Abstract
Background
Endothelial cell specific molecule-1, also called as endocan, is a dermatan sulfate proteoglycan, which is expressed by endothelial cells in alveolar walls of the lung and kidney. High endocan levels are found associated with endothelial dysfunction and inflammation. We hypothesize that endocan level is also high in COPD due to systemic inflammation and endothelial dysfunction. We aimed to investigate the expression of endocan in patients with stable COPD.
Material and methods
The study included patients with COPD and control subjects. COPD patients were classified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 criteria. Demographics, body mass index, smoking history, and comorbidities were recorded. Endocan levels of COPD patients and controls were compared.
Results
Totally, 88 subjects (47 stable COPD patients, 41 controls) were evaluated. Endocan levels were significantly higher in COPD patients than control group (860.1±259.8 vs 647.3±316.9 pg/mL, P=0.001). There was no relationship between GOLD COPD categories and endocan levels. Also endocan levels were similar between COPD patients with or without hypoxemia.
Conclusion
Serum endocan level was significantly higher in patients with stable COPD. Further studies should be performed to better understand the relationship between endocan and COPD.
Keywords: chronic obstructive pulmonary disease, endothelial cell specific molecule-1, endothelial dysfunction, systemic inflammation
Introduction
COPD is a chronic inflammatory lung disease that affects small airways, lung parenchyma, and vascular endothelium.1–4 COPD is not a disease that affects only the lungs; it is a systemic inflammatory and endothelial disease.1,5–8
Endothelial cell specific molecule-1, also called as endocan, is a dermatan sulfate proteoglycan, which is expressed by endothelial cells in alveolar walls of the lung and kidney.9,10 Endocan plays an important role in many endothelial-dependent pathophysiological situations, such as inflammation, tumor progression, cell proliferation and adhesion, migration, and angiogenesis.11–15 In certain studies, high endocan levels were associated with endothelial dysfunction and inflammation.16–34 Therefore, endocan was considered as a potential marker for endothelial dysfunction.11,15 There are several studies showing increased endocan levels in lung diseases such as lung cancer, acute lung injury, acute respiratory distress syndrome, community-acquired pneumonia, pulmonary embolism, obstructive sleep apnea (OSA), sarcoidosis, and pleural effusion.25–34 In the literature, there is no study about endocan level in patients with COPD. We hypothesize that endocan level is high in COPD, which is associated with systemic inflammation and endothelial dysfunction.
This study aimed to investigate the serum endocan levels in COPD. Secondary aim was to evaluate the relationship between endocan levels and severity of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD categories, and hypoxemia.
Materials and methods
This prospective study included stable COPD patients admitted to the outpatient clinic of Istanbul University Istanbul Medical Faculty from January 2017 to January 2018. Age-(40–80 years) and gender-matched subjects without COPD or uncontrolled chronic diseases were included as a control group. Control subjects were selected from the relatives of the subjects who were admitted to our outpatient clinic. All of the subjects voluntarily signed their informed consent. The study was carried out according to the principles of the Helsinki Declaration. It was approved by Istanbul University Istanbul Medical Faculty Institutional Board (Ethic no 2014/1715).
We included adult patients (>18 years) with COPD diagnosis for at least 1 year and stable disease for 3 months without any change in the medication. The exclusion criteria were restrictive pulmonary diseases, significant cardiac failure, acute coronary syndromes (unstable angina pectoris and myocardial infarction), valvular heart diseases, congenital heart disease, renal or hepatic dysfunction, active inflammatory diseases, acute infections, malignancies, and medication that can potentially interfere with level of endocan (lipid lowering therapy, vitamins, or antioxidants).
Demographics such as body mass index (BMI), smoking history, spirometric parameters, and comorbidities were recorded. COPD patients were classified according to the GOLD 2017 recommendations considering symptoms and exacerbation risk to grade disease severity into categories A–D.35 Significant hypoxemia was considered when partial arterial oxygen pressure <60 mmHg.
