ABSTRACT
The determination of time since death or the postmortem interval (PMI) is one of the most important and frequently asked questions in forensic medicine. Medicolegal scholars and forensic pathologists around the world have studied the estimation of PMI extensively in the past, and many novel methods and advanced technologies have now been applied in the field. For several centuries, Chinese forensic examiners have also worked on the estimation of the PMI, and there are a large number of excellent studies published in Chinese rather than in English, and these are not easily accessible or known internationally. Therefore we have conducted a review of relevant studies published by Chinese forensic scholars in the last few decades. The scope of this review is to provide a concise summary of the current progress in the estimation of PMI by Chinese forensic researchers using molecular biology, spectroscopic technology, entomological methods, energy changes, thanatochemistry and other methods.
KEYWORDS: Forensic science, forensic pathology, postmortem interval, methods, Chinese
Introduction
The determination of the time since death or postmortem interval (PMI) is one of the most important and most frequently asked questions in forensic practice [1]. It is also one of the fundamental tasks of the forensic pathologist who is consulted when a body is found. From the point of view of criminal law, a precise estimation of the PMI enables verification of witness statements, thus limiting the number of suspects and possible alibis. Imprecise or incorrect estimation of PMI may confuse and complicate an investigation [2]. From 1984 to 2015, Chinese forensic scholars have made great progress in improving the estimation of PMI. Many methods have been applied to help determine the time since death, and these can be divided into the following categories: molecular biology methods (degradation of DNA, RNA or proteins); spectroscopic technology (Fourier transform infrared or Raman microspectroscopy); entomological methods (either a carrion insect development or a succession model [3]); estimation of energy changes in the body after death (cooling or blood ATP levels); thanatochemistry methods (describing changes in the chemical composition of various body fluids [4]); and other methods such as imaging technology, electrophysiological methods and enzyme activity. Despite the fact that studies on the estimation of PMI span decades, there is still a long way to go before many of these can be applied definitively in forensic practice. The scope of this review is to provide a concise summary of the progress made by Chinese forensic scholars in improving PMI estimation methods.
Molecular biology methods
Studies on the estimation of PMI based on RNA degradation
After an organism's death, RNA is degraded by ribonucleases present in the cell, as well as those originating from bacteria or other environmental sources [5]. In general, mRNA is thought to be more unstable than DNA and other proteins. However, after crucial methodological advances in RNA extraction, reverse transcription, and the invention of real-time quantitative PCR, a number of forensic laboratories have monitored RNA degradation to estimate PMI [6]. In 2013, Young et al. studied the time-dependent differences in RNA decay rates [7]. They found that a segment of β-actin RNA from tooth pulp can be used to estimate PMI for pigs buried within a shallow grave for up to 84 days. In recent years, Chinese forensic scholars have also investigated PMI estimation based on RNA degradation, and their work is summarized in Table 1.
Table 1.
Studies by Chinese forensic scholars on PMI estimation based on RNA degradation.
| Year | Authors [reference number] | Sample source | Tissues and organs | Temperature (°C) | RNA | PMI |
|---|---|---|---|---|---|---|
| 2005 | Xiao Junhui et al. [8] | Rat | Myocardium and diaphragm muscle | 21 | β-actin mRNA | 12 d |
| 2007 | Liu Ji et al. [9] | Rat | Brain | 15, 20 | GAPDH mRNA,18S rRNA | 7 d |
| 2007 | Chen Xiaorui et al. [10] | Rat | Retina | 20 | β-actin,Pgk1,and Rpl4 mRNA | 28 h |
| 2009 | Ren Guangmu et al. [11] | Rat | Brain and spleen | 20 | GAPDH mRNA, β-actin mRNA | 12 d |
| 2010 | Wu Hongyan et al. [12] | Rat | Liver | 10, 25 | GAPDH mRNA | 48 h |
| 2011 | Liu Yuelin et al. [13] | Rat | Brain,heart and kidney | 20 | β-actin mRNA | 96 h |
| 2013 | Wang Hui et al. [14] | Rat | Cardiac muscle, liver, brain and skeletal muscle | — | miR-122, miR-133a, miR-150, miR-195, miR-206 | 48 h |
| 2014 | Li Wencan et al. [15] | Rat | Heart | — | 18S rRNA and microRNA | 7 d |
| 2014 | Lv Yehui et al. [6] | Rat | Spleen | 4 or 25 | mRNA, microRNA, 18S rRNA and U6 snRNA | 260 h |
In the past, few studies were conducted using RNA degradation to estimate PMI because it was difficult to extract the RNA. However, as we show in Table 1, Chinese researchers working on this subject for the last 10 years have found that measuring RNA degradation after death is especially useful for precise PMI estimation, but as all the experimental samples have come from rats, we do not know if this data can be extrapolated to humans. Another limitation is that RNA degradation studies are often carried out at a fixed temperature, so this does not reflect the effects of changing environmental conditions on RNA degradation. Furthermore, all of these studies were conducted over relatively short time-frames, using soft tissue samples that gave highly variable results [7]. The scope for the application of RNA degradation in forensic practice is still unresolved.
Studies on the estimation of PMI based on DNA degradation
When an organism dies, internal cellular nucleases cause chromosomal DNA to degrade into smaller fragments over time. As the PMI lengthens, chromatin is degraded until no high molecular weight DNA (HMW-DNA) remains [16]. DNA degradation as a predictor of the PMI has been studied for more than 40 years [17]. In Table 2, we summarize the studies by Chinese scientists on the use of DNA degradation to estimate PMI.
Table 2.
Studies by Chinese forensic scholars on PMI estimation based on DNA degradation.
| Year | Authors [reference number] | Sample source | Tissues and organs | Temperature (°C) | Detection methods | PMI |
|---|---|---|---|---|---|---|
| 2000 | Liu Liang et al. [18] | Rat | Brain | 22–28 | Image analysis | 24 h |
| 2001 | Liu Liang et al. [19] | Rat | Kidney | 16–22 | Auto-TV-image system | 48 h |
| 2002 | Chen Yuchuan et al. [20] | Human | Marrow in bosom bone | 20–25 | Feulgen staining and computerized image analysis | 7 d |
| 2005 | Zhang Lan et al. [21] | Rat | Liver, kidney and spleen | 20 | Terminal deoxynucleotide transferase | 48 h |
| 2005 | He Fanggang et al. [22] | Human | Spleen | 4, 17–28 | Feulgen staining and image analysis technology | 7–36 h |
| 2008 | Hu Jun et al. [23] | Rat | Bone marrow and brain | 10, 20 | Single cell gel electrophoresis | 40 h |
| 2011 | Li Shanshan et al. [24] | Human | Liver | 10, 20, 30 | Image analysis technique | 13–34 h |
DNA degradation rates are influenced by many factors such as temperature, pH values and diseases among others. To overcome the effect of temperature, Larkin et al. investigated the effect of accumulated degree-days on the DNA yield from skeletal muscle and its possible application to estimating the PMI [16]. The rate of DNA degradation varies in different tissue (liver, kidney and spleen) and in samples from different organisms (rat or human). Unfortunately, in such studies the extraction and quantitative analysis of DNA are usually performed under strictly controlled conditions and a small error can lead to inaccurate results. This work is also time-consuming and expensive. Additionally, most of the data obtained can only be used effectively for short PMI estimations. Therefore, DNA degradation is considered to be of limited value to forensic investigations requiring an estimation of PMI.
