Abstract
Transgenic mice overexpressing the type I isoform of neuregulin 1 (Nrg1; NRG1) have alterations in hippocampal gamma oscillations and an age-emergent deficit in hippocampus-dependent spatial working memory. Here, we examined the molecular and morphological correlates of these findings. Microarrays showed over 100 hippocampal transcripts differentially expressed in Nrg1tg-type I mice, with enrichment of genes related to neuromodulation and, in older mice, of genes involved in inflammation and immunity. Nrg1tg-type I mice had an enlarged hippocampus with a widened dentate gyrus. The results show that Nrg1 type I impacts on hippocampal gene expression and structure in a multifaceted and partly age-related way, complementing the evidence implicating Nrg1 signaling in aspects of hippocampal function. The findings are also relevant to the possible role of NRG1 signaling in the pathophysiology of schizophrenia or other disorders affecting this brain region.
Introduction
Neuregulin 1 (Nrg1; NRG1) is a growth factor, signaling via Erbb3 and Erbb4 receptor tyrosine kinases. Nrg1 plays diverse roles in the development, plasticity, and diseases of the nervous system1–5. Its pleiotropy arises, in part, from a family of structurally and functionally distinct isoforms (types I to VI), transcribed from different 5’ exons6. In humans, allelic variation can affect NRG1 isoform expression7–9, and polymorphisms in NRG1 may be a risk gene for schizophrenia10–12, although this has not been confirmed in genome-wide association studies13.
The type I isoform is affected in schizophrenia, with increased expression in hippocampus7 and prefrontal cortex14 compared with controls, and representing one of the abnormalities of NRG1-ErbB4 signaling observed in the disorder15–18. Reflecting the interest in the functional and pathological roles of NRG1 type I, a transgenic mouse selectively overexpressing this isoform (Nrg1tg-type I) was created19, and shown to have alterations in myelination20 and some aspects of behavior21, including an age-emergent deficit in hippocampus-dependent spatial working memory22. Nrg1tg-type I mice also exhibit a reduced frequency of carbachol-induced hippocampal gamma oscillations22. These findings complement a broad body of evidence linking Nrg1 to hippocampal function and plasticity23–28. Differing phenotypic profiles are seen in other genetic mouse models of Nrg1, highlighting the existence of isoform-specific properties29–34.
In the current study we examined two other aspects of the hippocampus in Nrg1tg-type I mice: its transcriptome, and its structure, in comparison with wildtype (wt) littermates. Because of the age-dependent features of the hippocampal phenotype observed previously, the transcriptomic analysis was performed at two ages.
Materials and methods
All experiments were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986, and had local ethical approval.
Generation and genotyping of Nrg1tg-type I mice
The generation and genotyping of the Nrg1tg-type I mice has been described19. The mice overexpress Nrg1 type I (β1a-isoform) under a Thy-1 promoter, with robust overexpression in multiple brain regions, including the hippocampus, with no alteration in Nrg1 types II or III20,21. The experiments reported here were performed in F6-F9 generations of backcross of heterozygous Nrg1tg-type I males with wt C57BL/6 J females, comparing Nrg1tg-type I mice with their wt littermates.
Microarrays and quantitative real-time PCR (RT-qPCR)
Two microarray experiments were performed, one in ‘young adult’ mice (2.5–4 months), the other in ‘old’ mice (14–15 months). Each comprised 24 animals, 6 of each genotype and sex.
RNA extraction and preparation for microarrays
Brains were frozen in isopentane cooled on dry ice. The left hemisphere was placed into RNAlater®-ICE Frozen Tissue Transition Solution (Ambion) at − 20 °C for 18 h, after which the hippocampus was dissected and homogenized in Qiazol in a TissueLyser (Qiagen). Total RNA was extracted and purified with RNeasy lipid tissue Mini kits (Qiagen) according to manufacturer’s protocol. In total, 300 ng RNA was used for amplification and labeling with Illumina® TotalPrep™ RNA Amplification Kit (Ambion). Complementary RNA (cRNA) quality was determined with an Agilent 2100 Bioanalyser (Agilent Technologies, Palo Alto, CA).
A total of 1.5 µg cRNA from each brain was hybridized to an Illumina Mouse WG-6 v1.1 (young mice) or v2 (old mice) chip according to manufacturers’ protocol and scanned with a BeadStation 500 machine.
Microarray analysis
Standard quality control measures were performed with the BeadStudio program (Illumina, CA), including subtracting the background from each array from the raw signal intensity of each probe type. The raw signal intensity data underwent variance stabilizing normalization, which is a generalized log2 transformation of the signal. A quantile standardization procedure was used to centralize the mean signal in the distribution (i.e., of all the probes) and to equalize the variance between mice. Further quality control was performed such as hierarchical clustering and box plots of normalized intensity (robust multichip average; RMA) values for each chip.
A linear model with an empirical Bayes t-statistic was fitted to the data to generate lists of significant effects of genotype, using the Limma program35. We corrected for multiple testing with the Benjamini–Hochberg false discovery rate to give an adjusted p value36. The criteria for differential expression were an adjusted p value < 0.05 and an absolute (unlogged RMA) fold change in expression (FC) > 1.5.
