols. ELISA assays for IFN-γ, TNF-α, TGF-β, IL-10 and CXCL12 were purchased from R&D Systems (R&D Systems duo-set ELISA, Minneapolis, MN, USA), whereas ELISA assay for sB7-H6 was purchased from AVIVA Systems Biology (San Diego, CA, USA).
Collection of BM and PB samples for molecular analysis
Half mL of each BM aspirate and 2 mL of PB were collected at study entry and after two cycles of Imatinib mesylate. Samples were stored in PAX© tubes at −80°C and RNA was extracted according to manufacturer’ procedures. Total RNA from both PB and BM samples at study entry and after two cycles was available for 7 subjects, namely #4, 8, 14, 15, 16, 19 and 22,4 all included in the above list of 13 subjects.
Total RNA was reverse transcribed and then amplified in triplicate by RT-qPCR, as described,14 with the CXCL12 and CXCR4 specific TaqMan© human gene expression assays (Thermo Fisher, catalog# Hs00171022_m1 and Hs00607978_s1, respectively), or with the primers and probe for NKp30 isoforms A, B and C and for the housekeeping gene β2-microblobulin (B2M), described by Delahaye et al.9 Results were expressed as Log 2−delta Cq by subtracting the Cq value obtained for the housekeeping gene B2M from the Cq value of the target RNA.15 Ratio of the three isoforms was calculated according to the formula ∆NKp30x NKp30y = CqNKp30y-CqNKp30x.10
Statistical methods
Potential associations between pharmacokinetics data, cytokine and sB7-H6 plasma levels, mRNA levels and NKp30 mRNA isoforms’ ratio with response/outcome were evaluated by the Mann-Whitney U test for continuous variables and the two-sided Fisher’s exact tests for binary (categorized) variables. Statistical analysis was performed by using GraphPad Prism 5.00 Software (GraphPad, Inc. La Jolla, CA). A p < 0.05 was considered significant.
Overall survival (OS) and progression-free survival (PFS) were analyzed by the Kaplan-Meier method using Stata for Windows statistical package (release 13.1, Stata Corporation, College Station, TX).
Results
Clinical update
Figure 1 shows the 10-year OS and the PFS of the 24 subjects enrolled in the Imatinib mesylate trial. Ten subjects had progressive disease (PD) during the first and second cycles and died shortly afterward; 5 subjects achieved complete response (CR), 2 partial response (PR) and 7 stable disease (SD) (Table 1). After the last Imatinib cycle, one died of a myelo-displastic syndrome with evolution to acute leukemia and 12 progressed; of these seven were treated with oral etoposide, two with intravenous chemotherapy and one with surgery (Table 1).
Figure 1.
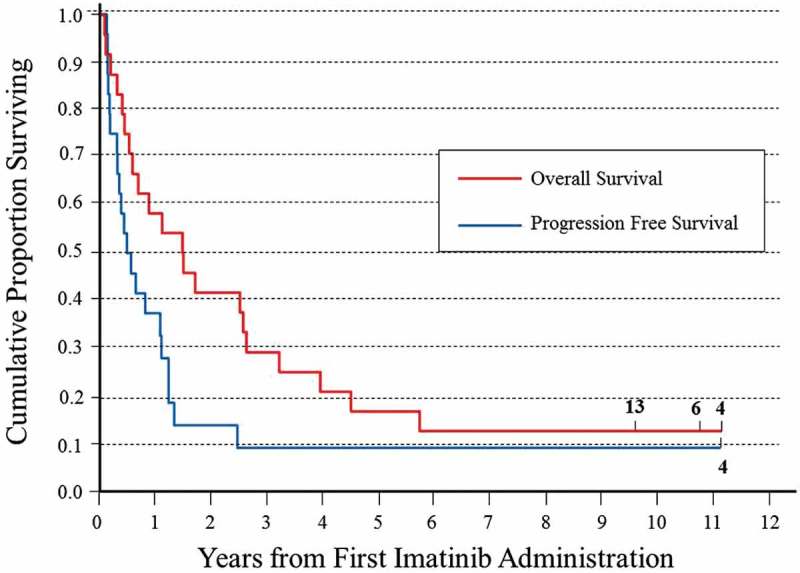
Kaplan-Meier plot of overall and progression-free survival after the first imatinib mesylate of the 24 subjects with relapsed/refractory NB enrolled in the EudraCT: 2005–005778-63 trial.
Table 1.