Serum endocan measurements
Blood samples were collected using EDTA as anticoagulant in the morning between 7.00 and 9.00 am. Blood samples centrifuged within 30 minutes of sampling at 1,500 g for 15 minutes and stored at −80°C until testing. Serum endocan level was measured by enzyme-linked immunoassay (ELISA) using a commercially available kit (Elabscience Biotechnology Inc, Houston, TX, USA) according to the manufacturer’s instructions. The results are presented in pg/mL.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software (AIMS, Istanbul, Turkey). All continuous variables are presented as the mean ± SD and categorical variables as frequency (percentage). The concordance of normal distribution of all variables was calculated with the Shapiro–Wilk test. If the data were not normally distributed, we used nonparametric tests for dependent variables. Comparisons between two groups were carried out with Mann–Whitney U-test or Student’s t-test. Categorical variables were compared with the chi-squared test. Comparison between more than two groups was done with one-way analysis of variance test. The Spearman correlation coefficient was used to examine the relationship between endocan and age, BMI, spirometric measurements, and comorbidities. A P-value of <0.05 was considered statistically significant.
Results
Totally, 47 COPD patients and 41 controls were included in the study. According to GOLD COPD classification, 38.3% (n=18) of our patients were in category B, 31.9% (n=15) in category D, and 29.8% (n=14) in category C. Comparison of the demographics and endocan levels of COPD patients and controls are presented in Table 1.
Table 1.
Differences between COPD patients and controls
| Characteristics | COPD patients (n=47) |
Control subjects (n=41) |
P-value |
|---|---|---|---|
| Age (years) | 61.5±8.7 | 59.7±9.7 | 0.4 |
| Range: 40–75 | Range: 43–79 | ||
| Gender (female/male) | 4/43 | 3/38 | 0.9 |
| BMI (kg/m2) | 25.0±2.9 | 26.0±1.9 | 0.06 |
| Smoking history (pack years) | 36.3±17.2 | 30.0±20.1 | 0.09 |
| Comorbidities (%) (hypertension and/or diabetes) | 25.5 | 19.5 | 0.6 |
| Endocan level (pg/mL) | 860.1±259.9 | 647.3±316.9 | 0.001 |
Abbreviations: BMI, body mass index; Endocan, endothelial cell specific molecule-1.
The distribution of endocan levels for COPD patients and control subjects was given in Figure 1.
Figure 1.
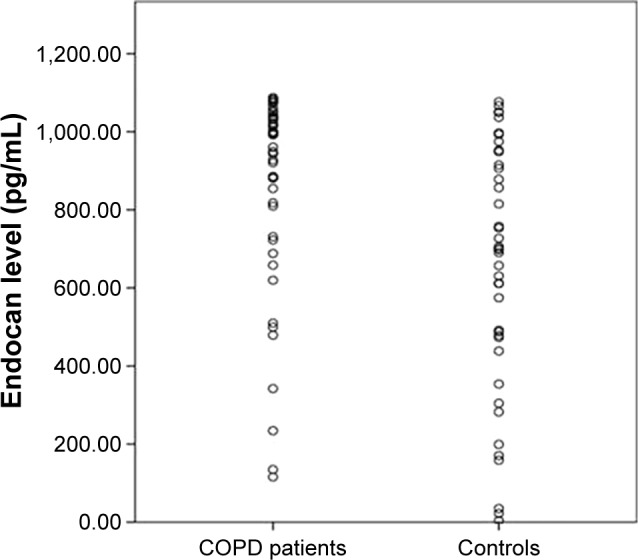
The distribution of endocan levels in COPD patients and control subjects.
Serum endocan levels were significantly higher in COPD patients than controls (860.1±259.9 vs 647.3±316.9 pg/mL P=0.001). There were seven females (four COPD patients and three control subjects) in our study. When we excluded female patients and control subjects, endocan levels were still significantly different between patients with COPD and control subjects (847.1±267.2 vs 654.2±307.8 pg/mL P=0.002). When COPD category progresses toward B to D, endocan levels tended to increase, but there was no statistically significant difference between COPD GOLD categories (group B: 820.9±280.8 pg/mL, group C: 867.6±236.8 pg/mL, group D: 900.3±265.2 pg/mL). Comparison of COPD GOLD categories are presented in Table 2.
Table 2.