Differentially expressed probe lists were condensed into lists of differentially expressed transcripts, including known splice variants, identified with PubMed basic local alignment search tool (BLAST). Probes with no accession number were identified by nucleotide sequence with PubMed Nucleotide BLAST. Results from each array were compared with obtain lists of genes that were differentially expressed in NRG1tg-type I mice at both ages, or only at one of the two ages. These three lists of genes were used for further investigation with Ingenuity Pathways Analysis (IPA; Ingenuity Systems), which generated networks of up to 35 genes based on their known functional links37.
RT-qPCR
Hippocampal RNA was treated with 1 μl (1 unit) RQ1 RNase-free DNAse (Promega) and 0.6 μl (24 units) RNasin ribonuclease inhibitor (Promega) at 37 °C for 30 min and then heated to 72 °C for 10 min. DNAse-treated RNA was reverse-transcribed using 1 μl (200 units) MMLV reverse transcriptase, Promega), 1xMMLV buffer, 0.8 μl of 10 mM dNTPs, 0.6 μl (24 units) RNasin, 0.6 μl of 10 mM oligoDTs. The reaction mix was incubated at 42 °C for 1 h and then heated to 72 °C for 10 min. The reverse-transcribed RNA (complementary DNA; cDNA) was diluted with nuclease-free water and stored at − 80 °C.
TaqMan assays were ordered from Applied Biosystems (Npy, Mm00445771_m1; Gfap, Mm01253034_m1; Inhba, Mm00434339_m1; Cntfr, Mm00516697_m1; C1qa, Mm00432142_m1; Rbbp4, Mm00771401_g1). Bdnf was detected using pan-BDNF primers and a TaqMan probe (Forward, 5′-GGGTCACAG CGGCAGATAAA-3′, Reverse 5′-GCCTTTGGATACCGGGACTT-3′; Probe, TCTGGCGGGACGGTCACAGTCCTA)38. Bdnf v1-specific probes39 were: Forward, 5′-CACATTACCTTCCTGCATCTGTTG-3′, reverse 5′-ACCATAGTAAGGAAAAGGATGGTCAT-3′, probe AAGCCACAATGTTCCACCAG. The PCR reaction mix included 15 ng cDNA, 1xTaqMan Universal PCR Master Mix (Applied Biosystems) and the assay in a final volume (with nuclease-free water) of 15 μl in 384-well plates. Nrg1 mRNA was quantified using a SyBr green assay, with the primers designed so that the PCR product would span the exon boundary between the type I-specific exon and the immunoglobulin-like domain, and checked in the BLAST database for binding specificity (Forward, 5′-AAGGGGAAGGGCAAGAAGAA-3′, Reverse 5′-TCTTTCAATCTGGGAGGCAAT-3′; Eurogentech). The reaction mix for Nrg1 type I was 1xSyBr Green Mix, 15 ng cDNA, 200 nM of each primer and nuclease-free water up to a final volume of 15 μl.
Standard curves of pooled cDNA from all samples were set up in triplicate with the starting amount of cDNA ranging from 100 ng to 0.54 pg (Nrg1), 5 pg (Npy), 0.01 ng (Gfap), or 0.39 ng (Inhba, Cntfr, C1qa, Rbbp4). The R2 of all standard curves was > 0.99. All experimental samples fell within the standard curve. Samples were run in triplicate on the same plate as the standard curve. No-template controls and RT-negative controls were also run in triplicate to test for any contamination of the reaction mix or cDNA, respectively. Cycling conditions for all qRT-PCR reactions were 50 °C for 2 min, 95 °C for 10 min and then 40 repeats of 95 °C for 15 seconds to denature and 60 °C for 1 min.
Morphology and histology
Snap-frozen brains from 10-month-old wt and Nrg1tg-type I mice were coronally cryosectioned at 20 μm thickness. Hippocampal area was measured by point counting on multiple cresyl violet-stained sections throughout the dorsoventral extent, and hippocampal volume estimated using Cavalieri’s theorem as described40. Whole brain volume was estimated in the same way. We also measured the cross-sectional area of subfields (dentate gyrus, CA3, CA1) by manual tracing using a Nikon Eclipse 3600 microscope coupled to an MCID Elite image analysis system (Interfocus, Haverhill, UK). Other sections were immunostained for parvalbumin (PV27, 1:100; Swant, Switzerland), detected with diaminobenzidine, by standard methods. All measurements were made blind to genotype.
