ABSTRACT
Chrysomya megacephala (Fabricius, 1794) occurs on every continent and is closely associated with carrion and decaying material in human environments. Its abilities to find dead bodies and carry pathogens give it a prominence in human affairs that may involve prosecution or litigation, and therefore forensic entomologists. The identification, geographical distribution and biology of the species are reviewed to provide a background for approaches that four branches of forensic entomology (urban, stored-product, medico-criminal and environmental) might take to investigations involving this fly.
KEYWORDS: Forensic sciences, forensic entomology, Chrysomya megacephala, morphology, taxonomy, biogeography, life cycle, ecology
Introduction
The blow fly Chrysomya megacephala (Fabricius, 1784), commonly called the oriental latrine fly [1], has significance for public health, food industries, medical entomology, investigations of deaths and, most recently, industrial recycling of organic waste. All of these concerns may become the subject of prosecution or litigation, and can therefore involve forensic entomology. Large populations of C. megacephala may inhabit human settlements, and adult flies visit moist foodstuffs and decaying carrion and faeces, where they may breed very abundantly. To compound its significance, this synanthropic species has spread almost globally from its native geographical distribution. The wide range and global ubiquity of human concerns associated with C. megacephala merits a review of the species’ biology as a foundation for research into its management and applications, particularly for forensic entomology. Therefore, this paper reviews published literature about this species in relation to each area of concern; a comprehensive bibliography is beyond the scope of this review.
Taxonomy
Correct identification of an organism is a cornerstone of forensic entomology, primarily because it facilitates access to relevant information stored in published literature and allows scientists to communicate and contextualize their findings effectively. Chrysomya megacephala is a case in point: it has 15 primary synonyms (Table 1), 3 putative forms and a history of being confused with other members of the megacephala species-group in scientific publications.
Table 1.
Primary synonyms of Chrysomya megacephala (Fabricius, 1794) (from Pape and Thompson 1915).
| No | Synonym | Taxonomic author |
|---|---|---|
| 1 | Musca megacephala | Fabricius, 1794: 317 |
| 2 | Musca dux | Eschscholtz, 1822: 114 |
| 3 | Chrysomya duvaucelii | Robineau-Desvoidy, 1830: 451 |
| 4 | Chrysomya gratiosa | Robineau-Desvoidy, 1830: 451 |
| 5 | Lucilia flaviceps | Macquart, 1843: 302 |
| 6 | Musca flaviceps | Walker, 1849: 870 |
| 7 | Musca remuria | Walker, 1849: 871 |
| 8 | Musca bata | Walker, 1849: 875 |
| 9 | Musca combrea | Walker, 1849: 876 |
| 10 | Pollenia basalis | Smith, 1876: 449 |
| 11 | Somomya pfefferi | Bigot, 1877: 257 |
| 12 | Somomyia saffranea | Bigot, 1877: 257 |
| 13 | Somomyia dives | Bigot, 1888: 600 |
| 14 | Somomyia cyaneocincta | Bigot, 1888: 604 |
| 15 | Somomya cyaneocincta | Bigot, 1888: 604 |
The species was divided into three morphological forms with distinct ecologies [2,3]: a “normal” southern Pacific, forest-dwelling form with relatively small dorsal ommatidia in the male; a “derived”, synanthropic and now cosmopolitan form with relatively large dorsal ommatidia in the male; and a “feral derived” Himalayan form with intermediately sized dorsal ommatidia in the male [3]. However, the “normal” form was subsequently described as Chrysomya pacifica Kurahashi, 1991 [4] and molecular evidence suggests that it is a distinct species [5]. The status of the feral derived form awaits molecular evidence, but seems likely on morphological grounds to be an independent species too, cf. [3].
In older publications, C. megacephala was notably confused with the closely related Chrysomya saffranea (Bigot, 1877) [6] and the less-closely related Chrysomya bezziana Villeneuve, 1914 [1,7], both of which belong to the megacephala clade or species-group [5]. Both of these species may occur in forensic cases involving death or neglect of people or animals. Care should be taken to verify the taxonomy of older studies before using their findings. Kurahashi's [2] defixa-subgroup appears to be a grade defined by plesiomorphic characters [2,5], and should be included in the megacephala-group clade.
Morphological identification
Adult
A morphological identification key to adults, including the named species of the megacephala species-group, has been published [8]. The adults of C. megacephala have been illustrated with drawings [1,9–11] and photographs [12–14]. The differentiation of males’ eye facets (Figure 1(A,B)), the shape of females’ fronses (Figure 1(C,D)) and the colours of the anterior thoracic spiracle and the lower calypter are particularly important in the morphological differentiation of this species from its close relatives [1,8]. The diagnostic males’ genitalia are illustrated by Zumpt [1] and Chaiwong et al. [15], and the females’ by Bansal and Murad [16].
Figure 1.
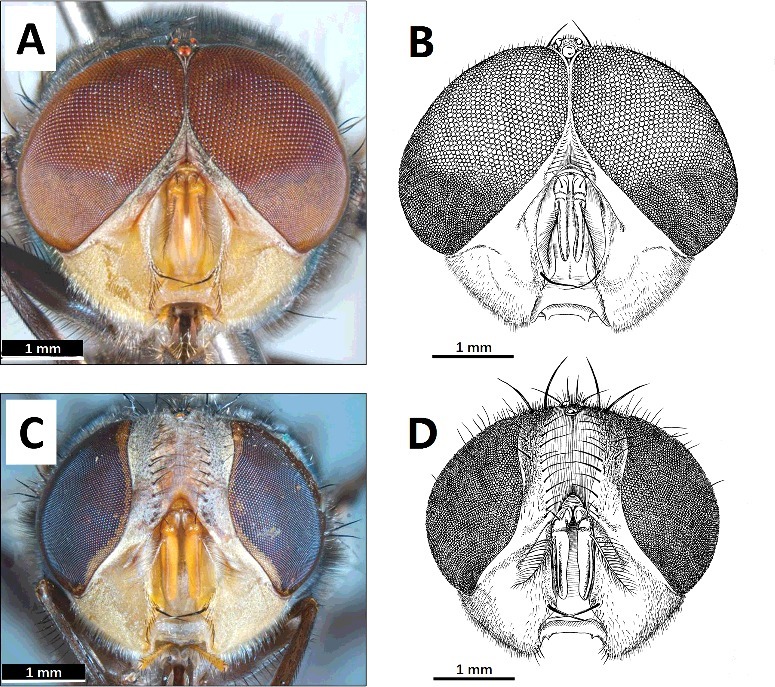
Anterior view of the head of a male (A and B) and female (C and D) adult of Chrysomya megacephala, showing the sharply differentiated sizes of the upper and lower facets of the male's eyes, and the shape of the female's frons, which has convex sides. Images A and C by Ken Walker via PaDIL - http://www.padil.gov.au under the Creative Commons Attribution 3.0 Australia License.
Spermatid
Comparative studies that would aid the identification of spermatozoa of C. megacephala have not been published. The spermatozoa are typically brachyceran, about 0.6 mm long and very thin; the head forms about 10% of the total length [17].
Egg
Comparative studies that would aid identification of the eggs of C. megacephala have not been published. The eggs [1] are 1.5–1.6 mm long, sausage-shaped and whitish, turning cream. Unlike the eggs of other blow flies [18], and particularly the closely related C. pacifica [19], they are reported to have a wide, flattened strip running the length of one side that bears leaf-like projections [20]. If these observations are correct, the projections would be taxonomically diagnostic.
Larva
The larvae have been illustrated with drawings and photographs, and there are studies of the ultrastructure of the larval exoskeleton [21–24]. Identification usually involves the mouthparts, the anterior spiracle and the bands of spines on each segment.
First-instar larvae [1,21,24] are 1.7–3.5 mm long, and the posterior spiracles have one slit. Keys are available that differentiate the first-instar larva of this species from many blow flies from Africa and Europe [24,25].
Second-instar larvae [1,21] are 6–8 mm long, and the posterior spiracles have two slits. There is no specific identification key for this instar, which differs slightly in morphology (particularly its mouthparts) from the first and third instar.
Third-instar larvae [1,21,26,27] grow to about 16 mm long, depending on the temperatures they experience, but contract rapidly just before pupariation [28], and the posterior spiracles have three slits. The band of spinules on the last segment is incomplete dorsally. The anterior spiracle has 11–13 branches.
Puparium
The puparium is formed from the exoskeleton of the third-instar larva, and thus bears the same identifying surface structures as the mature larva. It is brown with yellow anterior spiracles, as in most blow flies [1,24,29,30]. The mouth hooks of the third-instar larva, which can assist identification of pupae, can usually be found adhering to the inside of the eclosed puparium.
Molecular identification
DNA sequences can identify samples, but the choice of marker molecule is important. Mitochondrial DNA (mtDNA) markers evolve four times faster than nuclear DNA markers and are present in many times more copies per cell; they are therefore preferred for identifying species. However, mtDNA can fail to distinguish closely related species under some conditions, including recent hybridization and incomplete lineage sorting [31]. Introgression (ancient hybridization) may also pose problems for forensic entomology, especially if the species has not been studied before [31,32].
The whole mitochondrial genome of C. megacephala has been sequenced [33]. The mitochondrial control region could be used to identify conspecific specimens in phylogenetic analyses, but can be difficult to align consistently, which creates problems for identification by standard phylogenetic methods [34]. Dynamic homology analysis (implemented in, e.g. POY: [35]) or alignment-free analysis (implemented in, e.g. kSNP: [36]) may resolve this issue in a future study.
