Abstract
Objective
The aim of the present study was to evaluate the effect of PRP on the repair of spinal cord injury in rat model.
Material and methods
Rats were randomly divided into three groups with six rats in each group. Then, spinal cord injury was performed under general anesthesia using “weight dropping” method. Control group included rats receiving normal saline, group two received PRP 1 week after injury; group three received PRP 24 h after injury. The motor function was assessed weekly using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale. Anterograde tracing was performed for evaluation of axon regeneration.
Result
Motor recovery was significantly better in the rats treated with PRP 24 h after injury than the control group. In the rats treated with PRP 1 week after injury and rats treated with PRP 24 h after injury, the average numbers of BDA-labeled axons were statistically different from the control group.
Conclusion
Our experimental study demonstrated positive effects of platelet rich plasma on nerve regeneration after spinal cord injury.
Keywords: Spinal cord injury, Platelet rich plasma, Locomotor function
Introduction
Spinal cord injury (SCI) often causes permanent neurological deficits, mostly because injured neurons lack regenerative ability, and a series of pathological events following SCI results in a second wave of cell death and spreading tissue loss. Accordingly, axonal regeneration and neuro-protection to restore functional recovery after SCI become crucial. The majority of previous studies focused upon growth and neurotrophic factors for recovery of SCI.1, 2
Blood platelets contain many different growth and neurotrophic factors that are released when activated, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and neurotrophin-3 (NT3).3, 4, 5 There is a great interest in utilizing platelet rich plasma (PRP) in oral and maxillofacial bone grafting procedures, non-healing wounds, ulcers, fistula, skin rejuvenation, and peripheral nerve regeneration.6, 7, 8, 9, 10, 11, 12 However, there is no literature on the effect of PRP on the regeneration of central nerve fibers, particularly in SCI. The enhancing effect of PRP is based on the premises that a large number of platelets in PRP release significant quantities of growth factors that aid the healing process. These growth factors act locally to recruit undifferentiated cells to the site of injury, trigger mitosis in these cells, and stimulate angiogenesis.13
The aim of this study was to show that PRP can enhance nerve regeneration and functional recovery when locally applied in rat SCI model. Possible effects were evaluated using behavioral and histological methods.
Materials and methods
Animals
Male Wistar rats weighing 210–230 g were obtained from the Animal Facilities of the School of Medicine, Mashhad University of Medical Sciences (MUMS). All animal treated in accordance with the National Institutes of Health Guidance for the Care and Use of Laboratory Animals, and their use was approved by the Animal Ethics Committee of Mashhad University of Medical Sciences (910856). These rats underwent spinal cord contusion (see below) and were treated with antibiotics (cefazolin 50 mg/kg). Moreover, they were kept one per cage and underwent urinary bladder massage at least twice a day until the recovery of spontaneous micturition.
Spinal cord surgery
The rats were randomly divided into three groups, six rats each.14 Then, the SCI was induced under general anesthesia using the drop weight method. In group one, the rats received normal saline (control group); in group two, they received PRP a week after the injury; and in Group three, they received PRP 24 h after the injury. The rats were anesthetized (70 mg/kg ketamine and 10 mg/kg xylazine), laminectomized at T10, and contused by dropping a 10 g metal rod from the height of 50 mm onto the exposed spinal cord.15 Afterward, the dorsal musculature and skin was sutured. Locomotor function was observed and recorded using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale16 to ensure that complete loss of locomotion in both hindlimbs occurred. Animals that showed a movement in hindlimbs, were excluded from the study.
PRP preparation
One day before the operation, 10 ml of the peripheral blood was collected from two inbred rats in a tube containing 1 ml clinical grade citrate phosphate dextrose buffer. The donor rats were anesthetized, and their blood was collected by cardiac puncture under anesthesia. The animals were then killed by an intra-peritoneal injection of an overdose of ketamine and xylazine. PRP was then prepared by the first centrifugation at 2000 g for 2 min and second centrifugation at 4000 g for 8 min.7
PRP injection
Five μl PRP were stereotaxically injected into 1.5 mm depth of the caudal border of the lesion using Hamilton syringes fitted with 30G needles at a rate of 0.5 μl/min. Control spinally contused rats received the injection of normal saline in the same manner. After each injection, the 30 gauge needle was maintained in the spinal cord for an additional 5 min to reduce the possibility of leakage of the injected fluid from the site.
