Abstract
Introduction
Untreatable foot problems in diabetics may require lower extremity amputation, which has a high level of patient mortality. This high mortality rate is worse than most malignancies. The present study aimed to identify parameters that can be used to estimate survival in DM patients undergoing below-knee amputations for diabetic foot problems.
Materials and methods
A total of 470 patients (299 males, 171 females) with a mean age of 64.32 years who underwent below-knee amputation for diabetic foot problems between 2004 and 2014 were enrolled in the study. The length of time from the operation to time of death was recorded in days. Patient details were obtained, including age during surgery, BMI, oral antidiabetic and insulin usage, dialysis therapy history, lower extremity endovascular intervention, previous amputation at the same extremity, the need for stump revision surgery during follow-up, and above-knee amputation at the same site. Biochemical test results of pre-operative HbA1c, ESR, and levels of CRP, BUN, and creatinine were also obtained.
Results
A total of 333 patients (70.9%) died and 137 (29.1%) survived post-surgery. Survival rates were 90% in the first 7 days, 84% in the first 30 days, and 64% after the first year. Patient median life expectancy post-surgery was 930 ± 106 days. Hemodialysis treatment (p = 0.001), endovascular intervention (p = 0.04), sex (p = 0.004), age (p = 0.001), BUN level (p = 0.001), and duration of insulin use (p = 0.003) were shown to be effective predictors of mortality.
Conclusions
Life expectancy is low (<3 years) in DM patients requiring below-knee amputations for untreatable foot problems. Survival could be predicted by duration of insulin use, age, sex, and renal insufficiency.
Level of evidence
Level IV, Therapeutic study.
Keywords: Diabetic foot, Hemodialysis, Below-knee amputation, Life expectancy
Introduction
The diabetic foot syndrome encompasses a number of pathologies, including diabetic neuropathy, peripheral vascular disease, Charcot's neuroarthropathy, foot ulceration, osteomyelitis, and the potentially preventable end point amputation.1 Epidemiological studies have shown that each year 2.5% of patients with diabetes are affected by diabetic foot ulcers, and that 15% of patients with diabetes will ultimately be affected by diabetic foot ulcers.2, 3 The incidence of foot problems ranges from 10% to 25% throughout the lifetime of a Diabetes mellitus (DM) patient,4 and is strongly correlated with mortality and lover extremity major amputations (LEMAs).5, 6
Mortality following amputation ranges from 13 to 40% in 1 year, 35–65% in 3 years, and 39–80% in 5 years, being worse than most malignancies.7 Therefore, amputation-free survival is important in assessing the management of diabetic foot problems.
The aim of the present study was to determine parameters that can be used to estimate survival in DM patients with a planned below-knee amputation for a diabetic foot problem.
Patients and methods
Patient selection
Retrospective review of prospectively collected data from Başkent University research and training hospital Orthopaedic department database was performed. A total of 1134 patients who had undergone the major joint bone amputation between 2004 and 2014 were evaluated. Of the 608 diabetic transtibial amputated patients who were enrolled with the help of International Classification of Disease (ICD) codes, 470 were included in the study, the remainder were excluded for the following criteria's; if they had disarticulation at or above the knee joint (n = 101), if they were attending another center for diabetes and for whom inadequate information was available (n = 25), if they had received a diagnosis of malignant cancer before the surgery (n = 5), and if they died from unnatural reasons according to Social Security Institution data (n = 3; traffic accident, n = 1; firearm injury, n = 1; falling from a height).
We also obtained the following biochemical data: glycated hemoglobin (HbA1c) levels, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), blood urea nitrogen (BUN), and creatinine levels. Patient data acquired were: age during the operation (the date of the first amputation was used if bilateral amputation was performed on different dates), history of dialysis therapy, the lower extremity endovascular intervention procedures, history of previous amputations at the same extremity, the need for stump revision surgery during follow-up, and above-knee amputation at the same site. Body mass index (BMI), DM, and periods of oral antidiabetic and insulin use were also recorded. Data are presented in Table 1, Table 2.
Table 1.