Results
The hippocampal transcriptome of Nrg1tg-type I mice: age-related effects on genes involved in myelination, neurotransmission, and immunity
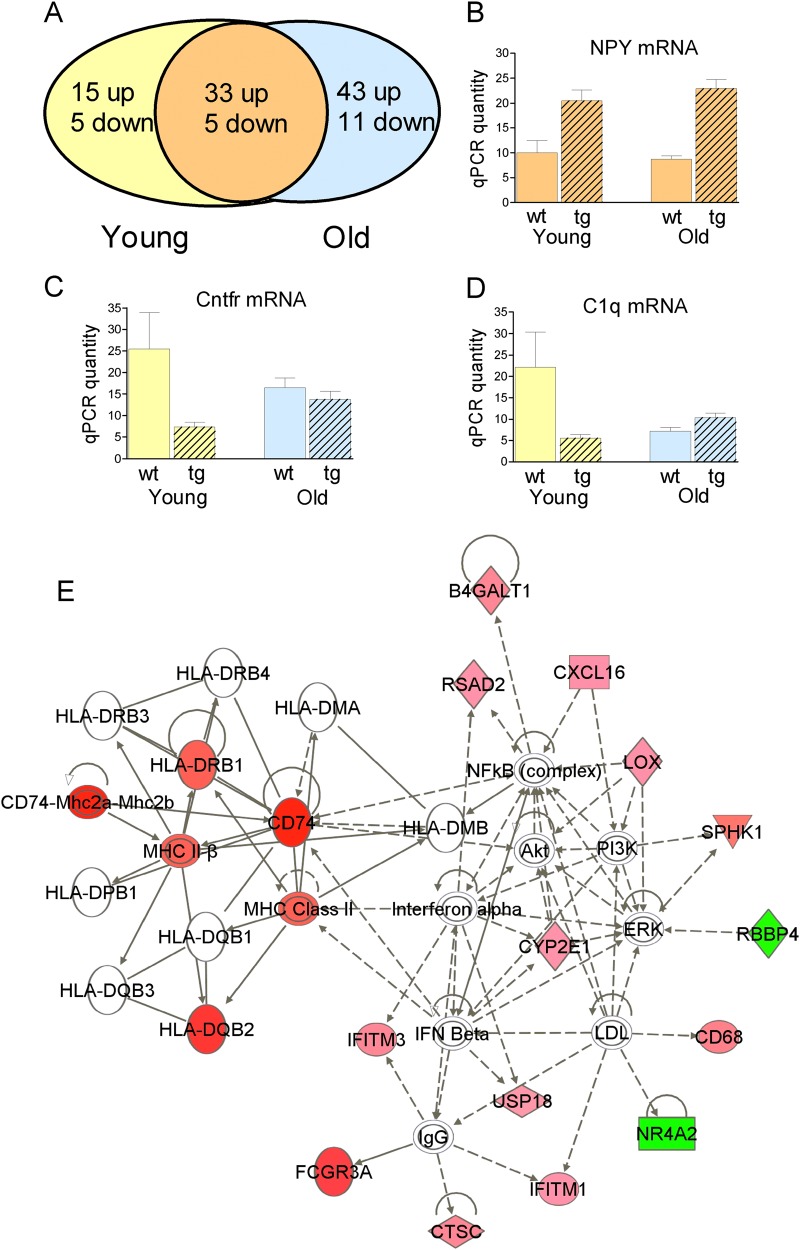
Nrg1 impacts upon the expression of many individual genes1,3. Here we used microarrays as an unbiased method to identify transcripts and networks altered in the hippocampus of Nrg1tg-type I mice. Adopting a stringent statistical approach, and with a 1.5-fold change threshold, we identified over 100 differentially expressed genes, of which ~ 80% were increased in Nrg1tg-type I mice compared with wt (Fig. 1a, and Supplementary Tables 1–3). Thirty-eight transcripts were altered in the same direction at both ages (Supplementary Table 1), 20 were differentially expressed only in the young adult (2.5–4 month) Nrg1tg-type I mice (Supplementary Table 2) and 54 only in old (14–15 month) Nrg1tg-type I mice (Supplementary Table 3). Eight transcripts were selected for qPCR validation, based on the microarray results as well as what was known about their functions and interactions with Nrg1 (Fig. 1b–d; and Table 1).
Fig. 1. Hippocampal gene expression in Nrg1tg-type I mice.
a Genes meeting the criteria described in text for differential expression in young (yellow) or old (blue) adult Nrg1tg-type I mice, or Nrg1tg-type I mice of both ages (orange). b–d examples of RT-qPCR validation of transcripts from each group. b Npy mRNA, increased in Nrg1tg-type I mice of both ages; c Cntfr mRNA, decreased in young but not old adult Nrg1tg-type I mice; d C1q mRNA, increased in old but not young adult Nrg1tg-type I mice. Statistics for the data shown in panels b–d are given in Table 1. e An IPA network of transcripts differentially expressed in old but not young adult Nrg1tg-type I mice. The network comprises nodes (genes) and their biological relationships shown by interconnecting lines. Red nodes are transcripts with increased expression, and the green nodes are transcripts with lower expression, in the old Nrg1tg-type I mice, compared with their age-matched wt controls. Increasing color intensity indicates a greater fold change. White nodes show genes that are functionally related to the other differentially expressed genes in the network and added by IPA. Solid lines between nodes indicate a direct interaction between them and dashed lines indicate indirect relationships. A continuous line denotes “binding only”; pointed line, “acts upon” and blunt ended line, “inhibits”. For gene symbols and names, see Supplementary Table 4. For additional IPA networks identified in one or both age groups of Nrg1tg-type I mice, see Supplementary Tables 1–3 and Supplementary Figures 1–4
Table 1.