Studies on the estimation of PMI based on protein degradation
Protein is a basic cellular component of organisms, found in all tissues and organs. When life ends, cellular proteins degrade under the influence of various proteolytic enzymes. Technological advances have allowed researchers to apply a range of methods to study the relationship between protein degradation and PMI [25–29]. We have summarized the Chinese research on PMI estimation using protein degradation in Table 3.
Table 3.
Studies by Chinese forensic scholars on PMI estimation based on protein degradation.
| Year | Authors [reference number] | Sample source | Tissues and organs | Protein | Detection methods | PMI |
|---|---|---|---|---|---|---|
| 1994 | Huang Qiuju et al. [30] | Human | Blood | Complement 3 (C3) | Cross immunoelectrophoresis | 40 h |
| 2004 | Lv Jiangming et al. [31] | Rat | Cardiac and skeletal muscle | Actin | Immunohistochemical S-P method | 54 h |
| 2006 | Zheng Xudong et al. [32] | Human | Skeletal muscle | Troponin I | Western blot technique | 5 d |
| 2006 | Wu Rongqi et al. [33] | Rabbit | Skeletal muscle | Myofibril fragmentation index | Biuret method | 48 h |
| 2007 | Bian Jie et al. [34] | Human | Cardiac and skeletal muscle | Myoglobin | Western blot and imaging technique | 72 h |
| 2008 | Kuai Jinxia et al. [35] | Rat | Cardiac muscle and lung | Tubulin | Western blot | 7 d |
| 2008 | Liu Yang et al. [36] | Rat | Cardiac muscle, liver, spleen, lung, kidney, brain and skeletal muscle | Actin | Western blot | 168 h |
Huang et al. studied the complement 3 (C3) cleavage of blood from human cadavers [30]. They found that the higher the temperature and the longer the time-frame, the faster the C3 cleavage; there was also a significant positive correlation between the C3 cleavage and the PMI. Lv et al. found that the extent of HHF35-staining depletion in cardiac and skeletal muscle cells increases with increased PMI within a certain range [31]. Zheng et al. described that troponin I content in the human pectoralis muscle gradually decreased with the extension of the PMI [32]. During the same year, Wu et al. reported that the myofibril fragmentation index significantly increased with prolonged PMI [33]. Bian et al. reported the rate of degradation of human myoglobin in both skeletal and cardiac muscles [34]. Kuai et al. found that the levels of tubulin in cardiac muscle and lung tissue of rats varied with PMI [35]. However, Liu et al. found that there was a strong correlation between actin degradation and PMI, and the coefficient of determination (R 2) exceeded 0.75 in the cardiac muscle, liver, spleen, lung, kidney, brain and skeletal muscle of rats [36].
The rate of protein degradation is similar for DNA and RNA, and the protein degradation curve often follows a parabola or a straight line. Because of their intrinsically stable structure, some proteins are commonly used as markers for PMI estimation, including actin, tubulin and thyroglobulin [35–37]. Though specific proteins have a significant correlation with PMI, the process of protein degradation is still affected by environment temperature and putrefying bacteria, which complicates the application of this method in forensic practice.
Spectroscopic techniques
Fourier transform infrared (FTIR) spectroscopy is one of the most powerful methods for recording IR spectra of biological materials. It is rapid and yields a strong signal with only a few micrograms of sample, because the penetration depth of IR is independent from sample thickness [38]. Because of recent technological developments in instrumentation, Raman microscopy has emerged as a powerful analytical tool in biology [39]. The resolution of confocal Raman microscopy (CRM) is on the submicron scale, close to 200 nm using a laser in the visible wavelength region [40]. In addition, it is not sensitive to water content in samples [39]. Many Chinese forensic scholars have also applied spectroscopy to PMI estimation (Table 4).
Table 4.
Application of spectroscopy technology in PMI estimation by Chinese forensic scholars.
| Year | Authors [reference number] | Sample source | Tissues and organs | Spectroscopic technology | PMI |
|---|---|---|---|---|---|
| 2009 | Huang Ping et al. [41] | Rat | Liver and spleen | FTIR | 144 h |
| 2010 | Huang Ping et al. [42] | Rat | Cardiac muscle | FTIR | 168 h |
| 2010 | Huang Ping et al. [43] | Rat | Spleen | FTIR | 72 h |
| 2010 | Xiong Ping et al. [44] | Human | Kidney and liver | Raman microspectroscopy | 48–72 h |
| 2011 | Huang Ping et al. [45] | Human and rat | Liver, spleen, kidney, heart, etc. | FTIR | 168 h |
| 2011 | Xiong Ping et al. [46] | Human | Liver | Laser confocal micro-Raman | 48–72 h |
| 2011 | Guo Ping et al. [47] | Human | Spleen | Raman microspectroscopy | 48–72 h |
| 2012 | Li Shiying et al. [48] | Rat | Spleen | FTIR | 15 d |
| 2012 | Ke Yong et al. [38] | Rat | Brain | FTIR | 144 h |
Huang et al. reported that the quantitative analysis of FTIR spectra related to PMI shows a strong linear correlation between absorbance ratios and increasing time after death [41]. Following this, Huang et al. estimated PMI in cardiac muscle and spleen tissue using FTIR spectroscopy [42,43]. Xiong et al. found that the relative peak intensities (I 1094/I 2923) of confocal Raman microscopy for the tissue cells decreased gradually with increased PMI from 48 to 72 h after death [44]. They then analyzed rat and human tissues using FTIR spectroscopy [45]. They found no obvious changes in the position of the absorbance bands for either rat or human, which both provided similar results. Xiong et al. found that there was no significant change for the position of the main scattering peaks of the liver tissue within PMI between 48 and 72 h, but the intensity of these peaks were significantly different [46]. The nucleic acid-related peak (1094 cm−1) significantly decreased in intensity with increased PMI. The intensity of the lipid-related peaks (1454 cm−1, 2923 cm−1) showed no significant changes, but each relative peak (I 1094/I 2923) reduced intensity over time. Also using Raman spectroscopy, Guo et al. corroborated this, reporting that the relative peak intensities (I 1094/I 2923) for spleen tissue gradually decreased with increased PMI over a range of 48–72 h [47]. In particular, peaks related to nucleic acids (1094 cm−1) were observably reduced.
Li et al. examined rat's liver and spleen tissue using FTIR [48]. Ke et al. also described the changes in attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectra when analyzing rat brain from 0 to 144 h post mortem [38]. They found a significant linear correlation between the relative absorption intensity and PMI.
Spectroscopy presents several advantages as a new method for the estimation of PMI. It is more convenient and easier to carry out than other methods, and just a few micrograms of sample are enough for detection. Various types of spectroscopic techniques have been applied, such as fluorescence spectroscopy [49], spectrophotometric analysis [50], laser-induced breakdown spectroscopy [51] and UV-induced autofluorescence [52], but as we show in Table 4, much of this type of work has also been attempted by Chinese researchers to estimate early PMI. However, the results are highly influenced by environment factors such as temperature, humidity and wind. Therefore, further study is needed before spectroscopic technology could be used routinely in forensic practice.
