Abstract
Objective
Studies have assessed the association between aspartic acid (D)-repeat polymorphism in the gene encoding Asporin (ASPN) and knee osteoarthritis (KOA) risk, but the results were inconclusive and contradictory. Therefore, we performed a meta-analysis to investigate the association between ASPN gene D-repeat polymorphism and KOA risk.
Methods
Eligible studies were identified by searching several electronic databases for relevant reports published before September 2016. The pooled odds ratios (ORs) for the association between ASPN polymorphism and KOA and their corresponding 95% confidence intervals (CIs) were estimated using the random- or fixed-effect model.
Results
A total of eleven case-control studies in ten publications with 4610 KOA cases and 3621 controls were included for the ASPN D-repeat polymorphism. Overall, no significant association was detected for D14 allele carrier (D14 vs. D13: OR = 1.10, 95% CI = 0.90–1.36, p = 0.32). Meta-analysis of D14 vs. other alleles and D13 vs. other alleles showed the same pattern of KOA association as the D14 vs. D13 (OR = 1.30, 95% CI = 1.00–1.70, p = 0.06; OR = 0.93, 95% CI = 0.82–1.06, p = 0.33, respectively). Also, in the stratified analysis by ethnicity, no significant association of this polymorphism with risk of KOA was found in the European and Asians populations (OR = 1.05, 95% CI = 0.91–1.21, p = 0.49; OR = 0.98, 95% CI = 0.78–1.23, p = 0.88, respectively).
Conclusions
The present meta-analysis suggests that the ASPN D-repeat polymorphism is not associated with an increased KOA risk. However, future large studies with gene–gene and gene–environment interactions are needed to validate these findings.
Level of evidence
Level III diagnostic study.
Keywords: Osteoarthritis, Knee, Asporin, D13 and D14 alleles, Polymorphism
Introduction
Osteoarthritis (OA) is a widespread and degenerative, chronic disease that affects up to 80% of the older adults.1 According to the World Health Organization, more than 150 million people suffer from OA worldwide.2 Knee OA (KOA) is a common form of arthritis usually occurs in one or both knee joints. According to the studies, KOA is likely to become the fourth most common cause of disability in women and the eighth most common cause in men.3 KOA is more important not only for its high prevalence rate compared with other types of OA but also for its presentation at earlier age groups particularly in younger age groups of obese women.4, a), b) The KOA-related symptoms have a major impact on subject social and physical wellbeing, and KOA is expected to be the fourth leading cause of disability in 2020.5
The strongest most prevalent risk factors for the incidence of KOA are overweight or obesity, a higher age, and female sex.5, 6 However, in the recent years the model of KOA has changed dramatically and presently considered as a condition influenced by genetics and environmental factors.7 The heritability estimates showing that genetic components account for 39–65% of the risk for the development of KOA.8, 9 However, it shown that the genetic contribution to KOA in sibling pairs may be much less than at other sites, such as the hips, hands and spine.10 Specific locus such as GDF-5, 7q22 locus, MCF2L, DOT1L, NCOA3 has been reported to increase the incidence of KOA, and in recent studies the importance of few specific alleles was confirmed.8, 10, 11 However, identification of genetic variants robustly associated with KOA at a genome-wide significant level has been more difficult than for other diseases.12
A number of studies have investigated the association between aspartic acid (D) repeat polymorphism of Asporin (ASPN) gene and the risk of KOA in different populations,13, 14, 15, 16, 17, 18, 19, 20, 21 but the results are inconsistent. For instance, four studies from in Mexican, Iranian, Japanese, and Han Chinese Asian populations detect an association with KOA.13, 16, 19, 20 An earlier meta-analyses failed to detect any association between ASPN D-repeat polymorphism and the risk of KOA in Asians and Caucasians.22 Since then, multiple studies on the relationship of KOA with ASPN D-repeat have been published. Therefore, the aim of this study was to determine the overall association between ASPN D-repeat polymorphism and KOA risk and whether the association varies by ethnicity.