Quantitative RT-PCR validation of differentially expressed genes in Nrg1tg-type I mice
| Young adult (2.5–4 months) | Old (4–15 months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microarray | qRT-PCR | Microarray | qRT-PCR | ||||||
| Accession | Gene | FC | p | FC | p | FC | p | FC | p |
| NRG1 type I | 8.60 | 2.62E-19 | 801 | 0.001 | NA | NA | 466 | 0.001 | |
| NM_023456.2 | NPY | 2.22 | 1.01E-05 | 2.0 | 0.005 | 2.46 | 9.93E-06 | 2.6 | 0.001 |
| NM_010277 | GFAP | 1.59 | 1.69E-03 | NC | NC | 1.62 | 1.42E-02 | 1.5 | 0.036 |
| NM_007540.3 | BDNF v1 | 1.65 | 4.12E-04 | 3.5 | 0.001 | 2.76 | 8.00E-07 | NC | NC |
| NM_008380.1 | Inhba | 2.28 | 4.83E-06 | NC | NC | NC | NC | 2.7 | 0.001 |
| NM_016673.1 | Cntfr | −1.86 | 5.66E-03 | −3.4 | 0.068 | NC | NC | NC | NC |
| NM_007572 | C1qa | NC | NC | NC | NC | 1.60 | 6.14E-04 | 1.4 | 0.032 |
| NM_009030 | Rbbp4 | NC | NC | NC | NC | −1.89 | 6.14E-04 | NC | NC |
NA: probe not present on array. NC: no significant change. The statistical approach to the microarray data is described in text; p values for qRT-PCR are from unpaired t tests (two-tailed) comparing transgenic and wt mice of each age group
Genes upregulated at both ages in the NRG1tg-type I mice included neuropeptide Y (Npy), brain-derived neurotrophic factor (Bdnf), and glial fibrillary acidic protein (Gfap). Consistent with the Npy mRNA data, Npy-immunoreactive hippocampal interneurons were markedly more prominent in the Nrg1tg-type I mice (not shown). The Bdnf mRNA increase affected the V1 isoform selectively. IPA generated two networks with scores corresponding to significance values of p = 10−31 and p = 10−25 (Supplementary Figs. 1 and 2). Network 1 included, as well as Npy and Bdnf, several transcripts involved in neurotransmission and implicated in schizophrenia, such as dopamine D1 and D4 receptors (Drd1 and Drd4).
Of the genes differentially expressed in young but not old Nrg1tg-type I mice, three were involved in myelination (myelin basic protein (Mbp), myelin oligodendrocyte glycoprotein (Mog), and myelin-associated oligodendrocytic basic protein). IPA generated a network that included Mog and Mbp (p = 10−35; Supplementary Fig. 3).
Genes overexpressed in old but not young Nrg1tg-type I mice (Supplementary Table 3) included many with immune and inflammatory functions, and IPA generated two highly significant networks (p = 10−37 and p = 10−34; one is shown in Fig. 1e, the other in Supplementary Fig. 4). In particular, the network shown in Fig. 1e includes many upregulated HLA and major histocompatibility complex (MHC) genes (Supplementary Table 4).
Nrg1tg-type I mice have altered hippocampal morphology
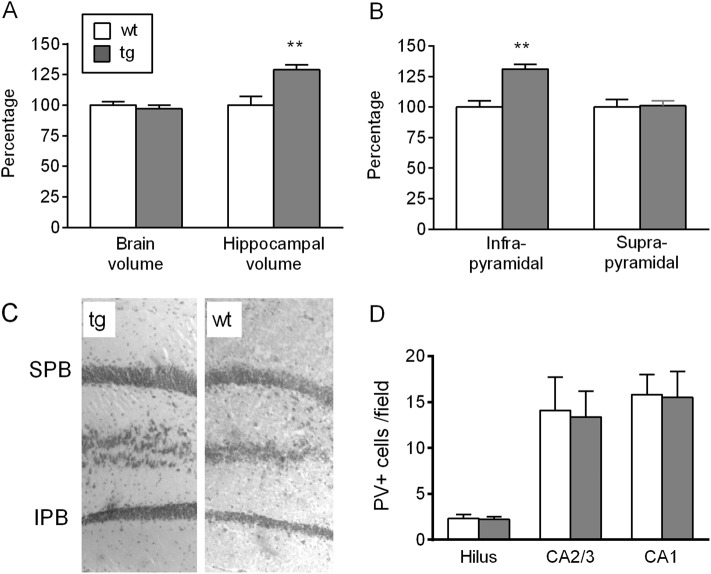
As shown in Fig. 2a, the hippocampus was enlarged (by ~ 25%) in Nrg1tg-type I mice, with brain volume unchanged. In terms of individual subfields, the only difference observed in Nrg1tg-type I mice was that the dentate gyrus granule cell layer was wider than in wt mice; this was primarily in the infra-pyramidal (external) blade rather than the supra-pyramidal (internal) blade (Fig. 2b, c). The density of PV + interneurons was unchanged in each subfield measured (Fig. 2d).