Clinical update on the fourteen subjects enrolled in the trial that did not progress during the first two cycle of Imatinib mesylate.
| Enrollment number | Status pre Imatinib | Disease status pre Imatinib (PT, B-BM, 123mIBG) * | Imatinib cycles | Best response | Time to progression from last Imatinib (months) | Rescue Therapy° | State at follow-up (2017) |
|---|---|---|---|---|---|---|---|
| 22 | PR after 1° line therapy | PT, B-BM, multiple | 14 | PR | 1 | VP16 | Dead |
| 9 | PR after 1° line therapy | BM, one | 12 | CR | 1 | VP16 | Dead |
| 10 | PR after 1° line therapy | PT, multiple | 8 | SD | - | none | Dead (a) |
| 8 | PR after 2° line therapy | PT, B-BM, multiple | 29 | PR | 1 | VP16 | Dead |
| 12 | PR after 2° line therapy | PT, B, multiple | 4 | SD | 4 | none | Dead |
| 13 | PR after 2° line therapy | PT, BM, multiple | 12 | CR | 3 | TVD | Alive |
| 15 | PR after 2° line therapy | PT, BM, multiple | 12 | CR | 1 | TEMIRI | Dead |
| 11 | PR after 2° line therapy | B/BM, two | 2 | SD | 1 | VP16 | Dead (b) |
| 7 | PR after 2° line therapy | B, multiple | 3 | SD | 1 | none | Dead |
| 14 | PR after 2° line therapy | B, one | 4 | SD | 1 | VP16 | Dead |
| 20 | Resistant relapse | PT, tumor | 4 | SD | 12 | VP16 | Dead |
| 17 | Resistant relapse | BM, one | 6 | CR | 1 | VP16 | Dead |
| 4 | Untreated relapse | PT, B-BM, multiple | 12 | CR | – | none | Alive |
| 6 | Untreated relapse | PT, tumor | 6 | SD | 4 | surgery | Alive |
Patients highlighted in blue were alive at the time of the first report of the trial 4.
* PT = presence of primary tumor, B = presence of bone metastasis, BM = presence of bone marrow metastasis, number of 123mIBG spots.
° VP16 = oral etoposide, TVD = Topotecan/Vincristine/Doxorubicine, TEMIRI = temozolomide-irinotecan
(a) This patient died of myelodisplastic syndrome with evolution to acute leukemia; (b) This patient died of a second malignancy (leukemia).
At the time of the first report of the trial,4 six subjects were alive (Table 1, blue rows); of these #22 died of NB progression at 30 months after last Imatinib and #20 died of unknown cause 27 months after last Imatinib. Both of them were treated with oral etoposide, whereas subject #10 never had NB progression but died of a second malignancy 62 months after last Imatinib. Presently, at 10 years after the last Imatinib, three subjects are alive and cured of NB. Subject #4 achieved CR during Imatinib treatment and never relapsed (Supplemental narrative), subject #6 relapsed locally and after surgery is in continuous CR and subject#13 is in continuous CR after TVD rescue therapy (#13, #4 and #6, Figure 1 and Table 1).
Plasma concentration of imatinib mesylate associated with response/outcome
In the first report of the trial, correlation between Imatinib mesylate exposure and response/outcome was established using the median maximal plasma concentration (Cmax) at first and second cycles.4 To better define the exposure associated with response/outcome and identify a cut-off value, we draw chart of the steady state area under the curve (ng*h/mL) at first and second cycle vs. a response score of 3 for CR, 2 for partial response (PR), 1 for stable disease (SD) and 0 for progression disease (PD). Recently, in fact subjects achieving SD have been considered as responder.16–18 As shown in Figure 2A and 2B, the charts suggested that response associated with a steady state value below 20.000 ng*h/mL at first cycle and below 35.000 ng*h/mL at second cycle, respectively.
Figure 2.
