Abstract
Group decision-making is required in early life in educational settings and central to a well-functioning society. However, there is little research on group decision-making in adolescence, despite the significant neuro-cognitive changes during this period. Researchers have studied adolescent decision-making in ‘static’ social contexts, such as risk-taking in the presence of peers, and largely deemed adolescent decision-making ‘sub-optimal’. It is not clear whether these findings generalise to more dynamic social contexts, such as the discussions required to reach a group decision. Here we test the optimality of group decision-making at different stages of adolescence. Pairs of male pre-to-early adolescents (8 to 13 years of age) and mid-to-late adolescents (14 to 17 years of age) together performed a low-level, perceptual decision-making task. Whenever their individual decisions differed, they were required to negotiate a joint decision. While there were developmental differences in individual performance, the joint performance of both adolescent groups was at adult levels (data obtained from a previous study). Both adolescent groups achieved a level of joint performance expected under optimal integration of their individual information into a joint decision. Young adolescents’ joint, but not individual, performance deteriorated over time. The results are consistent with recent findings attesting to the competencies, rather than the shortcomings, of adolescent social behaviour.
Introduction
Adolescence, spanning the second decade of life, is a period of profound physical and psychological development. It is characterised by continued brain maturation through synaptic pruning and myelination1,2, in particular of the prefrontal cortex, which is involved in higher-order functions such as decision-making, cognitive control and social cognition3–6. Indeed, the protracted development of prefrontal cortex has been linked to a range of ‘sub-optimal’ behaviours in adolescence, such as impaired monitoring of task performance and excessive risk-taking, especially in social contexts4. Surprisingly, there is little research on how the profound neuro-cognitive changes associated with adolescence affect the ability of adolescents to make decisions as part of a group. Group decision-making is required in early life in educational settings and, more generally, is central to a functioning society.
Here we report on a study which tested the optimality of group decision-making at different stages of adolescence using a well-established psychophysical paradigm7. While the choice problem in this paradigm is simple, the task captures important aspects of real-world social interaction: participants must resolve a disagreement with a same-aged friend through free, unconstrained verbal communication. Disagreements with peers over uncertain information play a central role in adolescents’ everyday life8. In addition, integrating and weighing social information is an important developmental task in adolescence, especially in the context of conflict resolution9,10.
Decision-making and Cognition in Adolescence
Research on social decision-making in adolescence has broadly pursued three avenues: (i) individual learning in social contexts (i.e., “collaborative learning”; e.g.11,12), (ii) decision-making, and in particular risk-taking, in the presence of peers (e.g.4,13–15); and (iii) social negotiations and exchanges, often in the context of economic games (e.g.16–18).
Research on collaborative learning has largely focused on educational contexts and individual learning outcomes (e.g.12,19). There is converging evidence that working as part of a group can boost individual learning20,21, with key factors being exchange of ideas through communication22,23, the ability of other group members24 and the type of task25. However, it remains unknown whether these individual benefits translate into benefits for the group as a whole.
Research on social decision-making has mainly been concerned with peer influence. Peers have a particularly salient, motivating effect on adolescent behaviour compared to other age groups. For example, peers are thought to increase adolescents’ proneness to engage in risky behaviours (e.g., risky behaviour in driving simulation games15). Recent studies have extended this work to suggest that the direction of these effects depend on the characteristics of the peer26–28 and the type of risk context (i.e., ambiguous vs known risk29). It has been proposed that increased susceptibility to peer influence is at least in part adaptive, resulting in important exploratory behaviour and formation of social networks outside of the family29,30. However, in the context of group decision-making, such increased susceptibility to peer influence may prevent a group from assigning appropriate weights to the opinions of its members31.
Research on social negotiations and exchanges has largely been concerned with economic behaviours. For instance, Guroglu et al.32 found that investment decisions in 8- to 18-year-olds in a version of the Ultimatum Game were increasingly modulated by the perceived tendency of another peer to reject a selfish offer. Burnett-Heyes et al.16 found developmental differences between mid- and late-adolescents in resource allocation: older adolescents were more likely to consider peers’ reciprocated feelings of friendship when deciding how to allocate resources in a modified Dictator Game. In the context of group decision-making, the protracted development of fairness and reciprocity norms may result in overly egocentric behaviour and discounting of others’ opinions in adolescence.
In addition to social decision-making, there is also a body of research concerned with cognitive functions, which are relevant to decision-making and social interaction more generally. For instance, using the same basic perceptual task as we use here, Weil et al.33 found that the ability to accurately monitor and report on one’s task performance – an ability known as metacognition – continues to develop in adolescence and only levels off toward adulthood. Similarly, there is evidence that cognitive and affective aspects of social cognition continue to develop in adolescence34,35.
Overall, research on decision-making and cognition in adolescence indicates that, in certain domains such as high-risk domains, social context has unique effects on adolescent behaviour. Additionally, with increasing age, as social-cognitive skills are refined, social computations increase in sophistication, affecting how youth relate to peers. To our knowledge, no study has yet examined the optimality of group decision-making in adolescence. Critically, it is not clear whether the findings obtained in more ‘static’ social contexts, such as the manipulation of peer presence, extend to more ‘dynamic’ social contexts, such as the discussion preceding a group decision. Additionally, no study has used a paradigm that can isolate developmental differences at the group level over and above developmental differences at the individual level – crucial when testing hypothesis about group-level developmental effects.