Results of univariate cox regression method to determine important factor on survival probabilities.
| Variables | Total | Total exitus (n (%)) | Exitus male (n (%)) | Exitus female (n (%)) | Median living day ± std. error | Mean age during surgery (min–max) | HR [95%CI] | P |
|---|---|---|---|---|---|---|---|---|
| Gender | 470 | 333 (70.1) | 201 (43) | 132 (28) | 64.32 (20–101) | 1.38 [1.112–1.728] | 0.004* | |
| Male | 299 | 201 (67) | 1171 ± 109.46 | 63.21 (29–88) | ||||
| Female | 171 | 132 (77) | 457 ± 153.76 | 66.32 (20–101) | ||||
| Dialysis | 1.535 [1.218–1.936] | 0.001* | ||||||
| + | 336 | 224 (67) | 128 (38) | 96 (29) | 674 ± 174.65 | 61.07 (29–84) | ||
| − | 134 | 109 (81) | 73 (54) | 36 (27) | 1132 ± 140.09 | 65.61 (20–101) | ||
| Revision surgery | 1.116 [0.868–1.436] | 0.392 | ||||||
| + | 115 | 80 (70) | 50 (43) | 30 (26) | 1147 ± 141.05 | 63.68 (20–86) | ||
| − | 355 | 253 (71) | 151 (43) | 102 (29) | 853 ± 118.07 | 64.52 (29–101) | ||
| Periferic arterial disease | 1.152 [0.868–1.436] | 0.365 | ||||||
| + | 74 | 48 (65) | 24 (32) | 24 (32) | 1239 ± 311415 | 67.20 (43–89) | ||
| − | 396 | 285 (72) | 177 (45) | 108 (27) | 888 ± 105.21 | 63.78 (20–101) | ||
| Embolectomy | 0.834 [0.496–1.401] | 0.492 | ||||||
| + | 24 | 15 (63) | 10 (42) | 5 (21) | 1567 ± 317.49 | 71.13 (46–89) | ||
| − | 446 | 318 (71) | 191 (43) | 127 (28) | 893 ± 104.52 | 63.95 (20–101) | ||
| Angiography | 1.262 [1.011–1.575] | 0.040* | ||||||
| + | 191 | 126 (66) | 71 (37) | 55 (29) | 1208 ± 116.15 | 64.72 (32–101) | ||
| − | 279 | 207 (74) | 130 (47) | 77 (28) | 734 ± 126.95 | 64.04 (20–90) | ||
| Previous amputation surgery | 240 | 94 (39) | 61 (25) | 33 (14) | 1029 ± 155.08 | 63.51 (31–89) | 1.060 [0.834–1.346] | 0.634 |
| Small bone and joint amputation | 93 | 65 (70) | 45 (48) | 20 (22) | 906 ± 182.61 | 62.77 (31–89) | 1.025 [0.779–1.350] | 0.859 |
| Middle bone and joint amputation | 47 | 29 (62) | 16 (34) | 13 (28) | 1196 ± 312.10 | 64.98 (40–84) | 0.800 [0.545–1.178] | 0.260 |
| Above to knee amputation | 132 | 96 (73) | 64 (48) | 32 (24) | 674 ± 190.66 | 64.28 (32–88) | 1.054 [0.830–1.337] | 0.667 |
| Side | ||||||||
| Right vs bilateral | 214 | 155 (73) | 89 (42) | 66 (31) | 906 ± 179.36 | 65.60 (20–90) | 0.837 [0.603–1.161] | 0.286 |
| Left vs bilateral | 188 | 120 (64) | 71 (38) | 49 (26) | 960 ± 160.77 | 63.40 (36–87) | 0.693 [0.494–0.972] | 0.034 |
| Bilateral | 56 | 47 (84) | 32 (57) | 15 (27) | 876 ± 264.44 | 62.25 (29–101) | ||
*Significant at 0.05 level.
Table 2.