Comparison of COPD GOLD categories
| Characteristics | COPD group B (n=18) | COPD group C (n=14) | COPD group D (n=15) | P-value |
|---|---|---|---|---|
| Age (years) | 60.5±9.1 | 63.4±9.1 | 60.9±9.8 | 0.6 |
| Gender (female/male) | 2/16 | 0/14 | 2/13 | 0.3 |
| BMI (kg/m2) | 26.4±2.2 | 24.7±2.4 | 23.7±3.3 | 0.01 |
| Smoking (pack years) | 33.4±14.7 | 33.6±15.6 | 43.3±21.0 | 0.3 |
| FEV1 (%) | 62.0±6.0 | 36.1±11.7 | 29.7±11.5 | <0.001 |
| FVC (%) | 74.4±8.2 | 52.9±12.3 | 44.9±16.9 | <0.001 |
| PaO2 (mmHg) | 76.7±6.3 | 68.7±12.3 | 61.0±1.0 | <0.001 |
| PaCO2 (mmHg) | 39.1±4.0 | 44.9±7.6 | 51.2±9.2 | <0.001 |
| Comorbidities (%) | 22.2 | 42.9 | 13.3 | 0.2 |
| Endocan level (pg/mL) | 820.9±280.8 | 867.6±236.8 | 900.3±265.2 | 0.6 |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PaCO2, partial arterial carbon dioxide pressure; PaO2, partial arterial oxygen pressure.
There were 17 patients with COPD who had significant hypoxemia. All of them were receiving long-term oxygen therapy (LTOT) and ten of them were also using noninvasive mechanical ventilation (NIMV) in addition to LTOT. There was no statistically significant difference between hypoxemic COPD patients and nonhypoxemic COPD patients in terms of endocan levels (921.2±252 vs 825.5±262.0 pg/mL, P=0.2). Comparisons of COPD patients with and without hypoxemia are given in Table 3. Endocan levels were similar in patients who were receiving NIMV and the others who were not (863.6±315.8 vs 859.2±247.7 pg/mL, P=0.9).
Table 3.
Differences between COPD patients with and without significant hypoxemia
| Characteristics | With hypoxemia (n=17) | Without hypoxemia (n=30) | P-value |
|---|---|---|---|
| Age (years) | 61.8±10.0 | 61.3±8.2 | 0.9 |
| Gender (female/male) | 2/15 | 2/28 | 0.2 |
| BMI (kg/m2) | 23.7±3.3 | 25.8±2.3 | 0.03 |
| Smoking (pack/years) | 37.1±18.2 | 35.9±17.0 | 0.2 |
| FEV1 (%) | 29.8±10.8 | 52.0±15.3 | <0.001 |
| FVC (%) | 44.3±15.0 | 66.6±14.2 | <0.001 |
| PaO2 (mmHg) | 59.1±9.8 | 75.0±7.6 | <0.001 |
| PaCO2 (mmHg) | 52.7±8.3 | 40.1±4.2 | <0.001 |
| Comorbidities (%) | 11.8 | 33.3 | 0.2 |
| Endocan level (pg/mL) | 921.2±252.0 | 825.5±262.0 | 0.2 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume 1 second; FVC, force vital capacity; PaCO2, partial arterial carbon dioxide pressure; PaO2, partial arterial oxygen pressure.
There were 12 COPD patients with comorbidities (8 had hypertension, 2 had diabetes mellitus, 2 of them had both hypertension and diabetes). COPD patients with comorbidities had slightly higher but not significantly different endocan levels than those without comorbidities (910.8±207.3 vs 842.8±276.2 pg/mL, P=0.4). Comparisons of COPD patients with and without comorbidities are given in Table 4.
Table 4.
Differences between COPD patients with and without comorbidities
| Characteristics | With comorbidities (n=12) | Without comorbidities (n=35) | P-value |
|---|---|---|---|
| Age (years) | 66.8±4.9 | 59.6±9.0 | 0.01 |
| Gender (female/male) | 1/11 | 3/32 | 0.9 |
| BMI (kg/m2) | 25.9±2.5 | 24.7±2.9 | 0.2 |
| Smoking (pack years) | 37.0±16.5 | 36.1±17.6 | 0.8 |
| FEV1 (%) | 43.6±15.4 | 44.1±18.4 | 0.9 |
| FVC (%) | 58.7±16.6 | 58.5±18.7 | 0.9 |
| PaO2 (mmHg) | 72.8±11.1 | 67.1±12.6 | 0.7 |
| PaCO2 (mmHg) | 41.3±5.1 | 45.8±9.1 | 0.1 |
| LTOT (%) | 16.7 | 42.9 | 0.2 |
| NIMV (%) | 8.3 | 25.7 | 0.4 |
| Endocan level (pg/mL) | 910.8±207.3 | 842.8±276.2 | 0.4 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume 1 second; FVC, forced vital capacity; LTOT, long-term oxygen therapy; NIMV, noninvasive mechanical ventilation; PaCO2, partial arterial carbon dioxide pressure; PaO2, partial arterial oxygen pressure.
There was no correlation between endocan level and forced expiratory volume 1 second percent (FEV1%), forced vital capacity percent, age, BMI, comorbidities, LTOT, and NIMV.
