ABSTRACT
Background: Chronic non-malignant lung diseases such as chronic obstructive pulmonary disease (COPD) and interstitial lung diseases (ILD) result in reduced quality of life (QoL), a high symptom burden and reduced survival. Patients with chronic non-malignant lung disease often have limited access to palliative care. The symptom burden and the QoL of these patients resembles patients with cancer and the general palliative approach is similar. However, the disease trajectory is often slow and unpredictable, and the palliative effort must be built on accessibility, continuity and professional competences. The Danish Health Authority as well as the WHO recommends that there is access to palliative care for all patients with life-threatening diseases regardless of diagnosis. In 2011, the Danish Health Authority requested that the national medical societies would to formulate guidelines for palliation.
Methods: In 2015, a group of members of the Danish Respiratory Society (DRS) was appointed for this purpose. It was composed of experienced ILD and COPD researchers as well as clinicians from different parts of Denmark. A literature review was made, a draft was prepared, and all recommendations were agreed upon unanimously.
Results: The Danish version of the position paper was finally submitted for review and accepted by all members of DRS.
Conclusion: In this position paper we provide recommendations on the terminology of chronic and terminal lung failure, rehabilitation and palliative care, advanced care planning, informal caregivers and bereavement, symptom management, the imminently dying patient, and organization of palliative care for patients with chronic non-malignant lung diseases.
KEYWORDS: Palliation, non-malignant, lung disease, end-of-life, terminal lung failure, chronic lung failure
Background
The World Health Organization (WHO) has defined palliative care as ‘ an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual [1]’.
Palliative care in patients with malignant lung diseases is well established and validated [2], but there is a knowledge gap concerning palliation in patients with chronic non-malignant lung diseases and their access to palliative care [3,4]. Chronic obstructive pulmonary disease (COPD) and interstitial lung diseases (ILD) may differ from cancer with respect to pathophysiology and prognosis, but in general palliative care practices remain similar. The treatment goals for all progressive lung diseases are to alleviate symptoms, to slow down disease progression and thereby improve QOL. These goals are best achieved in a collaboration between the informed, active patient, the primary caregiver(s) and a well-prepared pro-active multidisciplinary team [5,6].
It is a common misconception that palliation should only be initiated in the terminal phase of a disease. Palliation should be initiated early in the course of the disease alongside disease-specific treatment, and should aim at easing the disease burden when QOL is reduced [7–14]. The hallmark of chronic non-malignant lung disease is its unpredictable course, the considerable uncertainty that may result in prognostic paralysis [15] as well as delay in conversations between the patient and health care professionals regarding end-of-life issues, often due to a fear of addressing these topics too early.
The Danish Health Authority has called for national guidelines for palliative care in non-malignant chronic, progressive, symptomatic diseases [14]. A task force was appointed by the Danish Respiratory Society (DRS), and the present position paper describes the recommendations of the task force [16].
Methods
In 2015, DRS appointed a group of members including experienced COPD and ILD researchers as well as clinicians from different parts of Denmark.
A literature review was conducted and new literature searches were repeatedly updated with new or expanded search criteria. Thus, the current document is a position paper and not a systematic review.
A draft was prepared, and several meetings were held to clarify form and definitions. A primary author wrote the first draft that went through several reviews in the appointed DRS group; all definitions and recommendations were agreed upon unanimously. The position paper was finally submitted for review and accepted by all members of DRS. The Danish version is published on the DRS website (https://www.lungemedicin.dk/fagligt/klaringsrapporter.html).
|
Recommendation 1. Terminology Chronic lung failure is defined as a permanently reduced lung function and daily symptoms limiting daily activities despite optimal standard therapy. Palliative care can be started at this point. Terminal lung failure is present when a patient with chronic lung failure meets the criteria of being terminally ill and when life expectancy is limited to weeks or a few months. Imminently dying is when death is expected shortly i.e. within hours or a few days |
Terminology
When treating patients with life-threatening diseases, it is important to have a well-defined terminology. Therefore, DRS began by defining the three stages of disease where palliative care should be considered.
Chronic lung failure
Chronic lung failure is defined as a permanently reduced lung function and presence of daily symptoms despite optimal standard treatment. Chronic lung failure is present when the disease limits activities of daily living.
Patients may develop chronic lung failure due to different diseases such as COPD, ILD or bronchiectasis. In patients with chronic lung failure, the disease is so severe that palliative care, as described in this document, can be initiated. In patients with chronic lung failure prognostication may thus be difficult and the definition does not rely on expected time to death.
Terminal lung failure
Terminal lung failure is when death is anticipated to be close. There are no validated clinical tools for assessing the short-time risk of death within for instance 6 months. DRS recommends using the following criteria:
Chronic obstructive pulmonary disease (COPD)
The patient is considered to be in the terminal phase of the disease when dependent on help from others because of dyspnea or general weakness over a period of several months, and when at least two of the following criteria are fulfilled:
• At least two hospitalizations because of a COPD exacerbation and/or one hospitalization requiring treatment with non-invasive ventilation (NIV) or a ventilator within the past 6 months.
