Abstract
Background
Tendons and ligaments are common sites of musculoskeletal injuries especially during physical activity. The multifactorial etiology of tendon-ligament injury (TLI) includes both genetic and environmental factors. The genetic component could render influence on TLI risk to be either elevation or reduction.
Objective
Inconsistency of reported associations of the collagen type V alpha 1 chain (COL5A1) polymorphisms, mainly rs12722 (BstUI) and rs13946 (DpnII), with TLI warrant a meta-analysis to determine more precise pooled associations.
Methods
Multi-database literature search yielded eight articles (11 studies) for inclusion. Pooled odds ratios (ORs) and 95% confidence intervals were used to estimate associations. Heterogeneity of outcomes warranted examining their sources with outlier treatment.
Results
All rs12722 effects indicated reduced risk (OR < 1.0). The significant outcomes (ORs 0.59–0.77, p = 0.0009–0.04) in the pre-outlier analysis were non-heterogeneous (p > 0.10). The non-significant and heterogeneous (ORs 0.63–0.98, p = 0.13–0.95; up to I2 = 86%) pre-outlier rs12722 and rs13946 results became significant (ORs 0.32–0.78, p = 10−5−0.01) and heterogeneity eliminated (I2 = 0%) with outlier treatment. Significant associations (ORs 0.26–0.65, p = 0.002–0.03) were also observed in other COL5A1 polymorphisms (rs71746744 and rs16399). Sensitivity analysis deemed all significant outcomes to be robust.
Conclusions
In summary, COL5A1 polymorphisms reduce the risk of TLI among Caucasians. These findings are based on the evidence of significance, homogeneity, consistency, and robustness. Additional studies are warranted to draw more comprehensive conclusions.
Electronic supplementary material
The online version of this article (10.1186/s40798-018-0161-0) contains supplementary material, which is available to authorized users.
Keywords: COL5A1 polymorphisms, Tendon-ligament injury, Meta-analysis
Key points
COL5A1 polymorphisms reduce the risk of tendon-ligament injury among Caucasians.
Pre-outlier reduced risk effects on tendon-ligament injury were significant in rs12722 but not in rs13946.
Outlier treatment impacted upon the rs12722 and rs13946 polymorphism outcomes, with significance strengthened and gained, respectively.
Non-heterogeneous significant reduced risk of tendon injury observed in rs71746744 and rs16399 obviated the need for outlier treatment.
Background
Normal tendons and ligaments differ in function and are impacted under conditions of injury [1]. Tendon-ligament injury (TLI) includes Achilles tendon pathology (ATP), Achilles tendinopathy (AT), tennis elbow (TE), and anterior cruciate ligament rupture (ACLR). These common sites of musculoskeletal injuries are occupational and sports-related [2, 3]. ATP is a broad term that refers to AT which results from acute or repetitive mechanical loading during occupational and sporting activities [4]. TE (lateral epicondylitis) is a painful musculotendinous condition originating from the lateral epicondyle of the humerus related to overuse [5] and involves highly repetitive movements [6]. ACLR involves sprain or tear of the anterior cruciate ligament which scaffolds the bones within the knee helping to keep it stable. As over 70% of ACLR injuries are noncontact [7], it puts high risk (up to 10 times) on athletes performing sudden decelerations or changes in direction [8]. ACL ruptures are considered one of the most severe injuries sustained in sports [9]. Etiology of TLI involves genes and protein structure changes [3]. Changes in collagen composition and expression of the genes that encode for these proteins have been shown to be altered in TLI [10]. Collagen is the main component of tendons and ligaments [11]; its fibrils comprising collagen type I, III, V, VI, XI, and XIV [12]. Type V collagen may be a structurally minor player in the collagen hierarchy but is functionally prominent where it plays an important role in regulating fiber diameter as well as assembly (fibrillogenesis) of collagen fibers [13]. Type V collagen protein is encoded by the collagen type V alpha 1 chain (COL5A1) gene, located on the long (q) arm of chromosome 9 [14] and is expressed in both tendons and ligaments [1]. Polymorphisms in the COL5A1 gene have been found to impact upon TLI, the most prominent being rs12722 (BstUI) and rs13946 (DpnII) which are found within the 3′-untranslated region (UTR) [15]. Other COL5A1 polymorphisms (rs71746744, rs16399, and rs3196378) have been considered as risk modifiers [16]. Primary studies have been conducted to investigate genetic risk factors involving COL5A1 polymorphisms in TLI, but results have been inconsistent. Inconsistency of results may be attributed to lack of statistical power because of small sample sizes. Meta-analysis synthesizes primary study data yielding an aggregate sample size to indicate raised statistical power. Therefore, we perform a meta-analysis of all available data on COL5A1 polymorphisms and their relationship with TLI so that we could obtain better estimates of associations.
Methods
Selection of studies
We searched MEDLINE using PubMed, Science Direct, and Google Scholar for association studies as of April 15, 2018. The terms used were “type V collagen,” “COL5A1,” “ligament injury,” “tendon injury,” and “polymorphism” as medical subject heading and text, restricted to English. References cited in the retrieved articles were also screened manually to identify additional eligible studies. Inclusion criteria were (i) case–control studies evaluating the association between COL5A1 polymorphisms and risk for TLI, (ii) sufficient genotype/allele frequency data presented to calculate the odds ratios (ORs) and 95% confidence intervals (CIs), and (iii) participants were either athletes or non-athletes. Exclusion criteria were (i) non-English articles and (ii) studies whose genotype or allele frequencies were unavailable or, when they are, combined with other polymorphisms, preventing proper data extraction.
Data extraction
Two investigators (NP and PT) independently extracted data and arrived at a consensus. Extracted data were tabulated; when needed, we contacted authors of the original articles to request for additional information. The following information were obtained from each publication: first author’s name, published year, country of origin, ethnicity, TLI and COL5A1 polymorphism type, and basis for matching (Table 1). Departures of genotypic frequencies from the Hardy–Weinberg Equilibrium (HWE) in control subjects were determined with the χ2 test.
Table 1.