Discussion
In this study, serum endocan level was found significantly higher in stable COPD patients than the control group. To our knowledge, this is the first study in the literature that investigates the endocan levels in stable COPD patients. COPD is associated with vascular endothelial dysfunction and systemic inflammation. For this reason, we wanted to investigate endocan levels in COPD and speculate that higher levels of endocan in COPD might be associated with inflammation and/or endothelial dysfunction.
It is known that endocan is expressed by pulmonary and renal endothelial cells. It is secreted by vascular endothelial cells, and also expression of endocan is regulated by a number of proangiogenic factors such as vascular endothelial growth factor as well as cytokines.9–11,36 Previous studies mentioned that endocan not only plays a role in the inflammatory process but also plays a role in the vascular damage and endothelium-dependent pathological disorders. Therefore, it may represent a novel endothelial cell dysfunction marker.12–34 Since COPD is a systemic inflammatory disease that can affect vascular endothelium, increase of endocan it is expected. As expected, increased endocan level was shown in our study.
There are several studies in which endocan levels were investigated in lung diseases. Most of these previous studies were performed in patients with acute pulmonary diseases such as acute lung injury, acute respiratory distress syndrome, community-acquired pneumonia, pulmonary embolism, lung cancer, and pleural effusion.25–32 There are only two studies in stable lung diseases, sarcoidosis and OSA.33,34 These studies reported higher endocan levels in sarcoidosis and OSA.33,34 Aciksari et al34 reported that sarcoidosis is associated with high levels of endocan and lower flow-mediated dilation values, which may indicate endothelial dysfunction and an early stage of atherosclerosis. According to these results, they concluded that it may be a prognostic marker for both the inflammatory process and the endothelial function.34 However, they could not show any difference for endocan levels between stages of sarcoidosis. Also, Bingol et al33 reported higher endocan levels in OSA patients than controls. Similarly, they could not find any difference for endocan levels between mild, moderate, or severe OSA.33 In accordance with previous studies, we found higher endocan levels in stable COPD patients than controls. Endocan levels were not significantly different in COPD patients with different GOLD categories. Unlike ours and the other studies with stable lung diseases, the relationship between severity of disease and endocan has been shown in acute lung diseases. Güzel et al28 reported that serum endocan levels can be considered a practical biomarker to determine the severity of pulmonary embolism. Kao et al29 showed that high endocan values were associated with Pneumonia Severity Index, Confusion - Urea - Respiratory rate - Blood pressure (CURB-65), and Acute Physiology and Chronic Health Evaluation (APACHE II), which are the indicators of the disease severity. According to these results, we think that increased levels of endocan may represent a marker for severity of acute inflammatory lung diseases with endothelial dysfunction. Perhaps, endocan may not be a suitable biomarker for determining disease severity in stable lung diseases.
Except the study of Bingol et al,33 none of the previous studies evaluated the relationship between endocan levels and hypoxemia. Bingol et al33 showed no difference between endocan levels in OSA patients with and without nocturnal hypoxemia. Similarly, we did not find any difference between endocan levels in COPD patients with or without significant hypoxemia. Aciksari et al34 excluded the patients with hypoxemia and impaired pulmonary tests while investigating the endothelial function in sarcoidosis.
Another feature of our study is that we analyzed the relationship between endocan and spirometric parameters. We found no correlation between endocan level and FEV1%.
COPD is often associated with comorbid conditions such as cardiovascular and cerebrovascular disease, osteoporosis, depression, lung cancer, and diabetes.37,38 All these comorbidities are characterized by systemic inflammation, and it has been hypothesized that chronic systemic inflammation may be a key factor linking COPD and its comorbidities. So, it is accepted that comorbidities should be associated with increased systemic inflammation. Comorbidities such as hypertension and diabetes mellitus might be a reason for increased endocan levels in COPD. However, we could not find any difference between serum endocan levels of COPD patients with or without comorbidities. In addition, although comorbidities were similar in COPD and control groups, we found significantly higher endocan levels in patients with COPD than controls. Therefore, we cannot link high endocan levels to comorbidities in COPD patients. Similarly, Bingol et al33 found that endocan levels were similar in OSA subjects with and without hypertension or diabetes mellitus. Also, comorbidities were similar in OSA patients and controls. Güzel et al28 showed that endocan levels were not correlated with cardiac failure, hypertension, and pulmonary hypertension in patients with pulmonary thromboembolism.