• Need of long-term oxygen therapy (LTOT)
• Permanent NIV treatment at home
• Body mass index (BMI) <18 despite optimal nutrition, including nutritional supplementation
• Progressive or newly diagnosed severe comorbidity
Interstitial lung diseases (ILD)
The patient is considered to be in the terminal phase of the disease when dependent on help from others because of dyspnea or general weakness over a period of several months, and when fulfilling at least two of the following criteria:
• Two or more respiratory hospitalizations (e.g., due to infection or exacerbation) within the last year
• Peripheral oxygen saturation <88% at rest
• Reduced physical activity level (6-minute walk distance <212 m)
• Pulmonary hypertension
• Forced vital capacity (FVC) <50% of predicted or %FVC decline >10% of predicted or % predicted diffusion capacity for carbon monoxide (DLCO) decline >15% of predicted during the last 6 months
• Progressive or newly diagnosed severe comorbidity
The life expectancy of patients with terminal lung disease is anticipated to span from weeks to a few months.
The imminently dying patient
The patient is imminently dying when the disease has progressed to a stage where only symptomatic treatment is warranted. All other treatment possibilities are futile, and death is expected within hours or few days.
|
Recommendation 2. Rehabilitation and palliative care Palliation and pulmonary rehabilitation aim to improve the ability of patients and caregivers to live an active life according to their individual values despite their chronic lung disease. Patients and caregivers should be involved in the design of an individualized treatment plan. |
Rehabilitation and palliative care
The American Thoracic Society (ATS)/European Respiratory Society (ERS) definition of pulmonary rehabilitation: ‘Pulmonary rehabilitation is a comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors [17].’
Pulmonary rehabilitation is a multidisciplinary effort aiming at, but not limited to, improving the physical function, nutrition, knowledge of the disease and change towards a more healthy and active lifestyle for the individual patient. Pulmonary rehabilitation is designed to improve both the physical and psychological state and to promote a continued health behavior change [18,19].
Ideally, palliation and rehabilitation are based on a comprehensive assessment of the needs and wishes of the individual patient and caregiver. Rehabilitation is an important component of palliative care and should be tailored to meet the individual patient’s needs. Some patients may need exercise programs to increase their physical capacity, while others may aim to reduce their decline in physical function and their need for continuous training and support. The concept of personalized management is a combination of rehabilitation and palliative care (Figure 1).
Figure 1.
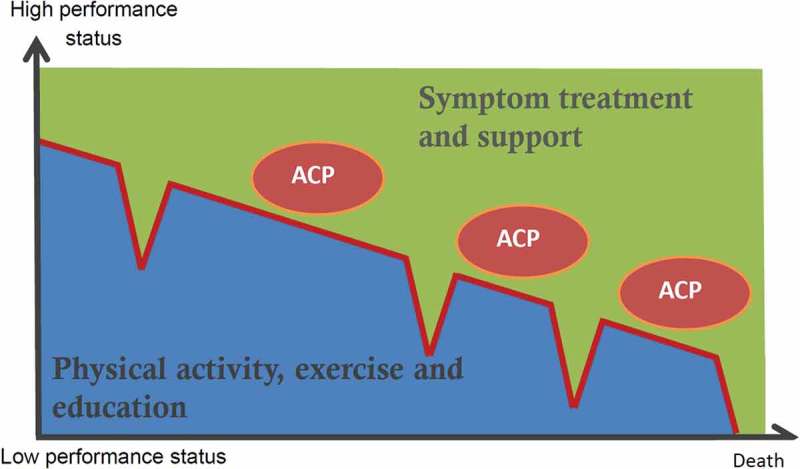
Personalized management and palliation includes symptom treatment, rehabilitation, patient education and support. Palliative support, including psychological, social and existential support, may continue into care for the informal caregiver after the patient’s death. Routine and early Advance Care Planning (ACP) provide the ability to adjust treatment goals during the disease trajectory.
Rehabilitation is expected to result in improved symptom control and functional capacity, and thus improved overall QOL [20]. Rehabilitation should be offered to all patients with symptomatic chronic lung diseases, even to those in the terminal phase of the disease [18].
|
Recommendation 3. Advance care planning (ACP) ACP is one or preferably several conversations where the patient, the multidisciplinary team and the informal caregiver discuss realistic treatment goals and the patient’s perceptions of living with the disease. Communication is the key in active management of patients with chronic lung failure. All patients with chronic lung failure should be offered ACP conversations once a year. ACP topics should focus on the patient’s needs and wishes and should never develop into a checklist. |
Definition
Advance care planning (ACP) is not a well-defined concept in the international literature. Therefore, DRS started by making a Danish guide [21] for ACP. The DRS defines ACP as: A communication process between the patient, the informal caregiver and the multidisciplinary team where focus is on living with a disease and symptoms. In this process issues like treatment limitations including end-of-life decisions can be discussed; however, ACP should not be limited to these topics.