Characteristics of the included studies that examined the association of COL5A1 polymorphisms with TLI studies
| K | First author | [R] | n | Year | Country | TLI | COL5A1 Polymorphisms | Tissue Sources | UM | BM | CB Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abraham | [25] | 2 | 2013 | SA/AU | AT | rs71746744/rs16399 | Blood | Yes | Ag/He | 8 |
| 2 | Altinisik | [26] | 1 | 2015 | TUR | TE | rs12722, rs13946 | Blood | Yes | Ag/Sx | 7 |
| 3 | Brown | [27] | 1 | 2016 | UK | ATP | rs12722/rs3196378/rs71746744 | Oral | Yes | Ag/G | 8 |
| 4 | Mokone | [28] | 1 | 2006 | SA | ATP | rs12722 | Blood | Yes | Ag/Sx | 8 |
| 5 | O’Connell | [29] | 2 | 2014 | SA/POL | ACLR | rs12722 | Blood | Yes | Ag/Sx/We | 9 |
| 6 | Posthumus | [30] | 1 | 2009 | SA | ACLR | rs12722/rs13946 | Blood | Yes | Ag | 6 |
| 7 | September | [31] | 2 | 2009 | SA/AU | AT | rs12722/rs13946/rs3196378 | Blood | Yes | Ag/G | 8 |
| 8 | Stepien–Slodkowska | [32] | 1 | 2015 | POL | ACLR | rs12722/rs13946 | Oral | Yes | NM | 9 |
K number designation of the article, [R] reference, n number of studies per article, SA South Africa, AU Australia, TUR Turkey, UK United Kingdom, POL Poland, TLI tendon-ligament injury, AT achilles tendinopathy, TE tennis elbow, ATP achilles tendon pathology, ACLR anterior cruciate ligament rupture, rs12722 BstUI, rs13946 DpnII, UM used matching, BM basis for matching, Ag age, He height, Sx sex, G geography, We weight, NM no mention, CB Clark–Baudouin
Modifier treatment and subgrouping
Additional file 1: Table S1 shows the sample sizes, number of cases and controls, and genotype frequencies, including the minor allele (maf) and p values for HWE. Confining the analyses to HWE-compliant studies constituted modifier treatment. Subgrouping was based on injury type (tendon or ligament).
Quality assessment of the studies
We used the Clark–Baudouin (CB) scale to evaluate methodological quality of the included studies [17]. We found this scale most appropriate because it uses criteria such as p values, power, corrections for multiplicity, comparative sample sizes between cases and controls, use of the HWE, and genotyping methods. CB scores range from 0 (worst) to 10 (best) where scoring is based on quality (low < 5, moderate 5–7, and high ≥ 8).
Meta-analysis
Examining five COL5A1 polymorphisms (rs12722, rs13946, rs71746744, rs16399, and rs3196378) warranted the use of a common notation indicating variant (var) and wild-type (wt) alleles. Additional file 1: Table S1, however, details the genotypes for each of the five COL5A1 polymorphisms. After estimating TLI risk (OR) for each study, pooled ORs with 95% CIs were calculated for the following genetic models: (i) homozygous: (var–var and wt–wt) genotypes compared with wt–wt, (ii) recessive: (var–var versus wt–var+wt–wt), (iii) dominant: (wt–wt versus wt–var+var–var), and (iv) codominant: (var versus wt). To compare effects on the same baseline, we used raw data for genotype frequencies to calculate pooled ORs, using either fixed [18] or random [19] effects models. Heterogeneity between studies was (i) estimated with the χ2-based Q test [20], (ii) quantified with the I2 statistic which measures degree of inconsistency among studies [21], and (iii) its sources (outliers) detected with the Galbraith plot [22], then subjected to outlier treatment which involves elimination of the outliers followed by reanalysis. Sensitivity analysis, which involves omitting one study at a time and recalculating the pooled ORs, was used to test for robustness of the summary effects. Robustness indicates that the pooled effects are stable, unaltered even when each study is removed. Publication bias was not examined because the qualitative and quantitative tests have low sensitivity when the number of studies is < 10 [23]. Data were analyzed using Review Manager 5.3 (Cochrane Collaboration, Oxford, England), SIGMASTAT 2.03 and SIGMAPLOT 11.0 (Systat Software, San Jose, CA, USA). Two-sided p values of < 0.05 were considered significant except in estimations of heterogeneity. Given the low power of the χ2-based Q test for heterogeneity, the p value was set at < 0.10 [24].
Results
Search outcomes
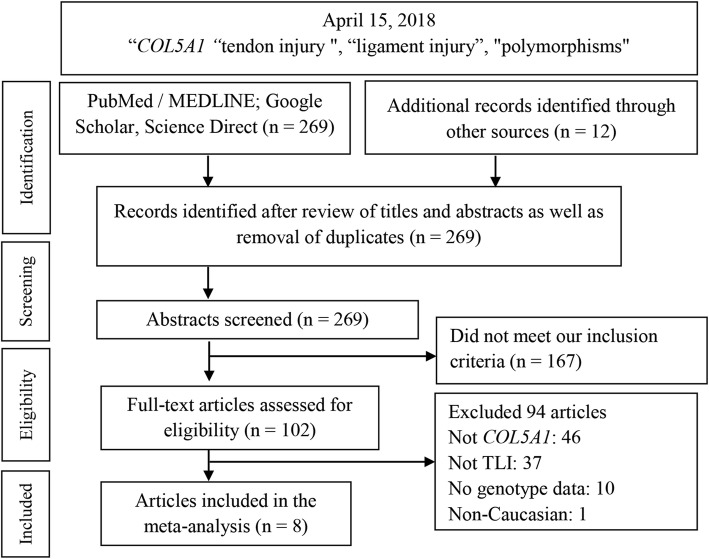
Figure 1 outlines the study selection process in a flowchart following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A total of 281 citations during the initial search was subjected to a series of omissions that eventually yielded eight articles for inclusion [25–32].
Fig. 1.
Summary flowchart of literature search. TLI tendon-ligament injury
Study characteristics
Table 1 shows the year range of studies from 2006 to 2016. We confined our meta-analysis to Caucasians only in order to reduce the likelihood of confounding by population stratification. Injury type subgroups were tendon (AT/ATP/TE) and ligament (ACLR). CB scores for the mean (7.88 ± 0.99), median (8.0), and range (6–9) indicate that methodological quality of the component studies was high. Additional file 1: Table S1 shows the quantitative features of the component studies under each of the five polymorphisms. Independent data from three articles [25, 29, 31] (on account of geography), put the number of studies to nine, five, three, two, and three for rs12722, rs13946, rs71746744, rs16399, and rs3196378, respectively. Respective aggregate sample sizes (case/control) for these polymorphisms are 1234/1667, 546/934, 191/299, 120/254, and 270/520. Assuming small effect size (d = 0.20) and an α level of 0.05 (two-tail), statistical powers are adequate for rs12722 (99%) and rs13946 (96%). Two studies [26, 28] reported data on rs12722 A1 and A2 alleles which are BstUI products of restriction fragment length polymorphism due to two single-nucleotide polymorphism substitutions, the A2 allele referring to the variant C allele [33]. Extracted separately, these data yielded highly significant p values for HWE (up to 10−5). We minimized this by combining the A1 and A2 data. Three studies from two articles were HWE-non-compliant [26, 31]. The PRISMA checklist provides detailed description of this meta-analysis (Additional file 2: Table S2).
rs12722 and rs13946 effects
Pooled effects (p < 0.05) indicating reduced (protective) risk (OR < 1.0) were observed in all significant ORs and non-significant ORs that were altered with outlier treatment as well as all post-outlier outcomes (Table 2). All post-outlier outcomes were also non-heterogeneous (fixed-effects). Outlier treatment induced the following outcomes: (i) significance either acquired or elevated, (ii) heterogeneity either reduced or eliminated, and (iii) narrowing of CIs indicating increased precision.