Entomological methods
Forensic entomologists have primarily used either a carrion insect development or an insect succession model to infer time since death [3]. The more common of the two is to use insect development, in which a model is used to determine the age of the carrion insects found on or near the corpse [53–57]. This value then provides a minimum PMI under the assumption that oviposition or larviposition occurred on the deceased immediately following death [3]. Chinese studies in this field are summarized in Table 5.
Table 5.
Studies on estimation of PMI by Chinese forensic scholars with entomology.
| Year | Authors [reference number] | Experimental premise |
|---|---|---|
| 1994 | Tang Zhizhou et al. [58] | Length of maggots |
| 2000 | Niu Qingshan et al. [59] | Relationship between the sum of effective temperature and the length of larvae or weight pupa of Lucilia sericata |
| 2002 | Wang Jiangfeng et al. [60] | Chronometrical morphology of Aldrichina grahami |
| 2005 | Liu Xingjia et al. [61] | Growth time of the maggot fly |
| 2007 | Zhu GH et al. [62] | Puparial case hydrocarbons of Chrysomya megacephala |
| 2010 | Shi Yanwei et al. [63] | Development of Chrysomya megacephala (Diptera: Calliphoridae) |
| 2010 | Chen Lushi et al. [64] | Necrophagous flies life cycle |
| 2011 | Chen Lushi et al. [65] | Sarcosaphagous flies community composition, seasonal variation and growth of the length |
| 2013 | Ma Ting et al. [66] | Growth and development patterns of Hydrotaea spinigera (Diptera: Muscidae) |
| 2013 | Liu Ying et al. [67] | Morphologic observation of Boettcherisca peregrina |
| 2014 | Xu Hong et al. [68] | Cuticular hydrocarbons of larvae in Aldrichina grahami |
| 2014 | Feng Dianxing et al. [69] | Pupal stage of Megaselia scalaris |
| 2015 | Yang Yongqiang et al. [70] | Development of Hemipyrellia ligurriens (Wiedemann) (Diptera: Calliphoridae) at constant temperatures |
Tang et al. formulated an equation for estimating the PMI by measuring the length of maggots [58], then in 2000 Niu et al. established three linear regression equations between the sum of effective temperature and the length of larvae or weight of pupa of Lucilia sericata (K 1 = 2.088 0 + 0.801 4X 1; K 2 = 54.091 7 − 2.881 4X 2; K 3 = 133.218 0 − 2.631 2X 3) [59]. Wang et al. found that structural traits of posterior spiracles, skin and digestive dust consistently changed with larval growth and could be used as larval age markers [60]. Liu et al. reported a direct connection between the length of maggot and the host's time of death [61]. They deduced a formula, with relevance ratio θ, for calculating the PMI using the length of the maggots. Zhu et al. reported that cuticular hydrocarbon was a potential indicator of the weathering time in Chrysomya megacephala, and possibly for other necrophagous flies, and might also be used to determine the PMI [62]. Shi et al. found that the maximum length of larvae and the weight of pupae increase stepwise with increasing malathion concentrations through the period of larval development [63]. Also in 2010, Chen et al. found that the abundance of necrophagous flies at high temperatures in summer was greater than at low temperatures in winter [64]. Later on, Chen et al. obtained base data of the sarcosaphagous fly community composition, and seasonal changes in maggot growth rates in the suburbs of Guiyang [65]. Ma et al. found that the relationship between larval body length and time, at four different constant temperatures could be simulated by a logistic function [66]. Xu et al. developed a mathematical model derived from multivariate linear regression analysis, for determining age of the larvae based on age-dependent changes in cuticular hydrocarbons [68]. The same year, Feng et al. found that some morphological features that changed during development within the puparium could be used as age markers [69]. Yang et al. constructed isomegalen and isomorphen diagrams depicting the time of larval length and developmental event, respectively, at different temperatures [70]. A thermal summation model was also constructed via regression analysis, by estimating the developmental threshold temperature (t) and the thermal summation constant (K).
Entomological methods are mainly used for the estimation of longer PMI. Though entomology plays an important role in the estimation of PMI, it still has some disadvantages. The results are often confounded by investigator subjectivity, such as seasonal and regional factors. International contribution of forensic entomologists has meant that many insects have been identified as being useful for determining PMI [54,55,71,72]. Many new techniques have also been employed for this research, including GC-MS [73], optical coherence tomography [56], artificial neural networks [74] and virtual forensic entomology [75] to explore the relationship between carrion insects and PMI.
Estimation of PMI based on energy changes
The changes in energy of a decaying organism can be monitored using corpse temperature or blood ATP levels. In general, the most accurate time-of-death estimates are obtained from the measurement of postmortem cooling [76]. Because of its central role in energy metabolism, adenosine-5′-triphosphate (ATP) is a conserved and highly specific marker that can be useful for determining the PMI with varying causes of death [77]. In Table 6 we list the Chinese studies which report using energy change measurements to estimate PMI.
Table 6.
Reports by Chinese forensic scholars in which PMI estimation depends on the changes in energy.
| Year | Authors [reference number] | Basic novelty |
|---|---|---|
| 1984 | The Chinese PMI estimation working group [78] | Relationship between rectal temperatures of 581 corpses and PMI |
| 2004 | Wang Qiong et al. [79] | Relationship between temperatures of liver, rectal and ear and PMI |
| 2011 | Sun Tingyi et al. [80] | Relationship between blood ATP level and PMI |
| 2012 | Yang Yulei et al. [81] | Correlation between factors related to body temperature and PMI |
| 2013 | Mao Shiwei et al. [77] | Estimation of PMI depending on the changes in ATP and its degradation products |
| 2013 | Yang Tiantong et al. [82] | Correlation between ATP concentration in human venous blood and PMI under various ambient temperatures |
| 2014 | Sun Tingyi et al. [83] | A mathematical model using rabbits to characterize the correlation between blood ATP levels in the right ventricle and PMI |
Chinese forensic scholars measured the rectal temperatures of 581 corpses aged 1–80 years [78]. They constructed numerous regression formulae for PMI estimation, and found that the environmental temperature, warm clothing and how the corpse was placed have important effects on the body temperature drop. A decade later, Wang et al. explored the relationship between PMI and the temperatures of the liver, rectum and ear [79]. They found that the measurement of rectal temperature is more precise than the others within 4 h, and the measurement of liver temperature is most reliable between 4 and 24 h. Sun et al. found that rabbit blood ATP levels at 25 °C rises in the early period after death, and it reaches its peak at 8 h after death [80]. It then decreases as PMI extends. There is clearly an effective correlation between blood ATP level and PMI in the range of 8–56 h after death. Yang et al. constructed a statistically significant regression model for the relationship between factors related to decreasing body temperature and PMI [81]. The equation they formulated is
where = fat thickness; = environment temperature; = warm clothing; = object for parking corpse; = ventilation conditions; = cause of death; = rectal temperature.
Mao et al. found that the K value is a robust index for the estimation of PMI (), based on highly significant linear correlations between PMI and concentrations of ATP breakdown products [77]. The same year, Yang et al. found that blood ATP level decreased with PMI extension [82]. They obtained six two-variable cubic curve equations (with R 2 from 0.976 to 0.990) after regression analysis, and formed a surface equation after interpolation analysis. And they also obtained the three-variable quadratic surface equation. Sun et al. developed a mathematical model using interpolation functions to characterize the correlation between the blood ATP level in the right ventricle of rabbit and PMI at different ambient temperatures [83].