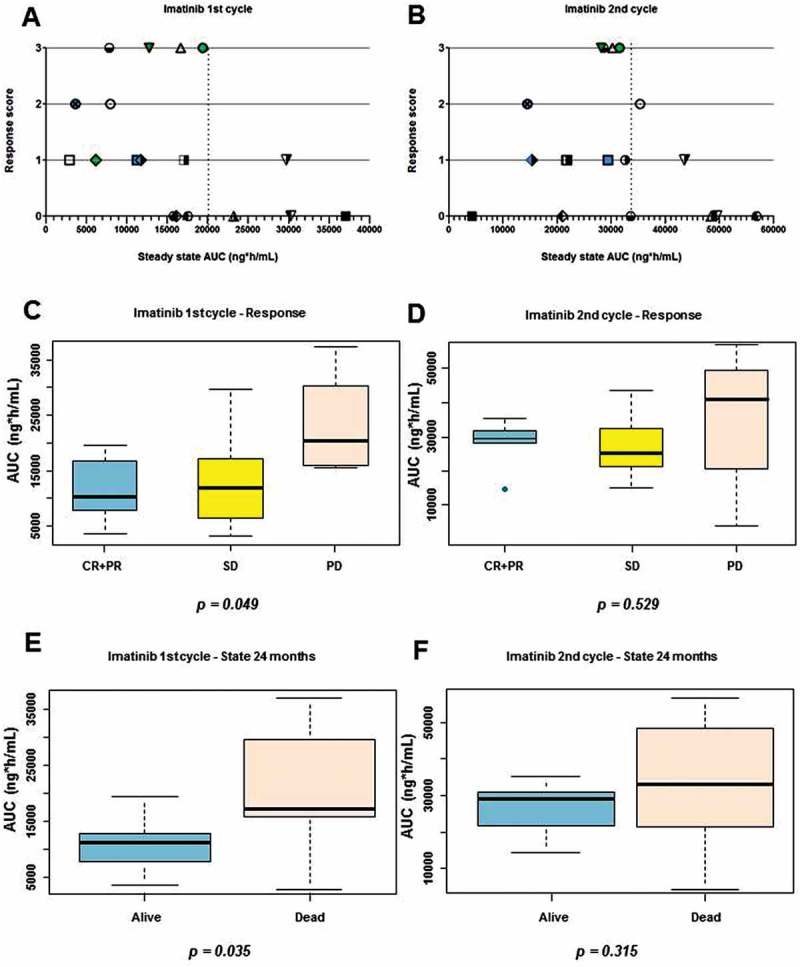
Association between imatinib mesylate exposure and response/outcome. A) Chart of steady state at first cycle of treatment versus response score (CR = 3, PR = 2, SD = 1 and PD = 0). B) Chart of steady state at second cycle of treatment versus response score. Each subject is indicated by the same symbol in the two graphs. C) Box-plot of at first cycle in responsive (CR+PR, blue), SD (yellow) and PD (red) subjects. D) Box-plot of at second cycle in responsive (CR+PR, blue), SD (yellow) and PD (red) subjects. E) Box-plot of at first cycle in subjects alive (blue) and dead (red) at 24 months after first Imatinib mesylate. F) Box-plot of at second cycle in subjects alive (blue) and dead (red) at 24 months after first imatinib mesylate.
Application of the cut-off values confirmed that subjects that achieved CR or PR had significantly lower levels at first cycle than subjects that progressed (PD) (p = 0.049, Figure 2C). It is interesting to note that subjects that achieved SD had levels similar to responders (Figure 2A and 2C). At the second cycles (Figure 2D), the differences in levels were not significant.
Since the median of the OS curve (Figure 1) is near 18 months, we then evaluated whether levels at first and second cycle associated with the 2-year survival. Subjects alive at 24 months after the first Imatinib cycle had significantly lower at the first cycle than those dead at that time point (Figure 2E p = 0.035). No significant difference in outcome occurred instead when considering levels at second cycle (Figure 2F). Taking together, these findings confirmed that low after an Imatinib mesylate dose of 170 mg/sqm BID associated with response and favorable outcome.
Plasma levels of cytokines, CXCL12 and sb7-h6 did not associate with clinical response
Based on the experience in subjects with GIST6,9,10 and on our in vitro data7 regarding the off-target effect of Imatinib mesylate on NK cell activity, we then measured plasma levels of the pro-inflammatory and immune-suppressive cytokines IFN-γ, TNF-α, TGF-β, IL-10 (secreted by activated NK cells and monocyte/macrophages), CXCL12 (the ligand of CXCR4) and of sB7-H6 (the ligand of the NK activating receptor NKp30).
Plasma samples collected at study entry, after the first and second Imatinib mesylate cycle were available from 13 subjects, whose exposures were representative of the entire cohort (Supplemental Figure 1). In all the 13 subjects, the measured plasma levels after Imatinib mesylate first and second cycle were not significantly different from those at study entry (Figure 3). The pro-inflammatory IFN-γ and TNF-α were undetectable in all except one subject, whereas TGF-β, IL-10, CXCL12 and sB7-H6 levels covered a wide range. However, no significant correlation between these parameters and response or outcome was found (Supplemental Figure 2 and 3, respectively).
Figure 3.