Table 2.
Summary effects in the overall, modifier (HWE), and subgroup (tendon/ligament) analyses of COL5A1 with TLI
| Comparison [R] genetic model | n | Test of association | Test of heterogeneity | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P a | P b | I2 (%) | AM | ||
| rs12722 All [26–32] | |||||||
| H | 9 | 0.69 | 0.54–0.89 | 0.003 | 0.25 | 21 | FE |
| R | 9 | 0.72 | 0.52–0.99 | 0.04 | 0.05 | 48 | RE |
| D | 9 | 0.90 | 0.68–1.21 | 0.49 | 0.001 | 69 | RE |
| C | 9 | 0.87 | 0.69–1.10 | 0.24 | 0.0001 | 75 | RE |
| rs13946 All [26, 30–32] | |||||||
| H | 5 | 0.83 | 0.33–2.08 | 0.69 | 0.006 | 72 | RE |
| R | 5 | 0.79 | 0.35–1.78 | 0.57 | 0.02 | 67 | RE |
| D | 5 | 1.12 | 0.78–1.63 | 0.54 | 0.02 | 65 | RE |
| C | 5 | 1.01 | 0.76–1.34 | 0.94 | 0.03 | 63 | RE |
| rs12722 HWE [27–32] | |||||||
| H | 7 | 0.68 | 0.52–0.88 | 0.004 | 0.13 | 39 | FE |
| R | 7 | 0.76 | 0.53–1.09 | 0.13 | 0.04 | 55 | RE |
| D | 7 | 0.86 | 0.66–1.12 | 0.27 | 0.05 | 52 | RE |
| C | 7 | 0.88 | 0.66–1.17 | 0.37 | 10−5 | 79 | RE |
| rs13946 HWE [26, 30–32] | |||||||
| H | 4 | 1.03 | 0.39–2.72 | 0.95 | 0.02 | 70 | RE |
| R | 4 | 1.00 | 0.44–2.27 | 1.00 | 0.05 | 61 | RE |
| D | 4 | 1.15 | 0.72–1.83 | 0.56 | 0.01 | 73 | RE |
| C | 4 | 1.06 | 0.76–1.46 | 0.74 | 0.03 | 68 | RE |
| rs12722 Tendon [26–28, 31] | |||||||
| H | 5 | 0.87 | 0.59–1.27 | 0.46 | 0.11 | 47 | FE |
| R | 5 | 0.81 | 0.42–1.57 | 0.53 | 0.009 | 70 | RE |
| D | 5 | 0.84 | 0.55–1.29 | 0.43 | 0.01 | 73 | RE |
| C | 5 | 0.98 | 0.62–1.55 | 0.95 | 10−5 | 86 | RE |
| rs13946 Tendon [26, 31] | |||||||
| H | 3 | 0.70 | 0.14–3.56 | 0.66 | 0.001 | 85 | RE |
| R | 3 | 0.63 | 0.16–2.58 | 0.52 | 0.005 | 81 | RE |
| D | 3 | 1.15 | 0.63–2.12 | 0.65 | 0.01 | 78 | RE |
| C | 3 | 0.97 | 0.58–1.61 | 0.90 | 0.006 | 81 | RE |
| rs12722 Ligament [29, 30, 32] | |||||||
| H | 4 | 0.59 | 0.43–0.82 | 0.001 | 0.96 | 0 | FE |
| R | 4 | 0.65 | 0.49–0.86 | 0.003 | 0.80 | 0 | FE |
| D | 4 | 0.99 | 0.66–1.50 | 0.96 | 0.03 | 68 | RE |
| C | 4 | 0.77 | 0.66–0.90 | 0.0009 | 0.95 | 0 | FE |
| rs13946 Ligament [30, 32] | |||||||
| H | 2 | 1.07 | 0.51–2.26 | 0.85 | 0.39 | 0 | FE |
| R | 2 | 1.10 | 0.54–2.24 | 0.80 | 0.22 | 34 | FE |
| D | 2 | 1.04 | 0.74–1.46 | 0.82 | 0.16 | 48 | FE |
| C | 2 | 1.04 | 0.80–1.35 | 0.79 | 0.53 | 0 | FE |
Cut-offs for Pa and Pb are < 0.05 and 0.10, respectively. Values in italics indicate significant associations
H homozygous, R recessive, D dominant, C codominant, n number of studies, [R] references, OR odds ratio, CI confidence interval, Pa p value for association, Pb p value for heterogeneity, AM analysis model, HWE Hardy–Weinberg Equilibrium, FE fixed-effects, RE random-effects
Overall
Our hypothesis-driven approach indicates associations of COL5A1 with TLI. Table 2 shows significant pre-outlier effects in rs12722 but not rs13946. These associations were observed in the overall (ORs 0.69–0.72, p = 0.003–0.04) and modifier (OR 0.68, p = 0.004) analyses. Non-significant heterogeneous pooled effects in rs12722 and rs13946 (overall and HWE: ORs 0.76–0.90, p = 0.13–0.69) were altered to significance with outlier treatment, outcomes of which are summarized in Table 3. These outcomes show gain in significance seen in the overall analysis of rs12722 (ORs 0.72–0.78, p = 0.0001–0.0003) and rs13946 (ORs 0.35–0.37, p = 0.009–0.01), modifier (HWE) outcomes in rs12722 (ORs 0.67–0.76, p = 10−5−0.004).
Table 3.