Behavioral analysis
Animals were assessed weekly for locomotor function by two blinded observers, using BBB hindlimb locomotor rating scale and the follow-up was continued for 5 weeks. All the results were assessed by two observers who were blind to the treatment. Locomotor activities were evaluated by placing animals for 4 min in an open field. Hindlimb locomotor recovery in animals was scored on a scale of 0 (no hindlimb movement) to 21 (normal mobility).
Anterograde tracing
Following the conclusion of behavioral experiments, three rats from each group were anaesthetized and an incision was made through the skin covering the skull. One hole was made at 2 mm lateral and 1.6 mm caudal to the bregma. Then, 1 μl anterograde tracer biotinylated dextran amine (BDA, Life Technologies, Cat No. D-1956) was slowly injected at the depth of 1.5 mm. Two weeks after the BDA injection, the animals were deeply anaesthetized and transcardially perfused with 100 ml of heparinized phosphate buffered saline (PBS), followed by 100 ml of 4% paraformaldehyde in phosphate buffer (pH 7.4). The vertebral column was dissected from each animal and post-fixed for 24 h. A 1 cm segment was cut from the spinal cord, with the lesion at the mid-point of this segment, and embedded in paraffin. The embedded spinal cords were transversely sectioned (5 μm thickness with 200 μm interval) using a microtome. The sections were washed in PBS containing 0.1% Triton X-100, incubated for 1 h with avidin and biotinylated horseradish peroxidase (HRP) (NeuroTrace TM BDA-10,000 Neuronal Tracer Kit, Cat No. N-7167), washed in PBS, and then reacted with 3,3′-diaminobenzidine (DAB) in 50 mM Tris buffer, pH 7.6, and 0.024% hydrogen peroxide. Following the DAB staining, which led to black deposit formation, ten sequential cross-sections, 5 μm apart, were randomly prepared. The cross-section blocks were used to determine the extent of corticospinal tract (CST) labeling in the lesion. The extent of the BDA labeled fibers of the thoracic spinal cord at the thoracic vertebrae (T10) in four sections from each animal were quantified (surface area 0.28 mm2) in a blind manner using Scion Image software (version 3.3, Germany).17
Statistical analysis
All the data were represented as mean ± SEM. Statistical analysis was performed using one-way ANOVA and two-way ANOVA. A Turkey test was used for post-hoc analysis for all the comparisons. The Statistical Package for Social Sciences (SPSS), version 16 (Chicago, Inc., USA) was used for all statistical comparisons.
Results
Recovery of hindlimb function
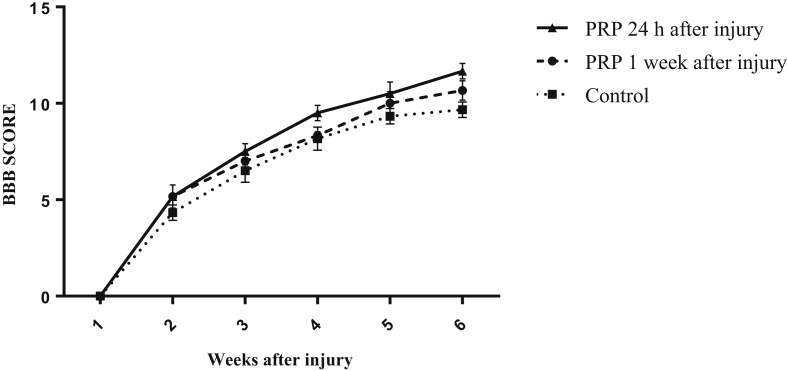
The locomotor function was assessed weekly using the BBB locomotor rating scale. As shown in Fig. 1, at week 5, the average score of recovery of hindlimb function in the rats treated with PRP 24 h after SCI significantly increased in comparison with control rats (p < 0.05). However, there was no significant difference in recovery of hindlimb function between rats treated with PRP 24 h after injury and rats treated with PRP 1 week after injury. The average score of recovery of hindlimb function in the rats treated with PRP 1 week after injury showed no significant difference in comparison with control rats. In the 5th week after SCI, the average score in the control group was 9.67 ± 0.42, while in the rats treated with PRP 1 week or 24 h after SCI were 10.67 ± 0.49 and 11.67 ± 0.42, respectively. At week 5 post spinal injury, most rats treated with PRP 24 h after injury showed plantar stepping with frequent to consistent weight bearing and occasional forelimb-hindlimb coordination (p < 0.05). On the contrary, control rats and those treated with PRP 1 week after injury showed plantar stepping with occasional weight bearing and no forelimb-hindlimb coordination.
Fig. 1.