There is no evidence that clearly defines the optimal timing of ACP; however, it is agreed that ACP should preferably be performed in a clinically stable phase of disease and not during hospitalization due to e.g. an acute exacerbation [22]. An important aspect of the ACP is to involve both the formal and informal caregivers [23].
It is recommended to allocate sufficient time for ACP conversation, as these are typically more time consuming than routine follow-up visits [24]. A Danish pilot study showed that ACP in patients with COPD lasted from 28 to 47 min and for up to 112 min in other diseases [25].
It must be kept in mind that ACP is not a checklist, but is intended to increase the understanding of the individual patient’s values and choices and to provide a better understanding of the disease for both the patient and the caregiver(s) [26,27]. The goal of ACP is not necessarily to reduce or stop treatment, but to ensure a balanced treatment based on an understanding of the needs and wishes of the individual patient.
Communication
During routine follow-up visits, health care providers do not always gain insight into what the patients perceive as their most troublesome symptoms [28,29]. Detailed knowledge of symptoms and disease burden is a prerequisite for targeted treatment and symptom relief. As in all patients with chronic diseases, communication is crucial for treatment adherence and lack of a common understanding of the disease, symptoms and treatment can hamper the benefits of palliation.
|
Recommendation 4. Informal caregivers and bereavement Severe disease affects not only the patient but also the informal caregiver(s), who should be offered to participate in ACP, rehabilitation, education and a follow-up after the patient’s death. |
Bereaved caregivers
A chronic disease affects not only the patient but also the caregiver(s). The feeling of being able to help a loved one is called caregiver benefits. However, living with a loved one who suffers from an increasingly symptomatic disease can be stressful; this is called the caregiver burden. Comparative studies have shown that it is not the underlying disease that determines the burden of disease on informal caregivers, but rather the psychosocial resources of the caregiver [30]. If the informal caregivers perceive themselves as sick or as economically poor, the feeling of the caregiver burden is increased [31].
It is therefore important to improve the ability of caregivers to cope and live with serious illness in the close family [32]. The active involvement of caregivers in pulmonary rehabilitation has been shown to result in improved coping strategies in patients and families and in psychosocial well-being [33]. It is common that the needs for information among caregivers and patients are not similar, and ACP can be one way to address this [34] and optimize communication between patient and caregiver(s) [34].
|
Recommendation 5. Symptom management Patients should be advised on and assisted in obtaining a lifestyle that helps to reduce the symptom burden. Comorbidities should be diagnosed and treated. Dyspnea and dyspnea-related anxiety may be difficult to separate and should be treated as a single symptom. Malnutrition is a marker of disease severity and must lead to special dietary advice. |
Dyspnea
Dyspnea is the patient’s perception of shortness of breath or air hunger. The perception of dyspnea is complex and depends on a variety of somatic and psychological factors such as deconditioning, underweight, anxiety, depression and other comorbidities such as cardiac disease [35]. Dyspnea is closely related to QOL and treatment and relief of dyspnea is of major importance. In pain management, the concept of ‘total pain’ describes the physical, mental, social, cultural and spiritual component of pain sensation. This broad understanding of ‘total dyspnea’ should be used in patients with chronic lung disease [36,37].
In addition to the pharmacological treatment of dyspnea, patient education, relaxation, breathing techniques and exercises are helpful [38,39]. Dyspnea may result in decreased physical activity and thus an inactive lifestyle, resulting in loss of muscle mass and deconditioning, which may further worsen dyspnea [40]. Physical rehabilitation is therefore an important way to improve dyspnea.
Dyspnea-related anxiety and depression
Dyspnea is terrifying for most patients and anxiety may worsen the situation. Dyspnea and dyspnea-related anxiety are difficult to distinguish and should therefore be considered as a single symptom. Anxiety in patients with COPD increases the risk of hospitalization and results in a self-reinforcing relationship [41,42]. In some patients, anxiety is seen as a direct part of the underlying disease and is therefore perceived as a marker of disease severity rather than a co-morbidity [42].
Opioids
There is a general consensus on the benefits of low-dose opioids to relieve dyspnea. This is supported by some evidence in COPD, while the evidence in ILD is limited [10,36,43–46]. When opioids are used, it is recommended to start with a low dose of morphine and to increase the dose slowly. Doses of 2.5 to 5 mg of oral morphine are often used as an initial dose, slowly increased until a beneficial effect is achieved. Opioid doses of up to 30 mg daily have not been associated with an increased rate of hospitalization or mortality in COPD, regardless of hypercapnia [47]. A small study on patients with IPF suggested the benefit of a single dose to relieve dyspnea without oxygen desaturation [48].