Current Study
We used a psychophysical task to study group decision-making in male pre-to-early adolescents (8- to 13-year-olds) and mid-to-late adolescents (14- to 17-year-olds). On each trial, pairs of participants viewed two brief displays serially, one of them containing a faint target. Participants privately indicated which display they thought contained the target. In the case of agreement (i.e., they privately selected the same display), they received feedback about choice accuracy and continued to the next trial. In the case of disagreement (i.e., they privately selected different displays), they were first required to reach a group decision. In contrast to commonly used social tasks based on abstract social scenarios (e.g., economic games or interactions with virtual agents), the paradigm involves free social interaction with a close peer and closely resembles a common type of everyday social experience, such as when two friends discuss whether a ball crossed the line in a football match. As the paradigm allows for standardisation of joint performance by individual performance, it is particularly suitable for studying group decision-making across development.
Bahrami et al.7 found that a Weighted Confidence Sharing (WCS) model provided the best fit to adult data collected on the above task. According to the WCS model, to resolve disagreement, participants communicate the level of confidence in their respective opinions and then weight each opinion by the communicated confidence. The model assumes that participants can accurately estimate the reliability of their private opinion and accurately communicate this information – a set of skills encompassing metacognition36 and social cognition37,38 – and thus provides an upper bound on joint performance. Critically, the ‘optimality’ measure derived from the WCS model takes into account individual performance and therefore provides a measure of joint performance that is unbiased by low-level developmental differences. What do we expect in adolescence? Given the protracted development of decision-making, social cognition and metacognition, we predicted joint performance to be lower and less optimal in the younger than in the older adolescent group – and that neither age group would reach an adult level of joint performance as estimated from the dataset collected by Bahrami et al.7.
Methods
Sample
We recruited seventy-four adolescent participants in pairs (37 dyads in total) via adverts in the local community. The members of each dyad were friends with one another. We recruited two age groups: 40 pre-to-early adolescents (20 dyads; mean age ± SD = 11.0 ± 1.11; age range: 8.5–12.6 years; school years: 5–8) and 34 mid-to-late adolescents (17 dyads; mean age ± SD = 15.5 ± 0.79; age range: 14.1–16.8 years; school years 9–11). All participants were healthy males (for consistency with Bahrami et al.7) with normal or corrected-to-normal vision. Participants were paid £20 for participation. We obtained written assent from participants and written informed consent from legal guardians. The University of Oxford Central University Research Ethics Committee (CUREC) approved the study (Title: Collective decision-making, Protocol Number: MSD/IDREC/C1/2012/16). The study was performed in accordance with all relevant guidelines and regulations. An adult dataset (14 dyads; mean age ± SD = 29.6 ± 7.7; age range: 18.3–50.2 years) was obtained from Experiment 1 in Bahrami et al.7 where participants also were recruited in pairs of friends.
Task
Participants performed a two-interval forced-choice contrast-discrimination task as part of a dyad (see Fig. 1 for a schematic of an experimental trial). Participants sat at right angles to each other in a dark room, with their own monitor and response device (keyboard or mouse). On each trial, participants were presented with two consecutive viewing displays, each containing six vertically oriented Gabor patches. In one of the two displays, the contrast level of one of the six Gabor patches (the target) was increased by adding one of four values (0.075, 0.15, 0.20, 0.30) to its baseline contrast (0.15). We chose the values for the current study based on four pilot adolescent participants. The values differ from those used for the adult data (baseline contrast: 0.10; added contrast: 0.015, 0.035, 0.07, 0.15), but we note that our key measures of joint performance are normalised relative to individual performance and thus comparable across datasets. Target location and display was randomized across trials and experimental sessions, contrast values were counterbalanced across trials such that each appeared equal number of times. Participants viewed identical visual stimuli on each trial.
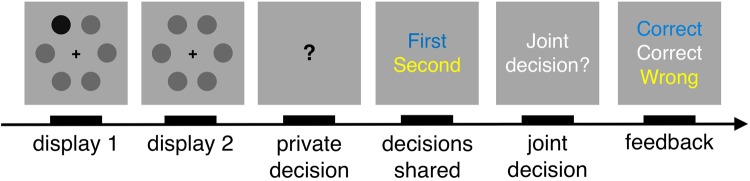
Figure 1.
Schematic of experimental procedure. On each trial, participants viewed two consecutive displays, each containing six contrast gratings (here shown as dots). There was a target with higher contrast (here the darker dot) in one of the two displays. Participants privately made a decision about which display they thought contained the target. The private responses were shared. In the case of disagreement, participants were required to make a joint decision; they took turns at indicating the joint decision. Participants received feedback about the accuracy of the individual and joint decisions, before continuing to the next trial. In the case of agreement, participants proceeded immediately to feedback. Individual responses (blue and yellow) and joint responses (white) were identified by colours.