Results of multivariate cox regression method to determine important factor on survival probabilities.
| Variables | Median value [min–max] | HR [95%CI] | P |
|---|---|---|---|
| Age | 65 [20–101] | 1.034 [1.024–1.045] | 0.001* |
| BMI (kg/m2) | 29 [18–49] | 1.019 [0.978–1.061] | 0.369 |
| HbA1c (%) | 8.8 [4.3–15.9] | 0.977 [0.920–1.038] | 0.448 |
| Sedimentation (mm/h) | 95 [8–204] | 0.997 [0.993–1.001] | 0.151 |
| CRP (mg/L) | 128 [3–469] | 0.999 [0.998–1.001] | 0.451 |
| BUN (mg/dL) | 32 [7–144] | 1.009 [1.005–1.014] | 0.001* |
| Creatinin (mg) | 1.34 [0.26–90] | 1.015 [1.000–1.031] | 0.051 |
| Oral antidiabetic using time (year) | 10 [1–30] | 0.972 [0.920–1.026] | 0.300 |
| Insulin using time (year) | 7 [1–25] | 1.109 [1.036–1.188] | 0.003* |
| DM time (year) | 18 [3–50] | 1.010 [0.992–1.028] | 0.273 |
*Significant at 0.05 level.
Statistical analysis
Normally distributed data were analyzed by the Shapiro–Wilk test. The Student's t-test was used to compare two independent groups of normally distributed variables, while the Mann–Whitney U test was used for non-normal data. Pearson's chi-squared test was used to investigate relationships between categorical variables. Logistic regression analysis estimated odds ratios and 95% confidence intervals (CIs). Survival probabilities were estimated using Kaplan–Meier curves. Cox regression analysis was performed to determine the important factors affecting overall survival probabilities. Statistical analyses were performed using SPSS v22.0 (Chicago, IL, USA). p values < 0.05 were deemed statistically significant.
Results
A total of 470 patients were enrolled (299 males and 171 females) in the study. The mean age of the patients was 64.32 years (range, 20–101 years). The mean age of male patients was 63.21 years (range, 29–88 years), whereas female patients were 66.26 years (range, 20–101 years). One unit of increase in age was associated with a 1.034-fold (95%CI, 1.024–1045) increase in mortality.
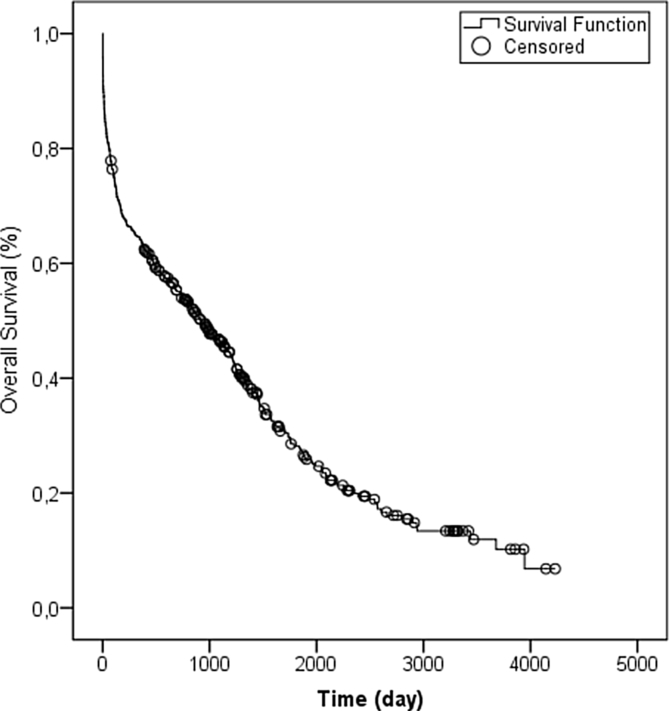
Following the below-knee amputation, a total of 137 patients (29.1%) survived, and 333 (70.9%) died (Table 2, Fig. 1). During the first 7 days post-surgery, the survival rate was 90%; this fell to 84% in the first 30 days, and to 64% by the end of the first year. The life expectancy of patients was median 930 ± 106 days. The mean age of women undergoing below-knee amputation was higher than that of males (Table 1), and their post-operative lifetime was significantly (1.38 times) shorter (95% CI, 1.113–1.728, p = 0.040; Table 2).
Fig. 1.
Kaplan Meier survival estimates after a first lower knee amputation.
The life expectancy of patients undergoing dialysis treatment was 674 ± 174 days (95% CI, 710–1038), whereas it was 1132 ± 140 days (95%CI, 1276–1628) in patients not receiving dialysis treatment, so hemodialysis was shown to increase mortality by 1.53 times (95% CI, 1.218–1.936) (Table 2). Only 96 (72.7%) of women and 128 (63.6%) of men losing their lives were receiving dialysis therapy. Also, one unit of increase in the BUN value was associated with a 1.009-fold (95%CI, 1.005–1.014) increase in mortality (Table 1).