Materials and methods
Study identification and selection
Eligible studies were identified by searching the relevant literature published before 20 September 2016 by searching databases of PubMed, EMBASE, Web of Science and Google Scholar. The main search terms were “Osteoarthritis”, “Knee osteoarthritis” or “KOA”, “Asporin gene”, “ASPN”, “D-repeat”, “genetic polymorphism”, and “variation”. The extracted publications were limited to English language and conducted on human subjects. We also have read completely all extracted articles and used a hand search of references of original studies or reviewed articles on this topic to identify additional studies.
Inclusion and exclusion criteria
Studies included to the meta-analysis had to be consistent with the following criteria: the study (1) evaluated the association between ASPN and the risk of KOA; (2) used a case-control and cohort design; (3) included a full-text article and; (4) offered the size of the sample and sufficient data (genotypes and allele distributions of both cases and controls) for estimating an odds ratio (OR) with a 95% confidence interval (CI); and (5) used the English language. Exclusion criteria were as follows: the study (1) used only case group data, (2) without definite information of genotypes and alleles (3) reviews, letters or case reports; (4) duplicate publications of data from the same study.
Data extraction
Based on the inclusion and exclusion criteria, the following data were extracted by two investigators independently from each study: the first author, year of publication, genotyping methods, number of KOA patients and controls, genotype and allele frequency. For conflicting evaluation, these two authors carried out discussions until a consensus was reached. If they could not reach a consensus, disagreements were resolved by discussion.
Statistical methods
The crude ORs and their 95% CIs were calculated to assess the strength of the association between ASPN and KOA risk. The pooled ORs were performed for D14 allele vs. D13 allele, D14 allele vs. other alleles combined and D13 allele vs. other alleles combined comparisons. Heterogeneity was assessed for each study using Cochrane's Q test and I2 measurement. The heterogeneity was considered significant if either the Q statistic had p < 0.1 or I2 > 50%. An I2 value of 0% represents no heterogeneity, with values of 25%, 50%, 75%, or more represent low, moderate, high, and extreme heterogeneity, respectively.23 A p value greater than 0.10 indicated a lack of heterogeneity among studies, so the fixed effect model (Mantel–Haenszel method) was used to calculate pooled OR. Otherwise, the fixed-effects model (Mantel–Haenszel approach) was used.24 One-way sensitivity analyses were carried out by consecutively omitting one study at a time to assess the power of the meta-analysis findings.25 Visual inspection of the asymmetry of funnel plots was carried out to assess potential publication bias. Begg's funnel plot, a scatter plot of effect against a measure of study size, was generated as a visual aid to detect bias or systematic heterogeneity.26 The Egger's linear regression test was used to evaluate possible publication bias of studies, and it was regarded as statistically significant when p < 0.05.27 All the statistical analyses were performed by comprehensive meta-analysis (CMA) 2.0 Software (Biostat, USA). Two-sided p values <0.05 were considered statistically significant.
Results
Study selection and characteristics
A total of 15 results were returned through PubMed and Google Scholar. Three articles were excluded after reviewing the titles and abstracts, one study excluded due to the in Chinese language28; another 1 study was removed owing to insufficient data after reviewing the full text29 (Fig. 1). Finally, a total of ten publications13, 14, 15, 16, 17, 18, 19, 20, 21, 30 (ten case-control studies and one cohort) examined the associations between ASPN gene D14 and D13 allele polymorphism with KOA risk, including 4610 cases and 3621 controls were selected for the meta-analysis. These studies were published from 2005 to 2015. Two out of 10 publications were conducted in the Mexico, 1 in Japan (included one case control and one cohort study), 1 in USA, 1 in UK, 1 in Spain, 1 in Greece, 1 in China, 1 in Korea and 1 in Iran. All of the articles were written in English. The study characteristics were presented in Table 1.