The popular DNA “barcode” (∼670 bp of the 5’ end of the mitochondrial cytochrome c oxidase subunit I [COI]) generally succeeds as an identification tool for blow flies [37–42], and benchmark sequences for C. megacephala are available on the National Center of Biotechnology Information and Barcode of Life databases (GenBank and BOLD, respectively). Phylogenetic analysis of COI sequences from adult specimens of C. saffranea and C. megacephala indicated hybridization and perhaps also incomplete lineage sorting [38,39]. Similar results were obtained from analysis of a 2 200 bp sequence encompassing the barcode fragment of COI and the cytochrome c oxidase subunit II (COII) and t-RNA leucine genes from larvae of Chrysomya pinguis (Walker, 1858) and C. megacephala [43]. Even whole mtDNA sequences might not provide unequivocal differentiation of C. megacephala from its near relatives when lineage sorting is far from complete. “Barcoding” of forensically important insects also has a surprisingly high failure rate for technical reasons [39–42,44]. “Barcoding” is problematic for forensics when there is no means to cross-validate an identification [45,46].
Because nuclear markers evolve at a quarter of the rate of mtDNA, they are not expected to perform better under incomplete lineage sorting. An example is that the nuclear bicoid gene shows paraphyly between C. megacephala and C. pinguis [32] that might be due to introgression.
Phylogenetic relationships
The more closely related two species are, the more likely they are to be confused in forensics investigations (and to hybridize naturally), and these species pairs can be identified by phylogenetic analysis. Phylogenetic analyses of DNA sequences of the mitochondrial COI and nuclear carbamoylphosphate synthetase genes showed that the probable sister species of C. megacephala is C. pacifica, and that this species pair is (increasingly distantly) related to Chrysomya cabrerai Kurahashi & Salazar, 1977, Chrysomya defixa (Walker, 1856), Chrysomya greenbergi Wells & Kurahashi, 1996, C. pinguis, Chrysomya thanomthini Kurahashi & Tumrasvin, 1977, C. bezziana, and Chrysomya chani Kurahashi, 1979 [5]. The exact relationship between C. megacephala and C. saffranea has not been established, but the hybridization and perhaps incomplete lineage sorting between them [38,39] indicates that they are about as close as C. megacephala and C. pinguis [43].
Several of these species are known to breed in carrion and can be quite common, emphasizing the need for correct identification in forensic entomology. All of these species inhabit the Oriental and Australasian Regions.
Biogeographical distribution
The original distribution of C. megacephala was in the Oriental and Australasian Regions where its close relatives occur; its closest relative, C. pacifica, inhabits the islands between New Guinea, Samoa and New Caledonia [2,4]. Chrysomya megacephala also occurs in the Indian Region and the Middle East [47]. Two of its synonyms, Pollenia basalis Smith, 1876 and Somomyia pfefferi Bigot, 1877, were described from the islands of Rodriguez and Mauritius, respectively [48,49], implying that the species was present there by the dates of those descriptions. Fabricius [50] gives the type locality of Musca megacephala as “Guinea” and the donor as “Dr Ifert”, who collected in the Danish Gold Coast (or, in Danish, Dansk Guinea), approximately in modern coastal Ghana (Thomas Pape, personal communication). These details imply that C. megacephala may in fact have occurred in West Africa before 1794, which would affect the interpretation of its historical geography.
Chrysomya megacephala has spread dramatically through Africa and the New World (Figure 2). It was first found on the African mainland in South Africa in 1971 [51] and (re)discovered abundantly in West Africa in 1977 [52]. Although it has invaded much of sub-Saharan Africa, it is largely absent from Asia Minor and Europe, except for Spain [53], Malta [54] and Portugal [55]. The earliest Neotropical locality records are from south-eastern Brazil in 1977 [56,57]. It was collected in North America at Baja California, Mexico, in 1987 and Florida in 1992 and appeared in Indiana, USA, in 2012 [58]. There is genetic evidence that the Florida population is drawn from two sources [59]. This species has also reached isolated oceanic localities like the Galapagos and Fernando de Noronha archipelagos [60] (Figure 2). Although it has not been reported from Morocco, Western Sahara, Chad, the Guianas and Chile, it probably occurs in those countries and therefore throughout sub-Saharan Africa and South America.
Figure 2.
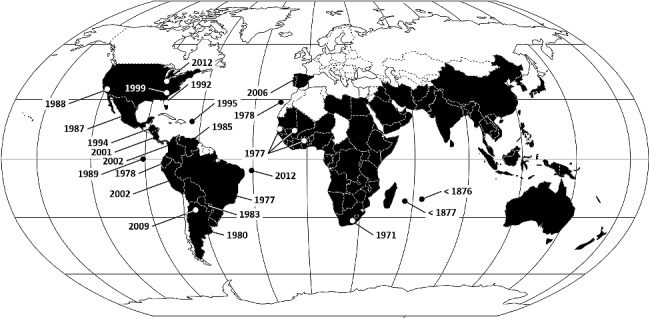
Geographical distribution of Chrysomya megacephala, by country, with year of discovery outside native range (after [14,47,51,52,58,60–63]).
New geographical appearances of pestilent blow flies quickly draw attention [7,10,53–56,58,60,64–70], allowing rough estimates of the rate of spread of the species. The species apparently arrived irruptively in Curitiba in 1976 [56] and spread promptly through coastal Brazil [57]. Recent Argentinian records of C. megacephala extend westward by approximately 500 km from the known distribution of the species [70]. Since blow flies are estimated to travel 2-3 km/day [71,72], such dispersal need not rely on human transport [51].
Chrysomya megacephala clearly inhabits tropical and temperate climates (Figure 2), although it was reported from Eastern Siberia, at about 50°N [73]. Although commonly termed synanthropic, it also occurs naturally in areas with little human presence [60]. With current trends in global climate, it seems likely that C. megacephala will colonize Europe and Canada, and therefore occur throughout the planet wherever climates are sufficiently moist. This supports its status as one of the most globally relevant insects in forensic entomology.
Biology
Habitat preferences
Chrysomya megacephala has been observed in all months of the year in its native range [6], in a wide range of natural and modified habitats, in a variety of climates [74], at altitudes from sea level to 2 667 meters above sea level [75]. The range of body temperatures that C. megacephala prefers is quite broad, even compared to eight other species of blow fly [76,77]. Such catholic preferences further explain why this species has spread around the world so successfully (Figure 2).
It is common around human dwellings where it can find dead fish, carcasses, human excrement, fruits and sweets. Although it does not usually come indoors, about three quarters (20/27) of human bodies found indoors in Malaysia hosted C. megacephala [78].
Diet
Adults of C. megacephala commonly collect on human excrement and sweet substances to feed. However, they preferred fish over faeces, mincemeat and banana [79–81].
Larvae feed on meat, liver and other soft tissues of mammals and birds, but thrive on fish [82], even if it is heavily salted. Artificial diets suitable for maintaining colonies for forensic entomotoxicological studies have not been developed specifically for C. megacephala [83].
Life cycle
The life cycle of C. megacephala is not unusual for a blow fly. In good weather, an adult female can arrive at carcasses or other decomposing organic matter within a few hours of their appearance, and will usually lay a mass of 220–325 (mean ≈ 254) eggs on under-surfaces of these substrates [1,82] on the same day, preferentially near recently laid flies’ eggs [84], and providing that larvae of Chrysomya albiceps (Wiedemann, 1819) and Chrysomya rufifacies (Macquart, 1842) are not present [82,85]. Females are predominantly active during daylight, but have been shown to lay eggs at night under warm conditions [86]. Females of C. megacephala apparently prefer to walk to nocturnal oviposition sites [87]. The occurrence of precocious eggs (eggs fertilized well before they are laid [88,89]) has not been established in C. megacephala. These points are especially important to qualifying estimates of minimum postmortem intervals in forensic entomology.
Since Richards and Villet [90] reviewed development rates in C. megacephala in relation to physiological age and environmental temperature, a number of studies have included relevant data (Table 2). Two studies in particular [91,92] have been sufficiently extensive to allow the calculation of thermal accumulation models (Tables 3 and 4). These models are based on developmental events or landmarks, which are usually observed by rearing forensic samples of live insects. When the forensic samples are preserved dead insects, the minimum postmortem interval is estimated using absolute growth models [22,91], which need to take into account that larvae reach larger sizes at optimal environmental temperatures and appear to trade-off growth against maintenance costs under more stressful conditions [66]. The relationship between body length and chronological age under different temperatures has been modelled statistically [28]; under increasingly suboptimal thermophysiological conditions, larvae mature at increasingly shorter body lengths. There are discrepancies between the published studies caused by varying temporal precision, differing descriptive statistics, geographical variation and differing rearing diets of the experiments measuring development rates [90]. These differences may have a genetic basis and be adaptive responses to local climes [93].
Table 2.