Benzodiazepines
Benzodiazepine treatment in COPD may be associated with a slightly increased mortality [47,49,50] and its effect on the perception of dyspnea is not fully elucidated [11,38,43]. Benzodiazepines reduce anxiety and can be tried when anxiety is a major component [11,50].
Corticosteroids
Low-dose prednisolone of 5–10 mg daily is sometimes used to relieve dyspnea. Corticosteroids have a beneficial effect on fatigue, but also significant side effects. There is a dose-effect relationship between adverse events but even at low doses, physical adverse events such as weight gain, gastrointestinal bleeding, osteoporosis, glaucoma, hypertension, increased risk of diabetes [51], and psychological side effects such as delirium, confusion, depression and mania have been observed [52]Corticosteroids may in selected cases be helpful to stabilize and increase wellbeing and appetite, but the risk of long-term adverse events should be balanced against disease severity and life expectancy.
Long-term oxygen treatment (LTOT)
LTOT may be a necessary and life-prolonging treatment in COPD and pulmonary fibrosis. However, LTOT may result in immobilization and dry out the mucous membranes, which may outweigh the benefits in some patients. In normoxic patients, it is questionable whether oxygen relieves dyspnea and it is therefore only prescribed as palliation after careful individual assessment [38].
Cough
Persistent cough is common in patients with COPD or ILD [53]. Patients should be evaluated and treated for underlying causes such as reflux or sinusitis. Moreover, attention should be paid to side effects from other drugs such as ACE inhibitors. In COPD, use of inhalers and inhalation medications should be optimized.
When no other cause for cough is found, low dose opioids can be tried. However, the scientific evidence is limited [53,54]. There is a growing interest in chronic cough and its similarity to neuropathic pain; gabapentin has been shown to improve the condition at acceptable side effects [55–57].
Malnutrition
Malnutrition and particularly loss of muscle mass is related to poor survival in COPD. While underweight is a bad prognostic sign, the same has not been shown for overweight. The relationship between obesity, the worsening of comorbidities and the symptom burden is poorly investigated [58]. Recent studies suggest that nutrition therapy may improve the condition of severely underweight patients, particularly in combination with a rehabilitation program [58–60].
|
Recommendation 6. Imminently dying patients with lung disease The presence of ‘Imminently dying’ is based on a clinical assessment of the patient’s current situation, comorbidity status, and the patient’s personal wishes. Treatment of the imminently dying patient focuses solely on symptom treatment and relief. Communication with the imminently dying patients and their caregivers requires sufficient time, an appropriate environment and an empathetic disclosure of the information. |
No reliable or definite prognostic signs can define when a patient is imminently dying. The assessment is based on thorough knowledge of
The patient’s overall disease burden, comorbidities and potentially reversible acute conditions.
Daily physical activities prior to hospitalization
Restrictions in treatment and discontinuation of treatment
Limitation of treatment covers the considerations on treatment such as transfer to an intensive care unit, non-invasive ventilation, ventilator therapy, or resuscitation in the case of cardiac arrest.
Central to the communication on treatment limitations is the description and explanation of the underlying medical reasons. Communication about limitations of treatment should always be disclosed with empathy and respect for the patient and caregivers.
When a patient is imminently dying, the treatment should be appropriately adjusted. It is important to regularly inform the patient and informal caregivers about changes in treatment goals i.e. from disease modifying treatment to symptom relief.
If the patient is awake, oxygen therapy can be adjusted in accordance with the patient’s perception of dyspnea. Oxygen dries out the mucous membranes and is therefore only indicated in the imminently dying patient to relieve dyspnea.
Fluid therapy and nutrition should be stopped after thoroughly informing the patient and informal caregivers.
In the imminently dying patient, it may be difficult to distinguish between anxiety, dyspnea or other disorders; opioids and also benzodiazepines may be helpful.
Communication about death with the dying patient and informal caregivers
Communication about death with the dying patient and the informal caregivers requires communicative skills and empathy. Conversations about these topics should be allowed sufficient time [24]. The need for information to both patient and caregivers is often underestimated by health professionals [54]. Caregivers may have difficulties realizing the severity of an exacerbation when their loved one has previously survived other exacerbations. In such cases, it may be helpful to discuss the daily activity and worsening over time before the actual hospitalization to allow the caregivers to formulate and accept that the patient’s underlying chronic lung disease has progressed and is worse than before.
|
Recommendation 7. Organization Palliative care in respiratory diseases should be organized as a multidisciplinary team approach. The case manager’s objective is to ensure intensified, personalized support for patients with severe and complex needs. A symptom screening tool may be helpful to obtain the best possible understanding of the overall symptomatology. |
Case manager
The goal of a case manager model is not to reduce the number of contacts to the health care system but to improve the patient’s feeling of security, to reduce anxiety and if possible also to reduce acute hospitalizations. A case manager model requires highly trained case managers, easy accessibility and frequent contacts between the patient and health care professionals [6,61].