One of the limitations of our study is the small sample size, but, this is the first study in the literature that has evaluated endocan levels in stable COPD. Another limitation is not evaluating inflammatory markers in our study. Most of the previous studies about endocan in lung diseases also did not evaluate inflammatory markers. Only Aciksari et al34 investigated the correlation between serum endocan and C-reactive protein levels in patients with sarcoidosis, and they could not find any relationship between the two.
Conclusion
Serum endocan level was significantly higher in patients with stable COPD than controls. Further large-scale studies should be performed to explain the reason behind this mechanism.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hogg JC, Paré PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev. 2017;97(2):529–552. doi: 10.1152/physrev.00025.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 3.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 4.O’Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155(3):852–857. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 5.Liebow AA. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis. 1959;80(1, Part 2):67–93. doi: 10.1164/arrd.1959.80.1P2.67. [DOI] [PubMed] [Google Scholar]
- 6.Peinado VI, Barbera JA, Ramirez J, et al. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998;274(6 Pt 1):908–913. doi: 10.1152/ajplung.1998.274.6.L908. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Mahler DA, Marcus P, Owen CA, Yawn B, Rennard S. Inflammation in COPD: implications for management. Am J Med. 2012;125(12):1162–1170. doi: 10.1016/j.amjmed.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Polverino F, Celli BR, Owen CA. COPD as an endothelial disorder: endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series) Pulm Circ. 2018;8(1):2045894018758528. doi: 10.1177/2045894018758528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lassalle P, Molet S, Janin A, et al. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271(34):20458–20464. doi: 10.1074/jbc.271.34.20458. [DOI] [PubMed] [Google Scholar]
- 10.Bechard D, Meignin V, Scherpereel A, et al. Characterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodies. J Vasc Res. 2000;37(5):417–425. doi: 10.1159/000025758. [DOI] [PubMed] [Google Scholar]
- 11.Béchard D, Scherpereel A, Hammad H, et al. Human endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1. J Immunol. 2001;167(6):3099–3106. doi: 10.4049/jimmunol.167.6.3099. [DOI] [PubMed] [Google Scholar]
- 12.Roudnicky F, Poyet C, Wild P, et al. Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesis. Cancer Res. 2013;73(3):1097–1106. doi: 10.1158/0008-5472.CAN-12-1855. [DOI] [PubMed] [Google Scholar]
- 13.Sarrazin S, Adam E, Lyon M, et al. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta. 2006;1765(1):25–37. doi: 10.1016/j.bbcan.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Béchard D, Gentina T, Delehedde M, et al. Endocan is a novel chondroitin sulfate/dermatan sulfate proteoglycan that promotes hepatocyte growth factor/scatter factor mitogenic activity. J Biol Chem. 2001;276(51):48341–48349. doi: 10.1074/jbc.M108395200. [DOI] [PubMed] [Google Scholar]
- 15.Aitkenhead M, Wang SJ, Nakatsu MN, Mestas J, Heard C, Hughes CC. Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: induction of ESM-1, (beta)ig-h3, and NrCAM. Microvasc Res. 2002;63(2):159–171. doi: 10.1006/mvre.2001.2380. [DOI] [PubMed] [Google Scholar]
- 16.Balta I, Balta S, Koryurek OM, et al. Serum endocan levels as a marker of disease activity in patients with Behçet disease. J Am Acad Dermatol. 2014;70(2):291–296. doi: 10.1016/j.jaad.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Balta I, Balta S, Demirkol S, et al. Elevated serum levels of endocan in patients with psoriasis vulgaris: correlations with cardiovascular risk and activity of disease. Br J Dermatol. 2013;169(5):1066–1070. doi: 10.1111/bjd.12525. [DOI] [PubMed] [Google Scholar]
- 18.Scherpereel A, Depontieu F, Grigoriu B, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34(2):532–537. doi: 10.1097/01.ccm.0000198525.82124.74. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz MI, Siriopol D, Saglam M, et al. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014;86(6):1213–1220. doi: 10.1038/ki.2014.227. [DOI] [PubMed] [Google Scholar]