Details of studies of development rates of Chrysomya megacephala at constant temperatures. The duration of development has been variously estimated using the minimum, mean, median and maximum time required to reach a developmental event. The food source used to culture the larvae in each study is listed because the diet of larvae can alter their development rate. Some studies have low temporal precision. Data for the wandering and pupariation phases are more variable than for the other stages, probably because disturbed larvae will wander longer, thus shortening the duration of pupariation.
| Developmental events |
Data format |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperatures (°C) | H | E1 | E2 | W | P | A | Raw | Min | Mean | Med | Max | Food Source | Locality | Source |
| 27, 30, 33, 36, 39 | X | X | X | Fish | Malaysia | [94] | ||||||||
| 27 | X | X | X | - | India | [95] | ||||||||
| 26 | X | X | X | X | X | Raw beef | Egypt | [96] | ||||||
| 29.4 | X | X | X | X | X* | C-ration stew | USA (Guam) | [79] | ||||||
| 28 | X | X | X | X* | X* | Pet's mince | Australia | [97] | ||||||
| 25.6 | X | X | X | X | X | X | X | X | Moistened meat | India | [98] | |||
| 26 | X | X | X | X | X | X | X | X | X | - | South Africa | [11] | ||
| 28 | X | X | X | X | X | X | Beef liver | Venezuela | [99] | |||||
| 28 | X | X | X | X | X | X | Sardines | Venezuela | [99] | |||||
| 27.5 | X | X | Ox liver | Australia | [100] | |||||||||
| 27 | X | X | X | X | X | X | X | Pig liver | India | [101] | ||||
| 25 | X | X | Rice bran | Brazil | [102] | |||||||||
| 24, 25, 30, 35 | X | X | X | X* | Horse meat | Japan | [103] | |||||||
| 23.5 | X | X | X | X | X | X | Cow liver | USA (Hawaii) | [104] | |||||
| 20, 30 | X | X | Fish | China | [105] | |||||||||
| 20, 25, 30 | X | X | X | X | X | X | X | - | Egypt | [106] | ||||
| 20, 25 | X | X | X | X | X | X | Chicken liver | South Africa | [107] | |||||
| 16, 19, 22, 25, 28, 31, 34 | X | X | X | X | X | X | X | Lean pork | China | |||||
| 15, 20, 25, 30, 35 | X | X | X | X | X | X | X | Beef liver agar | USA | [108] | ||||
| 5, 10, 13, 17, 20, 25, 30, 35 | X | X | (Eggs do not feed) | Brazil | [109] | |||||||||
| 17.5, 20, 22.5, 25, 27.5, 30, 32.5, 37.5, 42.5 | X | X | X | X | X | X | Chicken liver | South Africa | [90] | |||||
| 26 | X | - | - | Sri Lanka | [110] | |||||||||
| 22, 25, 28, 30 | X | X | X | X | X | X | - | Mutton | India | [111] | ||||
| 10 | X | X | X | X | X | X | - | Buffalo liver | India | [112] | ||||
H: hatching; E1: first ecdysis; E2: second ecdysis; W: onset of wandering; P: onset of pupariation; A: adult eclosion; * approximation. -: unstated.
Table 3.
Developmental threshold (D0) temperatures for Chrysomya megacephala, with standard errors.
|
D0 (°C) |
|||||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Hatching | Ecdysis 1 | Ecdysis 2 | Wandering | Pupariation | Eclosion | Source |
| 17.5, 20, 22.5, 25, 27.5, 30, 32.5, 37.5 and 42.5 | - | 12.49 ± 0.98 | 10.57 ± 1.12 | 10.68 ± 1.25 | 10.12 ± 1.67 | 10.40 ± 1.60 | [90] |
| 13, 17, 20, 25, 30 and 35 | 10.80 ± 0.82 | - | - | - | - | - | [109] |
| 16, 19, 22, 25, 28, 31 and 34 | 11.76 ± 0.26 | 12.72 ± 0.35 | 12.78 ± 0.44 | 11.06 ± 0.29 | 9.57 ± 0.61 | 9.07 ± 0.54 | [91] |
| 15, 20, 25, 30 and 35 | 9.07 ± 0.43 | 12.14 ± 0.51 | 12.48 ± 0.40 | 12.52 ± 0.34 | 11.80 ± 0.64 | 10.95 ± 0.71 | [108] |
-: unstated.
Table 4.
Thermal summation constants (K) for Chrysomya megacephala, with standard errors.
|
K (d°C) |
|||||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Hatching | Ecdysis 1 | Ecdysis 2 | Wandering | Pupariation | Eclosion | Source |
| 17.5, 20, 22.5, 25, 27.5, 30, 32.5, 37.5 and 42.5 | - | 0.61 ± 0.08 | 1.67 ± 0.16 | 4.00 ± 0.44 | 4.73 ± 0.69 | 8.66 ± 1.17 | [90] |
| 13, 17, 20, 25, 30 and 35 | 7.48 ± 1.11 | - | - | - | - | - | [109] |
| 16, 19, 22, 25, 28, 31 and 34 | 7.53 ± 0.23 | 9.85 ± 0.49 | 20.92 ± 1.30 | 55.89 ± 1.69 | 88.09 ± 4.99 | 166.29 ± 7.80 | [91] |
| 15, 20, 25, 30 and 35 | 11.24 ± 0.42 | 20.41 ± 1.38 | 30.88 ± 1.75 | 50.79 ± 2.52 | 70.42 ± 5.56 | 150.43 ± 11.4 | [108] |
-: unstated.
Male pupae tend to eclose two or three hours ahead of females, possibly because their genome is slightly smaller than females’ [113].
Mating occurs about two days after emergence and oviposition starts at 3–4 days. The longevity of adults is affected by temperature and humidity. The mean and maximum lifespans of adults at 40% relative humidity (RH) were 64 and 105 days, respectively; and at 75% RH, 54 and 95 days, respectively [110]; other studies found shorter lifespans [96], and that males lived seven days longer than females [114]. On salted cod, longevity was 47–55 days [115].
Predators and parasitoids
The eggs and larvae of C. megacephala are eaten by some carrion beetles, but their primary predators are larvae of the blow flies C. albiceps and C. rufifacies [82,85,116]. The ecological pressure exerted by these species has been sufficient that C. megacephala specifically avoids ovipositing near eggs and larvae of these blow flies, and larvae of C. megacephala emigrate earlier and further from breeding sites invaded by these intraguild predators. These adaptations mean that intraguild predation is unlikely to provide adequate biological control of populations of C. megacephala. They also need to be taken into account in qualifying estimates of minimum postmortem intervals.
Several species of non-specific parasitoid wasps also use C. megacephala as a host, including Brachymeria podagrica (Fabricius, 1787) (Chalcididae), Tachinaephagus zealandicus Ashmead, 1904 (Encyrtidae), Nasonia vitripennis (Walker, 1836) (Pteromalidae) and Pachycrepoideus vindemiae (Rondani, 1875) (Pteromalidae) [117–120]. Their potentials as biological control agents have not been explored, but they are likely to be substantial if effects on non-target hosts are not a concern. Parasitoids can also disrupt their host's rate of development, so their presence needs to be considered when qualifying estimates of minimum postmortem intervals [119,120].
Forensic significance
Forensic entomology has four sub-fields: medico-legal forensic entomology, urban forensic entomology, stored-product forensic entomology and environmental forensic entomology. These disciplines may be interested in C. megacephala for their own ends, rather than for its direct role in investigations, and it is particularly becoming a model species for developing techniques in medico-criminal forensics entomology [90,121]. Phylogenetically, it is a highly derived species [5], and ecologically it is an unusually catholic species, so attention needs to be given to how broadly representative it is of carrion flies in forensic contexts.
Urban forensic entomology
Because the association of C. megacephala with faeces and decay is a concern for public health [122,123], it can be involved in legal cases relating to urban forensic entomology, such as prosecution and litigation over health and sanitation laws and public nuisances.
Chrysomya megacephala propagates diseases like diarrhoea and parasite infestations [122,123]. The synanthropic habits of C. megacephala lead to mechanical transmission of pathogens such as Toxoplasma gondii [124], many bacteria [72,125–128], and eggs of parasitic helminth worms [129] via the adult flies’ mouthparts, feet and faeces.
Epidemiologically, populations of C. megacephala are likely to grow quickly because of their favourable intrinsic rate of natural increase (r ≈ 0.218 2/d at 26 °C), finite rate of natural increase (K ≈ 1.243 8/d), net reproduction rate (R0 ≈ 91.7 offspring/individual) and mean generation time (T ≈ 20.7 d) [96]. These figures illustrate another aspect of why this species spread around the planet so quickly, and why it is potentially the most significant insect in forensic entomology. The biotic potential (quantified as longevity and mean egg mass) of C. megacephala is greater than that of Cochliomyia macellaria (Fabricius, 1775) [114], but the shortage of data for other blow fly species under similar experimental conditions precludes wider comparison.
Stored-product forensic entomology
By traditional definition, stored-product forensic entomology is involved in litigation over infestations or contaminations of stored products, usually commercially distributed foods, by insects. As the use of insects to produce food and other goods is developing, insects are rapidly becoming a source of stored products themselves, and the traditional definition of stored-product forensic entomology may expand to include this new arena of litigation.
Chrysomya megacephala as a contaminant
Because of its ability to contaminate foodstuffs [122,123], C. megacephala can be the focus of regulatory enforcement and commercial insurance claims that require stored-product forensic entomologists to estimate when and where contaminations occurred. Two specific industries serve as illustrations here.
Because of their taste for sugary substances, adults of C. megacephala feed on the exudates of palm trees being tapped for toddy (sap forming the basis of palm wine), and contaminate the spathes and collecting pots with droppings, leading to fermentation and contamination of the toddy by pathogens.
The toddy industry is relatively small, but the sun-dried fish industry in many parts of south-east Asia, the Pacific and Africa sustains serious economic losses because of the dietary and breeding preferences of adults of C. megacephala, which lead to contamination of the fish with fly eggs and larvae [84,115,130]. Similar problems can arise in open-air meat markets and slaughter houses [1]. It appears that C. megacephala has evolved a tolerance to the use of salt to preserve fish in south-east Asia, where females will oviposit on salted fish, especially if conspecifics’ eggs are present or the salt concentration is below 40% (dry weight basis) [84]. Well-designed, fly-tight lids diminish infestation in salting tanks and physically screening fish during the initial drying period effectively controlled infestation [115], although blow flies sometimes oviposit through screens.
Chrysomya megacephala as a product
Stored-product forensic entomology is also likely to become involved in litigation around quality assurance of stored products for recently developed industries that are based on the ability of C. megacephala to convert organic waste into protein- and lipid-rich live tissue that eventually becomes products like fishmeal substitutes and biodiesel.