Fig. 1.
Flow diagram of the study selection process.
Table 1.
Characteristics of the individual studies included in the meta-analysis.
| First author | Country (Ethnicity) | Case/Control | Cases |
Control |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count |
Frequency |
Count |
Frequency |
|||||||||
| D14 | D13 | Others | D14 | D13 | D14 | D13 | Others | D14 | D13 | |||
| Kizawa-1 et al. 2005 (Cohort)13 | Japan (Asian) | 137/234 | 30 | 163 | 81 | 0.109 | 0.595 | 22 | 314 | 132 | 0.047 | 0.671 |
| Kizawa-2 et al. 2005 (Case-control)13 | Japan (Asian) | 986/374 | 61 | 459 | 266 | 0.078 | 0,584 | 36 | 479 | 233 | 0.048 | 0.640 |
| Mustafa et al. 200514 | UK (Caucasian) | 1247/748 | 76 | 258 | 222 | 0.137 | 0.464 | 190 | 752 | 554 | 0.127 | 0.503 |
| Kaliakatsos et al. 200615 | Greece (Caucasian) | 155/190 | 47 | 119 | 146 | 0.151 | 0.381 | 51 | 192 | 141 | 0.133 | 0.500 |
| Jiang et al. 200616 | China (Asian) | 218/454 | 41 | 300 | 95 | 0.094 | 0.689 | 44 | 604 | 260 | 0.050 | 0.665 |
| Rodriguez et al. 200630 | Spain (Caucasian) | 491/294 | 56 | 156 | 164 | 0.149 | 0.415 | 74 | 248 | 266 | 0.126 | 0.422 |
| Atif et al. 200817 | USA (Caucasian) | 775/511 | 206 | 749 | 595 | 0.133 | 0.483 | 142 | 496 | 384 | 0.139 | 0.485 |
| Song et al. 200818 | Korea (Asian) | 190/376 | 22 | 265 | 93 | 0.058 | 0.697 | 65 | 483 | 204 | 0.087 | 0.643 |
| Arelano et al. 201319 | Mexico (Caucasian) | 218/222 | 91 | 205 | 140 | 0.208 | 0.470 | 107 | 204 | 133 | 0.240 | 0.459 |
| Jazayeri et al. 201320 | Iran (Asian) | 100/100 | 32 | 82 | 86 | 0.16 | 0.41 | 40 | 91 | 69 | 0.200 | 0.455 |
| Gonzalez-Huerta et al. 201521 | Mexico (Caucasian) | 93/118 | 123 | 7 | 56 | 0.661 | 0.038 | 134 | 6 | 96 | 0.568 | 0.025 |
ASPN D13 and D14 allele polymorphism
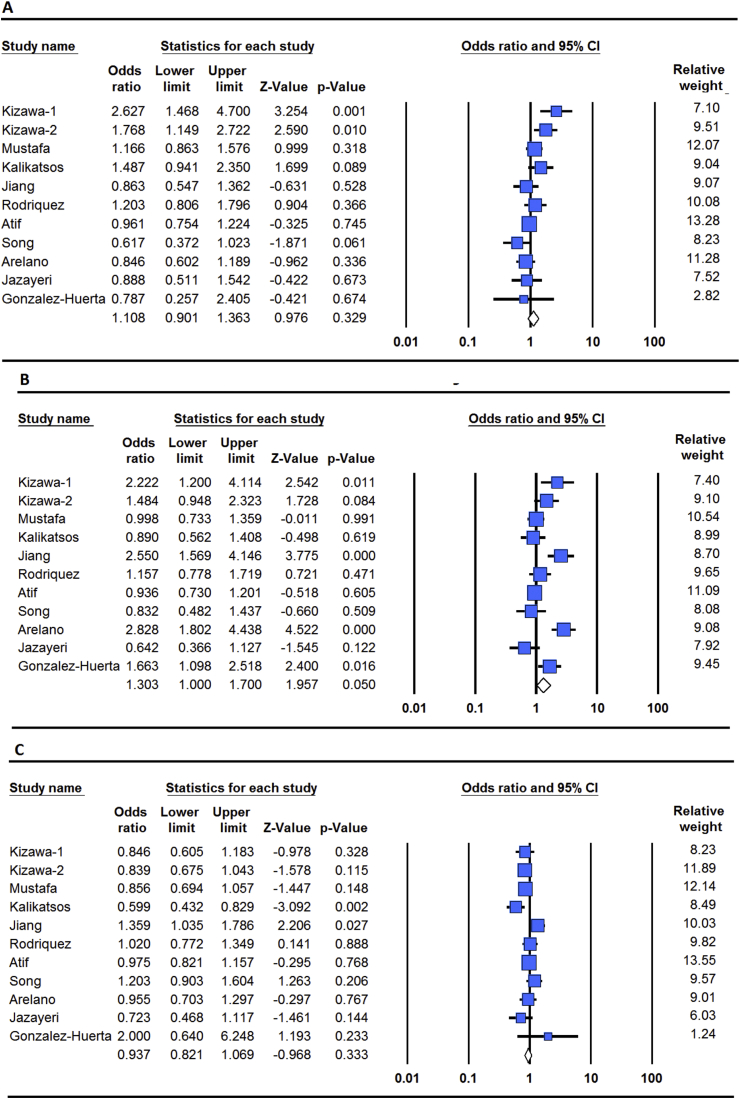
The distributions of allele frequencies in each case-control study are shown in Table 1. Meta-analysis showed no association between KOA and the ASPN D14 allele in the overall population (OR 1.10, 95% CI 0.90–1.36, p = 0.32). Furthermore, stratification by ethnicity failed to identify any association between this polymorphism and KOA in the European and Asians populations (OR 1.05, 95% CI 