Chinese forensic scholars have also constructed numerous regression formulae for the estimation of PMI based on the body cooling process. Yet environmental factors, especially ambient temperature, are still central problems to be solved for this method to be used in future forensic practice. Considering these problems, researchers in other countries have taken advantage of climatic-control chambers to explore the environmental effects on the estimation of PMI [76]. In addition, they have also made use of accumulated degree-days to account for the effect of frequent temperature changes [84]. Blood ATP levels are also affected by physical health, cause of death and environmental temperature. Thus, discovering a more precise method for using energy changes in the estimation of the PMI is still the goal for all forensic scholars.
Thanatochemistry methods
In the past, a variety of chemical methods have been employed to determine time since death [85–90]. Thanatochemistry refers to changes that occur in the chemical composition of various body fluids immediately after death [17]. A number of forensic scholars have tried to define the relationship between PMI and postmortem biochemical changes in various body fluids such as blood, serum, cerebrospinal fluid, vitreous humour and synovial fluid [4]. In Table 7 we show the Chinese forensic studies on the estimation of PMI with thanatochemistry methods.
Table 7.
Studies by Chinese forensic scholars on PMI estimation based on thanatochemistry methods.
| Year | Authors [reference number] | Sample source | Tissues and organs | Test material | Temperature (°C) | PMI |
|---|---|---|---|---|---|---|
| 2002 | Zhang Yanling et al. [91] | Human | Liver | Organic amines | 8, 15, 23, 32 | 69 h |
| 2005 | Dang Yonghui et al. [92] | Rat | Skeletal muscle | pH value | 24–28 | 24 h |
| 2008 | Jin Junfeng et al. [93] | Human | Bone marrow of sternum | Lipid | 32 | 9 d |
| 2008 | Yang Tiantong et al. [94] | Rabbit | Brain | Nacetylaspartate(Naa)/creatine(Cr), choline(Ch)/Cr | 10, 30 | 24 h |
| 2008 | Yang Tiantong et al. [95] | Rabbit | Brain | Naa, Ch, Cr | 30 | 24 h |
| 2012 | Yang Tiantong et al. [96] | Rabbit | Blood of right ventricle | pH value | 10, 15, 20, 25, 30, 35 | 66 h |
Throughout the world, forensic scientists have employed thanatochemical methods for PMI determination. To this end, they have studied a range of substances, including urea nitrogen, creatinine, uric acid, potassium, magnesium, sodium, chloride, calcium, hypoxanthine levels in postmortem serum, vitreous humour, cerebrospinal fluid and even pericardial fluid [4, 86–90]. Chinese researchers have also explored thanatochemistry as a means to estimate PMI.
Zhang et al. found that the organic amines produced during putrefaction under temperatures of 8 °C, 15 °C, 23 °C and 32 °C increased as PMI extended until attaining their peak values [91]. Meanwhile, other amino acid components decreased gradually over time after death. Dang et al. reported that the pH of rat quadriceps femoris muscle decreased with increasing PMI (within 12 h), which provided a useful linear negative correlation [92]. The formula describing this relationship between PMI and pH value is . Jin et al. found that the amount of lipid decreased gradually in a linear relationship with time after death, reaching a minimum value at 10 days post mortem [93]. In a different study, Yang et al. reported that the Naa/Cr ratio decreased, while the Ch/Cr ration increased as the PMI extended through the first 24 h [94]. Using Naa/Cr as the independent variable, the quadratic polynomial regression equation was expressed as
With Ch/Cr as the independent variable instead, the quadratic polynomial regression equation was expressed as
More recently, Yang et al. used a different sample type and found that pH values in the right ventricle correlated with increased PMI at different ambient temperatures (= 0.974–0.982) [96].
Using thanatochemistry principles, Chinese forensic scholars have constructed regression equations and provided some basic data for the estimation of PMI. Thanatochemical applications for PMI estimation can be problematic, as the compounds of interest are also influenced by environment factors. Scientists have found that the best fluids to study are the vitreous humour and the cerebrospinal fluid, to minimize the effects of the environment. However, these fluids are difficult to obtain, limiting the progress of this approach in the field of forensic practice.
Other methods
Chinese forensic scholars have also applied a range of novel methods to the estimation of PMI. Wang et al. studied the correlation between PMI and both cholinesterase (ChE) and glutamic oxaloacetic transaminase (GOT) activity in rabbit vitreous humour using spectrophotometry [97]. Results showed initial ChE and GOT activities were approximately 850 and 220 IU/L, respectively, decreasing to 0 IU/L after 54 h post mortem. The relationship can be expressed by simple and multiple regression equations (Table 8).
Table 8.
Simple and multiple regression equations [97].
| Temperature (°C) | Simple and multiple regression equation | Coefficient of determination, | Significance, P |
|---|---|---|---|
| 10–15 | –0.917 | 0.01 | |
| 10–15 | –0.948 | 0.01 | |
| 25–30 | –0.896 | 0.01 | |
| 25–30 | –0.944 | 0.01 | |
| 10–15 | 0.970 | 0.01 | |
| 25–30 | 0.959 | 0.01 |
Xue studied the relationship between albino rat imbibitions and PMI, using γ radiation from the autoscaler FH463A [98]. The main result is showed in Figure 1. It demonstrated that the imbibitions of γ radiation may be a useful estimator of PMI.
Fig 1.
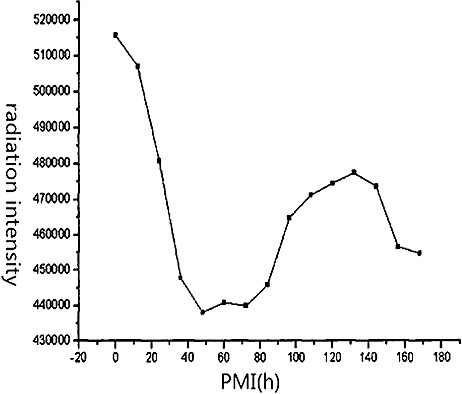
The relationship between radiation intensity and PMI in albino rats [98].
Mao et al. developed a rapid method for the estimation of PMI using electrical impedance spectroscopy [99]. Linear regression analysis between the maximal absolute value of Im Z″ (capacitive reactance component) and PMI were performed in every group. Results are presented in Table 9. It is demonstrated that the maximum absolute value of Im Z″ (capacitive reactance component) in electrical impedance per sample of each group gradually diminishes as time progresses.
Table 9.
Linear regression equations [99].
| Group | Linear regression equation | Coefficient of determination, | Significance, P |
|---|---|---|---|
| 10 °C (spleens in cabinet) | 0.956 | 0.01 | |
| 10 °C (spleens in vivo) | 0.963 | 0.01 | |
| 20 °C (spleens in cabinet) | 0.980 | 0.01 | |
| 20 °C (spleens in vivo) | 0.978 | 0.01 | |
| 30 °C (spleens in cabinet) | 0.962 | 0.01 | |
| 30 °C (spleens in vivo) | 0.964 | 0.01 |
Yang et al. investigated the correlation between the changes of oxidation reduction potential (ORP) values of heart blood in rabbits and the PMI at different temperatures after death [100]. Results showed the ORP values were highly correlated with the PMI at each of the temperatures studied (Table 10). As expected, the ORP values increased when the temperature was high, and decreased with lower temperatures.