Patients who underwent preoperative endovascular intervention also had a 1.26-fold higher in mortality than those who did not (hazards ratio (HR): 1.262, 95% CI, 1.011–1.575, p = 0.040).
Patients had been diagnosed with DM and had received oral antidiabetic drug (OAD) and insulin therapy for an average of 10 and 7 years, respectively (Table 1). A 1-year increase in the use of insulin increased the mortality rate 1.109-fold (95% CI, 1.036–1.188), whereas the use of OAD and the duration of DM had no effect on the mortality rate (Table 1).
It was found that performing revision surgery after a previous amputation of the same extremity, the inclusion of above-knee amputation, BMI, duration of diabetes, peripheral arterial disease (PAD), performing an embolectomy, and ESR, CRP, creatinine, and HbA1c blood levels had no significant effect on mortality (Table 1, Table 2). Previous small and/or middle joint amputation has no correlation with above or below knee amputation (Table 2).
Discussion
Against a background of changing treatment options for limb salvage, we aimed to review the effect this has on mortality rates for the population who go on to have a transtibial amputation. The mortality rates reported in this study demonstrated the frailty of the population, with 16% of people dying within 30-days. In a similar study, lower rates have been reported in Scandinavian studies with 19–30% of people dying in the first month after LEMA,8, 9, 10 while in other comparable western populations, this is reported to be much lower, around 10%.11, 12 It has been suggested that LEMA in people with vascular disease might be performed as pain relief at the final stages of care.10 Investigation of underlying influences from health services, surgical decisions and patient motivations behind decisions to amputate might help to explain some of the differences in post-operative mortality rates between studies. As an example, a poorer mortality outcome has been found when there are in-hospital delays in decision-making because the patients in our immediate region are mostly unwilling to have amputation surgery and resort to alternative therapies.
Diabetic nephropathy is the most important cause of renal insufficiency in both developed and developing countries.13 Moreover, the amputation rate of lower extremities is six times higher in patients receiving hemodialysis compared with those who do not,14 and it was also found that the life expectancy of patients receiving hemodialysis therapy to be 1.5 time shorter than those not receiving treatment. Previously, mortality rate was 1,56 fold increased with elevated serum levels of BUN (>35 mg/dl), which supports our current data.15
DM patients have a strong correlation between blood glucose control and the development of diabetic foot ulcers.16 Hyperglycemia is known to interfere with the migration, adhesion, phagocytosis, and opsonization of leucocytes.17 When other factors are eliminated, a 1% elevation in the level of HbA1c is reported to increase the PAD risk by 28% (95% CI, 12–46).18 This same study showed that when the main causes of death were considered to be vascular, a shorter lifespan was expected in patients with elevated HbA1c levels and PAD; however, they reported that neither parameter contributed to the expected lifespan. Previous studies reported that patients with HbA1c levels over 7.5% (58 mmol/mol) had increased amputation rates of 20%–54%.6, 19, 20 On the other hand Winskley et al found a relationship between high HbA1c levels and mortality (HR: 0.73; CI, 0.56–0.96).21 In the present study, no correlation was observed between HbA1c and mortality. However, the mean HbA1c value in the study was 8.8% (73 mmol/mol), which is 17% higher than 7.5% HbA1c value. This condition was commented on as severe fluctuations in the blood glucose regulation in the last three months of a patient.
Despite the known relationship between patient mortality and the occurrence of lower extremity major amputation for diabetic foot problems, data regarding the effects of amputation level on the mortality of a patient are limited. In the present study, no significant difference was found in mortality of 240 patients who underwent smaller and/or middle bone - joint amputation prior to below-knee amputation and the 132 patients in whom above-knee amputation was required. Several studies have investigated the efficacy of disease duration, regulation of blood glucose level, HbA1c level, blood levels of BUN, CRP, and ESR, BMI, additional comorbid diseases (renal insufficiency, congestive heath failure, and hypertension), smoking, sex, and age on estimating the progression of ulcer lesions in the feet of diabetic patients.22, 23, 24, 25, 26 In the present study, indicators such as ESR and CRP had no significant effect on mortality.