Trials were initiated by the participant with the keyboard after consulting with their partner. A black central fixation cross (width: 0.75 degrees visual angle) appeared on the screen for a variable period (500–1000 ms). After the stimulus presentation, separated by a blank display lasting 1000 ms, participants were asked to indicate which display they thought contained the target, without discussing their answers. A question mark prompt after the second display signalled to the participants to respond. The question mark remained on the screen until both participants had made a response. Once both of them had responded, the individual decisions were made public (keyboard: blue; mouse: yellow). In the case of agreement (i.e., they privately selected the same display), they received feedback (see below) and continued to the next trial. In the case of disagreement (i.e., they privately selected different displays), they were asked to agree on a joint decision through verbal discussion. Participants were free to discuss the joint decision as long as they wanted. Participants took turns at indicating the joint decision (keyboard: even trials; mouse: odd trials). Once the joint response had been made, they received feedback about the accuracy of each decision (keyboard: blue; mouse: yellow; joint: white) and continued to the next trial.
Participants first completed a practice block of 16 trials. They then performed two experimental sessions, each consisting of 8 blocks of 16 trials (128 trials in each session and 256 trials in total). The two sessions were separated by a short break (5–10 mins). Participants swapped response devices between the two sessions. See Bahrami et al.7 for details about display parameters, response mode and stimulus presentation.
Procedure
We introduced the task as a picture-book game akin to Where’s Wally, replacing the Gabor patches with cartoon figures. As in the main task, in one of the two displays, one of the cartoon figures had a higher level of contrast. We ensured that participants had understood the basic premise of the task (i.e., to respond whether the target was in the first or the second display), before introducing the Gabor patches. Participants were told that the task was about teamwork and they were encouraged to try to make as many correct joint decisions as possible. An experimenter was present throughout the entire study to ensure that all instructions were observed. Parents were not present during the task. The study lasted about two hours including breaks.
Measures
Individual performance
Accuracy: We calculated accuracy as fraction of correct individual decisions.
Reaction time: Reaction time was calculated as seconds taken to make a decision.
Sensitivity: To estimate sensitivity for each dyad member, we first plotted the proportion of trials on which the target was reported to be in the second display against the difference in contrast between the second and the first display at the target location. The data points were then fit with a cumulative Gaussian function whose parameters were bias, b, and variance, σ2 – the parameters were estimated using a probit regression model as implemented by MATLAB’s (Mathworks Inc.) glmfit function. A participant with bias b and variance σ2 would have a psychometric curve, denoted P(Δc), where Δc is the contrast difference between the second and first display at the target location, given by
where H(z) is the cumulative normal function
The psychometric curve, P(Δc), tells us how the probability of reporting that the target is in the second display changes with contrast difference. Given the above definition, the variance in responses is related to the maximum slope of the psychometric curve, denoted S, via
A steep slope indicates small variance and thus highly sensitive performance. In contrast to accuracy, sensitivity provides a bias-free measure of performance.
Joint performance
Joint accuracy: We calculated joint accuracy as fraction of correct joint decisions (both agreement and disagreement trials).
Joint reaction time: We calculated joint reaction time as seconds taken to reach a joint decision.
Egocentric bias: To quantify ‘egocentric bias’, we computed the proportion of joint-decision trials in which a dyad member indicated the joint decision and the joint decision was the same as that made by the dyad member39.
Similarity: We computed the similarity of dyad members’ sensitivities as the ratio of the sensitivity of the worse dyad member to that of the better dyad member, Smin/Smax, with values near zero corresponding to dyad members with very different sensitivities and values near one corresponding to dyad members of nearly equal sensitivity.
Joint sensitivity: Joint sensitivity was quantified using the same procedure as for individual sensitivity but this time relating joint responses to the stimulus.
Collective benefit: We computed the collective benefit accrued by a dyad as the ratio of the sensitivity of the dyad to that of the more sensitive dyad member, Sdyad/Smax, with values below 1 indicating a collective loss and values above 1 indicating a collective benefit.
Optimality: We estimated the ‘optimality’ of joint performance using the Weighted Confidence Sharing (WCS) model developed by Bahrami et al.7. The model assumes that participants on each trial can accurately estimate and communicate the reliability of their respective individual decisions – with the joint decision reached by weighting the individual decisions by the communicated reliabilities. The upper boundary on joint performance can thus be defined as
where s1 and s2 are the individual sensitivities calculated as above. We computed our optimality index as the ratio of the dyad’s sensitivity to that predicted by the WCS model, Sdyad/SWCS, with values above 1 indicating that the dyad is ‘supra-optimal’ and values below 1 indicating that the dyad is ‘sub-optimal’. See Bahrami et al.7 for mathematical details.
Data exclusion
We excluded dyads where one or both of the members performed at 55% accuracy or lower and/or had a negative sensitivity (18 pre-to-early adolescent and 16 mid-to-late adolescent dyads remaining after data exclusion).