Table 10.
Curvilinear regression equations [100].
| Temperature (°C) | Curvilinear regression equation | |
|---|---|---|
| 10 | 0.986 | |
| 15 | 0.983 | |
| 20 | 0.981 | |
| 25 | 0.984 | |
| 30 | 0.982 | |
| 35 | 0.974 |
Zhang et al. studied the time- and temperature-dependent survival of ovarian oocytes collected from a mouse carcass [101]. Results showed that at a constant temperature, the number of collected germinal vesicle oocytes in the ovary decreased with increasing PMI. Meanwhile, during this time the rate of germinal vesicle breakdown (GVBD) and the first polar body emission (PBE) gradually reduced as the temperature increased.
In more recent years, researchers in other countries have also taken advantage of novel ways for estimating time since death, including GC-MS/MS [73,102,103], micro-computed tomography, mid-infrared microscopic imaging, energy dispersive X-ray mapping [104], LC–MSMS [105], UPLC/Q-TOF MS and SELDI-TOF-MS [106,107]. While these methods are very convenient and efficient for use in the estimation of PMI, but the results obtained are still not precise enough for forensic practice.
Conclusion
As we have discussed here, there have been numerous attempts by Chinese forensic scholars during the last decades at finding methods to help estimate PMI more accurately. So far, none of them allows us to define the PMI with absolute precision if used alone. Currently, for the early PMI (up to 24 h), the Henssge nomogram is usually applied, complemented by an assessment of hypostasis and rigor mortis, sometimes including consideration of some supra-vital reactions [2]. However, recent Chinese forensic science studies, such as examinations of DNA or RNA degradation and measurements of pH value, have shown potential for new ways to estimate early PMI. There is also more work being done in Chinese research institutes to develop methods for longer PMI estimations, e.g. using entomological methods and spectroscopy techniques. The former is widely considered to be a standard method to estimate longer PMI, but it does have some disadvantages. First, it has no standard for verification and the methodologies for estimation are somewhat subjective, which means outside influencing factors can have a big effect on the resulting PMI estimation. Second, variable seasonal and regional factors also affect the accuracy of long PMI estimation. Conversely, spectroscopy is objectively measured, is convenient, efficient and usually can be performed easily. Therefore, it shows great potential for applications to PMI estimation. However, the obvious drawback is that the sample being measured is always affected by the environment temperature, which is difficult to account for using spectroscopic techniques.
With the continued development of new technology an increasing number of methods have been assessed for the estimation of PMI. Unfortunately, there is still no simple method that can provide a precise estimation of PMI in forensic practice. It appears that using a combination of different methods is the future trend for the estimation of PMI. Regardless of the approach, influential environment factors should always be taken into account when analyzing forensic casework samples.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Human Research Committee of Liaoning Medical University with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding Statement
This work was supported by National Sciences Foundation of China (NSFC) [grant numbers 81601645, 81671869, 81072509, 81273339 and 81273335] and the Science and Technology Commission of Shanghai Municipality [grant number 14DZ2270800].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Alibegovic A. Cartilage: a new parameter for the determination of the postmortem interval? J Forensic Leg Med. 2014;27:39–45. [DOI] [PubMed] [Google Scholar]
- [2]. Kaliszan M, Hauser R, Kernbach-Wighton G. Estimation of the time of death based on the assessment of post mortem processes with emphasis on body cooling. Leg Med (Tokyo). 2009;11:111–117. [DOI] [PubMed] [Google Scholar]
- [3]. Wells JD, Lecheta MC, Moura MO, et al. . An evaluation of sampling methods used to produce insect growth models for postmortem interval estimation. Int J Legal Med. 2015;129:405–410. [DOI] [PubMed] [Google Scholar]
- [4]. Tumram NK, Ambade VN, Dongre AP. Thanatochemistry: Study of synovial fluid potassium. AJM. 2014;50:369–372. [Google Scholar]
- [5]. Sara CZ, Menendez ST, Nunez P. Cell death proteins as markers of early postmortem interval. Cell Mol Life Sci. 2014;71:2957–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Lv YH, Ma KJ, Zhang H, et al. . A time course study demonstrating mRNA, microRNA, 18S rRNA, and U6 snRNA changes to estimate PMI in deceased rat's spleen. J Forensic Sci. 2014;59:1286–1294. [DOI] [PubMed] [Google Scholar]
- [7]. Young ST, Wells JD, Hobbs GR, et al. . Estimating postmortem interval using RNA degradation and morphological changes in tooth pulp. Forensic Sci Int. 2013;229:163 e1-6. [DOI] [PubMed] [Google Scholar]
- [8]. Xiao JH, Chen YC. Detection of β-actin mRNA in myocardium and diaphragm muscle at different postmortem intervals in rats. Chin J Forensic Med. 2005;20:196–198. [Google Scholar]
- [9]. Liu J, Song ZZ, Xie RH, et al. . A pilot study on cerebral RNA degradation and its applicability to estimation of postmortem interval in rats. Chin J Forensic Med. 2007;22:226–228. [Google Scholar]
- [10]. Chen XR, Yi SH, Yang LP, et al. . The relationship between mRNA degradation in retina and PMI of rat after death. Chin J Forensic Med. 2007;22:169–172. [Google Scholar]
- [11]. Ren GM, Liu J, Wang YY, et al. . The degradation of housekeeping mRNA in dead rats by real-time RT-PCR. Fa Yi Xue Za Zhi. 2009;25:33–36. [PubMed] [Google Scholar]
- [12]. Wu HY, Wang KJ, Zhang L, et al. . The relationship between GAPDH mRNA degradation in the mouse liver and postmortem interval. Fa Yi Xue Za Zhi. 2010;26:425–427. [PubMed] [Google Scholar]
- [13]. Liu YL, Ma KJ, Li WC, et al. . Estimation of early postmortem interval using β-actin mRNA in rat's brain, heart and kidney. Fa Yi Xue Za Zhi. 2011;27:5–8. [PubMed] [Google Scholar]
- [14]. Wang H, Mao J, Li Y, et al. . 5 miRNA expression analyze in post-mortem interval (PMI) within 48h. Forensic Sci Int – Gen Suppl Ser. 2013;4:e190–e191. [Google Scholar]
- [15]. Li WC, Ma KJ, Lv YH, et al. . Postmortem interval determination using 18S-rRNA and microRNA. Sci Justice. 2014;54:307–310. [DOI] [PubMed] [Google Scholar]