Symptom screening
An understanding of patients symptoms and needs is central to palliation and rehabilitation. Several validated questionnaires exist. In patients with COPD, we recommend use of the COPD Assessment Test (CAT). The CAT consists of eight questions covering the most common COPD symptoms. CAT is validated and correlates with the St. George’s Respiratory Questionnaire (SGRQ) [62]. CAT can also be used in patients with ILD.
Multidisciplinary team
The key participants in the pulmonary palliative team are physicians and nurses specialized in pulmonary medicine. Other relevant participants may include general practitioners, physiotherapists, palliative care specialists, occupational therapists, dieticians, psychologists, hospital chaplains and social workers.
The symptomatic, severely ill patient often consults different specialists. A multidisciplinary team should in addition to taking care of the patient, also coordinate those involved in treatment and care provision. Positive results with multidisciplinary team conferences within existing budgets have been reported in England [63].
Telemedicine
There is considerable political interest and optimism about the field of telemedicine, although evidence of the effect is still modest [64]. Obviously, the digitalization of our society will result in changes in future communication forms. Introduction of telemonitoring itself does not necessarily improve QOL or reduce the number of hospitalizations [65–67]. If introduced, it is important to adjust the tele-intervention to fit the patient’s needs and technical skills [66]. Telemedicine might be able to improve communication between health care providers and patients with severe dyspnea and reduce the need for physically attending appointments in the out-patient clinic.
Overall approach
Studies have shown that patients’ behavior can be changed by a case manager, patient education and self-management plans. In COPD, this approach has in some studies resulted in reduced healthcare costs [68], a lower rate of hospitalization due to COPD exacerbations as well as for other reasons [69]. In contrast, other well-designed studies have found excess mortality in self-help plan groups [70] and no effect of nurse-controlled interventions [71]. These contradictory results illustrate the complexity of severe chronic lung disease and highlight the need for personalized management.
There is limited knowledge of the optimal organization of a comprehensive palliative care program for patients with non-malignant chronic lung disease. Education and training in palliative care should be a part of the pulmonology training programs for both physicians and nurses.
Conclusion
Palliative care is based on a shared understanding of the individual patient’s symptoms, needs, fears and hopes. It is recommended to offer annual ACP conversations with the patient and, if possible, the caregivers. When a mutual understanding is achieved and a plan is formulated, the multidisciplinary team should start treatment according to the identified needs and resources.
Early palliation allows for better systematization of the different treatment needs, and for the development of a long-term strategy.
The goal of palliative care is to support the patient and caregivers to improve QOL throughout the disease trajectory.
Biographies
Kristoffer Marsaa, MD, Head of section Senior consultant palliative unite Copenhagen University Hospital Herlev and Gentofte, Denmark. Specialist in pulmonary medicine with clinical and research focus in COPD, pulmonary fibrosis, Chronic care, and palliative care.
Svend Gundestrup, MD, Specialist Registrar at Department of Respiratory Diseases, Bispebjerg University Hospital, Denmark. He is a specialist in training in pulmonary diseases, and his main focus is COPD and non-malignant palliative medicine.
Jens-Ulrik Jensen, MD, PhD, is Research Associate Professor at University of Copenhagen and consultant, Head of Respiratory Medicine Section at Herlev-Gentofte Hospital. He is a respiratory medicine specialist. Main research Area is COPD and complicated respiratory infections.
Peter Lange, MD, Dr Med Sciences, is consultant at Medical Department O at Herlev -Gentofte Hospital and professor at section of epidemiology, Department of Public Health, University of Copenhagen, Denmark. He is a specialist in respiratory medicine and his main research focus is COPD and asthma.
Anders Løkke, MD, is a consultant at The Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Denmark. He is a specialist in pulmonary diseases, and his main research focus is COPD.
Nassim Bazeghi Roberts MD, PhD, graduated medicin from Iran, got her PhD degree and specialty in pulmonary disease in Copenhagen Medical University, working as a consultant in Bispebjerg University Hospital. Her main interest of area is COPD.
Saher Shake, MD, PhD, is a consultant at the Department of Respiratory Diseases at Herlev-Gentofte Hospital, Copenhagen. He is a specialist i Respiratory Medicine and his main research interest is interstitial lung diseases.
Anita Rath Sørensen, MD, is a consultant at Department of Internal Medicine, Horsens Regional Hospital, Denmark. She is a specialist in pulmonary medicine and her main focus is in palliative medicine.
Ingrid Louise Titlestad, MD, PhD, Ass.Prof., is a consultant at Department of Respiratory Medicine, Odense University Hospital, Denmark. She is a specialist in pulmonary diseases, and her main research focus is lung function decline, biomarkers in COPD and non-invasive ventilation.
Laura Hohwü Thomsen, MD, PhD, is a consultant at Department of Respiratory Diseases and Palliative Care, Hvidovre University Hospital, Denmark. She is a specialist in pulmonary diseases, and her main research focus is on lung function and imaging as well as palliative care in patients with lung diseases.