Post-outlier analysis outcomes of the COL5A1 polymorphism associations with TLI
| Comparison genetic model | n | [R] | Test of association | Test of heterogeneity | Outlier treatment effect | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P a | P b | I2 (%) | AM | ||||
| rs12722 all | |||||||||
| R | 8 | [26, 27, 29, 31, 32] | 0.65 | 0.52–0.81 | 0.0002 | 0.19 | 30 | FE | RH, ES |
| D | 7 | [26–29, 31, 32] | 0.72 | 0.61–0.86 | 0.0003 | 0.88 | 0 | FE | EH, GS |
| C | 8 | [26, 28–32] | 0.78 | 0.69–0.87 | 0.0001 | 0.63 | 0 | FE | EH, GS |
| rs13946 all | |||||||||
| H | 3 | [30, 31] | 0.37 | 0.17–0.82 | 0.01 | 0.73 | 0 | FE | EH, GS |
| R | 3 | [30, 31] | 0.35 | 0.16–0.77 | 0.009 | 0.86 | 0 | FE | EH, GS |
| D | 4 | [30–32] | 0.95 | 0.74–1.22 | 0.69 | 0.36 | 8 | FE | RH |
| C | 4 | [30–32] | 0.90 | 0.74–1.10 | 0.30 | 0.36 | 6 | FE | RH |
| rs12722 HWE | |||||||||
| R | 6 | [27, 29–32] | 0.67 | 0.53–0.86 | 0.002 | 0.14 | 40 | FE | RH. GS |
| D | 6 | [27–32] | 0.76 | 0.63–0.92 | 0.004 | 0.96 | 0 | FE | EH, GS |
| C | 6 | [28–32] | 0.76 | 0.66–0.86 | 10 −5 | 0.94 | 0 | FE | EH, GS |
| rs13946 HWE | |||||||||
| H | 3 | [31] | 0.73 | 0.38–1.38 | 0.33 | 0.16 | 46 | FE | RH |
| R | 3 | [31] | 0.77 | 0.41–1.43 | 0.40 | 0.11 | 55 | FE | RH |
| D | 3 | [31] | 0.93 | 0.71–1.24 | 0.64 | 0.20 | 37 | FE | RH |
| C | 3 | [31] | 0.92 | 0.74–1.16 | 0.49 | 0.23 | 32 | FE | RH |
| rs12722 tendon | |||||||||
| R | 3 | [26, 31] | 0.45 | 0.28–0.72 | 0.0008 | 0.45 | 0 | FE | EH, GS |
| D | 4 | [26–28, 31] | 0.66 | 0.52–0.84 | 0.0006 | 0.76 | 0 | FE | EH, GS |
| C | 4 | [26–28, 31] | 0.78 | 0.65–0.94 | 0.008 | 0.18 | 38 | FE | RH, GS |
| rs13946 tendon | |||||||||
| H | 2 | [31] | 0.32 | 0.13–0.79 | 0.01 | 0.69 | 0 | FE | EH, GS |
| R | 2 | [31] | 0.32 | 0.13–0.78 | 0.01 | 0.79 | 0 | FE | EH, GS |
| D | 2 | [31] | 0.85 | 0.59–1.24 | 0.40 | 0.39 | 0 | FE | EH |
| C | 2 | [31] | 0.75 | 0.55–1.02 | 0.06 | 0.58 | 0 | FE | EH |
| rs12722 ligament | |||||||||
| D | 3 | [29, 30, 32] | 0.8 | 0.62–1.04 | 0.10 | 0.97 | 0 | FE | EH |
Cut-offs for Pa and Pb are < 0.05 and 0.10, respectively. Values in italics indicate significant associations
H homozygous, R recessive, D dominant, C codominant, n number of studies, [R] References, OR odds ratio, CI confidence interval, Pa p-value for association, Pb p value for heterogeneity, AM analysis model, HWE Hardy–Weinberg Equilibrium, FE fixed-effects, RH reduced heterogeneity, ES elevated significance, EH eliminated heterogeneity, GS gained significance
Mechanism of outlier treatment in rs12722
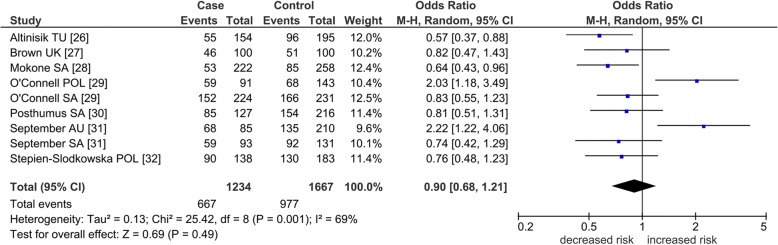
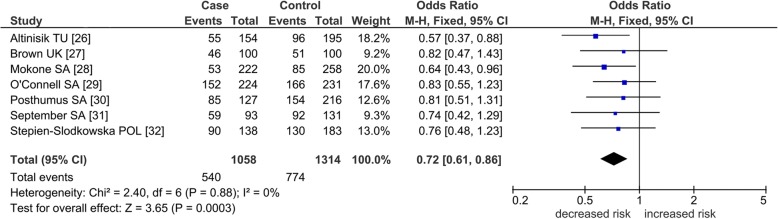
Operation of outlier treatment is visualized in Figs. 2, 3, and 4 for rs12722 in the dominant model. In Fig. 2, the pooled effect is non-significant (OR 0.90, p = 0.49) and heterogeneous (I2 = 69%). Sources of this heterogeneity were identified with the Galbraith plot visualized in Fig. 3 which shows the two outlying studies [29, 31] located above the + 2 confidence limit. Figure 4 shows the post-outlier value of acquired significance (OR 0.72, p = 0.0003) and eliminated heterogeneity (I2 = 0%).
Fig. 2.
Summary association of COL5A1 rs12722 polymorphism with TLI studies in the dominant model. Numbers in brackets under the study column indicate references. Squares indicate the odds ratio (OR) in each study, with square sizes directly proportional to the weight contribution (%) of the study. Horizontal lines on each side of the squares represent 95% confidence intervals (CIs). The random-effects model was used on account of the presence of heterogeneity (p = 0.001, I2 = 69%). M–H Mantel–Haenszel; df degree of freedom; TU Turkey; UK United Kingdom; POL Poland; SA South Africa; AU Australia
Fig. 3.
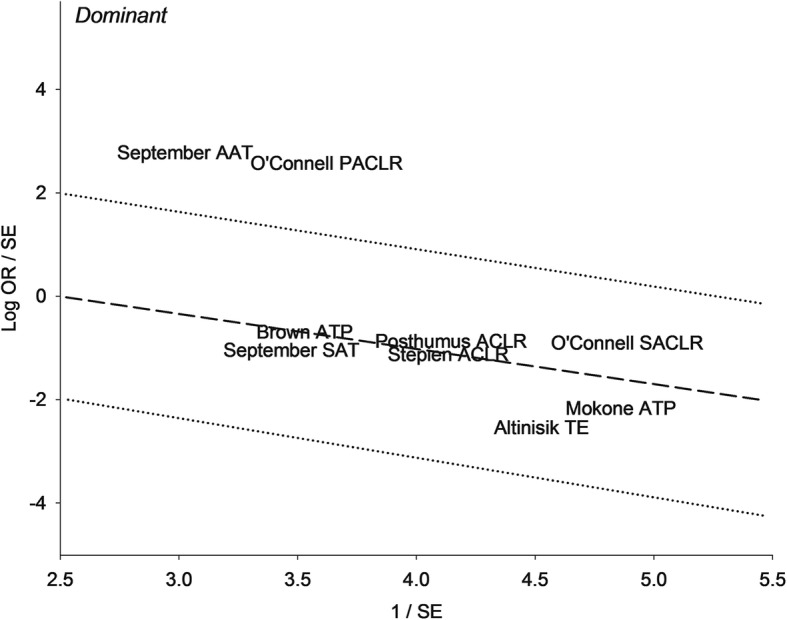
Galbraith plot analysis of COL5A1 rs12722 polymorphism in the dominant model. The two studies found above the + 2 confidence limit are identified as outliers. OR, odds ratio; SE, standard error; AAT, Australia Achilles Tendinopathy; PACLR, Poland Anterior Cruciate Ligament Rupture; SAT, South Africa Achilles Tendinopathy; SACLR, South Africa Anterior Cruciate Ligament Rupture; ATP, Achilles Tendinopathy; TE, Tennis elbow
Fig. 4.