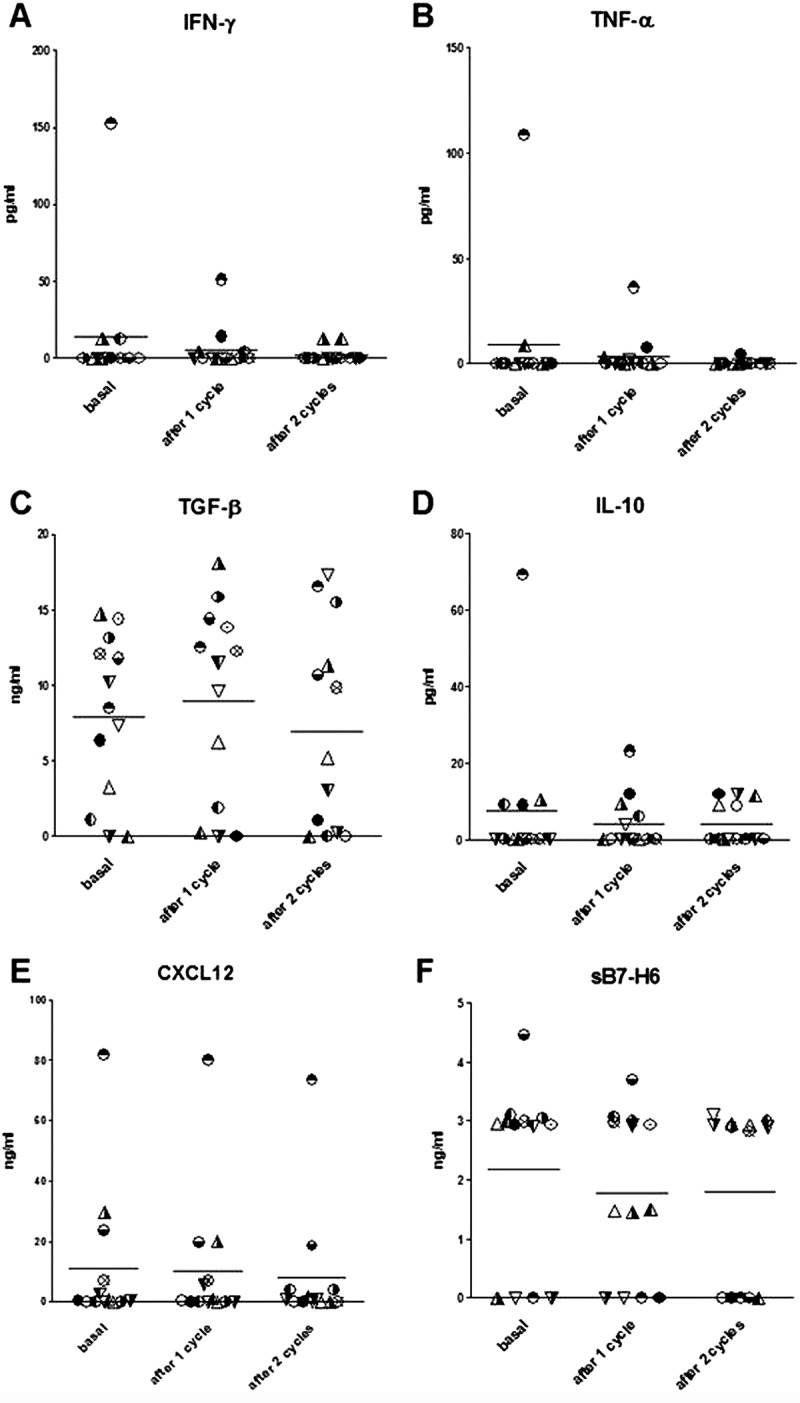
Plasma levels of IFN-γ (A), TNF-α (B), TGF-β (C), IL-10 (D), CXCL12 (E) and sB7-H6 (F) measured in 13 subjects, at study entry (basal) and after 1 or 2 cycles of imatinib mesylate. Each subject is indicated by the same symbol in all graphs.
Accordingly, CXCL12 and CXCR4 mRNA levels after Imatinib mesylate second cycle were not significantly different from those at study entry, in both PB and BM samples (Supplemental Figure 4A-D), and no association between mRNA levels and outcome was found (Supplemental Figure 4E-H).
Ratio of NKp30 isoforms did not associate with clinical response
The NKp30 activating receptor can be assembled starting from three different mRNA isoforms. NKp30 isoforms A and B deliver cytotoxic signals through secretion of IFN-γ and TNF-α, whereas isoform C deliver immune-suppressive signal through the secretion of IL-10.6 The expression levels of the NKp30 mRNA isoforms and the ratio between isoforms A or B and isoform C was shown to be predictive of Imatinib mesylate efficacy in subjects with GIST.9,10 We thus evaluated NKp30 isoform mRNAs in PB and BM samples taken at study entry and after two Imatinib mesylate cycles.
In PB samples, expression of NKp30 mRNA isoforms was not significantly modified by Imatinib mesylate treatment (Figure 4A, 4C and 4E), whereas in BM samples expression of all three isoforms significantly increased (Figure 4B, 4D and 4F), demonstrating an in vivo effect of Imatinib mesylate on the NK cells present in the BM compartment. It is interesting to note that at study entry all NKp30 isoform mRNA levels in BM were lower than in PB, confirming a previous observation in high-risk NB subjects.11
Figure 4.
