Abstract
Objective
The aim of the study were to evaluate the effect of sildenafil against avascular necrosis of femoral head (ANFH) in a rabbit model, and to study the role of protein kinase G (PKG) pathway and vascular endothelial growth factor (VEGF) in ANFH.
Methods
Three weeks after inducing ANFH with methylprednisolone injection, 45 female adult New Zealand white rabbits were divided into three groups and treated as follows: group SI received daily intraperitoneal sildenafil with a dose of 10 mg/kg per day; group SD received daily sildenafil identically to group SI plus auricular vein injection DT3 (a specific PKG inhibitor); group NS received only normal saline. The blood perfusion function in the femoral head was measured by perfusion MRI and ink artery infusion. Bilateral femora heads were examined histopathologically for the presence of osteonecrosis; VEGF of tissue was examined by Western blot analysis; cGMP level and PKG activity were also measured.
Results
The incidence of ANFH in SI group was significantly lower than that observed in NS and SD groups (p < 0.05). VEGF in SI group was increased compared to NS group. cGMP level and PKG activity were also significantly different between NS and SI group (p < 0.05). However, these effects of sildenafil in SD group were all markedly inhibited by the administration of DT3 compared to SI group.
Conclusion
Sildenafil appear to increase the perfusion of femoral head by up-regulating VEGF through PKG pathway. The increased perfusion of femoral head could prevent ANFH.
Keywords: Sildenafil, Steroid-induced avascular necrosis of femoral head, Protein kinase G
Introduction
Avascular necrosis of femoral head (ANFH) has been reported to occur in patients who have received steroid treatment for underlying diseases such as systemic lupus erythematosus and inflammatory bowel disease, and also for immunosuppression after organ transplants.1 The natural history of untreated ANFH generally involves a progressive collapse that often requires surgical treatments. Therefore, the prevention of ANFH is an optional strategy for such patients. ANFH is characterized through the impairment of osseous blood flow that leads to the collapse of femur head.2 Steroid-induced ANFH in rabbits is useful model to assess the efficacy of potential treatments on this disease.3
Sildenafil is a well-known phosphodiesterase type 5 (PDE5) antagonist that significantly attenuates catabolism of guanosine 3′, 5′-cyclic monophosphate (cGMP) thereby enhancing signaling pathways involving protein kinase G (PKG). As such, sildenafil has been shown effective for the treatment of erectile dysfunction, congestive heart failure, pulmonary hypertension, and diabetic neuropathy.4, 5, 6, 7 Moreover, sildenafil has also shown promise in the management of ischemia-reperfusion injury in the heart8 and limb9 and also has been useful in the treatment of fracture and bone defect.10 Importantly, all of these pathologies entail a significant ischemic component indicating the effectiveness of sildenafil in resolving these tissue pathologies. Sildenafil therapy could confer protection via PKG dependent pathway during ischemic disease including heart and limb ischemia.8, 9 However, it was reported that sildenafil can improve blood flow in ischemic diseases, yet the molecular mechanisms involved in this response are not well understood.8, 9, 11 Moreover, no studies have been performed examining the effects of PDE5 blockade in models of osteonecrosis. Thus, our study provides important new information regarding the effect of PDE5 inhibition on blood perfusion of bone tissue and reveals possible mechanisms necessary for this response.
In the present study here, we report the effects of sildenafil on the blood perfusion and angiogenesis of femoral head in steroid-induced model. VEGF is the only growth factor proven to be specific and critical for blood vessel formation.12, 13 We also examined VEGF expression with sildenafil treatment. Moreover, we blocked PKG dependent pathway to observe whether the effects of sildenafil therapy are dependent on PKG mediated activity.
Materials and methods
Animals, grouping, and treatment
Forty-five female 28-week-old healthy, pathogen-free, adult New Zealand white rabbits, weight (2.8–3.5 kg), were used. Animals were obtained from the Experimental Animal Center of Medical College of *** University. All experimental procedures were approved by the Ethics Committee of Medical College of *** University. ANFH model was induced as previously reported.14 All rabbits were injected one intravenous injection 10 μg/kg of lipopolysaccharide [(LPS), Sigma, St. Louis, MO, USA]. 24 h later, three injections of 20 mg/kg of methylprednisolone [(MPS), Pfizer, Inc., Brussels, Belgium] were given intramuscularly at a time interval of 24 h. Three weeks after final administration of MPS, rabbits were divided into three groups and treated as follows: one group received daily sildenafil (Prifer, Inc., Brussels, Belgium) mixed in normal saline by intraperitoneal injection with a dose of 10 mg/kg per day (Group SI; n = 15); One group received daily sildenafil identically to group SI plus DT3 (Sigma, St. Louis, MO, USA), a specific PKG inhibitor, which was administered 1 mg/kg by auricular vein injection (SD; n = 15); The other received only normal saline (NS; n = 15). Before and 6 weeks after final MPS administration, the blood perfusion function in the proximal femora was measured by perfusion MRI. Five rabbits in each group were used for intra-arterial ink perfusion and the others were sacrificed for measurement 6 weeks after final MPS administration.