Ulla Møller Weinreich, MD, PhD is a consultant at Department of Respiratory Diseases at Aalborg University Hospital, specialized in COPD and comorbidities and infectious lung diseases. Furthermore she is an Associate Professor at the Clinical Institute, Aalborg University.
Elisabeth Bendstrup, MD, PhD, Ass. Prof., is a consultant at Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Denmark. She is a specialist in pulmonary diseases, and her main research focus is interstitial lung diseases.
Torgny Wilcke, MD, Ph.D., is consultant at Medical Department O at Herlev -Gentofte Hospital and associate professor at clinical section of University of Copenhagen, Denmark. He is a specialist in respiratory medicine and his main research focus is COPD, rehabilitation, home NIV and palliation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. WHO Palliative care for older people: better practices. In: Hall S, Petkova H, Tsouros AD, Costantini M and Higginson IJ, editors. 2011, viii + 59 pages. ISBN 978 92 890 0224 0 [Google Scholar]
- [2]. Temel J, Greer J, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. Th E New Engl J O F Med. 2010. [DOI] [PubMed] [Google Scholar]
- [3]. Husted MG, Kriegbaum M, Kirkegaard N, et al. The use of healthcare resources in the last 3 years of life in patients with COPD and lung cancer in Denmark. A retrospective nationwide study. BMJ Support Palliat Care. 2014;4(2):1–10. [DOI] [PubMed] [Google Scholar]
- [4]. Kreuter M, Bendstrup E, Russell A-M, et al. Palliative care in interstitial lung disease: living well. Lancet Respir Med. 2017;2600:1–13. [DOI] [PubMed] [Google Scholar]
- [5]. Sundhedsstyrelsen Forløbsprogrammer for kronisk sygdom – generisk model. 2012.
- [6]. Lavesen M, Marsa KB-M, Bove DG.. A new way of organising palliative care for patients with severe chronic obstructive pulmonary disease. Int J Palliat Nurs. 2018;24:64–68. [DOI] [PubMed] [Google Scholar]
- [7]. Danoff SK, Schonhoft EH. Role of support measures and palliative care. Curr Opin Pulm Med. 2013;19:480–484. [DOI] [PubMed] [Google Scholar]
- [8]. Wuyts WA, Peccatori FA, Russell AM. Patient-centred management in idiopathic pulmonary fibrosis: similar themes in three communication models. Eur Respir Rev. 2014;23:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Bourke S, Peel E. Palliative care of chronic progressive lung disease. Clin Med. 2014;14:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Lanken PN, Terry PB, DeLisser HM, et al An official American thoracic society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–927. [DOI] [PubMed] [Google Scholar]
- [11]. Boland J, Martin J, Wells AU, et al. Palliative care for people with non-malignant lung disease.Summary of current evidence and future direction. Palliat Med. 2013;27:811–816. [DOI] [PubMed] [Google Scholar]
- [12]. Escarrabill J, Soler Cataluña JJ, Hernández C, et al. Recommendations for end-of-life care in patients with chronic obstructive pulmonary disease. Arch Bronconeumol. 2009;45:297–303. [DOI] [PubMed] [Google Scholar]
- [13]. GOLD. Global Initiative for Chronic Obstructive Lung Disease. 2013.
- [14]. Sundhedsstyrelsen Anbefalinger for den palliative indsats. 2011. http://sundhedsstyrelsen.dk/publ/Publ2011/SYB/Palliation/PalliativeIndsats_anbef.pdf.
- [15]. Epiphaniou E, Shipman C, Harding R, et al. Coordination of end-of-life care for patients with lung cancer and those with advanced COPD: are there transferable lessons? A longitudinal qualitative study. Prim Care Respir J. 2014;23:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Marså K, Knudsen T, Gundestrup S, et al. Dansk Lungemedicinsk Selskab : klaringsrapport om palliation til voksne med kronisk fremadskridende non-malign lungesygdom. https://www.lungemedicin.dk/fagligt/klaringsrapporter/189–dls–klaringsrapport–om–palliation–2015/file.html.
- [17]. Spruit MA, Singh SJ, Garvey C, et al. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188 DOI: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- [18]. Gilbert R, Dechman G. The effects of high intensity exercise during pulmonary rehabilitation on ventilatory parameters in people with moderate to severe stable COPD : a systematic review. Int J chronic obstructive pulmonary disease. 2014;9:1069–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Hill K, Vogiatzis I, Burtin C. The importance of components of pulmonary rehabilitation, other than exercise training, in COPD. Eur Respir Rev. 2013;22:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Reticker AL, Nici L, ZuWallack R. Pulmonary rehabilitation and palliative care in COPD: two sides of the same coin? Chron Respir Dis. 2012;9:107–116. [DOI] [PubMed] [Google Scholar]
- [21]. Marså K, Lavesen M, Knudsen T, et al. Samtalen om Fælles Planlægning af Behandlingsmål. https://www.lungemedicin.dk/fagligt/klaringsrapporter/188-dls-fælles-planlægning-af-behandlingsmål-2015/file.html.