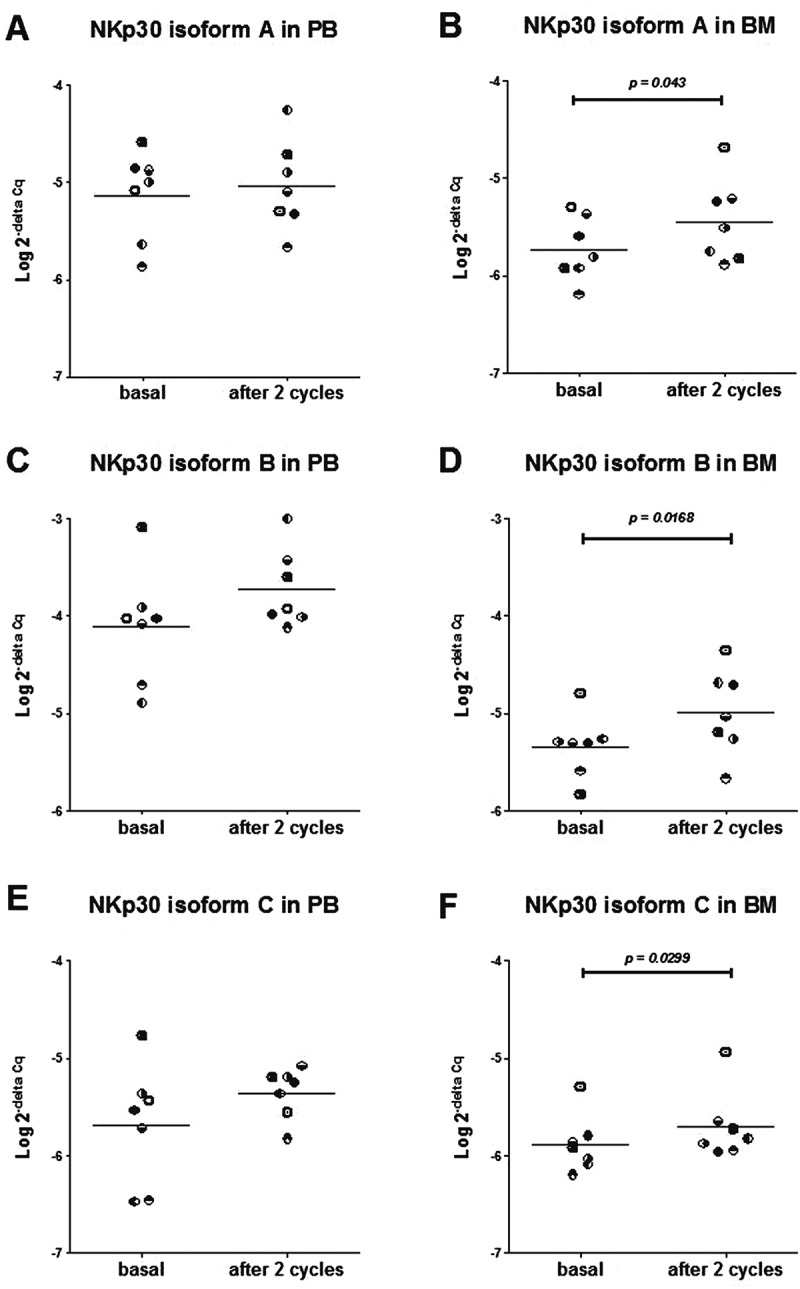
Expression levels (Log 2-delta Cq) of NKp30 receptor mRNA isoform A (A, B), isoform B (C, D) and isoform C (E, F) evaluated in PB samples (A, C, E) and BM samples (B, D, F), at study entry (basal) and after 2 cycles of Imatinib mesylate in 7 subjects. Each subject is indicated by the same symbol in all graphs.
NKp30 isoform levels did not associate with response (Supplemental Figure 5) in accordance with a previous observation in subjects with high-risk NB.11 The ratio of NKp30 isoforms (∆AB, ∆AC and ∆BC) was not modified by Imatinib mesylate treatment (Supplemental Figure 6), and no association with response was found, both in PB and BM samples (Figure 5).
Figure 5.