Fig. 2. Hippocampal morphology in Nrg1tg-type I mice.
a Hippocampal volume is increased in Nrg1tg-type I mice (n = 10) compared with wt (n = 9; two-tailed unpaired t test, t = 3.249, df = 17, p = 0.006), but whole brain volume is unchanged. b The width of the dentate gyrus granule cell layer is increased in Nrg1tg-type I mice in the infra-pyramidal blade (n = 6 in each group; two-tailed unpaired t test, t = 4.126, df = 10, p = 0.002) but not in the supra-pyramidal blade. c Illustration of the data in b, showing the wider infra-pyramidal blade (IPB) in a Nrg1tg-type I mouse compared with a wt mouse. The supra-pyramidal blade (SPB) is also shown, with CA3 in between. d The density of parvalbumin (PV)-immunoreactive cells did not differ in Nrg1tg-type I mice (n = 10) compared with wt (n = 12). Bars in a, b, and d show mean and standard deviation. All data in this Figure come from 10-month-old mice
Discussion
Mice selectively overexpressing the type I isoform of Nrg1 show differences in hippocampal function and age-emergent deficits in hippocampus-dependent behavior22. Here, we report that these changes are accompanied by an altered profile of gene expression which differs between young adult (2.5–4 month) and old (14–15 month) Nrg1tg-type I mice, and by an increase in hippocampal volume.
The hippocampal molecular and morphological profile of Nrg1tg-type I mice
Nrg1tg-type I mice exhibited differential expression of a number of genes (Fig. 1; Table 1; Supplementary Figures 1–4; Supplementary Tables 1–3). Of the genes overexpressed in Nrg1tg-type I mice at both ages, several are noteworthy. In particular, five transcripts (Npy, Gfap, Bdnf, Drd1, and Drd4) were part of the most significant gene network and all had been linked previously to Nrg1. The fact they were upregulated in both age cohorts, which were studied separately and with different versions of the microarray chip, strengthens the robustness of the findings.
Npy is expressed by a subpopulation of hippocampal interneurons, bistratified and ivy cells, which impose a strong inhibitory influence on pyramidal cell dendrites41,42. Its marked upregulation in the Nrg1tg-type I mice (Fig. 1b) is of interest for several reasons. First, it provides another hint that interneurons are affected and may contribute to the oscillatory and circuitry alterations that underlie the phenotype of the mice41–44. Second, Npy is anti-epileptic45–47, and enhanced Npy expression may help prevent the epileptiform predisposition of Nrg1tg-type I mice22 progressing to overt seizure activity. Third, the Npy mRNA elevation may be related to the morphological finding of a widened dentate gyrus. Preliminary data show an increase of hilar cells immunoreactive for doublecortin, a marker of newly formed neurons, in the Nrg1tg-type I mice (I.H.D. and P.J.H., unpublished observations), suggesting that the enlarged dentate gyrus might reflect increased adult neurogenesis—a process stimulated by Npy48–50, and influenced by Nrg151. Whether the persisting upregulation of Gfap mRNA in Nrg1tg-type I mice (Table 1) is also indicative of enhanced cell proliferation—as many neural precursors express Gfap52—remains to be seen; it might also be a remnant of the developmental role of Nrg1-ErbB signaling in neuron–astrocyte differentiation53,54. Bdnf is a regulator of hippocampal plasticity and function55, and its elevated expression may have many manifestations in the Nrg1tg-type I mice, including a contribution to their spatial working memory deficit22,56. Finally, the upregulation of Drd1 and Drd4 mRNAs complements evidence that hippocampal Drd4 mediates Nrg1-induced reversal of LTP24, and that Nrg1 application produces acute57 and sustained58 increases in dopamine release and dopamine neuron firing59. Another Nrg genetic mouse model also shows dopamine receptor alterations60.
The gene expression differences that occurred in the old but not young Nrg1tg-type I were striking, comprising many immune and inflammatory genes, such as HLA-DR, MHC class II CD74, and complement C1q. Similar changes have been reported in old vs. young wt rodents and in this respect there may be an ‘accelerated aging’ phenotype in Nrg1tg-type I mice61–64. C1q, like other complement factors, is also involved in neuronal and synaptic function65–67 and dysfunction68–70 and brain aging71. As such, the altered expression of the genes in the older Nrg1tg-type I mice may represent molecular correlates of, and might contribute to, their age-emergent memory impairment.
Fewer genes were differentially expressed in the young but not old Nrg1tg-type I mice, and they were primarily myelin-related. This is not unexpected, in that Nrg1 is a critical player in myelination72,73. Although the type I isoform has hitherto been implicated primarily in peripheral myelination19,74,75, these mice do show hypermyelination of small diameter axons in the central nervous system20. The upregulation of myelin-related transcripts in the Nrg1tg-type I mice may be a molecular indication that this process is also occurring in the hippocampus; any resulting hypermyelination may in turn contribute to the hippocampal volume increase. However, this remains speculative; indeed, more generally, the processes that link the morphological and molecular alterations reported here remain unknown.