Tissue preparation
Animals were sacrificed with an excessive i.v. dose of pentobarbital sodium, after which the bilateral femurs were rapidly removed and the femoral heads were harvested and divided into two parts through the coronal plane of the femoral head. Halves of the bilateral femoral heads were immediately fixed in 10% buffered formalin for 1 week and decalcified with 10% ethylenediaminetetraacetate for 3 weeks. After decalcification, femoral heads were embedded in paraffin, and 4-μm-thick coronal sections were prepared for histomorphological examination. Other halves were collected and stored at −80 °C for western blot analysis, enzyme-linked immunosorbent assay and PKG activity assay.
Dynamic MRI measurement
MRI examination was performed using a 1.5 T clinical whole-body MRI system (Intera NT, Philips Medical Systems, Best, Netherlands) with a maximum gradient strength of 30 mT/m in accordance with previous study.15 The rabbit was anesthetized by an intraperitoneal injection of pentobarbital sodium. We used a commercially available surface coil with a diameter of 4.7 cm (Micro 4.7, Philips Medical Systems) as the radiofrequency (RF) receiver, which was put under the hip, meanwhile we used body volume coil as the RF transmitter. After the positioning scan, a plane through the femoral head, greater trochanter, and proximal femur was performed for three-point planscan and T1-weighted anatomical images were obtained as in previous study.16 According to anatomical imaging, a dynamic MRI scan series was collected. We applied dimeglumine gadopentetate (Magnevist; Schering, Berlin, Germany) with a dose of 0.8 mmol/kg as MRI contrast agent. A series of dynamic images were collected to facilitate covering wash-out phase. For the dynamic MRI, the signal intensity (SI) values in predefined round regions of interest (ROI) in the femoral head were analyzed by the published protocol14 (Fig. 1). SI over time was recorded and drawn as the time-signal intensity curve. The perfusion index of maximum enhancement (Emax) was calculated from the curve as the previous literature.17 Ibase was defined as the mean signal intensity of the first 3 images, Imax was defined as the maximum value of the first quickly rising part of the time-signal intensity curve. Emax was calculated according to the following equation: Emax = [(Imax − Ibase)/Ibase]·100%.
Fig. 1.
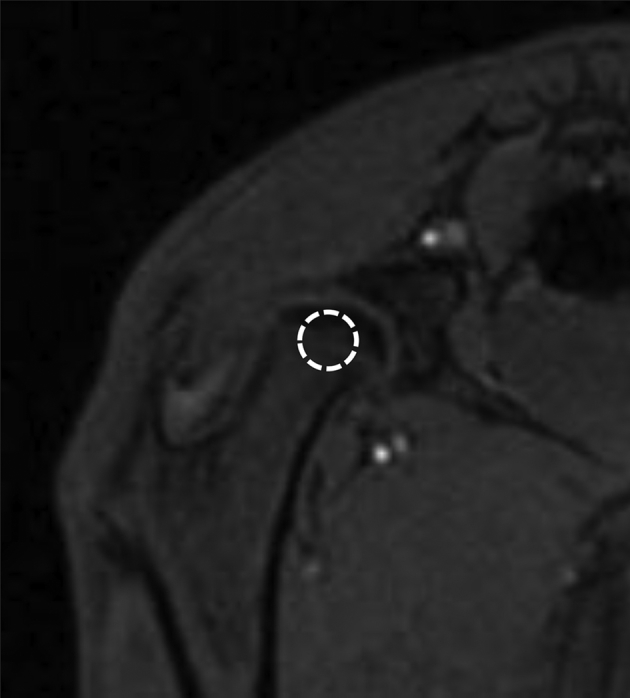
T1-weighted coronal MRI image of rabbit proximal femur. The region of interest (ROI) in the central part of femoral head (marked by the white circle) with a size of 8–10 pixels was defined for analysis of local intraosseous perfusion.