Post-outlier association of COL5A1 rs12722 polymorphism with TLI studies in the dominant model. Numbers in brackets under the study column indicate references. Squares indicate the odds ratio (OR) in each study, with square sizes directly proportional to the weight contribution (%) of the study. Horizontal lines on each side of the squares represent 95% confidence intervals (CIs). The fixed-effects model was used on account of zero heterogeneity (p = 0.88, I2 = 0%). M–H Mantel–Haenszel; df degree of freedom; TU, Turkey; UK, United Kingdom; SA, South Africa; POL, Poland
Tendon/ligament subgroups
Table 2 shows that rs12722 ligament pooled ORs were the only significant subgroup outcomes in the pre-outlier analyses (ORs 0.59–0.77, p = 0.0009–0.003). Non-significant tendon subgroup outcomes (rs12722: ORs 0.81–0.98, p = 0.43–0.95; rs13946: ORs 0.63–0.70, p = 0.52–0.66) were altered to significance (rs12722: ORs 0.45–0.78, p = 0.0006–0.008; rs13946: ORs 0.32, p = 0.01) following outlier treatment (Table 3).
Effects of the other COL5A1 polymorphisms
Table 4 shows associations of rs71746744 (ORs 0.45–0.65, p = 0.003–0.03) and rs16399 (ORs 0.26–0.58, p = 0.002–0.02) with tendon injuries only but not rs319378 (ORs 0.70–1.22, p = 0.06–0.77). These associations were either non-heterogeneous (I2 = 12–46%) or homogeneous (I2 = 0%).
Table 4.
Summary outcomes of associations in rs71746744, rs16399, and rs3196378 COL5A1 polymorphisms with tendon injury
| Comparison [R] genetic model | Test of association | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | P a | P b | I2 (%) | AM | |
| rs71746744 [25, 27] | |||||||
| H | 3 | 0.45 | 0.22–0.95 | 0.03 | 0.31 | 13 | FE |
| R | 3 | 0.57 | 0.28–1.17 | 0.13 | 0.32 | 12 | FE |
| D | 3 | 0.57 | 0.39–0.83 | 0.004 | 0.70 | 0 | FE |
| C | 3 | 0.65 | 0.48–0.87 | 0.003 | 0.41 | 0 | FE |
| rs16399 [25] | |||||||
| H | 2 | 0.26 | 0.10–0.69 | 0.007 | 0.38 | 0 | FE |
| R | 2 | 0.31 | 0.12–0.81 | 0.02 | 0.40 | 0 | FE |
| D | 2 | 0.57 | 0.37–0.88 | 0.01 | 0.42 | 0 | FE |
| C | 2 | 0.58 | 0.41–0.82 | 0.002 | 0.23 | 30 | FE |
| rs319378 [27, 31] | |||||||
| H | 3 | 0.93 | 0.58–1.49 | 0.77 | 0.76 | 0 | FE |
| R | 3 | 0.70 | 0.49–1.01 | 0.06 | 0.50 | 0 | FE |
| D | 3 | 1.22 | 0.84–1.77 | 0.29 | 0.16 | 46 | FE |
| C | 3 | 0.83 | 0.67–1.02 | 0.08 | 0.22 | 34 | FE |
Cut-offs for Pa and Pb are < 0.05 and 0.10, respectively. Values in italics indicate significant associations
H homozygous, R recessive, D dominant, C codominant, n number of studies, [R] References, OR odds ratio, CI confidence interval, Pa p value for association, Pb p value for heterogeneity, AM analysis model, FE fixed-effects
Heterogeneity analysis
Pre-outlier ORs were generally heterogeneous (pheterogeneity < 0.10). Outlier treatment impacts upon heterogeneity by reducing (pheterogeneity > 0.10) or eliminating it (I2 = 0%). Table 2 shows six significant pooled ORs, five of which are non-heterogeneous (fixed-effects). Of the five, three (60%) have zero heterogeneity (I2 = 0%). Table 3 shows 13 significant pooled ORs, all of which are non-heterogeneous (fixed-effects). Of the 13, 10 (77%) have zero heterogeneity (I2 = 0%).
Sensitivity analysis
This treatment was applied in all comparisons and stratified by genetic model with emphasis on significant effects (*).Table 5 summarizes our sensitivity analysis findings. The total number of significant effects across comparisons and genetic models is indicated by (S). Comparisons that were robust are labeled as such (robust) and those that were not are identified by reference number. Reference numbers reduce robustness of the comparisons. The total number of robust comparisons is indicated by (B), and the total number of identified reference numbers is indicated by (A). This approach to sensitivity treatment allows identification of the most and least robust comparisons and genetic models. Aggregate robustness was based on the least number of A and most counts for B and S. Thus, the most robust comparisons were rs12722 (pre- and post-outlier outcomes) and rs71746744 effects and in terms of genetic model, recessive and codominant effects.
Table 5.