The altered transcriptomic profile of the Nrg1tg-type I mice highlights an issue that pertains broadly to genetically modified animals: their phenotypes need not arise solely from the targeted gene(s) but also from the cascade of molecular changes which the manipulation induces. Moreover, these effects are not static but vary with age, and illustrate the value of going beyond the 3–6 month time-point at which characterization is often completed, even though capturing the temporal dynamic and longitudinal profile is demanding of resources and time.
Implications for NRG1 in schizophrenia
The evidence mentioned earlier showing Nrg1-dopamine interactions, and the increased D1 and D4 receptor expression seen here is notable, given that dopaminergic abnormalities are a final common pathway in schizophrenia pathophysiology76, and alterations in both receptors have been reported in the disorder77,78. The MHC complex is strongly implicated in schizophrenia79, in part through the complement C4 gene80, and several81–84 though not all85 studies report elevated expression of many immune and inflammatory genes, including C1q, especially in older, chronically ill patients86, reminiscent of the gene expression changes being restricted to the old Nrg1tg-type I mice. There is also some evidence for direct links between NRG1, immune function, and schizophrenia87–89.
However, there is less congruence when other findings are considered. Thus, in schizophrenia, in contrast to findings in the Nrg1tg-type I mice, hippocampal myelin-related transcripts are decreased90, hippocampal volume is unchanged or reduced91, and there is a lower density of hippocampal PV + neurons92. Thus, although the phenotype of Nrg1tg-type I mice may be seen as overlapping to an extent with that of schizophrenia, the differences are at least as striking as the similarities. One specific factor to consider is that the magnitude of overexpression in the Nrg1tg-type I mouse is far greater than the increased NRG1 type I expression reported in schizophrenia. More generally, these considerations draw attention to the need for cautious interpretation and extrapolation from any genetic mouse model to the human syndrome. Nevertheless, the results extend the evidence that Nrg1 type I is functional in the hippocampus, and hence may play a role in any disease in which its expression in this brain region is altered.
Electronic supplementary material
Acknowledgements
We thank P. Burnet, L. Chen, S. Eastwood, J. Flint K. Lamsa, H. Lockstone, W. Nissen, and J. Taylor for their contributions. I.H.D. was supported by a Wellcome Trust doctoral studentship. A.J.L. was a Medical Research Council (UK) Career Development Fellow. K.L. was a Wellcome Trust Career Development Fellow. Additional support from Stanley Medical Research Institute and Medical Research Council (UK) grants to P.J.H. M.H.S. holds a Heisenberg Fellowship from the Deutsche Forschungsgemeinschaft (DFG) and acknowleges funding by a DFG research grant (SCHW741/4-1).
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41398-018-0288-2).
References
- 1.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat. Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 2.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol. Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res. Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83:27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinthorsdottir V, et al. Multiple novel transcription initiation sites for NRG1. Gene. 2004;342:97–105. doi: 10.1016/j.gene.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Law AJ, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5’ SNPs associated with the disease. Proc. Natl. Acad. Sci. USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicodemus KK, et al. A 5 ‘promoter region SNP in NRG1 is associated with schizophrenia risk and type III isoform expression. Mol. Psychiatry. 2009;14:741–743. doi: 10.1038/mp.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weickert CS, Tiwari Y, Schofield PR, Mowry BJ, Fullerton JM. Schizophrenia-associated HapICE haplotype is associated with increased NRG1 type III expression and high nucleotide diversity. Transl. Psychiatry. 2012;2:e104. doi: 10.1038/tp.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mostaid MS, et al. Neuregulin-1 and schizophrenia in the genome-wide association study era. Neurosci. Biobehav Rev. 2016;68:387–409. doi: 10.1016/j.neubiorev.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Mostaid MS, et al. Elevated peripheral expression of neuregulin-1 (NRG1) mRNA isoforms in clozapine-treated schizophrenia patients. Transl. Psychiatry. 2017;7:1280. doi: 10.1038/s41398-017-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagannath V, Gerstenberg M, Correll CU, Walitza S, Grünblatt E. A systematic meta-analysis of the association of Neuregulin 1 (NRG1), D-amino acid oxidase (DAO), and DAO activator (DAOA)/G72 polymorphisms with schizophrenia. J. Neural Transm. (Vienna) 2018;125:89–102. doi: 10.1007/s00702-017-1782-z. [DOI] [PubMed] [Google Scholar]
- 13.Farrell MS, et al. Evaluating historical candidate genes for schizophrenia. Mol. Psychiatry. 2015;20:555–562. doi: 10.1038/mp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto R, et al. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol. Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- 15.Hahn CG, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat. Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 16.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum. Mol. Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 17.Law AJ, et al. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110δ inhibition as a potential therapeutic strategy. Proc. Natl. Acad. Sci. USA. 2012;109:12165–12170. doi: 10.1073/pnas.1206118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parlapani E, et al. Gene expression of neuregulin-1 isoforms in different brain regions of elderly schizophrenia patients. World J. Biol. Psychiatry. 2010;11:243–250. doi: 10.3109/15622970802022376. [DOI] [PubMed] [Google Scholar]