Intra-artery ink perfusion
We referred to the previous study18 to detect the static blood supply of the femoral head. After anesthetized by an intraperitoneal injection of pentobarbital sodium, all animals were performed intra-artery ink perfusion. The abdominal aorta and inferior vena cava were exposed and isolated, and then proximal part were ligated. A needle of vein transfusion device was distally inserted into the abdominal aorta for infusion. A drainage tube was inserted into the inferior vena cava distal to the site of ligation. Heparinized saline (25,000 units in 250 ml of 0.9% sodium chloride) was used for irrigation from the needle until the liquid from the drainage tube was clear. Then 10% gelatin/Chinese ink (20 g of gelatin in 100 ml of Chinese ink and 100 ml of water) at a pressure of 90 mmHg was performed to perfuse animals until the skin of the bilateral crura and nails were symmetrically black. All animals were sacrificed and retained in the refrigeration for 24 h at 4 °C. Then the bilateral femurs were removed and the samples of femoral heads were harvested, fixed, decalcified, embedded, cut into 25-mm-thick slices. The configuration of the femoral head and blood distribution were observed under a stereomicroscope. The perfusion ratio was calculated using the Image-Pro Plus 6.0 image analysis software. Ratio of perfusion was defined as the ratio of the area of inked artery to the area of the entire femoral head.19
Histopathology
The diagnosis of ANFH was determined at 6 weeks after the final steroid injection. The specimens stained with haematoxylin and eosin for the presence of ANFH. The presence of ANFH was assessed blindly by two authors based on the presence of diffuse empty lacunae or pyknotic nuclei of osteocytes within the bone trabeculae, accompanied by surrounding bone marrow cell necrosis or fat cell necrosis. If the diagnoses differed between the two investigators, a consensus was reached by discussion of the histological findings without knowledge of the group from which the specimen was obtained. Rabbits with at least one ANFH lesion among the eight areas were therefore considered to have ANFH. We used Image-Pro Plus 6.0 software. At 200 times magnification, 10 fields of the trabeculae were randomly chosen and 30 bone lacunae were counted in each field. The proportion of empty bone lacunae was calculated. 4 randomly-selected non-necrotic fields of bone marrow were randomly chosen and the average greatest diameters of 50 bone marrow fat cells were calculated.
Western blot analysis
Total protein was isolated by crushing and homogenizing the femoral head with RIPA buffer (Thermo Scientific, USA). Proteins were separated and transferred to membranes following standard protocols and probed using mouse monoclonal anti-VEGF (1:500; Abcam, USA) antibody. Protein expression was visualized on a film with an enhanced chemiluminescence kit (NEN Life Science Products Inc., Boston, MA, USA). Relative protein expression was determined using image analysis software (Media Cybernetics). For the quantitative analysis of protein, the intensity of each test band was expressed as the optical density (OD). β-actin was detected by mouse monoclonal anti-actin antibody (1:1000; Santa Cruz, USA) and was used as an internal control.
Measurement of Tissue cGMP and PKG Activity. Bone tissue cGMP level was measured by enzyme-linked immunosorbent assay using cGMP Enzyme Immunoassay Kit (R&D systems, Minneapolis, MN) according to the manufacturer's directions. PKG activity was measured according to the manufacturer's instructions (Cyclex, Nagano, Japan). Spectrophotometric absorbance was measured at 450 nm, and the results were normalized per mg of protein.
Statistical analysis
Statistical analyses were conducted using SPSS software 17.0 (SPSS, Inc., Chicago, IL, USA). Numerical data in each group were presented as mean ± standard deviation (SD). Data were analyzed using one-way analysis of variance (ANOVA), followed by the Newman–Keuls multiple comparison procedure. P < 0.05 was considered to indicate a statistically significant difference.