Effect of platelet rich plasma (PRP) on locomotor functions following spinal cord injury (SCI). BBB scores of the rats treated with PRP 24 h after injury were significantly higher than control animals, at 5th week. There was no significant difference between BBB scores of the rats treated with PRP 24 h after injury and those treated with PRP 1 week after SCI. Data are presented as mean ± SEM (n = 6). *p < 0.05, as compared with control group.
Quantification of CST axons
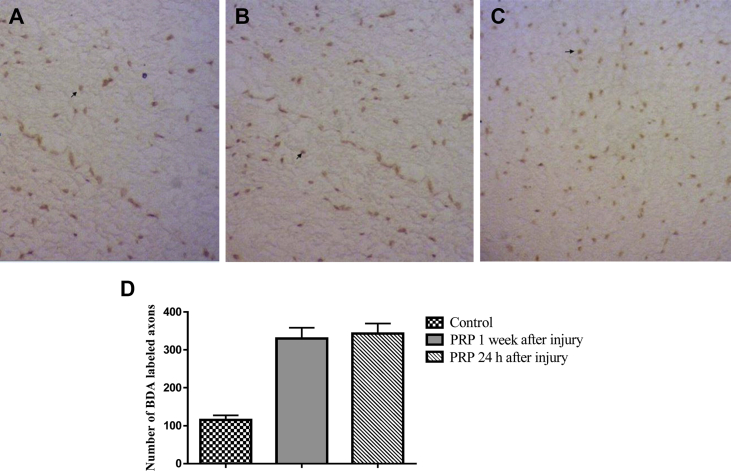
For verifying axon regeneration, we analyzed the spinal cords for the presence of descending fibers below in the lesion site, using stereotactic anterograde tracing of BDA administered to the motor cortexes. Representative images showing BDA labeled axons in the middle of the spinal lesion (T10) were illustrated in Fig. 2. The lowest count was found in the control group with the average of 115 ± 12 BDA-labeled axons (Fig. 2). In the rats treated with PRP 1 week after injury and rats treated with PRP 24 h after injury, the average numbers of BDA-labeled axons were 330 ± 28 and 343 ± 26, respectively, which were statistically different from the control group (p < 0.001). There was no significant difference in the average numbers of BDA-labeled axons between rats treated with PRP 1 week after injury and those treated with PRP 24 h after SCI.
Fig. 2.
Effect of platelet rich plasma (PRP) on axonal regeneration at 5 weeks after spinal cord injury (SCI). A–C: Representative photomicrographs of the thoracic transverse sections (T10) showing biotinylated dextran amine-labeled corticospinal tract (CST) fibers visualized by diaminobenzidine (DAB) staining technique (brown spots) in the control (A), rats treated with PRP 1 week after SCI (B) and rats treated with PRP 24 h after SCI (C). Arrows indicate biotinylated dextran amine (BDA) labeled CST fibers (magnification ×400). D: Quantitative data showing the numbers of BDA-labeled axons per section (n = 12/group). Data were shown as mean ± SEM. ***P < 0.001, as compared with control group.
Discussion
In this study, we found that the PRP injection promoted the axon regeneration in the rat model of spinal cord contusion. Moreover, in the 5th week of the post-spinal injury, the PRP administration resulted in significant functional recovery in the rats which received PRP 24 h post-lesion. This study is the first to report that PRP is able to improve the central nerve recovery after SCI.