Previous studies have shown that patients with high BMI levels have a high mortality rate. Ilavska et al reported that immunological dysfunction is higher in patients with high BMI. This dysfunction was reported to cause an increase in mortality as a result of delays in wound healing and being unable to prevent sepsis. In the same group of patients, it was found that major vascular problems were higher and contributed to mortality.27
Won et al reported 1-year mortality rates of patient with diabetic foot ulcers who did and did not undergo amputation as 34% (n = 114) and 3% (n = 167), respectively.6 Fortington et al determined life expectancy to be 25 months in patients who underwent lower extremity major amputations, compared with 20.7 months in patients with non-diabetic vascular disorders however, this is not statistically meaningful.5 They reported that 22% of patients died during the postoperative 30 days and that 44% died in the first year after surgery. They found that the patient's age, location, previous peripheral vascular interventions, and history of cerebrovascular disease negatively affected early and late mortality, while renal failure and cardiovascular disorders affected late period mortality. In the present study, survival rates were 90%, 84%, and 64% in the first 7 days, 30 days, and at the end of first year, respectively.
In the present study, the number of male patients was higher than that of female patients. Although some previous studies reported no difference between the occurrence of diabetic foot ulcers and sex, others state that the diabetes-related amputation rate is higher in male patients.22, 28, 29 The reason for the high rate in males has been suggested to be the negative effects of bad lifestyle habits such as alcohol consumption and smoking, and poor foot care, which are worse in men than in women.20, 22 So absenteeism is higher among men, ulcerations will take longer to recover, and ulcers are more likely to end in amputation.30 These results indicated the potential need for the health care system to devise a primary care strategy that will help ensure that men with diabetes receive good care.
PAD was present in approximately half of all patients admitted to hospital with diabetic foot problems in a previous study.31 The value of endovascular intervention in recanalization lower extremity arterial occlusion is currently under debate.32, 33 In the present study, the life expectancy was found to be 60% higher in patients who had undergone peripheral endovascular intervention compared with those who had not. The decision to take an endovascular intervention is made by the interventional radiologist.
Considering DM pathology at the cellular level, it was expected that the disease duration would have an effect on patient survive; however, this was not the case in the present study. The mean duration of DM was found to be 18 years. The patients' mean age was 64,2 years. It was diagnosed during their late 40s.
Previous studies have shown that the compliance with the treatment of diabetic patients has been steadily decreasing over the years, While OAD therapy, diet and exercise, a first step in diabetes treatment, cause fewer fluctuations in blood glucose levels, insulin therapy led much and higher and more common fluctuations in patients. We detected that a 1-year increase in the duration of 10-year OAD and 7-year insulin usage increased mortality by 0.9- and 1.1-fold, respectively. Age was another factor shown to increase mortality in the study. It was observed that a 1-year increase in age increased the mortality by 1.034-fold.
Diabetic foot problems are the visible effect of fluctuations in blood glucose on the all of the body over time. When this causes complications in the lower extremities, the effects on the cerebral and cardiac systems are greater, which is the main reason for mortality. Additionally, the strong relation between infection and hyperglycemia decreases the overall survival time.
The present study is the most extensive to date to investigate patients who underwent below-knee amputations because of diabetic foot. It shows the effect of preoperative parameters on patient life expectancy. Nevertheless, it had a number of limitations. First it is retrospective. The presence of co-morbid diseases, ASA risk values given by anesthesia, the duration of dialysis in patients receiving dialysis therapy, educational status, or the causes of death during amputation were not investigated. Further studies are therefore necessary to overcome these limitations.
Conclusion
Untreatable foot problems in diabetic patients that can be detected early while being indicative of a more serious problem. The present study shows that life expectancy was less than median 3 years in diabetic patients having clinical findings and requiring below-knee amputation. Age, sex, and renal insufficiency appear to be key factors in determining the length of the survival period of these patients.
Disclosure ethical approval
This study was approved by Başkent University Institutional Review Board and Ethics Committee (Project No.: KA16/172). This article does not contain any studies with animals performed by any of the authors.
Funding
This study was supported by the Başkent University Research Fund.