Results
Sildenafil treatment enhances blood perfusion
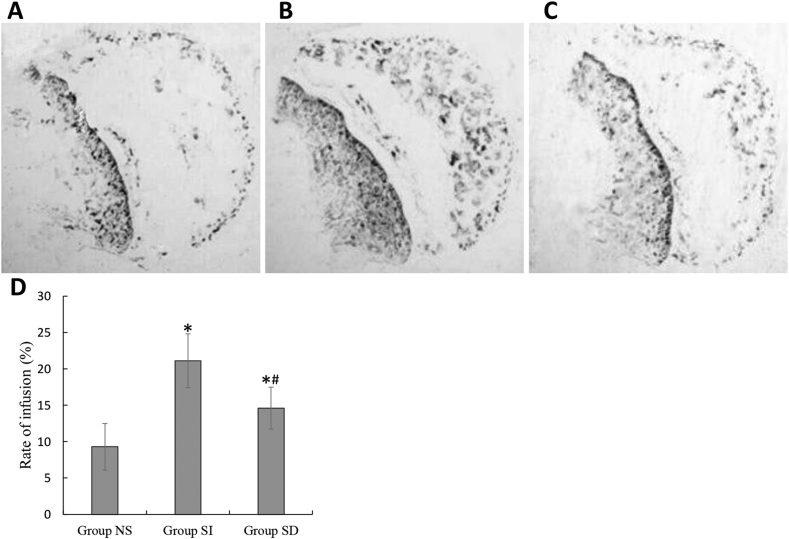
Blood perfusion of the femoral heads was monitored at 0 and 6 weeks by dynamic MRI, representative dynamic time–intensity curves for a rabbit with osteonecrosis showed a decrease in blood perfusion at 6 week compared with baseline (Fig. 2A). Emax in the femoral head of SI group with sildenafil treatment showed a significant increase at 6 week compared with group NS. In contrast, coadministration of DT-3 diminished the enhanced blood perfusion by sildenafil in SD group. A significant decrease in the Emax of femoral head was found in all rabbits with osteonecrosis, whereas no significant change in rabbits without osteonecrosis (Fig. 2B). Blood perfusion of the femoral heads was also detected by intra-artery ink perfusion. We could observed much more black granules in SI group (Fig. 3B), but there were just few granules in NS and SD groups (Fig. 3A and C). The rate of infusion in the femoral head were compared in three groups. The rate in SI group with sildenafil treatment showed a significant increase compared with NS group. However, the effect was blocked by DT-3 in SD group, though the rate in SD group still higher than NS group (Fig. 3D).
Fig. 2.
Blood perfusion of femoral head. (A) Representative dynamic MRI time–intensity curves for a rabbit with osteonecrosis of femoral head, showing a decrease in blood perfusion (Week 6) compared with baseline, based on MRI intensity. (B) Time-course changes (mean ± SD) in the perfusion index (maximum contrast enhancement) of femoral head showed a significantly decreased perfusion index (maximum contrast enhancement) at the femoral head of rabbits with osteonecrosis (ANFH+; n = 24) compared with controls without osteonecrosis (n = 12) (*P < 0.05, ANFH+ rabbits compared with baseline and control rabbits).
Fig. 3.
Ink artery infusion angiography of the femoral head and the ratio of perfusion. (A and C). Few black granules were found in NS and SD groups. (B). Compared with NS and SD groups, SI group had more ink-stained black granules. (D). Bar graph shows the ratio of perfusion in the femoral head. The ratio of perfusion in SI group was significantly higher than that in NS group; the ratio of perfusion in SD group was significantly lower than that in SI group *P < 0.05 vs group NS, #P < 0.05 vs group SI.