0.91–1.21, p = 0.49; OR 0.98, 95% CI 0.78–1.23, p = 0.88, respectively) (Fig. 2). However, high heterogeneity was found in Asians than European (I2 = 81.2, PH = 0.01; I2 = 8%, PH = 0.36, respectively). Meta-analysis of D14 vs. other alleles showed the same pattern of KOA association as the D14 vs. D13 (OR 1.30, 95% CI 1.00–1.70 p = 0.06). No association was found between the ASPN D13 vs. other alleles and risk of developing KOA by meta-analysis (OR 0.93, 95% CI 0.82–1.06, p = 0.33) (Table 2, Fig. 2).
Fig. 2.
Forest plot for overall KOA risk associated with the ASPN polymorphism for each of the 10 published studies. A: D14 vs. D13, B: D14 vs. other alleles, and C: D13 vs. other alleles.
Table 2.
Meta-analysis of associations between the D13 and D14 alleles of the ASPN polymorphisms and KOA risk.
| Comparison | Subgroup | Type of model | Heterogeneity |
Odds ratio |
|||
|---|---|---|---|---|---|---|---|
| I2 (%) | PH | OR | 95% CI | POR | |||
| D14 versus D13 | Overall | Random | 61 | 0.004 | 1.10 | 0.90–1.36 | 0.32 |
| Caucasian | Fixed | 8 | 0.36 | 1.05 | 0.91–1.21 | 0.49 | |
| Asian | Random | 67 | 0.01 | 0.98 | 0.78–1.23 | 0.88 | |
| D14 versus other alleles | |||||||
| Overall | Random | 77 | 0.00 | 1.30 | 1.00–1.70 | 0.06 | |
| Caucasian | Random | 78 | 0.00 | 1.26 | 0.91–1.73 | 0.15 | |
| Asian | Random | 79 | 0.001 | 1.35 | 0.81–2.26 | 0.24 | |
| D13 versus other alleles | |||||||
| Overall | Random | 57 | 0.01 | 0.93 | 0.82–1.06 | 0.33 | |
| Caucasian | Random | 50 | 0.07 | 0.90 | 0.81–1.00 | 0.06 | |
| Asian | Random | 67 | 0.01 | 0.98 | 0.78–1.23 | 0.88 | |
Heterogeneity and sensitivity analysis
A true heterogeneity existed in the D14 vs. D13 model (PH = 0.004, I2 = 61%), D14 versus other models (PH = 0.001, I2 = 77%) and D13 versus others (PH = 0.01, I2 = 57%) in overall populations. A high heterogeneity was found in Asians than European by ethnicity under all comparisons (Table 2). We deleted one single study involved in the meta-analysis at a time to reflect the impact of the individual data-set to the pooled ORs, and the corresponding pooled ORs were not materially altered (data not shown), indicating that our results were statistically robust.