The results of this study are consistent with the previous studies focusing on the effects of PRP on peripheral nerves injuries. Farrag et al reported that PRP promotes the peripheral nerve regeneration after the facial nerve transaction in rats.18 Furthermore, Cho et al applied PRP and mesenchymal stem cells (MSCs) in an animal model of facial nerve axotomy and concluded that PRP and MSCs enhanced the peripheral nerve regeneration of the acute nerve injury.19 In another study, Kucuk et al applied PRP in a sciatic nerve cut model and demonstrated the positive effects of PRP on the nerve regeneration. They concluded that the functional healing and histological parameters in the PRP group were significantly better than those in the control group.4 Similar investigations on peripheral nerve injuries have demonstrated the positive effects of PRP on the nerve regeneration and functional recovery.5, 20 Takeuchi et al applied a rat organ co-culture system (brain-spinal cord coculture) to evaluate the ability of PRP to promote the axonal growth in spinal cord tissues and identify the growth factors in PRP that contribute to the regulation of the axon growth. They concluded that PRP enhanced the axonal growth in the spinal cord tissues through mechanisms associated with IGF-1 and VEGF, while TGF-β1 in PRP exerted negative effects on the axonal growth.21 On the contrary, Piskin et al showed that sciatic nerve regeneration was not improved after microsurgical reconstruction of a nerve gap by platelet gel.22 In another study, Welch et al revealed that combined administration of PDGF and IGF-I did not improve peripheral nerve regeneration in a transection and anastomosis model.23
A higher density of axons appeared in the lesion site of PRP-treated rats, as compared with the control animals. In the rats treated with PRP 24 h post-lesion, the BBB score at the 5th week was significantly higher than control rats. However, no significant functional recovery was observed in the group treated with PRP 1 week after the SCI. This finding suggested that rescuing neurons at the first hours after SCI is crucial for the functional restoration. Therefore, PRP therapy should be performed before secondary injury is initiated. This finding was supported by a previous study, reporting that the hindlimb functional recovery failed when growth factors were infused into damaged spinal cords in the sub-acute phase.24 However, the number of regenerating axons might not correlate with the functional restoration of the axon, because the regeneration of the axons might occur outside the correct pathway, resulting in the innervations of both agonist and antagonist muscles.4, 25, 26, 27
A normal blood clot contains 95% red blood cells, 5% platelets, less than 1% white blood cells, and many fibrin strands. A PRP clot contains 4% red blood cells, 95% platelets, and 1% white blood cells. Alpha granules of platelets contain seven essential growth factors known to be actively secreted by platelets to initiate wound healing. These growth factors include the 3 isomers of the platelet-derived growth factor (PDGF-αα, PDGF-ββ, and PDGF-αβ), 2 of the numerous transforming growth factors-β (TGF-β1 and TGF-β2), a vascular endothelial growth factor, and an epithelial growth factor.3, 4 These factors are associated with repair processes after the CNS injury and act as a catalyst for enhancing the repair process of regenerating nerve fibers. In addition, platelets contain low concentrations of neurotrophic factors such as BDNF, NGF, and NT3, which are produced following the nerve injury by activated Schwann cells. PRP acts directly as a catalyst for accelerating the nerve regeneration and indirectly by activating Schwann cells to produce neurotrophic factors.4, 5, 28
In conclusion, our study showed that a single dose of PRP applied on the lesion site 24 h after SCI increases recovery of motor function by increasing the number of axons. The low number of subjects can be considered as main weakness of our study. However, we used the fewest number of animals necessary to produce significance for the sake of promoting the rights of animals. Based on the results of the present work, the authors concluded that PRP enhanced axonal regeneration and improved functional motor recovery when applied 24 h post-injury, which could indicate that PRP may have neurotrophic and neuroprotective effects in the rat SCI model. Further studies are recommended to support the present results and to explore the mechanisms of beneficial effects of PRP in the SCI.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Thuret S., Moon L.D.F., Gage F.H. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7(8):628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 2.Claire E. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;26(1–4):4. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- 3.Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kucuk L., Gunay H., Erbas O., Kucuk U., Atamaz F., Coskunol E. Effects of platelet-rich plasma on nerve regeneration in a rat model. Acta Orthop Traumatol Turc. 2014;48(4):449–454. doi: 10.3944/AOTT.2014.13.0029. [DOI] [PubMed] [Google Scholar]