Results
Individual performance
Accuracy
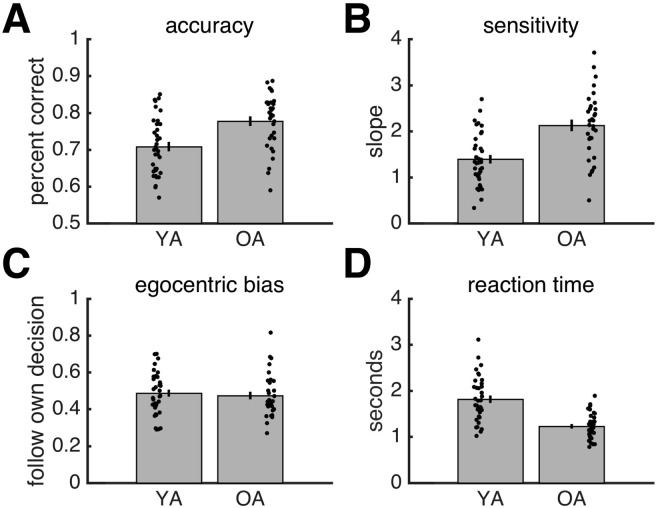
The mid-to-late adolescents (OA: older adolescents) reached higher levels of accuracy than the pre-to-early adolescents (YA: younger adolescents) (OAs: mean ± SD = 0.777 ± 0.074; YAs: mean ± SD = 0.701 ± 0.077) (Fig. 2A; t(66) = 3.745, p < 0.001, d = 0.930, independent-samples t-test). YAs, however, reached acceptable levels of performance (about 70%), indicating that the task was not too difficult. We emphasise that our main measures of joint performance are normalised by individual performance and therefore can be compared between age groups despite differences at the individual level.
Figure 2.
Results for individual behaviour. (A) Accuracy. (B) Sensitivity. (C) Egocentric bias. (D) Reaction time. (A–D) Grey bars are data averaged across individuals. Error bars reflect SEM. Each black dot is an individual. YA: younger adolescents. OA: older adolescents.
Reaction time
OAs made faster responses than YAs (OAs: mean ± SD = 1.227 ± 0.285 seconds; YAs: mean ± SD = 1.814 ± 0.488) (Fig. 2D; t(66) = −5.960, p < 0.001, d = −1.467, independent-samples t-test).
Sensitivity
OAs reached higher levels of sensitivity (i.e., steepness of psychometric function) than YAs (OAs: mean ± SD = 2.128 ± 0.716; YAs: mean ± SD = 1.394 ± 0.561) (Fig. 2B; t(66) = 4.731, p < 0.001, d = 1.165, independent-samples t-test).
Joint performance
Accuracy
As expected given the differences in individual performance, OA dyads (mean ± SD = 0.848 ± 0.060) reached higher levels of accuracy than YA dyads (mean ± SD = 0.755 ± 0.076) (t(32) = 3.897, p < 0.001, d = 1.378, independent samples t-test).
Reaction time
YA dyads (mean ± SD = 12.901 ± 4.928 secs) took slightly but significantly longer than OA dyads (mean ± SD = 9.564 ± 3.000 secs) to reach joint decisions (Fig. 3D; t(32) = 2.348, p = 0.025, d = 0.830, independent-samples t-test).
Figure 3.
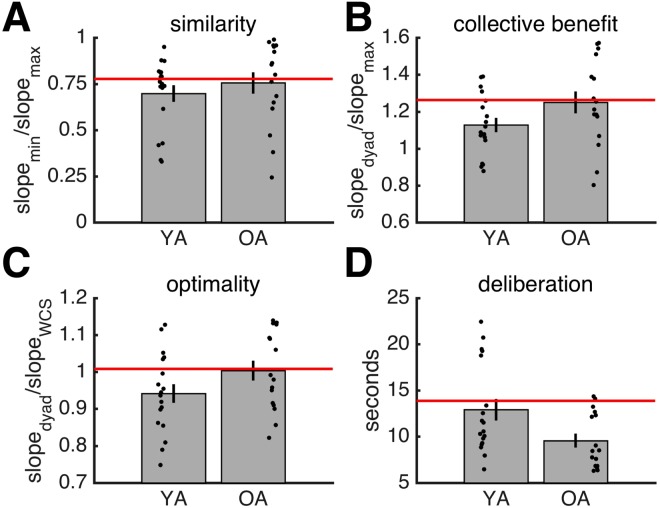
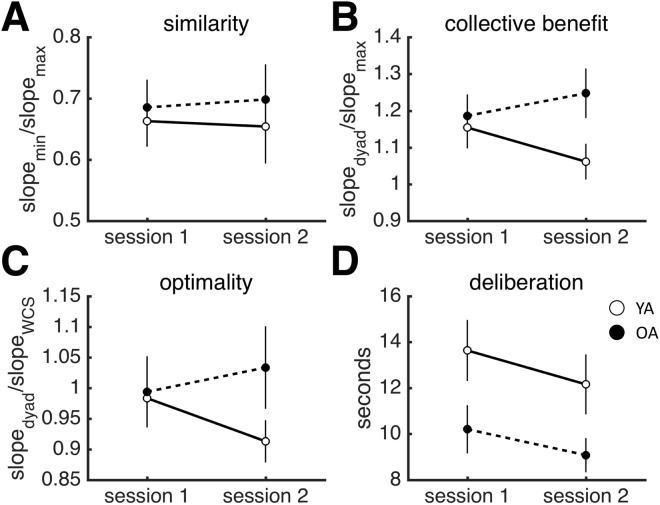
Results for joint behaviour. (A) Similarity of sensitivity. (B) Collective benefit. (C) Optimality. (D) Deliberation time. (A–D) Horizontal red lines indicate mean of adult data. Grey bars are data averaged across individuals. Error bars reflect SEM. Each black dot is a dyad. YA: younger adolescents. OA: older adolescents.