- [16]. Larkin B, Iaschi S, Dadour I, et al. . Using accumulated degree-days to estimate postmortem interval from the DNA yield of porcine skeletal muscle. Forensic Sci Med Pathol. 2010;6:83–92. [DOI] [PubMed] [Google Scholar]
- [17]. Madea B. Is there recent progress in the estimation of the postmortem interval by means of thanatochemistry? Forensic Sci Int. 2005;151:139–149. [DOI] [PubMed] [Google Scholar]
- [18]. Liu L, Zhang L, Liu YL, et al. . An experimental study on the relationship between the postmortem interval and the DNA content in rat's brain cells by image analysis. Chin J Forensic Med. 2000;15:1–3. [Google Scholar]
- [19]. Liu L, Peng DB, Liu Y, et al. . A study on the relationship between postmortem interval and the changes of DNA content in the kidney cellule of rat. Fa Yi Xue Za Zhi. 2001;17:65–68. [PubMed] [Google Scholar]
- [20]. Chen YH, Cheng JD. The relationship between postmortem degradation of marrow DNA in bosom bone and late postmortem interval estimation. Fa Yi Xue Za Zhi. 2002;18:144–145. [PubMed] [Google Scholar]
- [21]. Zhang L, Zhao ZQ, Shen YW, et al. . Estimation of postmortem intervals by using terminal deoxynucleotidy transferase. Fa Yi Xue Za Zhi. 2005;21: 113–114. [PubMed] [Google Scholar]
- [22]. He FG, Liu YL, Shu XJ, et al. . A study on the variability of DNA degradation of human ex vivo spleen cell at different temperature conditions. Chin J Forensic Med. 2005;20:321–324. [Google Scholar]
- [23]. Hu J, Zhao XH, Yi SH, et al. . Estimation of the postmortem interval by determining nuclear DNA degradation from bone marrows and brains in rats. Chin J Forensic Med. 2008;23:309–312. [Google Scholar]
- [24]. Li SS, Zhao CM, Liu Q, et al. . Early PMI estimation by DNA degradation of human hepatocytes at different temperatures. Chin J Forensic Med. 2011;26:4–6. [Google Scholar]
- [25]. Chen JH, Inamori-Kawamoto O, Michiue T, et al. . Cardiac biomarkers in blood, and pericardial and cerebrospinal fluids of forensic autopsy cases: A reassessment with special regard to postmortem interval. Leg Med (Tokyo). 2015;17:343–350. [DOI] [PubMed] [Google Scholar]
- [26]. Kikuchi K, Kawahara KI, Biswas KK, et al. . HMGB1: A new marker for estimation of the postmortem interval. Exp Ther Med. 2010;1:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Poloz YO, O'Day DH. Determining time of death: temperature-dependent postmortem changes in calcineurin A, MARCKS, CaMKII, and protein phosphatase 2A in mouse. Int J Legal Med. 2009;123:305–314. [DOI] [PubMed] [Google Scholar]
- [28]. Kumar S, Ali W, Singh US, et al. . The effect of elapsed time on the cardiac Troponin-T (cTnT) proteolysis in case of death due to burn: a study to evaluate the potential forensic use of cTnT to determine the postmortem interval. Sci Justice. 2015;55:189–194. [DOI] [PubMed] [Google Scholar]
- [29]. Kimura A, Ishida Y, Hayashi T, et al. . Estimating time of death based on the biological clock. Int J Legal Med. 2011;125:385–391. [DOI] [PubMed] [Google Scholar]
- [30]. Huang QJ, Yang QE, Dong LD. Estimation of postmortem interval from complement3 cleavage rates. Acta Univ Med Tongji. 1994;23:479–481. [Google Scholar]
- [31]. Lv JM, Yu JS, Chen MJ, et al. . An immunohistochemical study on relationship between the changes of actin in cardiac and skeletal muscle cells and postmortem interval in rats. Chin J Forensic Med. 2004;19:213–216. [Google Scholar]
- [32]. Zheng XD, Zhang YH, Zhi XM, et al. . Estimation of postmortem interval by determination of troponin I using Western blot technique in human pectoralis major. Chin J Forensic Med. 2006;21:146–148. [Google Scholar]
- [33]. Wu RQ, Zhao ZQ, Shen YW, et al. . Estimation of postmortem interval using myofibril fragmentation index. Fa Yi Xue Za Zhi. 2006;22:10–11. [PubMed] [Google Scholar]
- [34]. Bian J, Shen YW, Zhao ZQ, et al. . Relationship between the degradation of myoglobin and postmortem interval. Fa Yi Xue Za Zhi. 2017;23:90–91. [PubMed] [Google Scholar]
- [35]. Kuai JX, Liu Y, Zhang YW, et al. . A study on the relationship between the degradation of tubulin in cardiacmuscle and lung of rat and the postmortem interval. Chin J Forensic Med. 2008;23:96–98. [Google Scholar]
- [36]. Liu Y, Kuai JX, Zhang YW, et al. . The Relationship between the Degradation of Actin and the Postmortem Interval in Rats. Fa Yi Xue Za Zhi. 2008;24:165–167. [PubMed] [Google Scholar]
- [37]. Liu L, Cheng JY, Wang FL, et al. . An experimental study on relationship between degradation of thyroglobulin and postmortem Interval by immunohistochemistry. Chin J Forensic Med. 2005;20:265–267. [Google Scholar]
- [38]. Ke Y, Li Y, Wang ZY. The changes of Fourier transform infrared spectrum in rat brain. J Forensic Sci. 2012;57:794–798. [DOI] [PubMed] [Google Scholar]
- [39]. Notburga G, Manfred S. The potential of Raman microscopy and Raman imaging in plant research. Spectroscopy. 2007;21:69–89. [Google Scholar]
- [40]. Cabrales L, Abidi N, Manciu F. Characterization of developing cotton fibers by confocal Raman microscopy. Fibers. 2014;2:285–294. [Google Scholar]
- [41]. Huang P, Tian W, Tuo Y, et al. . Estimation of Postmortem Interval in Rat Liver and Spleen Using Fourier Transform Infrared Spectroscopy. Spectrosc Lett. 2009;42:108–116. [Google Scholar]
- [42]. Huang P, Su CP, Li SS, et al. . Estimation of postmortem interval using FTIR spectroscopy in rats’ cardiac muscle. Fa Yi Xue Za Zhi. 2010;26:1–5. [PubMed] [Google Scholar]
- [43]. Huang P, Yu RJ, Li L, et al. . Forensic medical analysis of FTIR spectral changes of rat spleen postmortem. Chin J Forensic Med. 2010;52:38–42. [Google Scholar]
- [44]. Xiong P, Guo P, Zhang J, et al. . Raman micro spectroscopic analysis of relationship between DNA degradation in tissue cells and postmortem interval. Spectrosc Spect Anal. 2010;30:1511–1515. [PubMed] [Google Scholar]
- [45]. Huang P, Wang SW, Bai J, et al. . To estimate the postmortem interval using FTIR spectroscopy. Chin J Forensic Med. 2011;26:104–109. [Google Scholar]
- [46]. Xiong P, Zhang J, Guo P. Analysis of postmortem human liver tissue by laser confocal micro-Raman spectroscopy. Chin J Forensic Med. 2011;26:7–9. [Google Scholar]
- [47]. Guo P, Xiong P, Zhang J. Raman microspectroscopic of relationship between DNA degradation in spleen tissue and postmortem interval. Chin J Med Phys. 2011;28:2809–2812. [Google Scholar]
