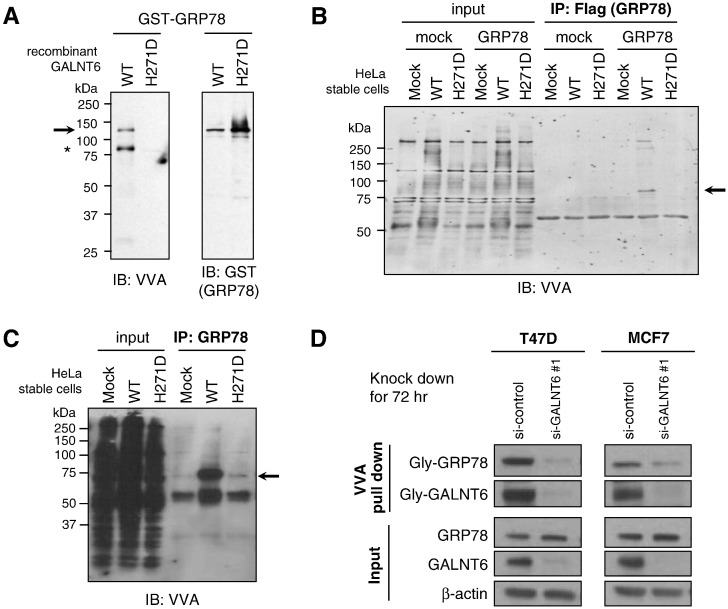
Figure 4.
GALNT6 O-glycosylates GRP78. (A) GST-tagged recombinant GRP78 protein (GST-GRP78) was incubated with UDP-GalNAc (sugar donor) and His-tagged recombinant wild-type GALNT6 (GALNT6-WT) or enzyme-dead GALNT6 (GALNT6-H271D) proteins. O-glycosylated proteins and GST-tagged GRP78 protein were detected by western blot analysis with VVA lectin and anti-GST antibody, respectively. GST-GRP78 was O-glycosylated by GALNT6-WT but not by enzyme activity-depleted GALNT6-H271D. We also observed that GALNT6-WT itself was auto-O-glycosylated (indicated by asterisk). In cell-based assay, we pulled down exogenous (B) or endogenous GRP78 (C), and detected its O-glycosylation level. GRP78 proteins had much higher level of O-glycosylation in HeLa-GALNT6-WT stable cells, when compared with -mock and -H271D stable cells (B and C). (D) GALNT6 was knocked down by using siRNA in T47D and MCF7 cells for 72 h, then VVA pull down assay and western blot were performed. The O-glycosylation level of GRP78 was significantly reduced after GALNT6 knockdown, suggesting that GALNT6 was critical for O-glycosylation of GRP78 in these cells.