Estimates of the food conversion efficiency of blow fly larvae are impressively high [131], and the protein content of the larvae is both high and rich in essential amino acids. The ability of larvae of C. megacephala to convert a broad spectrum of organic wastes, including excrement, abattoir waste and kitchen waste, makes it a strong alternative to Calliphora vicina (Robineau-Desvoidy, 1830) and Lucilia sericata (Meigen, 1826), which are used for industrial-scale recycling in Europe. The end product is dried fly meal for supplementing livestock feeds, and live larvae for fishing bait. The industrial cultivation of C. megacephala to produce a substitute for fishmeal has been explored in South Africa (Cameron Richards, personal communication).
Lipids extracted from C. megacephala larvae can transesterified to produce biodiesel [132,133], providing an industrial process that can convert organic waste to clean fuel. Chitosan may be derived from the cuticles of pupae and adults. As work on the transcriptomics of C. megacephala accumulates [134–136], more commercial applications derived from the genome can be expected.
Other economic applications of C. megacephala include enhancing mango pollination in Taiwan, Australia and Israel [137–140]. All of these industries may generate litigation over quality control of their products if their cultures become contaminated by other flies, and they may create cases for urban forensic entomology if flies or odours escape from breeding facilities.
Medicolegal forensic entomology
Estimating minimum postmortem intervals
The strong association of C. megacephala with decaying matter has led to its appearance in many forensic case reports [141–148] in forensic entomology. Its reputation as an early and dominant colonizer of carrion makes it a particularly attractive species for estimating minimum postmortem intervals [28,43].
The age of larvae can be estimated from the timing of developmental events or their growth [90–92]. In cases where parasitoids kill live samples of C. megacephala, a PMI can still be estimated using published thermal accumulation models for some of the parasitoids [119,120]. If larvae of facultative predatory blow flies (C. albiceps and C. rufifacies in particular) are also present, larvae of C. megacephala may pupate prematurely [85,116]. The age of puparia of C. megacephala has been estimated using cuticular hydrocarbons [149–151], while the age of adults has been estimated using the accumulation of pteridines in their eyes [152].
The presence of C. megacephala larvae on a corpse may be due to premortem myiasis, so it must be interpreted cautiously in framing a minimum postmortem interval [153]. Chrysomya megacephala larvae can facultatively infest traumatic lesions in living humans and other vertebrates [153,154], causing secondary myiasis that was initiated by another species [1]. Until Villeneuve distinguished C. bezziana from C. megacephala in 1914, many cases of primary wound myiasis were attributed to C. megacephala (as its synonym, C. dux) [1]. Most of these records are now ascribed to misidentifications of C. bezziana [1].
Growth may also continue under surprisingly cold conditions: live larvae of C. megacephala were found within the mouth of a corpse 12 days after it had been stored in a mortuary refrigerator [155]. The size of the mass of maggots is crucial in producing such cases [121].
Entomotoxicology
In common with a number of other carrion flies [83], immature specimens of C. megacephala have been used as secondary, qualitative toxicological samples to infer the presence of toxicants in bodies that are too decomposed to provide primary, quantitative samples [156–163]. Such information may provide forensic evidence of a cause of death. Modern drug detection instruments are now sufficiently sensitive that the condition of tissue samples is a dwindling technical concern [164], but late in decomposition, eclosed fly pupariae may be the only drug-containing tissue still available.
The presence of drugs in a corpse or carcass may alter the rate of development of insects feeding on it, which will affect the interpretation of PMIs estimated from these insects [83]. Although the dose-dependent effect of specific drugs on development has been investigated, it is extremely difficult to make more than a qualitative correction to PMI estimates [83,165].
Geographical relocation
Carrion insects can sometimes indicate the geographic origin of relocated corpses and products [166], but in practice this is uncommon [167,168]. Relevant data can be drawn from four primary sources: spatial distribution, temporal distribution, biology (behaviour and development) and molecular analyses; which of these will be significant in a particular case depends in part on the spatial scale of relocation [168]. Chrysomya megacephala is geographically and ecologically ubiquitous and highly mobile, active whenever weather and climate allow, and unspecialized in its general biology, so that it is unlikely to provide evidence of relocation from the first three sources listed; the most promising source is population genetics.
Relevant population genetic markers for C. megacephala have been explored. COI, COII and ISSR markers differentiated between eastern and western populations of C. megacephala in Malaysia [169], where orographic barriers occur. This study found less genetic variation between populations than expected (relative to related blow flies), and suggested that it was due to urbanization and intercity transport of flies by human activities [169]. Amplified fragment length polymorphisms of C. megacephala sampled across Florida and Alabama indicated some spatial differentiation, but also that its genetic diversity was diminished relative to both populations in its native range and populations of native blow flies in the USA [59]. It is likely that the population genetics of C. megacephala will provide evidence of relocation in rural parts of the species’ native range, but not in regions that it has invaded recently, especially if there are few geographical barriers to structure its populations.
Environmental forensic entomology
Environmental forensic entomology is in its infancy, but two examples illustrate its potential scope and the role for C. megacephala. One focus of this branch of forensic entomology is the detection and tracking environmental contaminants [83]. For example, blow flies have been used to monitor the movement of mercury through food chains, specifically one involving fish [170,171]. This forensic application has not yet been developed for C. megacephala, but is likely to have good potential. It is also now possible to detect the presence of mammals in an area through the presence for their DNA in the guts of blowflies [172], and C. megacephala has served as the model for such monitoring.
Conclusion
The near-global distribution of C. megacephala and its ubiquity and abundance in an unusually broad spectrum of situations that affect humans means that it may be the most important species of insect affecting forensic entomology. Its only rival may be the larder beetle, Dermestes maculatus De Geer, 1774, which has been distributed globally by humans, and which has relevance in urban, stored-product and medico-criminal forensics entomology because of its association with dried protein resources [166]. Lucilia sericata and Lucilia cuprina (Wiedemann, 1830) are also strong contenders because they have aspects of their biology and geographical distribution in common with C. megacephala, but are less often mentioned in forensic case reports at a global scale [141–148].
The second point that this review makes is that even though much forensically relevant information is available, much remains to be studied about C. megacephala, both as a species of pertinence, and as a model for techniques in forensic entomology. The growth of transcriptomics offers a particularly promising new method, but older methods are far from exhausted and there are many partially resolved questions that await closure. The ubiquity and abundance of C. megacephala offers opportunities for many laboratories to be involved in these developments.
Funding Statement
Rhodes University [grant number 37201].
Acknowledgments
We thank Jens Amendt for inviting this contribution and wisdom and patience in helping it to fruition; Thomas Pape for information about the type locality of C. megacephala; Shiying Li for very generously drawing Figure 1; two reviewers for their constructive feedback; and Rhodes University for access to library resources.
Disclosure statement
No potential conflict of interest was reported by the authors.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- [1].Zumpt F. Myiasis in man and animals in the old world: a textbook for physicians, veterinarians and zoologists. London: Butterworths; 1965. p. 1–267. [Google Scholar]
- [2].Kurahashi H. Probable origin of synanthropic fly, Chrysomya megacephala, in New Guinea (Diptera: Calliphoridae). In: Gressitt JL, editor Biogeography and ecology of New Guinea. Dordrecht: Springer; 1982. p. 689–698. [Google Scholar]