- 20.Kose M, Emet S, Akpinar TS, et al. Serum endocan level and the severity of coronary artery disease: a pilot study. Angiology. 2015;66(8):727–731. doi: 10.1177/0003319714548870. [DOI] [PubMed] [Google Scholar]
- 21.Balta S, Mikhailidis DP, Demirkol S, et al. Endocan – a novel inflammatory indicator in newly diagnosed patients with hypertension: a pilot study. Angiology. 2014;65(9):773–777. doi: 10.1177/0003319713513492. [DOI] [PubMed] [Google Scholar]
- 22.Schuitemaker JHN, Cremers T, van Pampus MG, Scherjon SA, Faas MM. Changes in endothelial cell specific molecule 1 plasma levels during preeclamptic pregnancies compared to healthy pregnancies. Pregnancy Hypertens. 2018;12:58–64. doi: 10.1016/j.preghy.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Toshikuni N, Ozaki K, George J, Tsutsumi M. Serum endocan as a survival predictor for patients with liver cirrhosis. Can J Gastroenterol Hepatol. 2015;29(8):427–430. doi: 10.1155/2015/153805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akarsu M, Atalay HA, Canat L, et al. Endocan is markedly overexpressed in severe erectile dysfunction. Andrologia. 2018;50(3):e12912. doi: 10.1111/and.12912. [DOI] [PubMed] [Google Scholar]
- 25.Grigoriu BD, Depontieu F, Scherpereel A, et al. Endocan expression and relationship with survival in human non-small cell lung cancer. Clin Cancer Res. 2006;12(15):4575–4582. doi: 10.1158/1078-0432.CCR-06-0185. [DOI] [PubMed] [Google Scholar]
- 26.Scherpereel A, Gentina T, Grigoriu B, et al. Overexpression of endocan induces tumor formation. Cancer Res. 2003;63(18):6084–6089. [PubMed] [Google Scholar]
- 27.Mikkelsen ME, Shah CV, Scherpereel A, et al. Lower serum endocan levels are associated with the development of acute lung injury after major trauma. J Crit Care. 2012;27(5):522.e11–7. doi: 10.1016/j.jcrc.2011.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Güzel A, Duran L, Köksal N, et al. Evaluation of serum endothelial cell specific molecule-1 (endocan) levels as a biomarker in patients with pulmonary thromboembolism. Blood Coagul Fibrinolysis. 2014;25(3):272–276. doi: 10.1097/MBC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 29.Kao SJ, Chuang CY, Tang CH, et al. Plasma endothelial cell-specific molecule-1 (ESM-1) in management of community-acquired pneumonia. Clin Chem Lab Med. 2014;52(3):445–451. doi: 10.1515/cclm-2013-0638. [DOI] [PubMed] [Google Scholar]
- 30.Tang L, Zhao Y, Wang D, et al. Endocan levels in peripheral blood predict outcomes of acute respiratory distress syndrome. Mediators Inflamm. 2014;2014:625180–189. doi: 10.1155/2014/625180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu GJ, Shao CJ, Zhang Y, Wei YY, Xie WP, Kong H. Diagnostic and prognostic values of endothelial-cell-specific molecule-1 with malignant pleural effusions in patients with non-small cell lung cancer. Oncotarget. 2017;8(30):49217–49223. doi: 10.18632/oncotarget.17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HR, Kim BR, Park RK, Yoon KH, Jeong ET, Hwang KE. Diagnostic significance of measuring vascular endothelial growth factor for the differentiation between malignant and tuberculous pleural effusion. Tohoku J Exp Med. 2017;242(2):137–142. doi: 10.1620/tjem.242.137. [DOI] [PubMed] [Google Scholar]
- 33.Bingol Z, Kose M, Pıhtılı A, Akpınar T, Tukek T, Kıyan E. Serum endothelial cell specific molecule-1 (endocan) levels in patients with obstructive sleep apnea. Biomark Med. 2016;10(2):177–184. doi: 10.2217/bmm.15.117. [DOI] [PubMed] [Google Scholar]
- 34.Aciksari G, Kavas M, Atici A, et al. Endocan Levels and Endothelial Dysfunction in Patients With Sarcoidosis. Angiology. 2018;1:3319718775283. doi: 10.1177/0003319718775283. [DOI] [PubMed] [Google Scholar]
- 35.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 36.Kechagia M, Papassotiriou I, Gourgoulianis KI. Endocan and the respiratory system: a review. Int J Chron Obstruct Pulmon Dis. 2016;11:3179–3187. doi: 10.2147/COPD.S118692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. doi: 10.1016/j.rmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Stockley RA. Progression of chronic obstructive pulmonary disease: impact of inflammation, comorbidities and therapeutic intervention. Curr Med Res Opin. 2009;25(5):1235–1245. doi: 10.1185/03007990902868971. [DOI] [PubMed] [Google Scholar]