Egocentric bias
Both YAs (mean ± SD = 0.487 ± 0.115) and the OAs (mean ± SD = 0.474 ± 0.117) opted for their own choice about half of the time. There was no significant difference in egocentric bias between the age groups (Fig. 2C; t(66) = 0.469, p = 0.641, d = 0.115, independent-samples t-test).
Similarity
The similarity of dyad members’ sensitivities was on average lower in the YA group (mean ± SD = 0.698 ± 0.191) than in the OA group (mean ± SD = 0.755 ± 0.230). However, due to the within-group heterogeneity, the difference in similarity was not significant (Fig. 3A; t(32) = −0.793, p = 0.433, d = −0.280, independent-samples t-test).
Joint sensitivity
OA dyads (mean ± SD = 3.014 ± 0.878) reached higher levels of sensitivity than YA dyads (mean ± SD = 1.832 ± 0.558) (t(32) = 12.134, p < 0.001; d = 4.290, independent-samples t-test).
Collective benefit
YA and OA dyads on average outperformed the more sensitive dyad members (Sdyad/Smax, Fig. 3B; YA: t(17) = 3.288, p = 0.004; OA: t(15) = 4.233, p < 0.001; one-sample t-test, null model mean is 1). The collective benefit was on average higher in the OA group (mean ± SD = 1.250 ± 0.236) than in the YA group (mean ± SD = 1.128 ± 0.165). However, this difference was not significant (Fig. 3B; t(32) = −1.767, p = 0.087, d = −0.625, independent-samples t-test).
Optimality
Comparison of the empirically observed joint sensitivity to that expected under optimal performance showed that YA dyads on average were ‘sub-optimal’ whereas OA dyads on average were ‘optimal’ (Fig. 3C; YA: t(17) = −2.321, p = 0.033; OA: t(15) = 0.127, p = 0.901; one-sample t-test, null model mean is 1). In line with the results for collective benefit, while OA dyads achieved a higher level of optimality (mean ± SD = 1.003 ± 0.108) than YA dyads (mean ± SD = 0.942 ± 0.107), this difference was not significant (Fig. 3C; t(32) = −1.681, p = 0.103, d = −0.594, independent-samples t-test).
Adult benchmark
Direct group-comparisons using the optimality index showed no differences between, on the one hand, YA dyads and OA dyads and, on the other hand, adult dyads (adults: mean ± SD = 1.008 ± 0.1432) (Fig. 3C; YA vs. adults: t(32) = −1.506, p = 0.143, d = −0.550; OA vs. adults: t(30) = −0.100, p = 0.921; d = −0.035, independent-samples t-test). Hence, the three age groups reached comparable levels of joint performance given their individual performance. In other words, no developmental difference emerged when individual performance was controlled for.
Session analyses
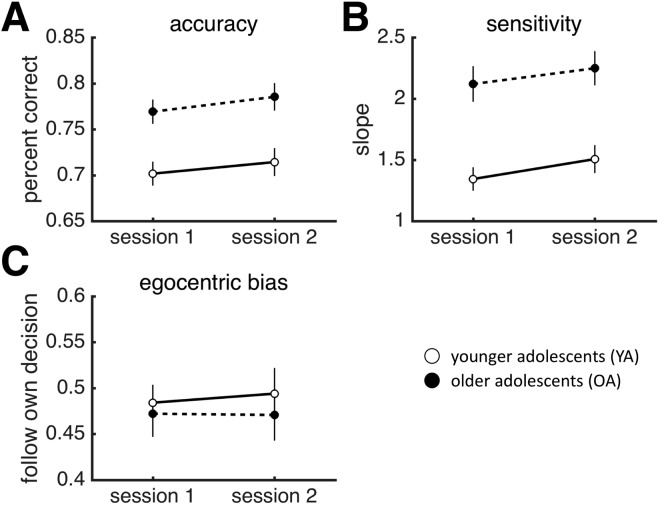
To test for group differences in the temporal profile of individual and joint behaviour, we split the data into two sessions (participants performed two sets of 128 trials divided by a short break), and applied repeated-measures ANOVAs with session as within-subject factor and age group as between-subject factor to our measures of individual and joint performance. No age group by session interaction emerged for individual or joint behaviour (Fig. 4; individual behaviour: accuracy: F1,66 = 0.047, p = 0.829; sensitivity: F1,66 = 0.060, p = 0.807; Fig. 5; joint behaviour: collective benefit F1,32 = 2. 458, p = 0.127; optimality F1,32 = 2.037, p = 0.163).
Figure 4.
Results for individual behaviour by session. (A) Similarity of sensitivity. (B) Collective benefit. (C) Optimality. (D) Deliberation time. Dots are data averaged across individuals. Error bars reflect SEM. YA: younger adolescents. OA: older adolescents.
Figure 5.