Publication bias
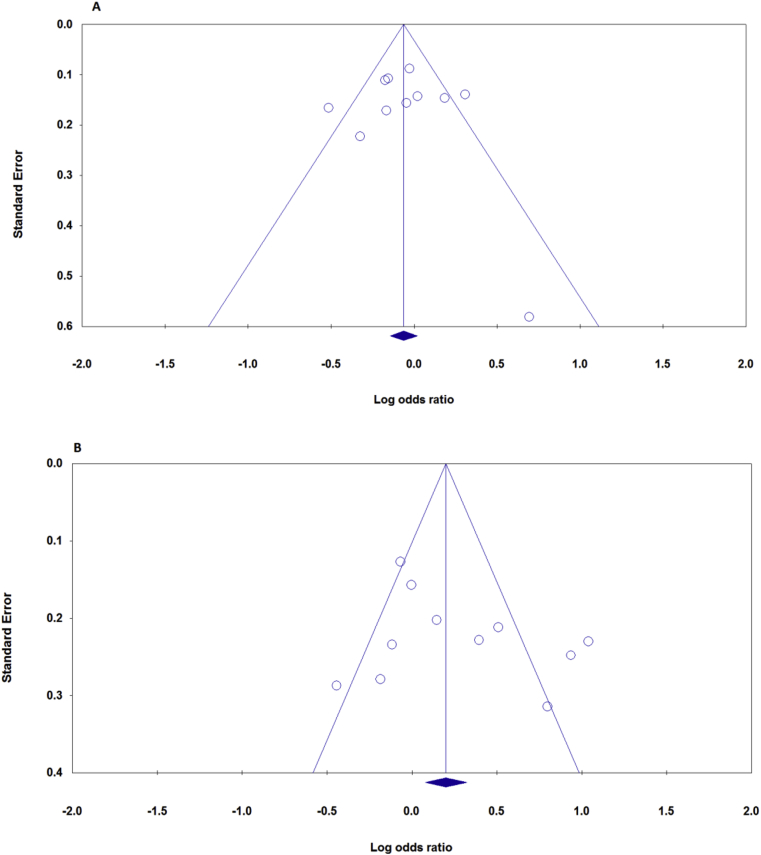
We used Begg's funnel plot and Egger's test to assess the publication bias of the studies. The shape of the funnel plot no revealed some evidence of obvious asymmetry in the meta-analyses of the APSN D13 and D14 alleles. In addition the Begg's tests and Egger's tests were greater than 0.05, providing statistical evidence of the funnel plot's symmetry. Ultimately, the results did not suggest any evidence of publication bias (Fig. 3).
Fig. 3.
Begg's funnel plots of ASPN polymorphism and KOA risk for publication bias test. Each point represents a separate study for the indicated association. Horizontal line, mean effect size. A: D14 vs. D13, B: D14 vs. other alleles.