Conflicts of interest
Salih Beyaz, Ümit Özgür Güler and Gülay Şimşek Bağır declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Rathur H.M., Boulton A.J. The diabetic foot. Clin Dermatol. 2007;25(1):109–120. doi: 10.1016/j.clindermatol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Shojaiefard A., Khorgami Z., Larijani B. Independent risk factors for amputation in diabetic foot. Int J Diabetes Dev Ctries. 2008;28(2):32–37. doi: 10.4103/0973-3930.43096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yesil S., Akinci B., Yener S. Predictors of amputation in diabetics with foot ulcer: single center experience in a large Turkish cohort. Horm (Athens) 2009;8(4):286–295. doi: 10.14310/horm.2002.1245. [DOI] [PubMed] [Google Scholar]
- 4.Frykberg R.G., Zgonis T., Armstrong D.G. Diabetic foot disorders. A clinical practice guideline (2006 revision) J Foot Ankle Surg. 2006;45(suppl 5):S1–S66. doi: 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 5.Fortington L.V., Geertzen J.H., van Netten J.J., Postema K., Rommers G.M., Dijkstra P.U. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg. 2013;46(1):124–131. doi: 10.1016/j.ejvs.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Won S.H., Chung C.Y., Park M.S. Risk factors associated with amputation-free survival in patient with diabetic foot ulcers. Yonsei Med J. 2014;55(5):1373–1378. doi: 10.3349/ymj.2014.55.5.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh N., Armstrong D.G., Lipsky B.A. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Eskelinen E., Lepantalo M., Hietala E.M. Lower limb amputations in Southern Finland in 2000 and trends up to 2001. Eur J Vasc Endovasc Surg. 2004;27(2):193–200. doi: 10.1016/j.ejvs.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen M.T., Holm G., Kirketerp-Moller K., Krasheninnikoff M., Gebuhr P. Very low survival rates after non-traumatic lower limb amputation in a consecutive series: what to do? Interact Cardiovasc Thorac Surg. 2012;14(5):543–547. doi: 10.1093/icvts/ivr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remes L., Isoaho R., Vahlberg T. Major lower extremity amputation in elderly patients with peripheral arterial disease: incidence and survival rates. Aging Clin Exp Res. 2008;20(5):385–393. doi: 10.1007/BF03325142. [DOI] [PubMed] [Google Scholar]
- 11.Davenport D.L., Ritchie J.D., Xenos E.S. Incidence and risk factors for 30-day postdischarge mortality in patients with vascular disease undergoing major lower extremity amputation. Ann Vasc Surg. 2012;26(2):219–224. doi: 10.1016/j.avsg.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Subramaniam B., Pomposelli F., Talmor D., Park K.W. Perioperative and long-term morbidity and mortality after above-knee and below-knee amputations in diabetics and nondiabetics. Anesth analgesia. 2005;100(5):1241–1247. doi: 10.1213/01.ANE.0000147705.94738.31. [table of contents] [DOI] [PubMed] [Google Scholar]
- 13.Atkins R.C., Zimmet P. Diabetic kidney disease: act now or pay later. Saudi J Kidney Dis Transpl. 2010;21(2):217–221. [PubMed] [Google Scholar]
- 14.Morbach S., Quante C., Ochs H.R., Gaschler F., Pallast J.M., Knevels U. Increased risk of lower-extremity amputation among Caucasian diabetic patients on dialysis. Diabetes Care. 2001;24(9):1689–1690. doi: 10.2337/diacare.24.9.1689. [DOI] [PubMed] [Google Scholar]
- 15.Kim T.G., Moon S.Y., Park M.S. Factors affecting length of hospital stay and mortality in infected diabetic foot ulcers undergoing surgical drainage without major amputation. J Korean Med Sci. 2016;31(1):120–124. doi: 10.3346/jkms.2016.31.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvin E., Wattanakit K., Steffes M.W., Coresh J., Sharrett A.R. HbA1c and peripheral arterial disease in diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2006;29(4):877–882. doi: 10.2337/diacare.29.04.06.dc05-2018. [DOI] [PubMed] [Google Scholar]