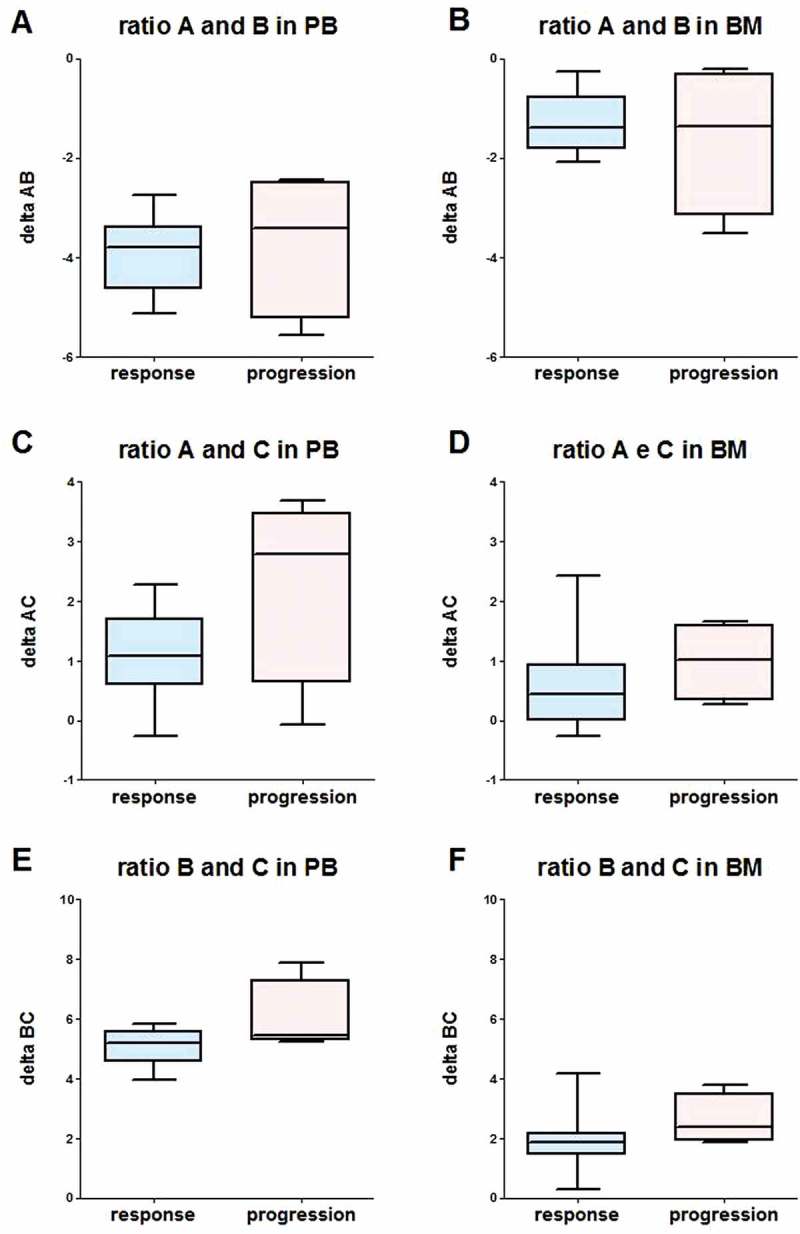
Box-plot of NKp30 ∆AB (A, B), ∆AC (C, D) and ∆BC (E, F), evaluated in PB samples (A, C, E) and BM samples (B, D, F) in responsive (CR+ PR, response, blue) and no responsive (SD + PD, progression, red) subjects, using data measured after 2 cycles of Imatinib mesylate. ∆ was calculated according to the formula ∆NKp30x NKp30y = CqNKp30y – CqNKp30x.
Discussion
Among the 24 subjects with relapsed/refractory NB enrolled in the Imatinib mesylate study EudraCT: 2005–005778-63, we obtained five CR (21%) and two PR (8%), with six subjects alive at 18 months after Imatinib mesylate, including one in complete remission (CR) without any further treatment.4 Here, we report that three of these subjects are still alive 10 years after the first Imatinib mesylate cycle. One never relapsed, one relapsed locally and after the surgical resection of the tumor is free of NB, and the third progressed, but after rescue therapy with topotecan+vincristine+doxorubicin, is also free of NB. A fourth subjects was cured from NB but died of a second malignancy. Thus, the 10-year overall survival achieved in this chemotherapeutic trial (12.5%) is suggestive of prolonged Imatinib efficacy in some subjects with relapsed/refractory NB.
At the time of the first report, response/outcome appeared to associate with low tumour burden and quite surprisingly with low Imatinib mesylate exposure.4 Here, the associations between low Imatinib mesylate exposure and response or favourable outcome have been confirmed by using a different method. It is interesting to note that the association was significant when the dose of 170 mg/sqm BID, used during the first cycle of treatment, was considered. These findings are in accordance with the in vitro study demonstrating that high doses of Imatinib mesylate have detrimental effects on cells of the innate immunity.7
Since in subjects with GIST Imatinib mesylate efficacy associated with high levels of IFN-γ6, low levels of sB7-H6 and low NKp30 mRNA isoform ∆BC ratio,9,10 we evaluated in samples collected during the EudraCT 2005–005778-63 trial whether these surrogate markers could predict response also in subjects with relapsed/refractory NB. Unfortunately, none of these surrogate markers was predictive of response/outcome.