Sensitivity analysis for all COL5A1 comparisons
| Comparison | Genetic model | S | A | B | |||
|---|---|---|---|---|---|---|---|
| Homozygous | Recessive | Dominant | Codominant | ||||
| All | |||||||
| rs12722 | Robust* | Robust* | Robust | Robust | 2 | 0 | 4 |
| rs13946 | [31]a [31]b | [31]b | [26] | [26, 30] | 0 | 4 | 0 |
| Modifier | |||||||
| rs12722 HWE | Robust* | Robust | Robust | Robust | 1 | 0 | 4 |
| rs13946 HWE | [26, 32] | [26, 32] | [26] | [31]a | 0 | 3 | 0 |
| Subgroup | |||||||
| rs12722 tendon | [31]b | [31]b | Robust | [26, 28] [31]b | 0 | 3 | 1 |
| rs13946 tendon | [31]a | [26] | Robust | [31]a [31]b | 0 | 3 | 1 |
| rs12722 ligament | Robust* | Robust* | [29, 30, 32] | Robust* | 3 | 3 | 3 |
| rs13946 ligament | Robust | Robust | [30] | [30] | 0 | 1 | 2 |
| Other COL5A1 polymorphisms | |||||||
| rs71746744 | Robust* | Robust | Robust* | Robust* | 3 | 0 | 4 |
| rs16399 | Robust* | Robust* | Robust* | Robust* | 4 | 0 | 4 |
| rs3196378 | [27] | Robust | Robust | Robust | 0 | 1 | 3 |
| Post-outlier | |||||||
| All | |||||||
| rs12722 | – | Robust* | Robust* | Robust* | 3 | 0 | 3 |
| rs13946 | Robust* | Robust* | [31]a | Robust | 2 | 1 | 3 |
| Modifier | |||||||
| rs12722 HWE | – | Robust* | Robust* | Robust* | 3 | 0 | 3 |
| rs13946 HWE | [31]a | [31]a | [31]a | [31]a | 0 | 1 | 0 |
| Subgroup | |||||||
| rs12722 tendon | [31]b | Robust* | Robust* | Robust* | 3 | 1 | 3 |
| rs13946 tendon | Robust* | Robust* | [31]a | Robust | 2 | 1 | 3 |
| rs12722 ligament | – | – | [29, 30, 32] | – | 0 | 3 | 0 |
| rs13946 ligament | [30] | [32] | [30] | [30] | 0 | 2 | 0 |
| S | 7 | 8 | 5 | 6 | |||
| A | 6 | 4 | 5 | 5 | |||
| B | 8 | 12 | 10 | 11 | |||
| Total number of comparisons | 16 | 18 | 19 | 18 | |||
HWE Hardy–Weinberg Equilibrium, S number of significant associations, A number of non-redundant references that contributed to instability, B number of robust findings
*Significant associations
[31]a AU; [31]b SA
Discussion
Summary of findings
Applying meta-analytical techniques is methodologically complex. This complexity arises from interpreting outcomes resulting from the combined applications of genetic modeling, modifier, outlier, and subgroup analyses as well sensitivity treatment. These considered, rs12722 was more significant (p < 0.05) than rs13946. For both polymorphisms, the most associated models with TLI were homozygous and recessive more than dominant/codominant. On the whole, this meta-analysis delineated which polymorphisms in the COL5A1 gene have associations with TLI (rs12722, rs71746744, rs16399) and those that do not (rs319378) as well as those that were altered (rs12722 and rs13946) with meta-analytical treatment (outlier analysis) such that significance was either intensified or gained. The main finding of this study points to significant reduced risk effects (all ORs < 1.0), up to 41% and 55%, pre- and post-outlier, respectively, in rs12722 and up to 68% in rs13946, post-outlier only. Outlier treatment impacted upon heterogeneity, significance, and precision of outcomes, seen in the overall, modifier, and subgroup analyses. Significant effects (up to 69%) of the other COL5A1 polymorphisms were consistent and homogeneous (most had I2 = 0%) indicating associations of rs71746744 and rs16399 with tendon injury. The combination of significance, consistency, homogeneity, and increased precision of pooled effects in these comparisons improved our findings.
Comparisons with other meta-analysis
We compare our findings with a recent (March 2018) meta-analysis [34] which examined rs12722 only, compared to five COL5A1 polymorphisms in ours. This additional array enabled us to contrast and compare effects of rs12722 with rs13946, not only in the overall analysis but also in the modifier and subgroup outcomes. Caucasian rs12722 comparisons between Lv et al. [34] and ours were based on sample sizes of 1381 and 2677, respectively. Recessive outcomes from these two meta-analyses had contrasting effects. This led us to examine qualitative and quantitative differences at the data level of the primary studies in rs12722 among Caucasians. First, both meta-analyses had six studies in common [26–30, 32]. The previous study [34] included Raleigh et al. [35] who examined interaction between matrix metalloproteinase 3 (MMP3) and COL5A1 but did not differentiate genotype data between these two genes, which was our reason for excluding it in our meta-analysis. Of note, MMP3 was genotyped but not COL5A1. Second, we had two added studies [25, 31] which were not in the previous study [34]. Third, the recessive model was defined differently, TT versus TC+CC for the previous study [34] and var–var versus wt–var+wt–wt in our study. Given this contrast, calculations of the ORs would inevitably have taken diverging outcomes. Thus, OR risks were increased for the previous study [34] and reduced for ours. Other major differences were as follows: (i) our use of outlier treatment but not the previous study [34]; (ii) they tested publication bias, we did not; and (iii) our use of standard genetic models versus model-free approach for Lv et al. [34].
Comparisons with primary studies
Comparing our reduced risk pooled findings with the component study-specific ORs shows the following for the rs12722 CC genotype: (i) decreased AT risk of up to 62% in different populations (Australia and South Africa) [31], (ii) reduced ACLR risk from three studies [29, 30, 32], and (iii) contrasting effects of the A2 and A1 alleles in rs12722 which elicited protective and increased risks in two studies, respectively [26, 28]. In rs12722, individuals with the CC genotype had a significantly decreased risk of developing AT compared with those with a T allele in either TT or TC genotypes [31].
Genetic variations in the COL5A1 gene 3′-UTR region affect mRNA stability and its export from the nucleus after transcription where regulatory sequences control gene expression at the posttranscriptional level [25]. Therefore, mutations or single-nucleotide variations within this region may alter mRNA secondary structure and thus protein characteristics [36]. Functionally, the rs12722 and rs71746744 variants are believed to alter stability of the COL5A1 mRNA [25]. Abrahams et al. [25] investigated other variants in the 3′-UTR of COL5A1 gene where rs71746744 and rs16399 were found to have significant association with AT. The rs71746744 variant del/del genotype was found to be associated with reduced risk of AT. Furthermore, they surmised linkage of rs12722 with rs71746744 and rs16399 [25] which suggest that the protective effects observed in this meta-analysis might be attributed to any or all of these three polymorphisms.
Reported findings on the role of genetic variants in TLI have differed between studies. Several methodological problems may explain the discrepancies, including limited statistical power, unrecognized confounding factors, misleading definition of phenotypes, and stratification of populations [17]. Reporting study-specific effects of COL5A1 polymorphisms ranged from presence to absence of associations. In their presence, risk effects for the variant genotype were increased or reduced, significant or not. Meta-analysis, however, gives more information in reporting effects for COL5A. These involve exploring magnitude and precision of effects, as well as consistency and stability, all in consideration of heterogeneous outcomes. These features raise the levels of evidence to support conclusions on the associations of COL5A1 polymorphisms with TLI.