Histological results
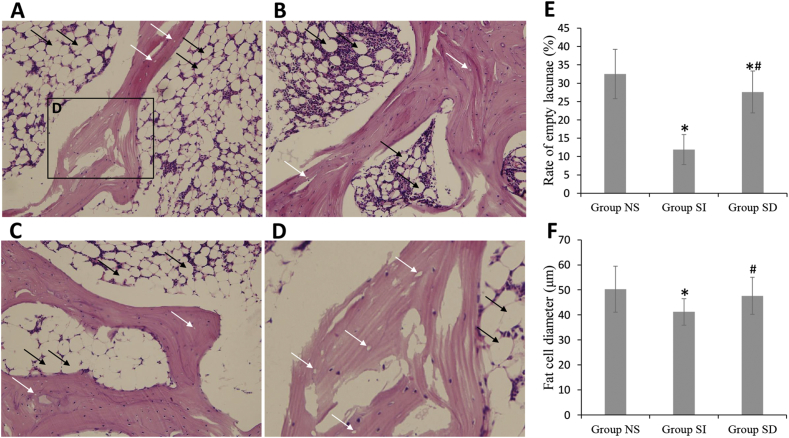
Throughout the experimental period, nine of the 45 rabbits died and were excluded from the evaluation: three were in NS group, four were in SI group and two were in SD group. Osteonecrosis in NS group was obvious (Fig. 4A and D). Many empty lacunae were found in bone trabeculae and the number and morphology of hematopoietic and fat cells in marrow tissue had necrotic changes. In SI group, slight osteonecrosis was observed with fewer empty lacunae and necrotic medullary hematopoietic cells and fat cells (Fig. 4B). However, osteonecrosis in SD group was still obvious (Fig. 4C). In total, 83.3% of the rabbits (10/12) in NS group developed ANFH 6 weeks after the last injection of MPS, while sildenafil reduced the incidence of osteonecrosis, only 36.7% of the rabbits (4/11) developed ANFH in SI group(P < 0.05, Pearson chi-square test). Although the incidence (76.9%, 10/13) in SD group was not significant compared with NS group (P > 0.05, Pearson chi-square test), it was still a tendency to ameliorate. The rate of empty lacunae was 11.9 ± 4.1% and the average bone marrow fat cell size was 41.2 ± 5.3 μm in SI group, which were significantly lower than those in NS group (37.5 ± 6.7%, 50.3 ± 9.2 μm). However, the effect was interdicted by DT3 in SD group (32.5 ± 6.7%, 47.6 ± 7.4 μm) (Fig. 4E and F).
Fig. 4.
H&E staining and data analysis. Group NS (n = 12) showed discrete trabecular bone with many empty lacunae, surrounded by few spindle-shaped osteoblasts and plenty of marrow fat cells (A, big view and D, local amplification); Group SI (n = 11) showed more trabecular bone and less empty lacunae in the trabecular bone was found (B); Group SD (n = 13) also showed more empty lacunae in the trabecular bone and enlarged fat cells were found (C). The ratio empty lacunae and the diameter of fat cells were decreased after sildenafil treatment and the affect was blocked by DT3 (E and F). Empty lacunae are shown by white arrows and enlarged fat cells are shown by black arrows. Magnification: ×200 (A, B and C), ×400 (D) *P < 0.05 vs group NS, #P < 0.05 vs group SI.
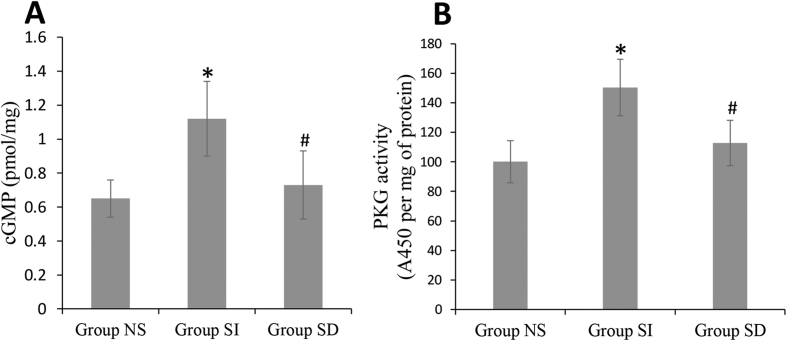
Tissue cGMP level and PKG Activity. Comparison revealed that the cGMP levels in sildenafil-treated femoral head were significantly higher than NS group (Fig. 5A). Treatment with sildenafil also caused increase in tissue PKG activity as compared with NS group (Fig. 5B). Together, these data indicated that sildenafil therapy augments ischemic tissue cGMP accumulation and PKG activity. However, the incensement were all markedly inhibited by the administration of DT3 (Fig. 5A and B).
Fig. 5.
cGMP level and PKG activity in femoral heads. cGMP level (A) and PKG activity (B) were increased after treatment of sildenafil in group SI, and the effects were blocked by DT3 in group SD. *P < 0.05 vs group NS, #P < 0.05 vs group SI.