- [3].Bharti M, Kurahashi H. Finding of feral derived form (fdf) of Chrysomya megacephala (Fabricius) from India with an evolutionary novelity [sic] (Diptera, Calliphoridae). Japan J Syst Ent. 2009;15:411–413. [Google Scholar]
- [4].Kurahashi H. Blow flies from Samoa with description of a new species of Chrysomya (Diptera, Calliphoridae). Japan J Ent. 1991;59:627–636. [Google Scholar]
- [5].Singh B, Kurahashi H, Wells JD. Molecular phylogeny of the blowfly genus Chrysomya. Med Vet Ent. 2011;25:126–134. [DOI] [PubMed] [Google Scholar]
- [6].James MT. Genus Chrysomya in New Guinea (Diptera: Calliphoridae). Pacific Insects. 1971;13:361–369. [Google Scholar]
- [7].Braack LEO. Spread in South Africa of the oriental latrine fly Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae), an introduced species closely resembling Chrysomya bezziana Villeneuve. Onderstepoort J Vet Res. 1991;58:311–312. [PubMed] [Google Scholar]
- [8].Wells J, Kurahashi H. New species of Chrysomya (Diptera: Calliphoridae) Sulawesi Indonesia with a key to the Oriental, Australasian and Oceanian species. Med Ent Zool. 1996;47:131–138. [Google Scholar]
- [9].James MT. The flies that cause myiasis in man. US Dept Agric Misc Publ. 1947;631:1–175. [Google Scholar]
- [10].Prins AJ. Discovery of the oriental latrine fly Chrysomya megacephala (Fabricius) along the south-western coast of South Africa. Ann South Afr Mus. 1979;78:39–47. [Google Scholar]
- [11].Prins AJ. Morphological and biological notes on six South African blowflies (Diptera, Calliphoridae) and their immature stages. Ann South Afr Mus. 1982;90:201–217. [Google Scholar]
- [12].Irish S, Lindsay T, Wyatt N. Key to adults of Afrotropical species of the genus Chrysomya Robineau-Desvoidy (Diptera: Calliphoridae). Afr Ent. 2014;22:297–306. [Google Scholar]
- [13].Prado e Castro C, Szpila K, Martínez-Sánchez A, et al. The blowflies of the Madeira Archipelago: species diversity, distribution and identification (Diptera, Calliphoridae s. l.). ZooKeys. 2016;634:101–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lutz L, Williams KA, Villet MH, et al. Species identification of African blowflies (Diptera: Calliphoridae) of forensic importance. Int J Legal Med. 2017[Aug 2017]; [12 p.] DOI: 10.1007/s00414-017-1654-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chaiwong T, Sukontason K, Olson JK, et al. Fine structure of the reproductive system of Chrysomya megacephala (Diptera: Calliphoridae): the external sexual organ. Parasitol Res. 2008;102:973–980. [DOI] [PubMed] [Google Scholar]
- [16].Bansal A, Murad H. Morphology of the female reproductive organ of the oriental latrine fly, Chrysomya megacephala F. (Diptera, Calliphoridae). Japan J Sanit Zool. 198;738:233–238. [Google Scholar]
- [17].Name KPO, Pujol Luz JR, Báo SN. Structure and ultrastructure of spermatozoa of Chrysomya megacephala (Diptera: Calliphoridae). Micron. 2010;41:853–560. [DOI] [PubMed] [Google Scholar]
- [18].Meskin I. The egg of the Highveld blowfly, Calliphora croceipalpis Jaennicke (Diptera: Calliphoridae), with a key to the eggs of five other Highveld species. J Ent Soc South Afr. 1991;54:185–190. [Google Scholar]
- [19].Sukontason K, Sukontason KL, Piangjai S, et al. Fine structure of the eggs of blowflies Aldrichina grahami and Chrysomya pacifica (Diptera: Calliphoridae). Biol Res. 2004;37:483–487. [DOI] [PubMed] [Google Scholar]
- [20].de Oliveira David JA, Rocha T, Caetano FH. Ultramorphological characteristics of Chrysomya megacephala (Diptera, Calliphoridae) eggs and its eclosion. Micron. 2008;39:1134–1137. [DOI] [PubMed] [Google Scholar]
- [21].Sukontason KL, Sukontason K, Piangjai S, et al. Larval morphology of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) using scanning electron microscopy. J Vector Ecol. 2003;28:47–52. [PubMed] [Google Scholar]
- [22].Sukontason K, Piangjai S, Siriwattanarungsee S, et al. Morphology and developmental rate of blowflies Chrysomya megacephala and Chrysomya rufifacies in Thailand: application in forensic entomology. Parasit Res. 2008;102:1207–1216. [DOI] [PubMed] [Google Scholar]
- [23].Mendonça PM, dos Santos-Mallet JR, de Carvalho Queiroz MM. Ultrastructure of larvae and puparia of the blowfly Chrysomya megacephala (Diptera: Calliphoridae). Microsc Res Tech. 2012;75:935–939. [DOI] [PubMed] [Google Scholar]
- [24].Szpila K, Hall MJR, Sukontason K, et al. Morphology and identification of first instars of the European and Mediterranean blowflies of forensic importance. Part I: Chrysomyinae. Med Vet Ent. 2013;27:181–193. [DOI] [PubMed] [Google Scholar]
- [25].Szpila K, Villet MH. Morphology and identification of first instar larvae of African blowflies (Diptera: Calliphoridae) commonly of forensic importance. Med Vet Ent. 2011;48:738–752. [DOI] [PubMed] [Google Scholar]
- [26].Patton WS, Evans AM. Insects, ticks, mites and venomous animals of medical and veterinary importance. Part I. Medical. Croydon: [publisher unknown]; 1929. p. 786. [Google Scholar]
- [27].Holloway BA. Identification of third-instar larvae of flystrike and carrion-associated blowflies in New Zealand (Diptera: Calliphoridae). N Z Ent. 1991;14:24–28. [Google Scholar]
- [28].Wang JF, Li ZG, Chen YC, et al. The succession and development of insects on pig carcasses and their significances in estimating PMI in south China. For Sci Int. 2008;179:11–18. [DOI] [PubMed] [Google Scholar]
- [29].Amorim JA, Ribeiro OB. Distinction among the puparia of three blowfly species (Diptera: Calliphoridae) frequently found on unburied corpses. Mem Inst Oswaldo Cruz. 2001;96:781–784. [DOI] [PubMed] [Google Scholar]
- [30].Siriwattanarungsee S, Sukontason K, Kuntalue B, et al. Morphology of the puparia of the housefly, Musca domestica (Diptera: Muscidae) and blowfly, Chrysomya megacephala (Diptera: Calliphoridae). Parasit Res. 2005;96:166–170. [DOI] [PubMed] [Google Scholar]
- [31].Tourle RA, Downie DA, Villet MH. Flies in the ointment: a morphological and molecular comparison of Lucilia cuprina and L. sericata (Diptera: Calliphoridae) in South Africa. Med Vet Ent. 2009;23:6–14. [DOI] [PubMed] [Google Scholar]
- [32].Park SH, Park CH, Zhang Y. Using the developmental gene bicoid to identify species of forensically important blowflies (Diptera: Calliphoridae). BioMed Res Int. 2013; [8 p.] DOI: 10.1155/2013/538051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stevens JR, West H, Wall R. Mitochondrial genomes of the sheep blowfly, Lucilia sericata, and the secondary blowfly, Chrysomya megacephala. Med Vet Ent. 2008;22:89–91. [DOI] [PubMed] [Google Scholar]
- [34].Lessinger AC, Azeredo-Espin AML. Evolution and structural organisation of mitochondrial DNA control region of myiasis-causing flies. Med Vet Ent. 2000;14:71–80. [DOI] [PubMed] [Google Scholar]
- [35].Varón A, Vinh LS, Wheeler WC. POY version 4: phylogenetic analysis using dynamic homologies. Cladistics. 2010;26:72–85. [DOI] [PubMed] [Google Scholar]
- [36].Gardner SN, Hall BG. When whole-genome alignments just won't work: kSNP3 v2 software for alignment-free SNP discovery and phylogenetics of hundreds of microbial genomes. PLoS ONE. 2013; [12 p.] DOI: 10.1371/journal.pone.0081760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Harvey ML, Mansell MW, Villet MH, et al. Molecular identification of some forensically important blowflies of southern Africa and Australia. Med Vet Ent. 2003;17:363–369. [DOI] [PubMed] [Google Scholar]
- [38].Harvey ML, Gaudieri S, Villet MH, et al. A global study of forensically significant calliphorids: implications for identification. For Sci Int. 2008;177:66–76. [DOI] [PubMed] [Google Scholar]
- [39].Nelson LA, Wallman JF, Dowton M. Using COI barcodes to identify forensically and medically important blowflies. Med Vet Ent. 2007;21:44–52. [DOI] [PubMed] [Google Scholar]
- [40].Sonet G, Jordaens K, Braet Y, et al. Utility of GenBank and the barcode of life data systems (BOLD) for the identification of forensically important Diptera from Belgium and France. Zookeys. 2013;365:307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Oliveira PV, Matos NS, Klippel AH, et al. Using DNA barcodes to identify forensically important species of Diptera in Espírito Santo State, Brazil. Braz Arch Biol Technol. 2017; [8 p.] DOI: 10.1590/1678-4324-2017160106 [DOI] [Google Scholar]
- [42].Zajac B, Sontigun N, Wannasan A, et al. Application of DNA barcoding for identifying forensically relevant Diptera from northern Thailand. Parasit Res. 2016;115:2307–2320. [DOI] [PubMed] [Google Scholar]
- [43].Kavitha R, Nazni WA, Tan TC, et al. Molecular identification of blow flies recovered from human cadavers during crime scene investigations in Malaysia. Malays J Pathol. 2012;34:127–132. [PubMed] [Google Scholar]
- [44].Ross HA, Murugan S, Sibon Li WL. Testing the reliability of genetic methods of species identification via simulation. Syst Biol. 2008;57:216–230. [DOI] [PubMed] [Google Scholar]