Results for joint behaviour by session. (A) Similarity of sensitivity. (B) Collective benefit. (C) Optimality. (D) Deliberation time. Dots are data averaged across dyads. Error bars reflect SEM. YA: younger adolescents. OA: older adolescents.
However, when examined per session, YA did not reap a collective benefit during the second session while OA outperformed the more sensitive member of the group in both sessions (YA: Session 1: t(17) = 2.707, p = 0.014; Session 2: t(17) = 1.269, p = 0.222; OA: Session1: t(15) = 3.208, p = 0.006; Session 2: t(15) = 3.679, p = 0.002; one-sample t-test, null model mean is 1). Additionally, YA dyads were ‘sub-optimal’ only during the second session – YA dyads were, in fact, ‘optimal’ during the first session, while OA remained ‘optimal’ across both sessions (Session 1: YA: t(17) = −0.383, p = 0.707; Session 2: t(17) = −2.533, p = 0.021; OA: t(15) = −0.167, p = 0.686; Session 2: t(15) = 0.850, p = 0.401; one-sample t-test, null model mean is 1). Hence, any developmental differences in the aggregate data are likely the result of YA’s performance during the second session, where YA performed worse as a group, but not individually. A direct comparison of the measures of joint performance separately for each session identified differences in the second session only (Session 1: Collective benefit: t(32) = 0.393, p = 0.697; Optimality: t(32) = 0.181, p = 0.858; Session 2: Collective benefit: t(32) = 2.275, p = 0.030; Optimality: t(32) = 2.317, p = 0.027).
Continuous age
See Supporting Information for details on the age of each participant and additional analyses of individual and joint performance using age as a continuous variable. While the age range was wider in the younger age group (8–13) compared to the older age group (14–17), only three participants in the younger age group were below the age of 10 (Fig. S1). Significant positive developmental gradients were found for individual performance (accuracy, sensitivity and reaction time, see Fig. S2). Developmental gradients for joint performance were not significant (collective benefit and optimality, see Fig. S3).
Discussion
Group decision-making, integrating and weighing social information, is an important developmental milestone in adolescence. The aim of this study was to investigate the optimality of group decision-making in adolescence (male sample). We used a psychophysical task, which allowed us to precisely separate developmental differences in joint performance from developmental differences in individual performance. We examined two measures of joint performance: collective benefit (performance of the group relative to the group) and optimality (WCS model). We observed significant developmental differences in individual performance, with pre-to-early adolescents performing significantly worse than mid-to-late adolescents. Mid-to-late adolescents were significantly more accurate, more sensitive and faster to reach consensus than the younger age group. However, we found that, in terms of joint performance, both groups were able to accrue collective benefits and perform at optimal, adult levels. Interestingly, young adolescents’ joint performance deteriorated over time (while older adolescents’ showed performance gains over time), prompting one to think of fatigue and lapse of attention as the most likely cause of this temporal deterioration. This account, however, is inconsistent with the observation that the individual performance of young adolescents did not deteriorate over time. If anything, individuals improved over time in both groups (Fig. 4A,B). A general loss of attention, drop in arousal or fatigue cannot explain the specific drop in collective performance across time in the younger adolescents (Fig. 5B,C). These results suggest, perhaps surprisingly, that whereas social decision-making per se plateaus relatively early on in development, the ability to maintain social activities shows a more protracted development.
Research on adolescent decision-making in social contexts has largely focused on how peers increase the propensity of youth to make risky decisions13,15. This focus on immaturity or sub-optimality in adolescent decision-making has perhaps neglected that adolescents in fact display a range of sophisticated social behaviours outside the laboratory. In addition, studies examining both individual differences and age effects in cognitive performance have found that variance attributable to individual differences (e.g. variation in IQ within an age bracket) is often much larger than variance attributable to age. For instance, Roalf et al.40 found that, while performance on an N-back working memory task improved with age from 8 to 22 years, many late adolescents performed above the average young adult. In other words, age effects, while significant, may be relatively subtle in some domains. In order to build integrative and comprehensive developmental models of social decision-making, we need to use approaches which are not only naturalistic but also allow for precise quantification of behaviour and strategies.
The current findings can be the starting point for a line of research with direct implications for educational settings. While limited to simple perceptual decisions, the current findings indicate that even individuals as young as ten years of age can benefit from social interaction to improve joint performance. Future research should examine whether the current findings translate to tasks used in educational settings, and examine whether benefits obtained from group decision-making can feed back into individual learning and improve individual learning outcomes.
The current study has several strengths. First, it adds to the limited literature on group decision-making in adolescence, which predominantly has been concerned with individual outcomes. In addition, it is important to extend our understanding of social decision-making in adolescence beyond the effects of peers on individual behaviour41. Second, the paradigm used in this study balances tight experimental control (i.e., precise and independent measurement of individual and joint performance) with ecological validity (i.e., naturalistic, unconstrained interaction in the setting of real-world friendships). Third, using a computational approach (i.e., the WCS model) is uniquely suited to further test hypotheses about specific decision-making strategies. In particular, the WCS model assumes that individuals in a dyad can accurately estimate and communicate the reliability of their decision on each trial – an effective strategy for individuals with relatively similar sensitivities. Using such an approach allows us to go beyond asking whether adolescents can achieve collective benefits to understand how adolescents are able to do so.