Expression of VEGF in femoral heads
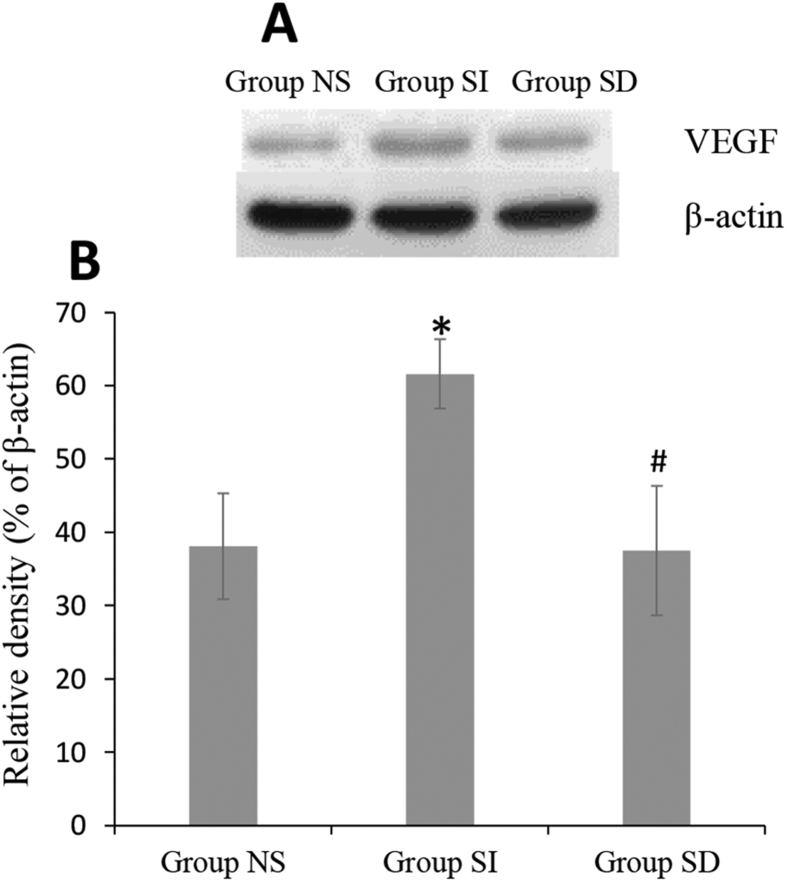
Different protein bands of VEGF measured by western blot with β-actin as internal control were displayed in Fig. 6A. According to quantification by Western blot, relative optical density of bands boosted in SI group when compared with NS group. Meanwhile, the response of protein expression was arrested by DT3 in SD group (Fig. 6B).
Fig. 6.
Western blot and analysis of VEGF protein. (A). Protein expression bands of VEGF in three groups with β-actin as internal control. (B). The relative density of the bands in group SI was higher than that in group NS and SD groups. *P < 0.05 vs group NS, #P < 0.05 vs group SI.
Discussion
As the exact pathogenesis of osteonecrosis remains to be elucidated, it is believed that the combined effects of metabolic factors, local factors affecting blood flow including thrombosis, increased intraosseous pressure, and mechanical stresses may give rise to osteonecrosis. Therapeutic angiogenesis and flow recovery remain important clinical goals for osteonecrosis of femoral head with the objective of avoiding further tissue dysfunction and total hip replacement. As such, several experimental approaches have been explored such as VEGF, bFGF, and other therapies.20, 21, 22, 23 However, successful clinical results are limited, thus highlighting the need for continued investigation of alternative approaches to restore vascular function and to recover the blood flow during tissue ischemia.
According to dynamic MRI and static ink perfusion, in this study, we provide novel information that PDE5 inhibitor sildenafil therapy significantly enhances blood flow recovery in necrotic femoral head with ANFH, along with the upregulation of VEGF protein expressions. Meanwhile, number of empty bone lacunae and bone marrow fat cells diameter decreased with the incidence decreasing. These effects suggested that sildenafil exerted a positive protective benefit to ANFH.
VEGF loss play an important role in the initial stage of femoral head ANFH. Since VEGF is a potent angiogenic peptide, it is likely responsible for the neovascularization observed in the penumbra of ARCO Stage IV ANFH of the femoral head.24 Previous study indicated that VEGF protein and mRNA levels were significantly lower in ANFH+ rabbits compared with controls.25 The down regulation of VEGF may play a critical role in the disease process of ANFH. However, in present study, VEGF increased after administration of sildenafil, which was consistent with previous studies.8, 26 The perfusion of tissue could increase after added neovascularization.