- [22]. Seamark D, Blake S, Seamark C, et al. Is hospitalisation for COPD an opportunity for advance care planning? A qualitative study. Prim Care Respir J. 2012;21:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Patel K, DJ A J, Jr C. Advance care planning in COPD. Respirology. 2012;17:72–78. [DOI] [PubMed] [Google Scholar]
- [24]. Parry R, Seymour J, Whittaker B, et al. Rapid evidence review: pathways focused on the dying phase in end of life care and their key components. Department of Health, University of Nottingham; 2013. . [Google Scholar]
- [25]. Andreassen P, Neergaard MA, Brogaard T, et al. Talking about sensitive topics during the advance care planning discussion: a peek into the black box. Palliat Support Care. 2015;13(6):1–8. [DOI] [PubMed] [Google Scholar]
- [26]. Russell S. Advance care planning: whose agenda is it anyway? Palliat Med. 2014;28:997–999. [DOI] [PubMed] [Google Scholar]
- [27]. Prendergast TJ. Advance care planning: pitfalls, progress, promise. Crit Care Med. 2001;29:N34–9. [DOI] [PubMed] [Google Scholar]
- [28]. Strömgren AS, Groenvold M, Sorensen A, et al. Symptom recognition in advanced cancer. A Comparison of Nursing Records against Patient Self-Rating. Acta Anaesthesiol Scand. 2001;45(9):1080–1085.. [DOI] [PubMed] [Google Scholar]
- [29]. Strömgren AS, Groenvold M, Pedersen L, et al. Does the medical record cover the symptoms experienced by cancer patients receiving palliative care? A comparison of the record and patient self-rating. J Pain Symptom Manage. 2001;21:189–196. [DOI] [PubMed] [Google Scholar]
- [30]. Burton AM, Sautter JM, Tulsky JA, et al. Burden and well-being among a diverse sample of cancer, congestive heart failure, and chronic obstructive pulmonary disease caregivers. J Pain Symptom Manage. 2012;44:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Cutrino A, Santamaria J. Research on family caregivers understanding levels of burden and how to provide assistance. Home Healthc Nurse. 2013;31:331–337. [DOI] [PubMed] [Google Scholar]
- [32]. Zakrisson A, Theander K, Anderzén-Carlsson A, et al. The experience of a multidisciplinary programme of pulmonary rehabilitation in primary health care from the next of kin ’ s perspective : a qualitative study Copyright PCRS-UK - reproduction prohibited. Prim Care Respir J. 2013;22:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Marques A, J C, C J, et al. Family-based pulmonary rehabilitation in COPD. Chest. 2015 Mar;147(3):662–672. [DOI] [PubMed] [Google Scholar]
- [34]. Overgaard D, Kaldan G, Marsaa K, et al. The lived experience with idiopathic pulmonary fibrosis: a qualitative study. doi: 10.1183/13993003.01566-2015 [DOI] [PubMed] [Google Scholar]
- [35]. Parshall MB, Schwartzstein RM, Adams L, et al. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64:910–915. [DOI] [PubMed] [Google Scholar]
- [37]. Johnson MJ, Bland JM, Oxberry SG, et al. Opioids for chronic refractory breathlessness: patient predictors of beneficial response. Eur Respir J. 2013;42:758–766. [DOI] [PubMed] [Google Scholar]
- [38]. Disler RT, Currow DC, Phillips JL, et al. Interventions to support a palliative care approach in patients with chronic obstructive pulmonary disease: an integrative review. Int J Nurs Stud. 2012;49:1443–1458. [DOI] [PubMed] [Google Scholar]
- [39]. Marianne S, Helle G, Annemarie S, et al. Klinisk retningslinie for lindring af dyspnø hos voksne uhelbredeligt syge kræft patienter. http://www.dmcgpal.dk/661/godkendte-retningslinjer.