- [45].Meier R, Shiyang K, Vaidya G, et al. DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Syst Biol. 2006;55:715–728. [DOI] [PubMed] [Google Scholar]
- [46].Wells JD, Wall R, Stevens JR. Phylogenetic analysis of forensically important Lucilia flies based on cytochrome oxidase 1 sequence: a cautionary tale of forensic species determination. Int J Legal Med. 2007;12:229–233. [DOI] [PubMed] [Google Scholar]
- [47].Akbarzadeh K, Wallman JF, Sulakova H, et al. Species identification of Middle Eastern blowflies (Diptera: Calliphoridae) of forensic importance. Parasitol Res. 2015;114:1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pape T, Thompson FC, editors.. 2013. Chrysomya megacephala. Systema Dipterorum [Internet]. Copenhagen: Natural History Museum of Denmark; [updated 2013 Jun 13; cited 2017 Aug 28] Available from: http://www.diptera.org/ [Google Scholar]
- [49].Pont AC. Family Calliphoridae. In: Crosskey RW, editor Catalogue of the Diptera of the Afrotropical region. London: British Museum (Natural History); 1980. p. 779–800. [Google Scholar]
- [50].Fabricius JC. Entomologia systematica emendata et aucta. Secundum classes, ordines, genera, species, adjectis synonymis, locis, descriptionibus, observationibus. Vol. 4, CG. Hafniae: Proft; 1794. [Google Scholar]
- [51].Williams KA, Villet MH. A new and earlier record of Chrysomya megacephala in South Africa, with notes on another exotic species, Calliphora vicina (Diptera: Calliphoridae). Afr Invert. 2006;47:347–350. [Google Scholar]
- [52].Kurahashi H. The oriental latrine fly: Chrysomya megacephala (Fabricius) newly recorded from Ghana and Senegal, West Africa. Kontyû. 1978;46:432. [Google Scholar]
- [53].Martínez-Sánchez A, Marcos-García MA, Rojo S. First collection of Chrysomya megacephala (Fabr.) in Europe (Diptera: Calliphoridae). Pan-Pac Ent. 2001;77:240–243. [Google Scholar]
- [54].Ebejer MJ. The occurrence of Chrysomya megacephala (Fabricius) (Diptera, Brachycera) in Malta and records of other Calliphoridae from the Maltese Islands. Ent Mon Mag. 2007;143:165–170. [Google Scholar]
- [55].Castro CP, García MD. First record of Chrysomya megacephala (Fabricius, 1794) (Diptera, Calliphoridae) from Portugal. Graellsia. 2009;65:75–77. [Google Scholar]
- [56].Imbiriba AS, Izutani DT, Milhoretto IT, et al. Introdução de Chrysomya chloropyga (Wiedemann, 1818) na Região Neotropical (Diptera: Calliphoridae). Arq Biol Technol. 1977;20:35–39. [Google Scholar]
- [57].Guimarães J, do Prado A, Linhares AX. Three newly introduced blowfly species in southern Brazil (Diptera, Calliphoridae). Rev Bras Entomol. 1978;22:53–60. [Google Scholar]
- [58].Picard CJ. First record of Chrysomya megacephala Fabricius. (Diptera: Calliphoridae) in Indiana, U.S.A. Proc Ent Soc Wash. 2013;115:265–267. [Google Scholar]
- [59].Bao F, Wells JD. Population genetic structure of an invasive forensically important insect. Electrophoresis. 2014;35:3193–3200. [DOI] [PubMed] [Google Scholar]
- [60].Carmo RFR, Vasconcelos SD. First record of the blow fly Chrysomya megacephala (Diptera: Calliphoridae) on a southern Atlantic island: implications for disease transmission in a protected environment. J Vector Ecol. 2014;39:228–230. [DOI] [PubMed] [Google Scholar]
- [61].Baez M, Ortega G, Kurahashi H. Immigration of the oriental latrine fly, Chrysomya megacephala (Fabricius) and the Afrotropical Filth Fly, Ch. chloropyga (Wiedemann), into the Canary Islands (Diptera, Calliphoridae). Kontyû. 1981;49:712–714. [Google Scholar]
- [62].Kosmann C, Pinto de Mello R, Harterreiten-Souza ES, et al. A list of current valid blow fly names (Diptera: Calliphoridae) in the Americas south of Mexico with key to the Brazilian species. EntomoBrasilis. 2013;6:74–85. [Google Scholar]
- [63].Tantawi TI, Sinclair BJ. An update of the blow flies (Diptera: Calliphoridae) of the Galápagos Islands, and first record of Chrysomya rufifacies (Macquart) from mainland Ecuador. Zootaxa. 2013;3750:237–250. [DOI] [PubMed] [Google Scholar]
- [64].Gill RJ, editor Chrysomya blowflies. California: (CA: ): Department of Food and Agriculture, Division of Plant Industry; 1988. [Google Scholar]
- [65].Greenberg B.Chrysomya megacephala (F.) (Diptera: Calliphoridae) collected in North America and notes on Chrysomya species present in the New World. J Med Ent. 1988;25:199–200. [DOI] [PubMed] [Google Scholar]
- [66].Wells J.Chrysomya megacephala (Diptera: Calliphoridae) has reached the continental united states: review of its biology, pest status, and spread around the world. J Med Ent. 1991;28:471–473. [DOI] [PubMed] [Google Scholar]
- [67].Wallman JF. First record of the oriental latrine fly, Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae), from South Australia. Trans R Soc S Austr. 1993;121:163–164. [Google Scholar]
- [68].Kurahashi H, Wells J, Ogino K. The oriental latrine fly, Chrysomya megacephala (Fabricius), newly recorded from Honduras, Central America. Japan J Ent. 1994;62:860. [Google Scholar]
- [69].DeJong GD. Report of Chrysomya megacephala (Diptera: Calliphoridae) in Northern New Mexico. Ent News. 1995;106:192. [Google Scholar]
- [70].Olea MS, Juri MJD, Centeno N. First report of Chrysomya megacephala (Diptera: Calliphoridae) in northwestern Argentina. Fla Ent. 2011;94:345–346. [Google Scholar]
- [71].Braack LEO, Retief PF. Dispersal, density and habitat preference of the blowflies Chrysomya albiceps (Wd.) and Chrysomya marginalis (Wd.) (Diptera: Calliphoridae). Onderstepoort J Vet Res. 1986;53:13–18. [PubMed] [Google Scholar]
- [72].Braack LEO, de Vos V. Feeding habits and flight range of blow-flies (Chrysomyia spp.) in relation to anthrax transmission in the Kruger National Park. Onderstepoort J Vet Res. 1990;57:141–142. [PubMed] [Google Scholar]
- [73].Schumann H. Family Calliphoridae. In: Soos A, Papp L, Catalog of Palearctic Diptera, Calliphoridae – Sarcophagidae. Budapest: [publisher unknown]; 1986. p. 11–58. [Google Scholar]
- [74].Ngoen-Klan R, Moophayak K, Klong-Klaew T, et al. Do climatic and physical factors affect populations of the blow fly Chrysomya megacephala and house fly Musca domestica? Parasit Res. 2011;109:1279–1292. [DOI] [PubMed] [Google Scholar]
- [75].Moophayak K, Klong-Klaew T, Sukontason K, et al. Species composition of carrion blow flies in northern Thailand: altitude appraisal. Rev Inst Med Trop São Paulo. 2014;56:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Nelson LA, Dowton M, Wallman JF. Thermal attributes of Chrysomya species. Ent Exp Appl. 2009;133:260–275. [Google Scholar]
- [77].Richards CS, Price BW, Villet MH. Thermal ecophysiology of seven carrion-feeding blowflies in Southern Africa. Ent Exp Appl. 2009;131:11–19. [Google Scholar]
- [78].Kumara TK, Disney RHL, Hassan AA, et al. Occurrence of oriental flies associated with indoor and outdoor human remains in the tropical climate of north Malaysia. J Vect Ecol. 2012;37:62–68. [DOI] [PubMed] [Google Scholar]
- [79].Bohart GE, Gressitt JL. Filth-inhabiting flies of Guam. Bull Bernice P Bishop Mus. 1951;204:1–151. [Google Scholar]
- [80].d'Almeida JM, Salviano RJB. Feeding preference of the larvae of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) and Ravinia belforti (Prado e Fonseca) (Diptera: Sarcophagidae) concerning different diets. Mem Inst Oswaldo Cruz. 1996;91:137–138. [DOI] [PubMed] [Google Scholar]
- [81].Bunchu N, Sukontason KL, Olson JK, et al. Behavioral responses of Chrysomya megacephala to natural products. Parasitol Res. 2008;102:419–429. [DOI] [PubMed] [Google Scholar]
- [82].Yang ST, Shiao SF. Oviposition preferences of two forensically important blow fly species, Chrysomya megacephala and C. rufifacies (Diptera: Calliphoridae), and implications for postmortem interval estimation. J Med Ent. 2012;49:424–435. [DOI] [PubMed] [Google Scholar]
- [83].da Silva E, Wilhelmi B, Villet MH. Forensic entomotoxicology revisited – towards professional standardization of study designs. Int J Leg Med. 2017; [13 p.] DOI: 10.1007/s00414-017-1603-9 [DOI] [PubMed] [Google Scholar]
- [84].Esser JR. Factors influencing oviposition, larval growth and mortality in Chrysomya megacephala. Bull Ent Res. 1990;80:369–376. [Google Scholar]
- [85].Galindo LA, Moral RA, Moretti TC, et al. Intraguild predation influences oviposition behavior of blow flies (Diptera: Calliphoridae). Parasitol Res. 2016;115:2079–2102. [DOI] [PubMed] [Google Scholar]
- [86].Williams KA, Wallman JF, Lessard BD, et al. Nocturnal oviposition behavior of carrion flies (Diptera: Calliphoridae, Sarcophagidae) in the southern hemisphere (South Africa and Australia) and its forensic implications. Forens Sci Med Path. 2017; [10 p.] DOI: 10.1007/s12024-017-9861-x [DOI] [PubMed] [Google Scholar]
- [87].Smith JL, Palermo NA, Theobald JC, et al. The forensically important blow fly, Chrysomya megacephala (Diptera: Calliphoridae), is more likely to walk than fly to carrion at low light levels. For Sci Int. 2016;266:245–249. [DOI] [PubMed] [Google Scholar]
- [88].Erzinçlioglu YZ. On the interpretation of maggot evidence in forensic cases. Med Sci Law. 1990;30:65–66. [DOI] [PubMed] [Google Scholar]