- 19.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 20.Brinkmann BG, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deakin IH, et al. Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport. 2009;20:1523–1528. doi: 10.1097/WNR.0b013e328330f6e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deakin IH, et al. Transgenic overexpression of the type I isoform of neuregulin 1 affects working memory and hippocampal oscillations but not long-term potentiation. Cereb. Cortex. 2012;22:1520–1529. doi: 10.1093/cercor/bhr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J. Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon OB, et al. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc. Natl. Acad. Sci. USA. 2008;105:15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neddens J, Vullhorst D, Paredes D, Buonanno A. Neuregulin links dopaminergic and glutamatergic neurotransmission to control hippocampal synaptic plasticity. Commun. Integr. Biol. 2009;2:261–264. doi: 10.4161/cib.2.3.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nason MW, Jr, Adhikari A, Bozinoski M, Gordon JA, Role LW. Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology. 2011;36:488–496. doi: 10.1038/npp.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura H, Kawata M, Hamaguchi S, Ishikawa Y, Shiosaka S. Processing of neuregulin-1 by neuropsin regulates GABAergic neuron to control neural plasticity of the mouse hippocampus. J. Neurosci. 2012;32:12657–12672. doi: 10.1523/JNEUROSCI.2542-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawata M, Morikawa S, Shiosaka S, Tamura H. Ablation of neuropsin-neuregulin 1 signaling imbalances ErbB4 inhibitory networks and disrupts hippocampal gamma oscillation. Transl. Psychiatry. 2017;7:e1052. doi: 10.1038/tp.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Tuathaigh CMP, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 30.Ehrlichman RS, et al. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Res. 2009;1294:116–127. doi: 10.1016/j.brainres.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy L, Cappas E, Lai D, Boucher AA, Karl T. Cognition in transmembrane domaine neuregulin 1 mutant mice. Neurosci. 2010;170:800–807. doi: 10.1016/j.neuroscience.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 32.Yin DM, et al. Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron. 2013;78:644–657. doi: 10.1016/j.neuron.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo X, He W, Hu X, Yan R. Reversible overexpression of bace1-cleaved neuregulin-1 N-terminal fragment induces schizophrenia-like phenotypes in mice. Biol. Psychiatry. 2014;76:120–127. doi: 10.1016/j.biopsych.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papaleo F, et al. Behavioral, neurophysiological, and synaptic impairment in a transgenic neuregulin1 (NRG1-IV) murine schizophrenia model. J. Neurosci. 2016;36:4859–4875. doi: 10.1523/JNEUROSCI.4632-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth GK. Limma: Linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- 37.Calvano SE, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 38.Burbach GJ, Dehn D, Del Turco D, Deller T. Quantification of layer-specific gene expression in the hippocampus: effective use of laser microdissection in combination with quantitative RT-PCR. J. Neurosci. Methods. 2003;131:83–91. doi: 10.1016/S0165-0270(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 39.Liu QR, et al. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Walker MA, et al. Estimated neuronal populations and volumes of the hippocampus and its subfields in schizophrenia. Am. J. Psychiatry. 2002;159:821–828. doi: 10.1176/appi.ajp.159.5.821. [DOI] [PubMed] [Google Scholar]
- 41.Klausberger T, et al. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat. Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- 42.Fuentealba P, et al. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J. Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulyas AI, et al. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J. Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y reduces orthodromically evoked population spike in rat hippocampal CA1 by a possibly presynaptic mechanism. Brain Res. 1985;346:404–408. doi: 10.1016/0006-8993(85)90880-7. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen AT, et al. Hippocampal NPY gene transfer attenuates seizures without affecting epilepsy-induced impairment of LTP. Exp. Neurol. 2009;215:328–333. doi: 10.1016/j.expneurol.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gøtzsche CR, et al. Combined gene overexpression of neuropeptide Y and its receptor Y5 in the hippocampus suppresses seizures. Neurobiol. Dis. 2012;45:288–296. doi: 10.1016/j.nbd.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Gray WP. Neuropeptide Y signaling on hippocampal stem cells in health and disease. Mol. Cell Endocrinol. 2008;288:52–62. doi: 10.1016/j.mce.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 49.Decressac M, et al. Neuropeptide Y stimulates proliferation, migration and differentiation of neural precursors from the subventricular zone in adult mice. Neurobiol. Dis. 2009;34:441–449. doi: 10.1016/j.nbd.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Geloso MC, Corvino V, Di Maria V, Marchese E, Michetti F. Cellular targets for neuropeptide Y-mediated control of adult neurogenesis. Front. Cell Neurosci. 2015;9:85. doi: 10.3389/fncel.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghashghaei HT, et al. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc. Natl. Acad. Sci. USA. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmid RS, et al. Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc. Natl. Acad. Sci. USA. 2003;100:4251–4256. doi: 10.1073/pnas.0630496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 55.Waterhouse EG, Xu BJ. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunha C, et al. Brain-derived neurotrophic factor (BDNF) overexpression in the forebrain results in learning and memory impairments. Neurobiol. Dis. 2009;33:358–368. doi: 10.1016/j.nbd.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Yurek DM, Zhang L, Fletcher-Turner A, Seroogy KB. Supranigral injection of neuregulin1-[beta] induces striatal dopamine overflow. Brain Res. 2004;1028:116–119. doi: 10.1016/j.brainres.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 58.Kato T, et al. Transient exposure of neonatal mice to neuregulin-1 results in hyperdopaminergic states in adulthood: implication in neurodevelopment hypothesis for schizophrenia. Mol. Psychiatry. 2011;16:307–320. doi: 10.1038/mp.2010.10. [DOI] [PubMed] [Google Scholar]