- 5.Elgazzar R., Mutabagani M., Abdelaal S., Sadakah A. Platelet rich plasma may enhance peripheral nerve regeneration after cyanoacrylate reanastomosis: a controlled blind study on rats. Int J Oral Maxillofac Surg. 2008;37(8):748–755. doi: 10.1016/j.ijom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Sariguney Y., Yavuzer R., Elmas C., Yenicesu I., Bolay H., Atabay K. Effect of platelet-rich plasma on peripheral nerve regeneration. J Reconstr Microsurg. 2008;24(3):159–167. doi: 10.1055/s-2008-1076752. [DOI] [PubMed] [Google Scholar]
- 7.Shirvan M.K., Alamdar D.H., Ghorifi A., Rahimi H.R. A novel treatment for urethrovaginal fistula: autologous platelet-rich–plasma injection and platelet-rich–fibrin-glue interposition. J Gynecol Surg. 2013;29(5):268–270. [Google Scholar]
- 8.Tashnizi M.A., Alamdari D.H., Khayami M.E. Treatment of non-healing sternum wound after open-heart surgery with allogenic platelet-rich plasma and fibrin glue-preliminary outcomes. Indian J Plast Surg Off Publ Assoc Plast Surg India. 2013;46(3):538. doi: 10.4103/0970-0358.122011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavakolinejad S., Hamidi Alamdari D., Khajehahmadi S., Ebrahimzadeh Bidskan A. Histological evidences after platelet-rich-plasma and adipose drived stem cells injection on critical size cleft palate. Int J Pediatr. 2014;2(2.3):88. [Google Scholar]
- 10.Tavakolinejad S., Khosravi M., Mashkani B. The effect of human platelet-rich plasma on adipose-derived stem cell proliferation and osteogenic differentiation. Iran Biomed J. 2014;18(3):151. doi: 10.6091/ibj.1301.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter C.A., Jolly D.G., Worden C.E., Hendren D.G., Kane C.J. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol. 2003;74(3):244–255. doi: 10.1016/s0014-4800(03)00017-0. [DOI] [PubMed] [Google Scholar]
- 12.Oyama T., Nishimoto S., Tsugawa T., Shimizu F. Efficacy of platelet-rich plasma in alveolar bone grafting. J Oral Maxillofac Surg. 2004;62(5):555–558. doi: 10.1016/j.joms.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Yu W., Wang J., Yin J. Platelet-rich plasma: a promising product for treatment of peripheral nerve regeneration after nerve injury. Int J Neurosci. 2011;121(4):176–180. doi: 10.3109/00207454.2010.544432. [DOI] [PubMed] [Google Scholar]
- 14.Beaumont E., Guevara E., Dubeau S., Lesage F., Nagai M., Popovic M. Functional electrical stimulation post-spinal cord injury improves locomotion and increases afferent input into the central nervous system in rats. J Spinal Cord Med. 2014;37(1):93–100. doi: 10.1179/2045772313Y.0000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metz G.A., Curt A., Van De Meent H., Klusman I., Schwab M.E., Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17(1):1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- 16.Basso D.M., Beattie M.S., Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 17.Fouad K., Schnell L., Bunge M.B., Schwab M.E., Liebscher T., Pearse D.D. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;2;25(5):1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrag T.Y., Lehar M., Verhaegen P., Carson K.A., Byrne P.J. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117(1):157–165. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- 19.Cho H.H., Jang S., Lee S.C. Effect of neural-induced mesenchymal stem cells and platelet-rich plasma on facial nerve regeneration in an acute nerve injury model. Laryngoscope. 2010;120(5):907–913. doi: 10.1002/lary.20860. [DOI] [PubMed] [Google Scholar]
- 20.Ye F., Li H., Qiao G. Platelet-rich plasma gel in combination with Schwann cells for repair of sciatic nerve injury. Neural Regen Res. 2012;7(29):2286. doi: 10.3969/j.issn.1673-5374.2012.29.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi M., Kamei N., Shinomiya R. Human platelet-rich plasma promotes axon growth in brain–spinal cord coculture. Neuroreport. 2012;23(12):712–716. doi: 10.1097/WNR.0b013e3283567196. [DOI] [PubMed] [Google Scholar]
- 22.Piskin A., Kaplan S., Aktaş A. Platelet gel does not improve peripheral nerve regeneration: an electrophysiological, stereological, and electron microscopic study. Microsurgery. 2009;29(2):144–153. doi: 10.1002/micr.20599. [DOI] [PubMed] [Google Scholar]
- 23.Welch J., Kraus K., Wells M., Blunt D., Weremowitz J. Effect of combined administration of insulin-like growth factor and platelet-derived growth factor on the regeneration of transected and anastomosed sciatic nerve in rats. Am J Vet Res. 1997;58(9):1033–1037. [PubMed] [Google Scholar]
- 24.Cheng H., Wu J.P., Tzeng S.F. Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J Neurosci Res. 2002;69(3):397–405. doi: 10.1002/jnr.10303. [DOI] [PubMed] [Google Scholar]
- 25.Guo J., Zeng Y., Li H. Cotransplant of neural stem cells and NT-3 gene modified Schwann cells promote the recovery of transected spinal cord injury. Spinal Cord. 2007;45(1):15–24. doi: 10.1038/sj.sc.3101943. [DOI] [PubMed] [Google Scholar]
- 26.Martins R.S., Siqueira M.G., Da Silva C.F., Plese J.P. Overall assessment of regeneration in peripheral nerve lesion repair using fibrin glue, suture, or a combination of the 2 techniques in a rat model. Which is the ideal choice? Surg Neurol. 2005;64(1):S10–S16. doi: 10.1016/j.surneu.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Dellon A.L., Mackinnon S.E. Selection of the appropriate parameter to measure neural regeneration. Ann Plast Surg. 1989;23(3):197–202. doi: 10.1097/00000637-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Oudega M., Xu X.-M. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23(3-4):453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]