Discussion
The association of genetic polymorphism and KOA has recently attracted growing attention. Candidate gene-association studies showed an association between ASPN gene and OA.13, 31 The ASPN gene lies within a gene cluster on human chromosome 9q22-9q21.3, which encodes a cartilage extracellular protein that is member of the small leucine-rich (LRR) proteoglycan family.13, 21 The encoded protein (Asporin) may regulate chondrogenesis by inhibiting transforming growth factor beta 1 (TGF-β) induced gene expression in cartilage.21 Asporin possesses a unique stretch of aspartic acid residues (D-repeat) in its N-terminal region.32 Polymorphisms in the aspartic acid repeat region of this gene are associated with a susceptibility to osteoarthritis, and also with intervertebral disc disease.21, 32 It is estimated the number of D-repeats varies from 9 (D9) to 20 (D20), and each variant with a different number of D-repeats may play a different role in OA pathogenesis.32 In Japanese and Chinese Han populations, D14 was found to be a risk factor in development of KOA while, D13 was found to be a protective factor against OA in some Japanese, and D12, D15, or D16 alleles were associated with OA either as a risk factor or a protective factor in Mexican and Iranian study populations.31, 32, 33
Meta-analysis is a useful method for investigating associations of KOA with genetic variants because it has the advantage of combining results of similar studies on the same topic through a quantitative approach.34 To avoid the disadvantage of a low power in a single study, we performed this meta-analysis to reveal the actual association between ASPN polymorphism and KOA risk. However, the current meta-analysis results suggest that ASPN polymorphism is not associated with increased risk of KOA. In addition, our data suggest that also the asporin polymorphism is not a major influence on OA etiology by ethnicity. Similarly, Xing et al in meta analysis of seven studies (2334 KOA patients and 3181 controls) have suggested that the D-repeat of asporin gene (ASPN) may not be a major susceptibility locus in the Caucasian and Asian populations with KOA.22 However, in a meta analysis of six studies, Nakamura et al suggested a strong association between KOA and the ASPN D-repeat alleles.35 We suggest such differences may be explained due to the limited number of studies included by Nakamura et al. It seems low statistical power due to the low sample size of studies negatively affects the likelihood that a nominally statistically significant finding actually reflects a true effect.36
D13 allele is the most common allele both in cases and control group, the frequencies are 29.9% and 53.4% and D14 allele frequency was estimated 8.5% and 12.5%, respectively. However, Mustafa et al have reported that the D13 allele was more common in controls, and the D14 allele was more common in patients.14 In study by Shi et al, reported that in the developmental dysplasia of the hip (DDH) group, a significantly higher frequency of the D14 allele and significantly lower frequency of D13 was observed.29
Between-study heterogeneity is a multifactorial phoneme, which is a potential problem when interpreting the results of all meta-analyses. It seems different factors such as source and selection of controls, race variation, age, gender and prevalence of lifestyle factors might also generate the heterogeneities.37 When in the current meta analysis subgroup analyses by ethnicity performed high between-study heterogeneity was observed in most comparisons, so sources of between study heterogeneity were able to be investigated by stratifying analyses.
To the best of our knowledge, our work has provided most robust evidence of association between ASPN D-repeat polymorphism and risk of KOA. However, when explaining the results, some limitations of this meta-analysis should be considered. First, the number of published studies was not sufficiently large for a comprehensive analysis, especially for pooled estimate of the effect size. Both fixed effect and random effects models showed significantly increased risk in substitution carriers, but we are not stressing any conclusion using either of these models owing to the small number of studies available at present. Second, the subgroup analyses concerned only Caucasians and Asians. Data regarding other ethnicities such as Africans were not found. Studies conducted on other ethnicity such as Africans are needed. Third, in this meta-analysis, most published studies written in English were searched. It is possible that some important published or unpublished studies written in other languages that meet the inclusion criteria were missed and ignored in the literature search. Hence, inevitable publication bias might exist. This might help to explain the possible existence of differences between current meta-analysis and Nakamura et al study results. Fourth, subgroup analyses regarding age, gender and other risk factors have not been conducted in the present meta-analysis because insufficient data were available from the primary literature. Hence, investigations containing large sample sizes in view of these factors are also desired. Finally, it is clear that KOA is a complex and multifactorial condition, there is much complexity in the etiology of KOA, and there are complex gene–environment and gene–gene interactions in the KOA pathogenesis. However, we did not perform analyses on gene–environment or gene–gene interactions because those included studies did not provide usable data. Therefore, further studies with good design are needed to provide a more precise estimation on the gene–gene or gene–environment interactions in the KOA pathogenesis.