- 59.Namba H, Okubo T, Nawa H. Perinatal exposure to neuregulin-1 results in disinhibition of adult midbrain dopaminergic neurons: implication in schizophrenia modeling. Sci. Rep. 2016;6:22606. doi: 10.1038/srep22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newell KA, Karl T, Huang XF. A neuregulin 1 transmembrane domain mutation causes imbalanced glutamatergic and dopaminergic receptor expression in mice. Neuroscience. 2013;248:670–680. doi: 10.1016/j.neuroscience.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 61.Verbitsky M, et al. Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice. Learn Mem. 2004;11:253–260. doi: 10.1101/lm.68204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frank MG, et al. mRNA upregulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol. Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 63.Kadish I, et al. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J. Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pawlowski TL, et al. Hippocampal gene expression changes during age-related cognitive decline. Brain Res. 2009;1256:101–110. doi: 10.1016/j.brainres.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 65.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 66.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Di Filippo M, Sarchielli P, Picconi B, Calabresi P. Neuroinflammation and synaptic plasticity: theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol. Sci. 2008;29:402–412. doi: 10.1016/j.tips.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Chu YX, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl. Acad. Sci. USA. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michailidou I, et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann. Neurol. 2015;77:1007–1026. doi: 10.1002/ana.24398. [DOI] [PubMed] [Google Scholar]
- 71.Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 72.Chen S, et al. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J. Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krishnan A. Neuregulin-1 type I: a hidden power within Schwann cells for triggering peripheral nerve remyelination. Sci. Signal. 2013;6:270. doi: 10.1126/scisignal.2003945. [DOI] [PubMed] [Google Scholar]
- 76.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr. Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harrison PJ. Neurochemical alterations in schizophrenia affecting the putative receptor targets of atypical antipsychotics: Focus on dopamine (D1, D3, D4) and 5-HT2A receptors. Br. J. Psychiatry. 1999;174:12–22. doi: 10.1192/S0007125000298073. [DOI] [PubMed] [Google Scholar]
- 78.Abi-Dargham A, et al. Increased prefrontal cortical D-1 receptors in drug-naïve patients with schizophrenia: a PET study with [C-11]NNC112. J. Psychopharmacol. 2012;26:794–805. doi: 10.1177/0269881111409265. [DOI] [PubMed] [Google Scholar]
- 79.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sekar A, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol. Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saetre P, et al. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim S, Hwang Y, Webster MJ, Lee D. Differential activation of immune/inflammatory response-related co-expression modules in the hippocampus across the major psychiatric disorders. Mol. Psychiatry. 2016;21:376–385. doi: 10.1038/mp.2015.79. [DOI] [PubMed] [Google Scholar]
- 84.Sanders AR, et al. Transcriptome sequencing study implicates immune-related genes differentially expressed in schizophrenia: new data and a meta-analysis. Transl. Psychiatry. 2017;7:e1093. doi: 10.1038/tp.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birnbaum R, et al. Investigating the immunogenetic architecture of schizophrenia. Mol. Psychiatry. 2017;23:1251–1260. doi: 10.1038/mp.2017.89. [DOI] [PubMed] [Google Scholar]
- 86.Narayan S, et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008;1239:235–248. doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanakry CG, Li Z, Nakai Y, Sei Y, Weinberger DR. Neuregulin-1 regulates cell adhesion via an ErbB2/phosphoinositide-3-kinase/Akt-dependent pathway: potential implications for schizophrenia and cancer. PLoS ONE. 2007;2:e1369. doi: 10.1371/journal.pone.0001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marballi K, et al. In vivo and in vitro genetic evidence of involvement of neuregulin 1 in immune system dysregulation. J. Mol. Med. 2010;88:1133–1141. doi: 10.1007/s00109-010-0653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Leary C, et al. Phenotypic effects of maternal immune activation and early postnatal milieu in mice mutant for the schizophrenia risk gene neuregulin-1. Neuroscience. 2014;277:294–305. doi: 10.1016/j.neuroscience.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 90.Dracheva S, et al. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol. Dis. 2006;21:531–540. doi: 10.1016/j.nbd.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 92.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr. Res. 2002;55:1–10. doi: 10.1016/S0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.