- [89].Wells JD, King J. Incidence of precocious egg development in flies of forensic importance (Calliphoridae). Pan-Pac Ent. 2001;77:235–239. [Google Scholar]
- [90].Richards CS, Villet MH. Data quality in thermal summation models of development of forensically important blowflies (Diptera: Calliphoridae): a case study. Med Vet Ent. 2009;23:269–276. [DOI] [PubMed] [Google Scholar]
- [91].Yang YQ, Li XB, Shao RY, et al. Developmental times of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) at constant temperatures and applications in forensic entomology. J Forensic Sci. 2016;61:1278–1284. [DOI] [PubMed] [Google Scholar]
- [92].Gruner SV, Slone DH, Capinera JL, et al. Development of the Oriental latrine fly, Chrysomya megacephala (Diptera: Calliphoridae), at five constant temperatures. J Med Entomol. 2017;54:290–298. [DOI] [PubMed] [Google Scholar]
- [93].Hu Y, Yuan X, Zhu F, et al. Development time and size-related traits in the Oriental blowfly, Chrysomya megacephala along a latitudinal gradient from China. J Them Biol. 2010;35:366–371. [Google Scholar]
- [94].Mohd II, Khairul O, Ong H-K, et al. Accelerating Chrysomya megacephala maggot growth for forensic entomology cases. Jurnal Sains Kesihatan Malaysia. 2007;5:17–26. [Google Scholar]
- [95].Khole V. Studies on metabolism in relation to post embryonic development of some calliphorid flies (Diptera: Insecta). Entomon. 1979;4:61–63. [Google Scholar]
- [96].Gabre RM, Adham FK, Chi H. Life table of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae). Acta Oecol. 2005; [4 p.] DOI: 10.1016/j.actao.2004.12.002 [DOI] [Google Scholar]
- [97].O'Flynn MA. The succession and rate of development of blowflies in carrion in southern Queensland and the application of these data to forensic entomology. J Aust Entomol Soc. 1983;22:137–147. [Google Scholar]
- [98].Subramanian K, Mohan KR. Biology of the blowflies Chrysomyia megacephala, Chrysomyia rufifacies and Lucillia cuprina. Kerala J Vet Sci. 1980;11:252–261. [Google Scholar]
- [99].Arias-Di Donato L, Liria J. Vital statistics of Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae) under different diets from Venezuela. J Entomol Zool Stud. 2016;4:247–251. [Google Scholar]
- [100].Levot GW, Brown KR, Shipp E. Larval growth of some calliphorid and sarcophagid Diptera. Bull Entomol Res. 1979;69:469–475. [Google Scholar]
- [101].Wells JD, Kurahashi H. Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) development: rate, variation and the implications for forensic entomology. Japan J Sanitary Zool. 1994;45:303–309. [Google Scholar]
- [102].Aguirre-Gil OJ, Valente FI, Santos LS, et al. Desarrollo larval de Chrysomya megacephala (Diptera: Calliphoridae) en diferentes dietas y densidades larvales. Rev Colomb Entomol. 2015;41:48–57. [Google Scholar]
- [103].Nishida K. Experimental studies on the estimation of postmortem intervals by means of fly larvae infesting human cadavers. Japan J Forensic Med. 1984;38:24–41. [PubMed] [Google Scholar]
- [104].Goodbrod JR, Goff ML. Effects of larval population density on rates of development and interactions between two species of Chrysomya (Diptera: Calliphoridae) in laboratory culture. J Med Entomol. 1990;27:338–343. [DOI] [PubMed] [Google Scholar]
- [105].Hu Y, Yuan X, Zhu F, et al. Development time and size-related traits in the oriental blowfly, Chrysomya megacephala along a latitudinal gradient from China. J Therm Biol. 2010;35:366–371. [Google Scholar]
- [106].Kamel AS, Helmy NM, Marwan DA, et al. Influence of temperature and larval food oncertain biological aspectsof Chrysomyamegacephala (Diptera: Calliphoridae). IOSR J Pharm Biol Sci. 2016;11:05–11. [Google Scholar]
- [107].Lunt N. Applied studies of some southern African blowflies (Diptera: Calliphoridae) of forensic importance [unpublished MSc thesis]. Grahamstown: Rhodes University; 2002. p. 224. [Google Scholar]
- [108].Gruner SV, Slone DH, Capinera JL, et al. Development of the oriental latrine fly, Chrysomya megacephala (Diptera: Calliphoridae), at five constant temperatures. J Med Entomol. 2017;54:290–298. [DOI] [PubMed] [Google Scholar]
- [109].Alonso MA, Souza CM, Linhares AX, et al. Egg developmental time and survival of Chrysomya megacephala and Chrysomya putoria (Diptera: Calliphoridae) under different temperatures. J. Med. Entomol. 2015;52:551–556. DOI: 10.1093/jme/tjv066 [DOI] [PubMed] [Google Scholar]
- [110].Wijesundara DP. The life history and bionomics of Chrysomyia megacephala (Fab.). Ceylon J Sci. 1957;25:169–185. [Google Scholar]
- [111].Bharti M, Singh D, Sharma YP. Effect of temperature on the development of forensically important blowfly. Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae). Entomon. 2007;32:149–151. [Google Scholar]
- [112].Abd Algalil FM, Zambare SP. Effects of temperature on the development of calliphorid fly of forensic importance Chrysomya megacephala (Fabricius, 1794). Indian J Appl Res. 2015;5:767–769. [Google Scholar]
- [113].Picard CJ, Johnston JS, Tarone AM. Genome sizes of forensically relevant Diptera. J Med Ent. 2012; [5 p.] DOI: 10.1603/ME11075 [DOI] [PubMed] [Google Scholar]
- [114].Barbosa LS, Couri MS, Valéria MA, et al. The classic episode of biological invasion: Cochliomyia macellaria (Fabricius, 1775) versus Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae) – evaluation of the biotic potential. Ann Braz Acad Sci. 2016;88:1401–1406. [DOI] [PubMed] [Google Scholar]
- [115].Esser JR. Biology of Chrysomya megacephala (Diptera: Calliphoridae) and reduction of losses caused to the salted-dried fish industry in south-east Asia. Bull Ent Res. 1991; [8 p.] DOI: 10.1017/S0007485300053219 [DOI] [Google Scholar]
- [116].Shiao SF, Yeh TC. Larval competition of Chrysomya megacephala and Chrysomya rufifacies (Diptera: Calliphoridae): behavior and ecological studies of two blow fly species of forensic significance. J Med Ent. 2008;45:785–799. [DOI] [PubMed] [Google Scholar]
- [117].de Carvalho A, Pinto De Mello R, d'Almeida JM. Microhimenópteros parasitóides de Chrysomya megacephala. Rev Saude Publica. 2003;37:810–812. [DOI] [PubMed] [Google Scholar]
- [118].Marchiori CH. Parasitoids of Chrysomya megacephala (Fabricius) collected in Itumbiara, Goias, Brazil. Rev Saúde Pública. 2004;38:323–325. [DOI] [PubMed] [Google Scholar]
- [119].Voss SC, Spafford H, Dadour IR. Temperature-dependant development of Nasonia vitripennis on five forensically important carrion fly species. Ent Exp Appl. 2010;24:189–198. [DOI] [PubMed] [Google Scholar]
- [120].Voss SC, Spafford H, Dadour IR. Temperature-dependent development of the parasitoid Tachinaephagus zealandicus on five forensically important carrion fly species. Med Vet Ent. 2010;24:189–198. [DOI] [PubMed] [Google Scholar]
- [121].Gruner SV, Slone DH, Capinera JL, et al. Volume of larvae is the most important single predictor of mass temperatures in the forensically important calliphorid, Chrysomya megacephala (Diptera: Calliphoridae). J Med Ent. 2017;54:30–34. [DOI] [PubMed] [Google Scholar]
- [122].Olsen AR, Sidebottom TH, Bennett SG. The oriental latrine fly, Chrysomya megacephala (Fabricius 1794) (Diptera: Calliphoridae), as an invading blow fly of public health importance. J Soc Vector Ecol. 1993;18:133–146. [Google Scholar]
- [123].Olsen AR, Gecan JS, Ziobro GC, et al. Regulatory action criteria for filth and other extraneous materials V. Strategy for evaluating hazardous and nonhazardous filth. Reg Tox Pharm. 2001;33:363–392. [DOI] [PubMed] [Google Scholar]
- [124].Graczyk TK, Knight R, Gilman RH, et al. The role of non-biting flies in the epidemiology of human infectious diseases. Microb Infect. 2001;3:231–235. [DOI] [PubMed] [Google Scholar]
- [125].Sinha SK, Nandi BC. Notes on calliphorid flies (Diptera: Calliphoridae) from Sunderbans Biosphere Reserve and their impact on man and animals. J Bombay Nat Hist Soc. 2004;101:415–420. [Google Scholar]
- [126].Sukontason KL, Bunchoo M, Khantawa B, et al. Comparison between Musca dometica and Chrysomya megacephala as carriers of bacteria in northern Thailand. Southeast Asia J Trop Med Public Health. 2007;38:38–44. [PubMed] [Google Scholar]
- [127].Chaiwong T, Srivoramas T, Sueabsamran P, et al. The blow fly, Chrysomya megacephala, and the house fly, Musca domestica, as mechanical vectors of pathogenic bacteria in Northeast Thailand. Trop Biomed. 2014;31:336–346. [PubMed] [Google Scholar]
- [128].Brits D, Brooks M, Villet MH. Diversity of bacteria isolated from the flies Musca domestica (Muscidae) and Chrysomya megacephala (Calliphoridae) with emphasis on vectored pathogens. Afr Ent. 2016; [10 p.] DOI: 10.4001/003.024.0000 [DOI] [Google Scholar]
- [129].Sulaiman S, Sohadi AR, Yunus H, et al. The role of some cyclorrhaphan flies as carriers of human helminths in Malaysia. Med Vet Ent. 1988;2:1–6. [DOI] [PubMed] [Google Scholar]
- [130].Wall R, Howard JJ, Bindu J. The seasonal abundance of blowflies infesting drying fish in South-West India. J Appl Ecol. 2001; [9 p.] DOI: 10.1046/j.1365-2664.2001.00588.x [DOI] [Google Scholar]