- [48]. Li SY, Shao Y, Li ZD, et al. . Relation between PMI and FTIR Spectral Changes in Asphyxiated Rat ’ s Liver and Spleen. Fa Yi Xue Za Zhi. 2012;28:321–326. [PubMed] [Google Scholar]
- [49]. Éverton SE, Cristina K, José RV, et al. . Determination of post-mortem interval using in situ tissue optical fluorescence. Opt Exp. 2009;17:8185–8192. [DOI] [PubMed] [Google Scholar]
- [50]. Usumoto Y, Hikiji W, Sameshima N, et al. . Estimation of postmortem interval based on the spectrophotometric analysis of postmortem lividity. Leg Med (Tokyo). 2010;12:19–22. [DOI] [PubMed] [Google Scholar]
- [51]. Marín-Roldan A, Manzoor S, Moncayo S, et al. . Determination of the postmortem interval by Laser Induced Breakdown Spectroscopy using swine skeletal muscles. Spectrochim Acta B: Atom Spectrosc. 2013;88:186–191. [Google Scholar]
- [52]. Hoke N, Grigat A, Grupe G, et al. . Reconsideration of bone postmortem interval estimation by UV-induced autofluorescence. Forensic Sci Int. 2013;228:176 e1-6. [DOI] [PubMed] [Google Scholar]
- [53]. Szelecz I, Fournier B, Seppey C, et al. . Can soil testate amoebae be used for estimating the time since death? A field experiment in a deciduous forest. Forensic Sci Int. 2014;236:90–98. [DOI] [PubMed] [Google Scholar]
- [54]. Ridgeway JA, Midgley JM, Collett IJ, et al. . Advantages of using development models of the carrion beetles Thanatophilus micans (Fabricius) and T. mutilatus (Castelneau) (Coleoptera: Silphidae) for estimating minimum post mortem intervals, verified with case data. Int J Legal Med. 2014;128:207–220. [DOI] [PubMed] [Google Scholar]
- [55]. Brown K, Thorne A, Harvey M. Calliphora vicina (Diptera: Calliphoridae) pupae: a timeline of external morphological development and a new age and PMI estimation tool. Int J Legal Med. 2015;129:835–850. [DOI] [PubMed] [Google Scholar]
- [56]. Brown K, Harvey M. Optical coherence tomography: age estimation of Calliphora vicina pupae in vivo? Forensic Sci Int. 2014;242:157–161. [DOI] [PubMed] [Google Scholar]
- [57]. Reibe S, Doetinchem PV, Madea B. A new simulation-based model for calculating post-mortem intervals using developmental data for Lucilia sericata (Dipt.: Calliphoridae). Parasitol Res. 2010;107:9–16. [DOI] [PubMed] [Google Scholar]
- [58]. Tang ZZ. Estimation death time by the length of maggots. Fa Yi Xue Za Zhi. 1994;10:113. [Google Scholar]
- [59]. Niu QS, Pan YF, Wen ZC, et al. . Study on the growth and development of fly (Lucilia Sericata) under natural temperature and its application on forensic medicine practice. Chin J Forensic Med. 2000;15:214–216. [Google Scholar]
- [60]. Wang JF, Hu C, Chen YC, et al. . Chronometrical morphology of Aldrichina grahami and its application in the determination of postmortem interval. Acta Entomologica Sinica. 2002;45:265–270. [Google Scholar]
- [61]. Liu XJ, Zhang HC, Wang S, et al. . Estimation of postmortem interval and time of death by maggot fly. Forensic Sci Technol. 2005;6:15–17. [Google Scholar]
- [62]. Zhu GH, Xu XH, Yu XJ, et al. . Puparial case hydrocarbons of Chrysomya megacephala as an indicator of the postmortem interval. Forensic Sci Int. 2007;169:1–5. [DOI] [PubMed] [Google Scholar]
- [63]. Shi YW, Liu XS, Wang HY, et al. . Effects of malathion on the insect succession and the development of Chrysomya megacephala (Diptera: Calliphoridae) in the field and implications for estimating postmortem interval. Am J Forensic Med Pathol. 2010;31:46–51. [DOI] [PubMed] [Google Scholar]
- [64]. Chen LS, Xu Q, Shi F, et al. . Estimation time of death by necrophagous flies life cycle. Fa Yi Xue Za Zhi. 2010;26:332–335. [PubMed] [Google Scholar]
- [65]. Chen LS. The observation of sarcosaphagous flies community composition, seasonal variation and growth of the length in the suburbs of Guiyang. Chin J Forensic Med. 2011;26:204–206. [Google Scholar]
- [66]. Ma T, Gui LF, Wang JF, et al. . Growth and development patterns of hydrotaea spinigera (Ditpera:Muscidae) and their significance for estimating postmortem interval. Acta Entomol Sinica. 2013;56:890–895. [Google Scholar]
- [67]. Liu Y, Chen YQ, Guo YD, et al. . Estimation of post-mortem interval for a drowning case by using flies (Diptera) in Central-South China: implications for forensic entomology. Rom J Leg Med. 2013;4:293–298. [Google Scholar]
- [68]. Xu H, Ye GY, Xu Y, et al. . Age-dependent changes in cuticular hydrocarbons of larvae in Aldrichina grahami (Aldrich) (Diptera: Calliphoridae). Forensic Sci Int. 2014;242:236–241. [DOI] [PubMed] [Google Scholar]
- [69]. Feng DX, Liu GC. Pupal age estimation of forensically important Megaselia scalaris (Loew) (Diptera: Phoridae). Forensic Sci Int. 2014;236:133–137. [DOI] [PubMed] [Google Scholar]
- [70]. Yang YQ, Lyu Z, Li XB, et al. . Technical note: development of Hemipyrellia ligurriens (Wiedemann) (Diptera: Calliphoridae) at constant temperatures: applications in estimating postmortem interval. Forensic Sci Int. 2015;253:48–54. [DOI] [PubMed] [Google Scholar]
- [71]. Reibe S, Madea B. Use of Megaselia scalaris (Diptera: Phoridae) for post-mortem interval estimation indoors. Parasitol Res. 2010;106:637–640. [DOI] [PubMed] [Google Scholar]
- [72]. Cecília K, Marcos PM, Thiago AFB, et al. . Chrysomya albiceps (Wiedemann) and Hemilucilia segmentaria (Fabricius) (Diptera, Calliphoridae) used to estimate the postmortem interval in a forensic case in Minas Gerais, Brazil. Revista Brasileira de Entomologia. 2011;55:621–623. [Google Scholar]
- [73]. Frere B, Suchaud F, Bernier G, et al. . GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal Bioanal Chem. 2014;406:1081–1088. [DOI] [PubMed] [Google Scholar]
- [74]. Brown K, Harvey M. Optical coherence tomography: age estimation of Calliphora vicina pupae in vivo? Forensic Sci Int. 2014;242:157–161. [DOI] [PubMed] [Google Scholar]
- [75]. Richards CS, Simonsen TJ, Abel RL, et al. . Virtual forensic entomology: improving estimates of minimum post-mortem interval with 3D micro-computed tomography. Forensic Sci Int. 2012;220:251–264. [DOI] [PubMed] [Google Scholar]
- [76]. Muggenthaler H, Sinicina I, Hubig M, et al. . Database of post-mortem rectal cooling cases under strictly controlled conditions: a useful tool in death time estimation. Int J Legal Med. 2012;126:79–87. [DOI] [PubMed] [Google Scholar]
- [77]. Mao S, Fu G, Seese RR, et al. . Estimation of PMI depends on the changes in ATP and its degradation products. Leg Med (Tokyo). 2013;15:235–238. [DOI] [PubMed] [Google Scholar]