- [40]. Johnson MJ, Hui D, Currow DC. Opioids, exertion, and dyspnea: a review of the evidence. Am J Hosp Palliat Med. 2014. published online Oct 7. doi: 10.1177/1049909114552692 [DOI] [PubMed] [Google Scholar]
- [41]. Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133): p. 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Pumar MI, Gray CR, Walsh JR, et al. Anxiety and depression — important psychological comorbidities of COPD. J thoracic disease. 2014;6:1615–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Runo J, Ely W. Treating dyspnea in a patient with advanced chronic obstructive pulmonary disease. West j Med. 2001;175:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Koblizek V, Chlumsky J, Zindr V, et al. Chronic Obstructive Pulmonary Disease : official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Papers Med Faculty Palacky Univ in Olomouc. 2013;157:189–201. [DOI] [PubMed] [Google Scholar]
- [45]. Raghu G, Collard HR, Egan JJ, et al An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Kohberg C, Andersen CU, Bendstrup E. Opioids: an unexplored option for treatment of dyspnea in IPF. Eur Clin Respir J. 2016;3:30629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Ekström MP, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease : national prospective study. Bmj. 2014;445:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Allen S, Raut S, Woollard J, et al. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med. 2005;19:128–130. [DOI] [PubMed] [Google Scholar]
- [49]. Vozoris N. Benzodiazepines and opioids need to be prescribed with caution in advanced COPD. Evid Based Med. 2014;19:2014. [DOI] [PubMed] [Google Scholar]
- [50]. Battaglia S, Bezzi M, Papa GFS. Are benzodiazepines and opioids really safe in patients with severe COPD? Minerva Med. 2014;105:1–7. [PubMed] [Google Scholar]
- [51]. Kavanaugh A, Wells AF. Benefits and risks of low-dose glucocorticoid treatment in the patient with rheumatoid arthritis. Rheumatol. 2014;53(10)1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Schettler PJ, Ph D, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045–1051. [DOI] [PubMed] [Google Scholar]
- [53]. Wee B, Browning J, Adams A, et al Management of chronic cough in patients receiving palliative care: review of evidence and recommendations by a task group of the Association for Palliative Medicine of Great Britain and Ireland. Palliat Med. 2012;26:780–787. [DOI] [PubMed] [Google Scholar]
- [54]. Neergaard MA, Larsen H. Palliativ medicin – en lærebog. Copenhagen, Denmark: Munksgaard; 2015. [Google Scholar]
- [55]. Ryan NM. Drug Evaluation A review on the efficacy and safety of gabapentin in the treatment of chronic cough. Expert Opin Pharmacother. 2015;135–145. [DOI] [PubMed] [Google Scholar]
- [56]. Ryan NM, Gibson PG. Recent additions in the treatment of cough. J Thorac Dis. 2014;6:S739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Altman KW, Noordzij JP, Rosen CA, et al. Neurogenic cough. Laryngoscope. 2015;125:1675–1681. [DOI] [PubMed] [Google Scholar]
- [58]. Rutten EPA, Wouters EFM, Franssen FME. Chapter 6. Malnutrition and obesity in COPD. Eur Respir Monogr. 2013;80–92. [Google Scholar]
- [59]. Ferreira I, Brooks D, Lacasse Y, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease (Review). Cochrane Database Syst Rev. 2005;(2):CD000998. [DOI] [PubMed] [Google Scholar]
- [60]. Collins PF, Stratton RJ, Elia M. Nutritional support in chronic obstructive pulmonary disease : a systematic review and meta-analysis 1 –. The Am J Clin Nutrition. 2012;3:1385–1395. [DOI] [PubMed] [Google Scholar]
- [61]. Bourbeau J, Saad N. Integrated care model with self-management in chronic obstructive pulmonary disease: from family physicians to specialists. Chron Respir Dis. 2013;10:99–105. [DOI] [PubMed] [Google Scholar]
- [62]. Global Strategy for the Diagnosis, Management, and Prevention of COPD Global initiative for Chronic Obstructive Lung Diseases (GOLD). 2017; Available from: http://goldccopd.org 2013. [Google Scholar]
- [63]. Boland J, Owen J, Ainscough R, et al. Developing a service for patients with very severe chronic obstructive pulmonary disease (COPD) within resources. BMJ Support Palliat Care. 2013;4(2)196–201. [DOI] [PubMed] [Google Scholar]
- [64]. Currell R, Urquhart C, Wainwright P, et al. Telemedicine versus face to face patient care : effects on professional practice and health care outcomes (Review). Cochrane Database Syst Rev. 2000;(2):CD002098. [DOI] [PubMed] [Google Scholar]
- [65]. Pinnock H, Hanley J, Mccloughan L, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease : researcher blind, multicentre, randomised controlled trial. Bmj. 2013;6070:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Gottlibe M, Marsaa K, Andressessen H, et al. Feasibility of a telecare solution for patients admitted with COPD exacerbation: screening data from a pulmonary ward in a university hospital. Eur Clin Respir J. 2014;1:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Ulrik CS, Lomholt Gregersen T, Green A, et al. Do telemedical interventions improve quality of life in patients with COPD? A systematic review. Int J Chron Obstruct Pulmon Dis. 2016;11:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Bourbeau J, Collet J-P, Schwartzman K, et al. Economic benefits of self-management education in COPD. Chest. 2006;130:1704–1711. [DOI] [PubMed] [Google Scholar]
- [69]. Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(5):585–591. [DOI] [PubMed] [Google Scholar]
- [70]. Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156:673–683. [DOI] [PubMed] [Google Scholar]
- [71]. Coultas D, Randomized A. Trial of two types of nurse-assisted home care for patients with COPD. Chest J. 2005;128:2017. [DOI] [PubMed] [Google Scholar]