Strengths and limitations
Interpreting our findings should be contextualized in view of its strengths and limitations. Limitations of our study include the following: (i) we did not examine gender effects due to insufficiency of data. Nevertheless, Posthumus et al. [30] examined gender differences of the CC genotype among ACLR participants; (ii) A1 and A2 alleles for BstUI were not examined separately, but instead, we combined them which may have sacrificed precision of outcomes, given the reported contrasting effects of these two alleles in the literature [26, 28]; (iii) linkage disequilibrium was reported for the COL5A1 polymorphisms in the component studies [25, 31, 32], which may have introduced bias [37] by masking identity of the true causal variant; and (iv) the low number of studies (n = 2–3) and underpowered status (58% and 44%) warrant caution in interpreting the significant effects of rs71746744 and rs16399.
On the other hand, these are the following strengths of the meta-analysis: (i) confining our meta-analysis to Caucasians rendered epidemiological homogeneity to the study which thus excludes potential confounding effects of population stratification; (ii) non-HWE-compliant studies were a minority which minimizes the issue of genotyping errors thus avoiding methodological weaknesses in the summary outputs [38]. Besides, confining our analyses to HWE-compliant studies did not materially alter the outcomes in all genetic models; (iii) overall methodological quality (determined by CB) of the included studies was high; (iv) aggregate case/control totals for rs12722 and rs13946 show that the significant findings from these two polymorphisms have statistical powers of 99% and 96%, respectively; (v) all controls were defined as healthy; (vi) most (75%) tissue sources were blood; (vii) all controls were matched with cases, with 88% based on age; and (viii) sensitivity treatment deemed the significant outcomes to be robust.
Conclusions
The importance of our results is underpinned by the fact that each component study in this meta-analysis lacked adequate statistical power, but when combined using meta-analysis, clear reduced risk associations of COL5A1 polymorphisms with TLI are uncovered. Genetic structure of the homozygous and recessive models point to the variant allele as protectively associated with TLI. TLI is a complex condition involving interactions of several genetic and non-genetic risk factors. Gene-gene and gene-environment interactions have been reported to have roles in the associations of COL5A1 polymorphisms with TLI [3]. None of the eight included articles mentioned gene-environment interaction, but haplotype analysis has been addressed in the component studies [25, 27, 29, 31, 32]. However, it should be emphasized that phenotypic variations between tendon (AT, ATP, and TE) and ligament (ACLR) engender different aetiologies. Additional well-designed studies that explore other ethnic groups based on sample sizes commensurate with detection of small genotypic risks would allow more definitive conclusions about the association of COL5A1 polymorphisms and TLI. Injury-related issues in sports medicine beg comprehensive investigation of its risks. Our contribution is the synthesis approach offered by meta-analysis in elevating the level of evidence. Given the focus of this study, we hope to have clarified the genetic risks posed by COL5A1 polymorphisms to TLI. The mainly protective findings of COL5A1 rs12722 and rs13946 polymorphisms may be modest given our focus on just one gene. However, the evidence we present hopes to contribute to better understanding of the genetic nature of TLI.
Additional files
Table S1. Quantitative characteristics of the included studies that examined the association of COL5A1 polymorphisms with TLI studies. (DOCX 57 kb)
Table S2. PRISMA checklist. (DOCX 30 kb)
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Availability of data and materials
Please contact author for data requests.
Abbreviations
- *
Significant associations
- [R]
References
- 3′ UTR
3′ Untranslated region
- A
Number of non-redundant references that contributed to instability
- AAT
Australia Achilles Tendinopathy
- ACLR
Anterior Cruciate Ligament Rupture
- Ag
Age
- AM
Analysis model
- AT
Achilles tendinopathy
- ATP
Achilles tendon pathology
- AU
Australia
- B
Number of robust findings
- BM
Basis for matching
- C
Codominant
- CB
Clark–Baudouin
- CI
Confidence interval
- COL5A1
Collagen type V alpha 1 chain
- D
Dominant
- d
Measure of the effect side
- EH
Eliminated heterogeneity
- ES
Elevated significance
- FE
Fixed-effects
- G
Geography
- GS
Gained significance
- H
Homozygous
- He
Height
- HWE
Hardy–Weinberg Equilibrium
- I2
Measure of variability between studies
- K
Number designation of the article
- maf
Minor allele frequency
- MMP3
Matrix metalloproteinase 3
- n
Number of studies
- NM
No mention
- OR
Odds ratio
- p
p value
- Pa
p value for association
- PACLR
Poland Anterior Cruciate Ligament Rupture
- Pb
p value for heterogeneity
- POL
Poland
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- R
Recessive
- RE
Random-effects
- RH
Reduced heterogeneity
- rs12722
BstUI
- rs13946
DpnII
- S
Number of significant associations
- SA
South Africa
- SACLR
South Africa Anterior Cruciate Ligament Rupture
- SAT
South Africa Achilles Tendinopathy
- SE
Standard error
- Sx
Sex
- TE
Tennis elbow
- TLI
Tendon-ligament injury
- TUR
Turkey
- UK
United Kingdom
- UM
Used matching
- var
Variant
- We
Weight
- wt
Wild-type
Authors’ contributions
NP and PT conceived and designed the experiments. NP, PT, SP, and HJ analyzed the data. NP, PT, SP, and HJ wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study did not directly involve human participants, human data, or human tissue. Not applicable.
Consent for publication
This manuscript does not contain any individual person’s data in any form; therefore, consent to publish is not applicable.
Competing interests
The authors, Noel Pabalan, Phuntila Tharabenjasin, Suphawadee Phababpha, and Hamdi Jarjanazi, declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Noel Pabalan, Email: npabalan@alumni.yorku.ca.
Phuntila Tharabenjasin, Email: pacezen@yahoo.com.
Suphawadee Phababpha, Email: suphapha9p21@gmail.com.
Hamdi Jarjanazi, Email: hamdi@hamdi.ca.