The current study also has its limitations. First, the rich but largely uncontrolled setting of the study (i.e., free discussion, real-world relationships) may have contributed to the absence of developmental effects. While a previous study in adults on a similar psychosocial task found no effect of familiarity on joint performance42, familiarity may play a role in efficient communication in social interactions in younger age groups. Future research should assess the effects of relationship type and communication format on joint performance in adolescents. It may be that unconstrained communication with a peer is key for younger individuals – with developmental effects becoming apparent when individuals are required to rely on abstract indicators to arrive at a joint decision (e.g., confidence ratings made on a scale) instead of freely communicating it. Second, the sample was entirely male. Because same sex friendships during adolescence may be qualitatively different in males and females43, it remains to be seen whether the current results extend to adolescent females. We note, however, that two adult studies using a similar task found comparable behavioural results across male-male, male-female and female-female groups39,44. Lastly, session-by-session analyses showed that joint performance declined in younger adolescents. Restricting trial number in future developmental studies may help make results more comparable, unless temporal aspects of performance are of primary interest.
While the task relies on unconstrained social interactions, other aspects of the task are constrained (i.e., stimuli, number of participants, two choices) in order to provide experimental control and allow for precise measurement and computational modelling. Future work may want to expand on the basic paradigm. Developmental effects in how information is integrated in group decisions may become apparent under increased social-cognitive load (i.e., different stimuli or multiple group members) or in a more affectively-laden context (i.e., dyads selected for specific interactive dynamics).
Adolescents are experts in their own social worlds, showing remarkable flexibility and abilities to adapt and learn in social contexts: they operate complex social networks and are often quick adopters of new social trends from fashion to music and social media30. Here we observed that group decision-making is largely optimal in adolescents, with even very young adolescents being able to perform at adult levels. These results underscore the importance of examining different aspects of social decision-making in adolescence and studying adolescent social interaction within the setting of authentic relationships.
Electronic supplementary material
Acknowledgements
SH was supported by a studentship from Medical Research Council (Reference: 1242237) and a Scatcherd European Scholarship. DB was supported by the Calleva Research Centre for Evolution and Human Sciences. BB was supported by the European Research Council (NEUROCODEC 309865).
Author Contributions
D.B., B.B. and J.L. designed the study. D.B. collected the data. S.H. and D.B. analysed the data. S.H., D.B. and J.L. wrote the first draft of the manuscript. All authors reviewed and approved the final draft of the manuscript.
Data Availability
Deidentified raw data and code supporting main analyses are available on GitHub (https://github.com/danbang/article-adolescence-optimal/).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Simone P. W. Haller and Dan Bang contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33557-x.
References
- 1.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giorgio A, et al. Longitudinal changes in grey and white matter during adolescence. NeuroImage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 4.Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nature neurosci. 2012;15:1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- 5.Manes F, et al. Decision‐making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- 6.Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- 7.Bahrami B, et al. Optimally interacting minds. Science. 2010;329:1081–1085. doi: 10.1126/science.1185718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laursen, B. Closeness and conflict in adolescent peer relationships: Interdependence with friends and romantic partners. The company they keep: Friendship in childhood and adolescence, 186–210 (1996).
- 9.Laursen B, Collins WA. Interpersonal conflict during adolescence. Psychol Bull. 1994;115:197–209. doi: 10.1037/0033-2909.115.2.197. [DOI] [PubMed] [Google Scholar]
- 10.Nikitin, J. & Freund, A. M. Feeling loved and integrated or lonely and rejected in everyday life: The role of age and social motivation. Dev Psychol. (2018). [DOI] [PubMed]
- 11.Azmitia M. Peer interaction and problem solving: When are two heads better than one? Child Dev. 1988;59:87–96. doi: 10.2307/1130391. [DOI] [Google Scholar]
- 12.Fawcett LM, Garton AF. The effect of peer collaboration on children’s problem‐solving ability. Br J Educ Psychol. 2005;75:157–169. doi: 10.1348/000709904X23411. [DOI] [PubMed] [Google Scholar]
- 13.Albert D, Steinberg L. Judgment and decision making in adolescence. Journal of Research on Adolescence. 2011;21:211–224. doi: 10.1111/j.1532-7795.2010.00724.x. [DOI] [Google Scholar]
- 14.Pincham HL, Wu C, Killikelly C, Vuillier L, Fearon RP. Social provocation modulates decision making and feedback processing: Examining the trajectory of development in adolescent participants. Dev Cogn Neurosci. 2015;15:58–66. doi: 10.1016/j.dcn.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Dev Psychol. 2005;41:625. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- 16.Burnett Heyes S, et al. Relationship reciprocation modulates resource allocation in adolescent social networks: developmental effects. Child Dev. 2015;86:1489–1506. doi: 10.1111/cdev.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Güroğlu, B., van den Bos, W. & Crone, E. A. Sharing and giving across adolescence: an experimental study examining the development of prosocial behavior. Front Psychol. 5, (2014). [DOI] [PMC free article] [PubMed]
- 18.Gummerum M, Keller M, Takezawa M, Mata J. To give or not to give: Children’s and adolescents’ sharing and moral negotiations in economic decision situations. Child Dev. 2008;79:562–576. doi: 10.1111/j.1467-8624.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 19.Underwood J, Underwood G, Wood D. When does gender matter?: Interactions during computer-based problem solving. Learning and Instruction. 2000;10:447–462. doi: 10.1016/S0959-4752(00)00008-6. [DOI] [Google Scholar]
- 20.Levin I, Druyan S. When sociocognitive transaction among peers fails: The case of misconceptions in science. Child Dev. 1993;64:1571–1591. doi: 10.2307/1131553. [DOI] [Google Scholar]
- 21.Tudge J, Winterhoff P. Can young children benefit from collaborative problem solving? Tracing the effects of partner competence and feedback. Soc Dev. 1993;2:242–259. doi: 10.1111/j.1467-9507.1993.tb00016.x. [DOI] [Google Scholar]
- 22.Samaha NV, De Lisi R. Peer collaboration on a nonverbal reasoning task by urban, minority students. J Exp Educ. 2000;69:5–21. doi: 10.1080/00220970009600646. [DOI] [Google Scholar]
- 23.King, A. Discourse patterns for mediating peer learning (1999).