In conclusion, the results of the present meta-analysis suggest that ASPN D-repeat polymorphism might not confer susceptibility to KOA. Further studies with good design are needed to provide a more precise estimation on the gene–gene or gene–environment interactions in the KOA pathogenesis.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
Not applicable.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Loeser R. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26(3):371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2004. The Global Burden of Disease: 2004 Update. [Google Scholar]
- 3.Jia L., Wang Y., Chen J., Chen W. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: a randomized, double blind, placebo-controlled trial. Sci Rep. 2016;6:35453. doi: 10.1038/srep35453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: part I. Caspian J Intern Med. 2011;2(2):205–212. [PMC free article] [PubMed] [Google Scholar]; b) Zhu L., Yang S., Wang S., Gong H., Li L., Wei X. Effectiveness and safety of manufactured Chinese herbal formula for knee osteoarthritis: insights from a systematic review. Evid Based Complement Alternat Med. 2015;2015:1–19. doi: 10.1155/2015/328642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King Lauren K., March Lyn, Anandacoomarasamy Ananthila. Obesity & osteoarthritis. Indian J Med Res. 2013;138:185–193. [PMC free article] [PubMed] [Google Scholar]
- 7.Klußmann A., Gebhardt H., Liebers F. Individual and occupational risk factors for knee osteoarthritis – study protocol of a case control study. BMC Musculoskelet Disord. 2008;9(1) doi: 10.1186/1471-2474-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynard Loughlin J. The genetics and functional analysis of primary osteoarthritis susceptibility. Expert Rev Mol Med. 2013;15 doi: 10.1017/erm.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salter D., Su S., Lee H. Epidemiology and genetics of osteoarthritis. J Med Sci. 2014;34(6):252. [Google Scholar]
- 10.Spector T., Cicuttini F., Baker J., Loughlin J., Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312(7036):940–943. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg J., Zeggini E. Functional genomics in osteoarthritis: past, present, and future. J Orthop Res. 2016;34(7):1105–1110. doi: 10.1002/jor.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castano Betancourt M., Cailotto F., Kerkhof H. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci U S A. 2012;109(21):8218–8223. doi: 10.1073/pnas.1119899109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kizawa H., Kou I., Iida A. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37(2):138–144. doi: 10.1038/ng1496. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa Z., Dowling B., Chapman K., Sinsheimer J.S., Carr A., Loughlin J. Investigating the aspartic acid (D) repeat of asporin as a risk factor for osteoarthritis in a UK Caucasian population. Arthritis Rheum. 2005;52(11):3502–3506. doi: 10.1002/art.21399. [DOI] [PubMed] [Google Scholar]
- 15.Kaliakatsos M., Tzetis M., Kanavakis E. Asporin and knee osteoarthritis in patients of Greek origin. Osteoarthr Cartil. 2006;14(6):609–611. doi: 10.1016/j.joca.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Q., Shi D., Yi L. Replication of the association of the aspartic acid repeat polymorphism in the asporin gene with knee osteoarthritis susceptibility in Han Chinese. J Hum Genet. 2006;51(12):1068–1072. doi: 10.1007/s10038-006-0065-6. [DOI] [PubMed] [Google Scholar]