In most of the enrolled subjects, plasma levels of sB7-H6 were below the limit of detection of the commercial ELISA assay. Although NB cells express B7-H6 mRNA and sB7-H6 has been measured in a fraction of children with NB,11 it is unknown whether it is massively released from the cell membrane as occurring in GIST cells,10 or in variable way as occurring in ovarian cancer.19 However, the possibility that the sensitivity of the commercial ELISA was lower than the home-made one used for subjects with GIST10 cannot be ruled out.
As for the ratio of NKp30 mRNA isoforms, the levels of all three mRNA isoforms significantly increased in BM after treatment, demonstrating that the off-target effect of Imatinib mesylate on NK cells may occur in vivo. The lower BM expression levels measured at study entry as compared to PB levels, already described in subjects with metastatic NB,11 may be due to a lower expression of activating receptor by BM-derived NK cells or to a lower frequency of NK cells in the BM space. Since BM is the elective metastatic site for NB,8 the second hypothesis appears to be more likely, also because the expression of all three isoforms increased after Imatinib treatment, suggesting the occurrence of an increased recruitment of NK cells, each one harbouring a different NKp30 isoform. This event was possibly supported by the increased surface levels of CXCR4 protein in PB-derived NK cells, as observed in vitro.20
As for the lack of association between NKp30 ∆BC and response/outcome, this may be dependent on the small number of samples tested. In fact, NKp30 ∆BC was predictive of response/outcome in subject with high-risk NB without BM metastatic disease after induction chemotherapy.11 However, the possibility that BM disease and/or the occurrence of NB-mediated immunosuppressive mechanisms had damped NK cell function21 cannot be ruled out.
We also tested plasma for pro-inflammatory or immune-stimulatory cytokines released by cells of innate immunity, as well as for CXCL12, the main attractor of immune cells into the BM space20 and the ligand of CXCR4, up-regulated by low doses of Imatinib mesylate in vitro.7 None of these parameters was significantly modified by Imatinib mesylate treatment at either 170 or 300 mg/sqm BID. Most importantly, no association was found between plasma level and response/outcome for any of these parameters, thus excluding a predictive power of Imatinib efficacy in subjects with relapsed/refractory NB for all of them.
In conclusion, given the very limited number of new drugs available for pediatric subjects with solid tumors,22,23 the 10-year survival rate achieved with Imatinib mesylate in subjects with relapsed/refractory NB, together with the high tolerability and minor toxicity 4, supports its inclusion into new treatment programs for these subjects 18. A new trial with a dose of 170 mg/sqm BID would possibly allow cells of innate immunity to drive an effective anti-NB response, and a correct design of sample collection and analysis may eventually lead to the identification of predictive markers of Imatinib mesylate efficacy in subjects with relapsed/refractory NB.
Funding Statement
This study was supported by Fondazione Italiana per la lotta al neuroblastoma [Progetto clinico to MVC and AG]. Fabio Morandi was the recipient of a Fellowship from Fondazione Umberto Veronesi.
Acknowledgments
Imatinib was provided by Novartis Parma, Origgio, Italy.
Disclosure of potential conflicts of interest
AM was a founder and shareholder of Innate-Pharma (Marseille, France). The remaining authors declare no conflicts of interest.
Supplemental Material>
Data for this article can be accessed here
References
- 1.Garaventa A, Parodi S, De Bernardi B, Dau D, Manzitti C, Conte M, Casale F, Viscardi E, Bianchi M, D’Angelo P, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45(16):2835–2842. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 2.London WB, Castel V, Monclair T, Ambros PF, Pearson AD, Cohn SL, Berthold F, Nakagawara A, Ladenstein RL, Iehara T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol. 2011;29(24):3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno L, Rubie H, Varo A, Le Deley MC, Amoroso L, Chevance A, Garaventa A, Gambart M, Bautista F, Valteau-Couanet D, et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediat Blood Cancer. 2017;64(1):25–31. doi: 10.1002/pbc.26192. [DOI] [PubMed] [Google Scholar]
- 4.Calafiore L, Amoroso L, Della Casa Alberighi O, Luksch R, Zanazzo G, Castellano A, Podda M, Dominici C, Haupt R, Corrias MV, et al. Two-stage phase II study of imatinib mesylate in subjects with refractory or relapsing neuroblastoma. Ann Oncology. 2013;24(5):1406–1413. doi: 10.1093/annonc/mds648. [DOI] [PubMed] [Google Scholar]