- 24.Garton AF, Pratt C. Peer assistance in children’s problem solving. British Journal of Developmental Psychology. 2001;19:307–318. doi: 10.1348/026151001166092. [DOI] [Google Scholar]
- 25.Phelps E, Damon W. Problem solving with equals: Peer collaboration as a context for learning mathematics and spatial concepts. J Educ Psychol. 1989;81:639. doi: 10.1037/0022-0663.81.4.639. [DOI] [Google Scholar]
- 26.Romer D, Lee Y-C, McDonald CC, Winston FK. Adolescence, attention allocation, and driving safety. Journal of Adolescent Health. 2014;54:6–15. doi: 10.1016/j.jadohealth.2013.10.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bingham CR, et al. Peer passenger norms and pressure: experimental effects on simulated driving among teenage males. Transportation research part F: traffic psychology and behaviour. 2016;41:124–137. doi: 10.1016/j.trf.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons-Morton BG, et al. Experimental effects of injunctive norms on simulated risky driving among teenage males. Health Psychol. 2014;33:616. doi: 10.1037/a0034837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romer D, Reyna VF, Satterthwaite TD. Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Dev Cogn Neurosci. 2017;27:19–34. doi: 10.1016/j.dcn.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Neurosci. 2012;13:636. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoodi A, et al. Equality bias impairs collective decision-making across cultures. Proc Natl Acad Sci USA. 2015;112:3835–3840. doi: 10.1073/pnas.1421692112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Güroğlu B, van den Bos W, Crone EA. Fairness considerations: increasing understanding of intentionality during adolescence. J Exp Child Psychol. 2009;104:398–409. doi: 10.1016/j.jecp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Weil LG, et al. The development of metacognitive ability in adolescence. Conscious Cogn. 2013;22:264–271. doi: 10.1016/j.concog.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumontheil I, Apperly IA, Blakemore SJ. Online usage of theory of mind continues to develop in late adolescence. Dev Sci. 2010;13:331–338. doi: 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 35.Vetter NC, Leipold K, Kliegel M, Phillips LH, Altgassen M. Ongoing development of social cognition in adolescence. Child Neuropsychol. 2013;19:615–629. doi: 10.1080/09297049.2012.718324. [DOI] [PubMed] [Google Scholar]
- 36.Fleming SM, Dolan RJ, Frith CD. Metacognition: computation, biology and function. Philos Trans R Soc Lond B Biol Sci. 2012;367:1280–1286. doi: 10.1098/rstb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carruthers P. Mindreading underlies metacognition. Behav Brain Sci. 2009;32:164–182. doi: 10.1017/S0140525X09000831. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn D. Metacognitive development. Curr Dir Psychol Dir. 2000;9:178–181. doi: 10.1111/1467-8721.00088. [DOI] [Google Scholar]
- 39.Wright ND, et al. Testosterone disrupts human collaboration by increasing egocentric choices. Proc. R. Soc. B Biol. Sci. 2012;279:2275–2280. doi: 10.1098/rspb.2011.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roalf DR, et al. Within-individual variability in neurocognitive performance: Age-and sex-related differences in children and youths from ages 8 to 21. Neuropsychol. 2014;28:506. doi: 10.1037/neu0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haddad, A. D., Harrison, F., Norman, T. & Lau, J. Y. Adolescent and adult risk-taking in virtual social contexts. Front Psychol. 5 (2014). [DOI] [PMC free article] [PubMed]
- 42.Bahrami B, Didino D, Frith C, Butterworth B, Rees G. Collective enumeration. J. Exp. Psychol. Hum. Percept. Perform. 2013;39:338. doi: 10.1037/a0029717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: potential trade-offs for the emotional and behavioral development of girls and boys. Psychol Bull. 2006;132:98. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bang D, et al. Confidence matching in group decision-making. Nat. Hum. Behav. 2017;1:117. doi: 10.1038/s41562-017-0117. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified raw data and code supporting main analyses are available on GitHub (https://github.com/danbang/article-adolescence-optimal/).