The above responses may be all through PKG pathway as several previous studies with application of sildenafil.9, 26 The volume of cerebral infarction was significantly decreased by PDE5 inhibitor administration with selectively increasing blood flow in the ischemic brain via increased cGMP levels.27 In this study, PKG activity is also enhanced by PDE5 inhibitor sildenafil after increase of cGMP level, which is an important mediator of ischemia-induced angiogenesis and acts as a relaxant second messenger in the vessels. Therefore, we determined whether sildenafil-induced increasing blood flow and elevated expression of VEGF retentively occur through PKG pathways using the specific PKG inhibitor DT-3. Our study revealed that sildenafil exerts the effects through the PKG pathway. In addition, sildenafil-induced VEGF expression was blocked by DT-3 as well, suggesting that VEGF was very likely in the downstream of PKG, which was consistence with Sahara's study, whose study demonstrated another PDE5 vardenafil significantly enhanced blood flow recovery and augmented capillary collateral formation in ischemic muscle.26
There are some limitations in this study. First, we did not measure the blood vessel density but measured the activity of angiogenesis. After all neovascularization was an important component of the increasing blood flow. Second, we did not measure the change of NO, and we were just concerned about the downstream of NO/cGMP/PKG. Therefore, further studies about NO/cGMP/PKG pathway need to be further studied. Third, sildenafil can accelerate fracture healing including delayed union28, 29 and attenuate apoptosis and oxidative stress,30 which were greatly connected with osteonecrosis; however, our study did not involve those.
In conclusion, our findings suggest that the PDE5 inhibitor sildenafil increases blood flow of femoral head through a PKG-dependent pathway. Thus, PDE5 inhibition may have a therapeutic potential to treat ischemic conditions such as ANFH.
Conflict of interest
None declared.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Seguro L.P., Rosario C., Shoenfeld Y. Long-term complications of past glucocorticoid use. Autoimmun Rev. 2013;12:629–632. doi: 10.1016/j.autrev.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Shah K.N., Racine J., Jones L.C., Aaron R.K. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015;8:201–209. doi: 10.1007/s12178-015-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boss J., Misselevich I. Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol. 2003;40:345–354. doi: 10.1354/vp.40-4-345. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ameri H., Kloner R. Erectile dysfunction and heart failure: the role of phosphodiesterase type 5 inhibitors. Int J Impot Res. 2009;21:149–157. doi: 10.1038/ijir.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montani D., Chaumais M.-C., Savale L. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv Ther. 2009;26:813–825. doi: 10.1007/s12325-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Chopp M., Szalad A. Phosphodiesterase-5 is a therapeutic target for peripheral neuropathy in diabetic mice. Neuroscience. 2011;193:399–410. doi: 10.1016/j.neuroscience.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halcox J.P., Nour K.R., Zalos G. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40:1232–1240. doi: 10.1016/s0735-1097(02)02139-3. [DOI] [PubMed] [Google Scholar]
- 8.Koneru S., Varma Penumathsa S., Thirunavukkarasu M. Sildenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: role of VEGF/angiopoietin-1. J Cell Mol Med. 2008;12:2651–2664. doi: 10.1111/j.1582-4934.2008.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senthilkumar A., Smith R.D., Khitha J. Sildenafil promotes ischemia-induced angiogenesis through a PKG-dependent pathway. Arterioscler Thromb Vasc Biol. 2007;27:1947–1954. doi: 10.1161/ATVBAHA.107.147421. [DOI] [PubMed] [Google Scholar]
- 10.Smith K.M., Romanelli F. Recreational use and misuse of phosphodiesterase 5 inhibitors. J Am Pharm Assoc. 2005;45:63–72. [PubMed] [Google Scholar]
- 11.Li L., Jiang Q., Zhang L. Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by MRI after administration of sildenafil to rats with embolic stroke. Brain Res. 2007;1132:185–192. doi: 10.1016/j.brainres.2006.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persico M., Vincenti V., DiPalma T. Structure, expression and receptor-binding properties of placenta growth factor (PlGF) Curr Top Microbiol Immunol. 1999;237:31–40. doi: 10.1007/978-3-642-59953-8_2. [DOI] [PubMed] [Google Scholar]