- 17.Atif U., Philip A., Aponte J. Absence of association of asporin polymorphisms and osteoarthritis susceptibility in US Caucasians. Osteoarthr Cartil. 2008;16(10):1174–1177. doi: 10.1016/j.joca.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J.H., Lee H.S., Kim C.J. Aspartic acid repeat polymorphism of the asporin gene with susceptibility to osteoarthritis of the knee in a Korean population. Knee. 2008;15(3):191–195. doi: 10.1016/j.knee.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Arellano R.D., Hernandez F., Garcia-Sepulveda C.A., Velasco V.M., Loera C.R., Arguello J.R. The D-repeat polymorphism in the ASPN gene and primary knee osteoarthritis in a Mexican mestizo population: a case-control study. J Orthop Sci. 2013;18(5):826–831. doi: 10.1007/s00776-013-0414-1. [DOI] [PubMed] [Google Scholar]
- 20.Jazayeri R., Qoreishi M., Hoseinzadeh H.R. Investigation of the asporin gene polymorphism as a risk factor for knee osteoarthritis in Iran. Am J Orthop (Belle Mead NJ) 2013;42(7):313–316. [PubMed] [Google Scholar]
- 21.González-Huerta N., Borgonio-Cuadra V., Zenteno J., Cortés-González S., Duarte-Salazar C., Miranda-Duarte A. D14 repeat polymorphism of the asporin gene is associated with primary osteoarthritis of the knee in a Mexican Mestizo population. Int J Rheum Dis. 2015 Dec 1 doi: 10.1111/1756-185X.12797. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Xing D., Ma X., Ma J. Association between aspartic acid repeat polymorphism of the asporin gene and susceptibility to knee osteoarthritis: a genetic meta-analysis. Osteoarthr Cartil. 2013;21(11):1700–1706. doi: 10.1016/j.joca.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Thabane L., Mbuagbaw L., Zhang S. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013;13:92. doi: 10.1186/1471-2288-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 27.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji J., Dai J., Shi D., Jiang Q. Association of genetic and mechanical factors with age of onset of knee osteoarthritis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2010;27(6):672–674. doi: 10.3760/cma.j.issn.1003-9406.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Shi D., Nakamura T., Dai J. Association of the aspartic acid-repeat polymorphism in the asporin gene with age at onset of knee osteoarthritis in Han Chinese population. J Hum Genet. 2007;52(1):664–667. doi: 10.1007/s10038-007-0166-x. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Lopez J., Pombo-Suarez M., Liz M., Gomez-Reino J.J., Gonzalez A. Lack of association of a variable number of aspartic acid residues in the asporin gene with osteoarthritis susceptibility: case-control studies in Spanish Caucasians. Arthritis Res Ther. 2006;8(3):R55. doi: 10.1186/ar1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riyazi N., Rosendaal F., Slagboom E., Kroon H., Breedveld F., Kloppenburg M. Risk factors in familial osteoarthritis: the GARP sibling study. Osteoarthr Cartil. 2008;16(6):654–659. doi: 10.1016/j.joca.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Shen J., Li S., Chen D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014;2:14002. doi: 10.1038/boneres.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L., Li Z., Liu S., Xu S., Ni G. Asporin and osteoarthritis. Osteoarthr Cartil. 2015;23(6):933–939. doi: 10.1016/j.joca.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Ilea C., Lupan I., Leucuta D.C., Lucaciu D. Investigation of asporin gene polymorphism (rs387906276) as a risk factor for primary hip osteoarthritis in Romanian population. Med Con Oct. 2015;10(3):13–18. [Google Scholar]
- 35.Nakamura T., Shi D., Tzetis M. Meta-analysis of association between the ASPN D-repeat and osteoarthritis. Hum Mol Genet. 2007;16(14):1676–1681. doi: 10.1093/hmg/ddm115. [DOI] [PubMed] [Google Scholar]
- 36.Button K., Ioannidis J., Mokrysz C. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5) doi: 10.1038/nrn3475. 442–442. [DOI] [PubMed] [Google Scholar]
- 37.Haidich A.B. Meta-analysis in medical research. Hippokratia. 2010;14(suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]