References
- 1.Hildebrand KA, Frank CB, Hart DA. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene Ther. 2004;11(4):368–378. doi: 10.1038/sj.gt.3302198. [DOI] [PubMed] [Google Scholar]
- 2.Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006;45(5):508–521. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- 3.September AV, Schwellnus MP, Collins M. Tendon and ligament injuries: the genetic component. Br J Sports Med. 2007;41(4):241–246. doi: 10.1136/bjsm.2006.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins M, Raleigh SM. Genetic risk factors for musculoskeletal soft tissue injuries. Med Sport Sci. 2009;54:136–149. doi: 10.1159/000235701.. [DOI] [PubMed] [Google Scholar]
- 5.Savoie FH, 3rd, O'Brien MJ. Arthroscopic tennis elbow release. Instr Course Lect. 2015;64:225–230. [PubMed] [Google Scholar]
- 6.Weber C, Thai V, Neuheuser K, Groover K, Christ O. Efficacy of physical therapy for the treatment of lateral epicondylitis: a meta-analysis. BMC Musculoskelet Disord. 2015;16:223. doi: 10.1186/s12891-015-0665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 8.Parkkari J, Pasanen K, Mattila VM, Kannus P, Rimpela A. The risk for a cruciate ligament injury of the knee in adolescents and young adults: a population-based cohort study of 46 500 people with a 9 year follow-up. Br J Sports Med. 2008;42(6):422–426. doi: 10.1136/bjsm.2008.046185. [DOI] [PubMed] [Google Scholar]
- 9.Brooks JH, Fuller CW, Kemp SP, Reddin DB. Epidemiology of injuries in English professional rugby union: part 1 match injuries. Br J Sports Med. 2005;39(10):757–766. doi: 10.1136/bjsm.2005.018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466(7):1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connell K, Posthumus M, Schwellnus MP, Collins M. Collagen genes and exercise-associated muscle cramping. Clin J Sport Med. 2013;23(1):64–69. doi: 10.1097/JSM.0b013e3182686aa7.. [DOI] [PubMed] [Google Scholar]
- 12.Frank CB. Ligament structure, physiology and function. J Musculoskelet Neuronal Interact. 2004;4(2):199–201. [PubMed] [Google Scholar]
- 13.Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95(Pt 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 14.Collins M, Posthumus M. Type V collagen genotype and exercise-related phenotype relationships: a novel hypothesis. Exerc Sport Sci Rev. 2011;39(4):191–198. doi: 10.1097/JES.0b013e318224e853.. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan DS, BstUI PAE. DpnII RFLPs at the COL5A1 gene. Hum Mol Genet. 1994;3(2):385. doi: 10.1093/hmg/3.2.385-a. [DOI] [PubMed] [Google Scholar]
- 16.Maffulli N, Margiotti K, Longo UG, Loppini M, Fazio VM, Denaro V. The genetics of sports injuries and athletic performance. Muscles Ligam Tend J. 2013;3(3):173–189. [PMC free article] [PubMed] [Google Scholar]
- 17.Clark MF, Baudouin SV. A systematic review of the quality of genetic association studies in human sepsis. Intensive Care Med. 2006;32(11):1706–1712. doi: 10.1007/s00134-006-0327-y. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7(8):889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 23.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176(8):1091–1096. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrahams Y, Laguette MJ, Prince S, Collins M. Polymorphisms within the COL5A1 3′-UTR that alters mRNA structure and the MIR608 gene are associated with Achilles tendinopathy. Ann Hum Genet. 2013;77(3):204–214. doi: 10.1111/ahg.12013. [DOI] [PubMed] [Google Scholar]
- 26.Altinisik J, Meric G, Erduran M, Ates O, Ulusal AE, Akseki D. The BstUI and DpnII variants of the COL5A1 gene are associated with tennis elbow. Am J Sports Med. 2015;43(7):1784–1789. doi: 10.1177/0363546515578661. [DOI] [PubMed] [Google Scholar]
- 27.Brown KL, Seale KB, El Khoury LY, Posthumus M, Ribbans WJ, Raleigh SM, et al. Polymorphisms within the COL5A1 gene and regulators of the extracellular matrix modify the risk of Achilles tendon pathology in a British case-control study. J Sports Sci. 2017;35(15):1475–1483. doi: 10.1080/02640414.2016.1221524. [DOI] [PubMed] [Google Scholar]
- 28.Mokone GG, Schwellnus MP, Noakes TD, Collins M. The COL5A1 gene and Achilles tendon pathology. Scand J Med Sci Sports. 2006;16(1):19–26. doi: 10.1111/j.1600-0838.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 29.O'Connell K, Knight H, Ficek K, Leonska-Duniec A, Maciejewska-Karlowska A, Sawczuk M, et al. Interactions between collagen gene variants and risk of anterior cruciate ligament rupture. Eur J Sport Sci. 2015;15(4):341–350. doi: 10.1080/17461391.2014.936324. [DOI] [PubMed] [Google Scholar]
- 30.Posthumus M, September AV, O'Cuinneagain D, van der Merwe W, Schwellnus MP, Collins M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am J Sports Med. 2009;37(11):2234–2240. doi: 10.1177/0363546509338266.. [DOI] [PubMed] [Google Scholar]
- 31.September AV, Cook J, Handley CJ, van der Merwe L, Schwellnus MP, Collins M. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. Br J Sports Med. 2009;43(5):357–365. doi: 10.1136/bjsm.2008.048793. [DOI] [PubMed] [Google Scholar]
- 32.Stepien-Slodkowska M, Ficek K, Kaczmarczyk M, Maciejewska-Karlowska A, Sawczuk M, Leonska-Duniec A, et al. The variants within the COL5A1 gene are associated with reduced risk of anterior cruciate ligament injury in skiers. J Hum kinet. 2015;45:103–111. doi: 10.1515/hukin-2015-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longo UG, Loppini M, Margiotti K, Salvatore G, Berton A, Khan WS, et al. Unravelling the genetic susceptibility to develop ligament and tendon injuries. Curr Stem Cell Res Ther. 2015;10(1):56–63. doi: 10.2174/1574888X09666140710112535. [DOI] [PubMed] [Google Scholar]
- 34.Lv ZT, Gao ST, Cheng P, Liang S, Yu SY, Yang Q, et al. Association between polymorphism rs12722 in COL5A1 and musculoskeletal soft tissue injuries: a systematic review and meta-analysis. Oncotarget. 2018;9(20):15365–15374. doi: 10.18632/oncotarget.23805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raleigh SM, van der Merwe L, Ribbans WJ, Smith RK, Schwellnus MP, Collins M. Variants within the MMP3 gene are associated with Achilles tendinopathy: possible interaction with the COL5A1 gene. Br J Sports Med. 2009;43(7):514–520. doi: 10.1136/bjsm.2008.053892. [DOI] [PubMed] [Google Scholar]
- 36.Michalova E, Vojtesek B, Hrstka R. Impaired pre-mRNA processing and altered architecture of 3′ untranslated regions contribute to the development of human disorders. Int J Mol Sci. 2013;14(8):15681–15694. doi: 10.3390/ijms140815681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller B, Wilcke A, Czepezauer I, Ahnert P, Boltze J, Kirsten H, et al. Association, characterisation and meta-analysis of SNPs linked to general reading ability in a German dyslexia case-control cohort. Sci Rep. 2016;6:27901. doi: 10.1038/srep27901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thakkinstian A, McElduff P, D'Este C, Duffy D, Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2005;24(9):1291–1306. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Quantitative characteristics of the included studies that examined the association of COL5A1 polymorphisms with TLI studies. (DOCX 57 kb)
Table S2. PRISMA checklist. (DOCX 30 kb)
Data Availability Statement
Please contact author for data requests.