- 13.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 14.Qin L., Zhang G., Sheng H. Multiple bioimaging modalities in evaluation of an experimental osteonecrosis induced by a combination of lipopolysaccharide and methylprednisolone. Bone. 2006;39:863–871. doi: 10.1016/j.bone.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng H., Zhang G., Wang Y.X. Functional perfusion MRI predicts later occurrence of steroid-associated osteonecrosis: an experimental study in rabbits. J Orthop Res. 2009;27:742–747. doi: 10.1002/jor.20765. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y.F., Wang Y.X.J., Griffith J.F. Proximal femur bone marrow blood perfusion indices are reduced in hypertensive rats: a dynamic contrast-enhanced MRI study. J Magn Reson Imaging. 2009;30:1139–1144. doi: 10.1002/jmri.21954. [DOI] [PubMed] [Google Scholar]
- 17.Chen W.T., Shih T.T.F., Chen R.C. Blood perfusion of vertebral lesions evaluated with gadolinium-enhanced dynamic MRI: in comparison with compression fracture and metastasis. J Magn Reson Imaging. 2002;15:308–314. doi: 10.1002/jmri.10063. [DOI] [PubMed] [Google Scholar]
- 18.Fan L., Zhang C., Yu Z., Shi Z., Dang X., Wang K. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and osteogenesis in rabbit femoral head osteonecrosis. Bone. 2015;81:544–553. doi: 10.1016/j.bone.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Fan L., Li J., Yu Z., Dang X., Wang K. Hypoxia-Inducible factor prolyl hydroxylase inhibitor prevents steroid-associated osteonecrosis of the femoral head in rabbits by promoting angiogenesis and inhibiting apoptosis. PLoS One. 2014;9:e107774. doi: 10.1371/journal.pone.0107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hang D., Wang Q., Guo C., Chen Z., Yan Z. Treatment of osteonecrosis of the femoral head with VEGF165 transgenic bone marrow mesenchymal stem cells in mongrel dogs. Cells Tissues Organs. 2012;195:495–506. doi: 10.1159/000329502. [DOI] [PubMed] [Google Scholar]
- 21.Yang C., Yang S-h, Du J-y, Li J., Xu W., Xiong Y. Vascular endothelial growth factor gene transfection to enhance the repair of avascular necrosis of the femoral head of rabbit. Chin Med J. 2003;116:1544–1548. [PubMed] [Google Scholar]
- 22.Mont M.A., Jones L.C., Einhorn T.A., Hungerford D.S., Reddi A.H. Osteonecrosis of the femoral head: potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355:S314–S335. [PubMed] [Google Scholar]
- 23.Ma X., Cui D., Zhao D. Vascular endothelial growth factor/bone morphogenetic protein-2 bone marrow combined modification of the mesenchymal stem cells to repair the avascular necrosis of the femoral head. Int J Clin Exp Med. 2015;8:15528–15534. [PMC free article] [PubMed] [Google Scholar]
- 24.Namba T., Koike H., Murakami K. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation. 2003;108:2250–2257. doi: 10.1161/01.CIR.0000093190.53478.78. [DOI] [PubMed] [Google Scholar]
- 25.Wang G., Zhang C., Sun Y. Changes in femoral head blood supply and vascular endothelial growth factor in rabbits with steroid-induced osteonecrosis. J Int Med Res. 2010;38:1060–1069. doi: 10.1177/147323001003800333. [DOI] [PubMed] [Google Scholar]
- 26.Sahara M., Sata M., Morita T., Nakajima T., Hirata Y., Nagai R. A phosphodiesterase-5 inhibitor vardenafil enhances angiogenesis through a protein kinase G-dependent hypoxia-inducible factor-1/vascular endothelial growth factor pathway. Arterioscler Thromb Vasc Biol. 2010;30:1315–1324. doi: 10.1161/ATVBAHA.109.201327. [DOI] [PubMed] [Google Scholar]
- 27.Gao F., Sugita M., Nukui H. Phosphodiesterase 5 inhibitor, zaprinast, selectively increases cerebral blood flow in the ischemic penumbra in the rat brain. Neurol Res. 2005;27:638–643. doi: 10.1179/016164105X25135. [DOI] [PubMed] [Google Scholar]
- 28.Fauzi A., Kamal A., Kurniawan A., Kodrat E. Role of sildenafil in acceleration of delayed union fracture healing on Sprague-Dawley rats model. Br J Med Med Res. 2015;8:419–428. [Google Scholar]
- 29.Histing T., Marciniak K., Scheuer C. Sildenafil accelerates fracture healing in mice. J Orthop Res. 2011;29:867–873. doi: 10.1002/jor.21324. [DOI] [PubMed] [Google Scholar]
- 30.Ebrahimi F., Shafaroodi H., Asadi S. Sildenafil decreased cardiac cell apoptosis in diabetic mice: reduction of oxidative stress as a possible mechanism. Can J Physiol Pharmacol. 2009;87:556–564. doi: 10.1139/y09-036. [DOI] [PubMed] [Google Scholar]