- 5.Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114(3):379–388. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menard C, Blay JY, Borg C, Michiels S, Ghiringhelli F, Robert C, Nonn C, Chaput N, Taieb J, Delahaye NF, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69(8):3563–3569. doi: 10.1158/0008-5472.CAN-08-3807. [DOI] [PubMed] [Google Scholar]
- 7.Bellora F, Dondero A, Corrias MV, Casu B, Regis S, Caliendo F, Moretta A, Cazzola M, Elena C, Vinti L, et al. Imatinib and nilotinib off-target effects on human NK cells, monocytes, and M2 macrophages. J Immunol. 2017;199(4):1516–1525. doi: 10.4049/jimmunol.1601695. [DOI] [PubMed] [Google Scholar]
- 8.Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, Weiss WA.. Neuroblastoma. Nat Rev Dis Prim. 2016;2(16078). doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 9.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17(6):700–707. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 10.Rusakiewicz S, Perier A, Semeraro M, Pitt JM, Pogge Von Strandmann E, Reiners KS, Aspeslagh S, Piperoglou C, Vely F, Ivagnes A, et al. NKp30 isoforms and NKp30 ligands are predictive biomarkers of response to imatinib mesylate in metastatic GIST patients. Oncoimmunology. 2017;6(1):e1137418. doi: 10.1080/2162402x.2015.1137418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semeraro M, Rusakiewicz S, Minard-Colin V, Delahaye NF, Enot D, Vely F, Marabelle A, Papoular B, Piperoglou C, Ponzoni M, et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci Translat. 2015;7(283):283ra55. doi: 10.1126/scitranslmed.aaa2327. [DOI] [PubMed] [Google Scholar]
- 12.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Bryant J, Day R. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Biometrics. 1995;51(4):1372–1383. doi: 10.2307/2533268. [DOI] [PubMed] [Google Scholar]
- 14.Scaruffi P, Morandi F, Gallo F, Stigliani S, Parodi S, Moretti S, Bonassi S, Fardin P, Garaventa A, Zanazzo G, et al. Bone marrow of neuroblastoma patients shows downregulation of CXCL12 expression and presence of IFN signature. Pediat Blood Cancer. 2012;59(1):44–51. doi: 10.1002/pbc.23339. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Amoroso L, Erminio G, Makin G, Pearson ADJ, Brock P, Valteau-Couanet D, Castel V, Pasquet M, Laureys G, Thomas C, et al. Topotecan-vincristine-doxorubicin in stage 4 high-risk neuroblastoma patients failing to achieve a complete metastatic response to rapid COJEC: A SIOPEN study. Cancer Res Treat. 2018;50(1):148–155. doi: 10.4143/crt.2016.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Giannatale A, Dias-Gastellier N, Devos A, Mc Hugh K, Boubaker A, Courbon F, Verschuur A, Ducassoul S, Malekzadeh K, Casanova M, et al. Phase II study of temozolomide in combination with topotecan (TOTEM) in relapsed or refractory neuroblastoma: a european innovative therapies for children with cancer-SIOP-european neuroblastoma study. Eur J Cancer. 2014;50(1):170–177. doi: 10.1016/j.ejca.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Moreno L, Caron H, Geoerger B, Eggert A, Schleiermacher G, Brock P, Valteau-Couanet D, Chesler L, Schulte JH, De Preter K, et al. Accelerating drug development for neuroblastoma - new drug development strategy: an innovative therapies for children with cancer, european network for cancer research in children and adolescents and international society of paediatric oncology Europe neuroblastoma project. Expert Op Drug Disc. 2017;12(8):801–811. doi: 10.1080/17460441.2017.1340269. [DOI] [PubMed] [Google Scholar]
- 19.Pesce S, Tabellini G, Cantoni C, Patrizi O, Coltrini D, Rampinelli F, Matta J, Vivier E, Moretta A, Parolini S, et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. Oncoimmunology. 2015;4(4):e1001224. doi: 10.1080/2162402x.2014.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellora F, Castriconi R, Dondero A, Carrega P, Mantovani A, Ferlazzo G, Moretta A, Bottino C. HumanNK cells and NK receptors. Immunol Lett. 2014;161(2):168–173. doi: 10.1016/j.imlet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Bottino C, Dondero A, Bellora F, Moretta L, Locatelli F, Pistoia V, Moretta A, Castriconi R. Natural killer cells and neuroblastoma: tumor recognition, escape mechanisms, and possible novel immunotherapeutic approaches. Front Immunol. 2014;5:56. doi: 10.3389/fimmu.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vassal G, Kearns P, Blanc P, Scobie N, Heenen D, Pearson A. Orphan drug regulation: A missed opportunity for children and adolescents with cancer. Eur J Cancer. 2017;84:149–158. doi: 10.1016/j.ejca.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Amoroso L, Haupt R, Garaventa A, Ponzoni M. Investigational drugs in phase II clinical trials for the treatment of neuroblastoma. Exp Op Iinvestigat Drugs. 2017;26(11):1281–1293. doi: 10.1080/13543784.2017.1380625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