- 17.Bagdade J.D., Root R.K., Bulger R.J. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23(1):9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- 18.Adler A.I., Stevens R.J., Neil A., Stratton I.M., Boulton A.J., Holman R.R. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes care. 2002;25(5):894–899. doi: 10.2337/diacare.25.5.894. [DOI] [PubMed] [Google Scholar]
- 19.Papanas N., Maltezos E. Glycated hemoglobin as a risk factor for lower extremity amputations in diabetes: “success is counted sweetest”. Int J Low Extrem Wounds. 2015;14(2):106–107. doi: 10.1177/1534734615592313. [DOI] [PubMed] [Google Scholar]
- 20.Pscherer S., Dippel F.W., Lauterbach S., Kostev K. Amputation rate and risk factors in type 2 patients with diabetic foot syndrome under real-life conditions in Germany. Prim Care Diabetes. 2012;6(3):241–246. doi: 10.1016/j.pcd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Winkley K., Stahl D., Chalder T., Edmonds M.E., Ismail K. Risk factors associated with adverse outcomes in a population-based prospective cohort study of people with their first diabetic foot ulcer. J Diabetes Complicat. 2007;21(6):341–349. doi: 10.1016/j.jdiacomp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Amin L., Shah B.R., Bierman A.S. Gender differences in the impact of poverty on health: disparities in risk of diabetes-related amputation. Diabet Med. 2014;31(11):1410–1417. doi: 10.1111/dme.12507. [DOI] [PubMed] [Google Scholar]
- 23.Namgoong S., Jung S., Han S.K., Jeong S.H., Dhong E.S., Kim W.K. Risk factors for major amputation in hospitalised diabetic foot patients. Int Wound J. 2015 doi: 10.1111/iwj.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pemayun T.G., Naibaho R.M., Novitasari D., Amin N., Minuljo T.T. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a hospital-based case-control study. Diabet Foot Ankle. 2015;6 doi: 10.3402/dfa.v6.29629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ries Z., Rungprai C., Harpole B. Incidence, risk factors, and causes for thirty-day unplanned readmissions following primary lower-extremity amputation in patients with diabetes. J Bone Jt Surg Am. 2015;97(21):1774–1780. doi: 10.2106/JBJS.O.00449. [DOI] [PubMed] [Google Scholar]
- 26.Yusof N.M., Rahman J.A., Zulkifly A.H. Predictors of major lower limb amputation among type II diabetic patients admitted for diabetic foot problems. Singap Med J. 2015;56(11):626–631. doi: 10.11622/smedj.2015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilavska S., Horvathova M., Szabova M. Association between the human immune response and body mass index. Hum Immunol. 2012;73(5):480–485. doi: 10.1016/j.humimm.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Lavery L.A., Armstrong D.G., Wunderlich R.P., Tredwell J., Boulton A.J. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003;26(5):1435–1438. doi: 10.2337/diacare.26.5.1435. [DOI] [PubMed] [Google Scholar]
- 29.Tang Z.Q., Chen H.L., Zhao F.F. Gender differences of lower extremity amputation risk in patients with diabetic foot: a meta-analysis. Int J Low Extrem Wounds. 2014;13(3):197–204. doi: 10.1177/1534734614545872. [DOI] [PubMed] [Google Scholar]
- 30.Moura Neto A., Zantut-Wittmann D.E., Fernandes T.D., Nery M., Parisi M.C. Risk factors for ulceration and amputation in diabetic foot: study in a cohort of 496 patients. Endocrine. 2013;44(1):119–124. doi: 10.1007/s12020-012-9829-2. [DOI] [PubMed] [Google Scholar]
- 31.Brownrigg J.R., Schaper N.C., Hinchliffe R.J. Diagnosis and assessment of peripheral arterial disease in the diabetic foot. Diabet Med. 2015;32(6):738–747. doi: 10.1111/dme.12749. [DOI] [PubMed] [Google Scholar]
- 32.Courtois M.C., Sapoval M., Del Giudice C., Ducloux R., Mirault T., Messas E. Distal revascularization in diabetic patients with chronic limb ischemia. J Mal Vasc. 2015;40(1):24–36. doi: 10.1016/j.jmv.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Pernes J.M., Auguste M., Borie H. Infrapopliteal arterial recanalization: a true advance for limb salvage in diabetics. Diagn Interv Imaging. 2015;96(5):423–434. doi: 10.1016/j.diii.2014.09.002. [DOI] [PubMed] [Google Scholar]