- [78]. The Chinese PMI Estimation Working Group Study on the relationships between rectal temperature of 581 abnormal death bodies and PMI. Forensic Sci Technol. 1984;4:1–10. [Google Scholar]
- [79]. Wang Q, Lang BJ, Meng XZ, et al. . The relation between the corpse temperature and the postmortem interval. J Lanzhou Med Coll. 2004;30:13–15. [Google Scholar]
- [80]. Sun TY, Zhang HD, Yang TD, et al. . The degradation law of blood ATP in rabbit after death and its application in the estimation of PMI. Symp Forensic Theory Pract. 2011;2011:17–20. [Google Scholar]
- [81]. Yang YL, Wu RQ, Fei G, et al. . Study on estimating postmortem interval with factors related to body temperature. Chin J Forensic Med. 2012;5:75–78. [Google Scholar]
- [82]. Yang TD, Yu YG, Liao XB, et al. . Correlation between postmortem intervals and the changes of adenosine triphosphate value in human venous blood and in vitro after death under the condition of different temperature. Chin J Forensic Med. 2013;28:207–210. [Google Scholar]
- [83]. Sun T, Yang T, Zhang H, et al. . Interpolation function estimates post mortem interval under ambient temperature correlating with blood ATP level. Forensic Sci Int. 2014;238:47–52. [DOI] [PubMed] [Google Scholar]
- [84]. Granrud MA, Dabbs GR. A preliminary study of incisor exfoliation as an estimator of the postmortem interval using accumulated degree days. Forensic Sci Int. 2012;220:e29–e32. [DOI] [PubMed] [Google Scholar]
- [85]. Nishida A, Funaki H, Kobayashi M, et al. . Blood creatinine level in postmortem cases. Sci Justice. 2015;55:195–199. [DOI] [PubMed] [Google Scholar]
- [86]. Palmiere C, Mangin P, Urea nitrogen, creatinine, and uric acid levels in postmortem serum, vitreous humor, and pericardial fluid. Int J Legal Med. 2015;129:301–305. [DOI] [PubMed] [Google Scholar]
- [87]. Mihailovic Z, Atanasijevic T, Popovic V, et al. . The role of vitreous magnesium quantification in estimating the postmortem interval. J Forensic Sci. 2014;59:775–778. [DOI] [PubMed] [Google Scholar]
- [88]. Chandrakanth HV, Kanchan T, Balaraj BM, et al. . Postmortem vitreous chemistry – an evaluation of sodium, potassium and chloride levels in estimation of time since death (during the first 36 h after death). J Forensic Leg Med. 2013;20:211–216. [DOI] [PubMed] [Google Scholar]
- [89]. Ravindra BD, Ajay TS, Sachin SP. Estimation of time since death by means of changes in the eye – Vitreous humour calcium levels. Int J Healthcare Biomed Res. 2013;1:141–146. [Google Scholar]
- [90]. Passos ML, Santos AM, Pereira AI, et al. . Estimation of postmortem interval by hypoxanthine and potassium evaluation in vitreous humor with a sequential injection system. Talanta. 2009;79:1094–1099. [DOI] [PubMed] [Google Scholar]
- [91]. Zhang YL, Deng XJ, Lv YX, et al. . Study on the relationship between organic amines derived from putrefaction of isolated human hepatic tissues and the time of death under different environmental temperatures. Chin J Forensic Med. 2002;17:338–340. [Google Scholar]
- [92]. Dang YH, Wang ZY, Zhang LH, et al. . Preliminary study on determining pH value of postmortem skeletal muscle of rat with the Thermo Orion KNIpHE electrode to estimate the early PMI. Chin J Forensic Med. 2005;20:202–205. [Google Scholar]
- [93]. Jin JF, Luo GH, Gao CL, et al. . Study on the relationship between content of lipid in bone marrow of sternum and postmortem interval. Shaanxi Med J. 2008;37:336–338. [Google Scholar]
- [94]. Yang TD, Li ZW, Liu L, et al. . The study on estimation of time since death with multivoxel-voxel proton H-MR spectroscopy at different temperatures. Chin J Forensic Med. 2008;23:379–382. [Google Scholar]
- [95]. Yang TD, Li ZW, Liu L, et al. . A preliminary study on estimation of time since death by single-voxel proton H-MR spectroscopy. Chin J Forensic Med. 2008;23:220–222. [Google Scholar]
- [96]. Yang TD, Yu YG, Sun TY, et al. . A study on estimation of time since death with pH value by three dimensional curve fitting function at different ambient temperatures. Chin J Forensic Med. 2012;27:268–271. [Google Scholar]
- [97]. Wang WP, Long Z, Liu CQ, et al. . An experimental study on correlation between postmortem intervals and ChE and GOT activities in vitreous humor in rabbit. Chin J Forensic Med. 2005;20:199–201. [Google Scholar]
- [98]. Xue B. Study on the estimation of PMI based on γ radiation [Dissertation]. Zhengzhou University; 2007. [Google Scholar]
- [99]. Mao S, Dong X, Fu F, et al. . Estimation of postmortem interval using an electric impedance spectroscopy technique: a preliminary study. Sci Justice. 2011;51: 135–138. [DOI] [PubMed] [Google Scholar]
- [100]. Yang TD, Yu YG, Bai J, et al. . Correlation between the changes of oxidation reduction potential values and postmortem interval of heart blood in rabbits after death. Fa Yi Xue Za Zhi. 2013;29:321–324. [PubMed] [Google Scholar]
- [101]. Zhang GL, Ma JY, Sun Q, et al. . Effects of postmortem interval on mouse ovary oocyte survival and maturation. PloS one. 2014;9:e98384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Sato T, Zaitsu K, Tsuboi K, et al. . A preliminary study on postmortem interval estimation of suffocated rats by GC-MS/MS-based plasma metabolic profiling. Anal Bioanal Chem. 2015;407:3659–3665. [DOI] [PubMed] [Google Scholar]
- [103]. Donaldson AE, Lamont IL. Metabolomics of post-mortem blood: identifying potential markers of post-mortem interval. Metabolomics. 2014;11:237–245. [Google Scholar]
- [104]. Longato S, Woss C, Hatzer-Grubwieser P, et al. . Post-mortem interval estimation of human skeletal remains by micro-computed tomography, mid-infrared microscopic imaging and energy dispersive X-ray mapping. Anal Methods. 2015;7:2917–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Lendoiro E, Cordeiro C, Rodriguez-Calvo MS, et al. . Applications of Tandem Mass Spectrometry (LC-MSMS) in estimating the post-mortem interval using the biochemistry of the vitreous humour. Forensic Sci Int. 2012;223:160–164. [DOI] [PubMed] [Google Scholar]
- [106]. Kang Y-R, Park YS, Park YC, et al. . UPLC/Q-TOF MS based metabolomics approach to post-mortem-interval discrimination: mass spectrometry based metabolomics approach. J Pharm Invest. 2012;42:41–46. [Google Scholar]
- [107]. Machaalani R, Gozal E, Berger F, et al. . Effects of post-mortem intervals on regional brain protein profiles in rats using SELDI-TOF-MS analysis. Neurochem Int. 2010;57:655–661. [DOI] [PubMed] [Google Scholar]