- [131].Hanski I. An interpolation model of assimilation by larvae of the blowfly Lucilia illustris (Calliphoridae) in changing temperatures. Oikos. 1977;28:187–195. [Google Scholar]
- [132].Li ZD, Yang M, Huang X, et al. Chrysomya megacephala (Fabricius) larvae: a new biodiesel resource. Appl Energy. 2012;94:349–354. [Google Scholar]
- [133].Yang S, Liu Z. Pilot-scale biodegradation of swine manure via Chrysomya megacephala (Fabricius) for biodiesel production. Appl Energy. 2014;113:385–391. [Google Scholar]
- [134].Zhang M, Yu H, Yang Y, et al. Analysis of the transcriptome of blowfly Chrysomya megacephala (Fabricius) larvae in responses to different edible oils. PLoS ONE. 2013; [11 p.] DOI: 10.1371/journal.pone.0063168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wang X, Xiong M, Lei C, et al. The developmental transcriptome of the synanthropic fly Chrysomya megacephala and insights into olfactory proteins. BMC Genomics. 2015; [12 p.] DOI: 10.1186/s12864-014-1200-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang X, Xiong M, Wang J, et al. Reference gene stability of a synanthropic fly, Chrysomya megacephala. Parasit Vector. 2015; [10 p.] DOI: 10.1186/s13071-015-1175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Anderson DL, Sedgley M, Short JRT, et al. Insect pollination of mango in northern Australia Mangifera indica, includes Apis mellifera. Aust J Agr Res. 1982;33:541–548. [Google Scholar]
- [138].Hu T, Len CH, Lee BS. The laboratory rearing and radiation effects of gamma ray on the pupae of Chrysomya megacephala (Fabricius). China J Entomol. 1995;15:103–111. [Google Scholar]
- [139].Dag A, Gazit S. Mango pollinators in Israel. J Appl Hort. 2001;2:39–43. [Google Scholar]
- [140].Sung IH, Lin MY, Chang CH, et al. Pollinators and their behaviors on mango flowers in southern Taiwan. Formosan Ent. 2006;26:161–170. [Google Scholar]
- [141].Goff ML, Odom CB. Forensic entomology in the Hawaiian Islands: three case studies. Am J Forensic Med Path. 1987;8:45–50. [DOI] [PubMed] [Google Scholar]
- [142].Goff ML, Omori AI, Gunatilake K. Estimation of postmortem interval by arthropod succession. three case studies from the Hawaiian Islands. Am J Forens Med Path. 1988;9:220–225. [DOI] [PubMed] [Google Scholar]
- [143].Goff ML, Flynn MM. Determination of postmortem interval by arthropod succession: a case study from the Hawaiian Islands. J Forensic Sci. 1991;36:607–614. [PubMed] [Google Scholar]
- [144].Cheong WH, Mahadevan S, Inder SK. Three species of fly maggots found on a corpse. Southeast Asian J Trop Med Public Health. 1973;4:281. [PubMed] [Google Scholar]
- [145].Barreto M, Burbano ME, Barreto P. Flies (Calliphoridae, Muscidae) and beetles (Silphidae) from human cadavers in Cali, Colombia. Mem Inst Oswaldo Cruz. 2002;97:137–138. [DOI] [PubMed] [Google Scholar]
- [146].Lee HL, Krishnasamy M, Abdullah AG, et al. Review of forensically important entomological specimens in the period of 1972-2002. Trop Biomed. 2004;21:69–75. [PubMed] [Google Scholar]
- [147].Oliveira-Costa J, de Mello-Patiu CA. Application of forensic entomology to estimate the postmortem interval (PMI) in homicide investigations by the Rio de Janeiro police department in Brazil. Aggrawal Internet J Forens Med Tox. 2004;5:40–44. [Google Scholar]
- [148].Sukontason K, Narongchai P, Kanchai C, et al. Forensic entomology cases in Thailand: a review of cases from 2000 to 2006. Parasit Res. 2007;101:1417–1423. [DOI] [PubMed] [Google Scholar]
- [149].Ye G, Li K, Zhu J, et al. Cuticular hydrocarbon composition in pupal exuviae for taxonomic differentiation of six necrophagous flies. J Med Ent. 2007;44:450–456. [DOI] [PubMed] [Google Scholar]
- [150].Zhu GH, Xu XH, Yu XJ, et al. Puparial case hydrocarbons of Chrysomya megacephala as an indicator of the postmortem interval. Forensic Sci Int. 2007;169:1–5. [DOI] [PubMed] [Google Scholar]
- [151].Zhu GH, Yu XJ, Xie LX, et al. Time of death revealed by hydrocarbons of empty puparia of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae): a field experiment. PLoS ONE. 2013; [7 p.] DOI: 10.1371/journal.pone.0073043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhu GH, Ye G, Hu C. Determining the adult age of the oriental latrine fly, Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) by pteridine fluorescence analysis. Insect Sci. 2003;10:245–255. [Google Scholar]
- [153].Sukontason KL, Narongchai P, Sripakdee D, et al. First report of human myiasis caused by Chrysomya megacephala and Chrysomya rufifacies (Diptera: Calliphoridae) in Thailand, and its implications in forensic entomology. J Med Ent. 2005;42:702–704. [DOI] [PubMed] [Google Scholar]
- [154].Mondal PC, Mahato S, Chakraborty B, et al. First report of Oriental latrine flies causing vaginal myiasis in human. J Parasit Dis. 2016;40:1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Thevan K, Ahmad AH, Rawi CSM, et al. Growth of Chrysomya megacephala (Fabricius) maggots in a morgue cooler. J Forensic Sci. 2010;55:1656–1658. [DOI] [PubMed] [Google Scholar]
- [156].Gunatilake K, Goff ML. Detection of organophosphate poisoning in a putrefying body by analyzing arthropod larvae. J Forensic Sci. 1989;34:714–716. [PubMed] [Google Scholar]
- [157].Rumiza AR, Osman K, Mohd Iswadi I, et al. Determination of malathion levels and the effect of malathion on the growth of Chrysomya megacephala (Fabricius) in malathion-exposed rat carcass. Trop Biomed. 2008;25:184–190. [PubMed] [Google Scholar]
- [158].Oliveira HG, Gomes G, Morlin JJ, et al. The effect of Buscopan ® on the development of the blow fly Chrysomya megacephala (F.) (Diptera: Calliphoridae). J Forensic Sci. 2009;54:202–206. [DOI] [PubMed] [Google Scholar]
- [159].Oliveira JS, Baia TC, Gama RA, et al. Development of a novel non-destructive method based on spectral fingerprint for determination of abused drug in insects: an alternative entomotoxicology approach. Microchem J. 2014; [7 p.] DOI: 10.1016/j.microc.2014.02.009 [DOI] [Google Scholar]
- [160].Souza CM, Thyssen PJ, Linhares AX. Effect of nandrolone decanoate on the development of three species of Chrysomya (Diptera: Calliphoridae), flies of forensic importance in Brazil. J Med Ent. 2011;48:111–117. [DOI] [PubMed] [Google Scholar]
- [161].Bakr R, Ramadan R, El Sawy S, et al. Ultrastructure of the midgut of the third larval instar of Chrysomya megacephala (Diptera: Calliphoridae) fed on malathion treated diet. Egypt Acad J Biol Sci. 2012;3:13–26. [Google Scholar]
- [162].Rezende F, Alonso MA, Souza CM, et al. Developmental rates of immatures of three Chrysomya species (Diptera: Calliphoridae) under the effect of methylphenidate hydrochloride, phenobarbital, and methylphenidate hydrochloride associated with phenobarbital. Parasitol Res. 2014;113:1897–1907. [DOI] [PubMed] [Google Scholar]
- [163].Baia TC, Gama RA, Silva de Lima LA, et al. FTIR microspectroscopy coupled with variable selection methods for the identification of flunitrazepam in necrophagous flies. Anal Methods. 2016; [4 p.] DOI: 10.1039/C5AY02342D [DOI] [Google Scholar]
- [164].Tracqui A, Keyser-Tracqui C, Kintz P, et al. Entomotoxicology for the forensic toxicologist: much ado about nothing? Int J Legal Med. 2004;118:194–196. [DOI] [PubMed] [Google Scholar]
- [165].Musvasva E, Williams KA, Muller WJ, et al. Preliminary observations on the effects of hydrocortisone and sodium methohexital on development of Sarcophaga (Curranea) tibialis Macquart (Diptera: Sarcophagidae), and implications for estimating post mortem interval. Forensic Sci Int. 2001;120:37–41. [DOI] [PubMed] [Google Scholar]
- [166].Catts EP, Haskell NH. Entomology and death: a procedural guide. Clemson (SC): Joyce's Print Shop; 1990. [Google Scholar]
- [167].Zabala J, Diaz B, Saloña-Bordas MI. Seasonal blowfly distribution and abundance in fragmented landscapes. Is it useful in forensic inference about where a corpse has been decaying? PLoS ONE. 2014; [11 p.] DOI: 10.1371/journal.pone.0099668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Charabidze D, Gosselin M, Hedouin V. Use of necrophagous insects as evidence of cadaver relocation: myth or reality? PeerJ. 2017; [32 p.] DOI: 10.7717/peerj.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Chong Y, Chua T, Song B. Genetic variations of Chrysomya megacephala populations in Malaysia (Diptera: Calliphoridae). Adv Entomol. 2014; [7 p.] DOI: 10.4236/ae.2014.21009 [DOI] [Google Scholar]
- [170].Nuorteva P. Sarcophagous insects as forensic indicators. In: Tedeschi CG, Eckert WG, Tedeschi L, editors. Forensic medicine. Study in Trauma and environmental hazards. Philadelphia: (PA: ): W.B. Saunders Co; 1977. p. 1072–1095. [Google Scholar]
- [171].Nuorteva P, Nuorteva SL. The fate of mercury in sarcosaprophagous flies and in insects eating them. Ambio. 1982;11:34–37. [Google Scholar]
- [172].Lee PS, Sing KW, Wilson JJ. Reading mammal diversity from flies: the persistence period of amplifiable mammal mtDNA in blowfly guts (Chrysomya megacephala) and a new DNA mini-barcode target. PLoS ONE. 2015; [12 p.] DOI: 10.1371/journal.pone.0123871 [DOI] [PMC free article] [PubMed] [Google Scholar]


