Abstract
Background
Retrospective clinical studies suggest there is a risk for neurodevelopmental impairment following early childhood exposure to anaesthesia. In the developing animal brain, including those of non-human primates (NHPs), anaesthetics induce apoptotic cell death. We previously reported that a 5 h isoflurane (ISO) exposure in infant NHPs increases apoptosis 13-fold compared with control animals. However, the majority of paediatric surgeries requiring anaesthesia are of shorter durations. We examined whether 3 h ISO exposure similarly increases neuroapoptosis in the NHP developing brain.
Methods
Six-day-old NHP infants (Macaca mulatta) were exposed to 3 h of a surgical plane of ISO (n=6) or to room air (n=5). Following exposure, NHP brains were screened for neuronal and oligodendrocyte apoptosis using activated caspase-3 immunolabelling and unbiased stereology.
Results
ISO treatment increased apoptosis (neurones + oligodendrocyte) to greater than four times that in the control group [mean density of apoptotic profiles: 57 (SD 22) mm−3vs 14 (SD 5.2) mm−3, respectively]. Oligodendrocyte apoptosis was evenly distributed throughout the white matter whereas neuroapoptosis occurred primarily in the cortex (all regions), caudate, putamen and thalamus.
Conclusions
A 3 h exposure to ISO is sufficient to induce widespread neurotoxicity in the developing primate brain. These results are relevant for clinical medicine, as many surgical and diagnostic procedures in children require anaesthesia durations similar to those modelled here. Further research is necessary to identify long-term neurobehavioural consequences of 3 h ISO exposure.
Keywords: anaesthesia, apoptosis, growth and development, isoflurane
Editor's key points.
-
•
Previous studies in non-human primates show that isoflurane exposure for 5 h produces widespread apoptotic cell death in neurones and glia.
-
•
The effects of a shorter 3 h exposure, which is more relevant to paediatric anaesthesia, on apoptosis in neonatal macaque brain was analysed.
-
•
The shorter isoflurane exposure also produced widespread apoptosis; further studies are required to test for long-term neurobehavioural sequelae.
Exposure to general anaesthetics can cause structural injury1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and/or long-term neurodevelopmental impairment (NDI)2, 4 10, 11, 12, 13, 14 in several animal species, including non-human primates (NHPs).5, 6, 7, 8, 9 12, 13, 14 Currently, anaesthesia exposure in NHP infants is known to produce widespread apoptosis of two cell types: neurones and oligodendrocytes (oligos).5, 6, 7, 8, 9 15 16 Loss of either or both cell types might contribute to long-term NDI because the integrity of neurones is dependent on an intact myelin sheath, which is produced exclusively by oligos.
Retrospective clinical studies have found early childhood anaesthesia exposure is associated with an increased risk for NDI with a stronger association following repeated or more prolonged anaesthesia.17, 18, 19, 20, 21 However, whereas similar studies identified increased risk for NDI after a short anaesthetic exposure (1–4 h),22 23 other such studies produced ambiguous results24 or observed no increased risk,25 which was echoed most recently in two publications reporting data from prospective clinical trials.26 27 Therefore, it is important to determine whether a single, short exposure triggers brain injury in an experimental model that more closely resembles the human condition.
We previously reported significant neuronal and oligodendrocyte apoptosis following 5 h of isoflurane (ISO) in the NHP;6 15 however, this may have limited clinical relevance because the majority of anaesthetic exposures are of shorter duration. We recently assessed that at Doernbecher Children's Hospital (Oregon Health & Science University, OR, USA), about 30% of infant anaesthetic exposures lasted 3 h or longer28 (institutional electronic medical records data). At Texas Children's Hospital, about 10% of paediatric patients under 3 years of age undergo anesthesia for more than 3 h.29 This suggests that several hundred thousand of the 1.5 million American infants who undergo anaesthesia every year30 are exposed to anaesthesia for periods of 3 h or longer. Accordingly, we designed the present study to determine whether a 3 h exposure to ISO causes apoptotic cell death in the neonatal NHP brain.
Methods
All animal protocols received approval by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center, Oregon Health & Science University, and were conducted in full accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals. All procedures were performed according to the same methods and standards that are employed in a human paediatric surgical setting.
General anaesthesia
Six-day-old infant rhesus macaques received ISO anaesthesia for 3 h (n=6; 2 females and 4 males). ISO was administered as described,6 at a tightly regulated concentration to maintain a surgical plane of anaesthesia (no movement and not more than 10% increase in heart rate or blood pressure in response to a profound mosquito-clamp pinch at hand and foot; checked every 30 min). During ISO anaesthesia, animals were mechanically ventilated via a tracheal tube (FiO2=0.30), and their physiological status was extensively monitored and maintained as described.6 After anaesthetic exposure, animals were recovered for 3 h (n=4) or 5 h (n=2) in an intensive care unit (ICU) system (Snyder ICU cage; Snyder MFG, Centennial, CO, USA), were visually monitored and were fed milk formula as tolerated. At the end of the observation period, animals were immediately euthanized as described below and their brains were prepared for histopathological analysis. Other animals received no anaesthesia and served as controls (n=5; 3 females and 2 males).6 They underwent a similar procedure, including insertion of an i.v. catheter, physiological measurements and a period of handling to simulate the environment that the other animals experienced prior to induction of anaesthesia. Control animals were then monitored in the ICU cage until final measurements and euthanasia 8 h after time zero.
Histopathological analysis
All subjects were euthanized by methods approved by the American Veterinary Medical Association. In brief, animals were anaesthetized with ketamine, followed by high-dose pentobarbital to induce a deep surgical plane of anaesthesia, and were exsanguinated by incision of the right cardiac atrium. Paraformaldehyde (4% in phosphate buffered saline) fixative was then perfused via the left ventricle to prepare the brain for histopathological analysis. A battery of previously described histological procedures6, 7, 8, 9 was applied to characterize cell death, identify the cell types affected and evaluate the pattern of injury.
Quantitative assessment
For quantitative analysis, coronal 70 µm-thick sections (cut by vibratome) were cut across the entire rostro-caudal extent of the brain. From these sections, in an unbiased manner, sections at 4 mm intervals (approximately 15 sections per brain) were selected for antigen retrieval and immunohistochemical (IHC) staining with antibodies to activated caspase-3 (AC3), a well-established marker for detection of brain cells undergoing apoptosis.6, 7, 8, 9 The control group consisted of stored brain sections used in a prior study6 that was re-immunolabelled for AC3. To make sure the stored tissue does not lose AC3 antigenicity over time, counts were performed on both these newly stained control sections and previously stained sections from the same animals archived from the prior study. Re-using anaesthesia-naive NHP subjects from a prior study is justified in that it is not ethically defensible to sacrifice additional NHP infants to re-establish that naive animals display low levels of physiological apoptosis.
An experienced neurohistologist who was blinded to the experimental conditions counted all cellular profiles that stained positive for AC3 using a computer-assisted Microbrightfield Stereo-Investigator system (Microbrightfield, Inc., Williston, VT, USA) to record the location and number of dying cells and the quantitative dimensions of the counting field. It was possible to distinguish apoptotic neurones from apoptotic oligos,6, 7, 8 because the AC3 stain displays the full cell body and processes of both cell types, and they are morphologically dissimilar (see Figs. 1, Fig 2, Fig 3). Moreover, dying oligos are confined almost exclusively to white matter regions and dying neurones are confined exclusively to grey matter regions (see Fig. 4).
Fig 1.
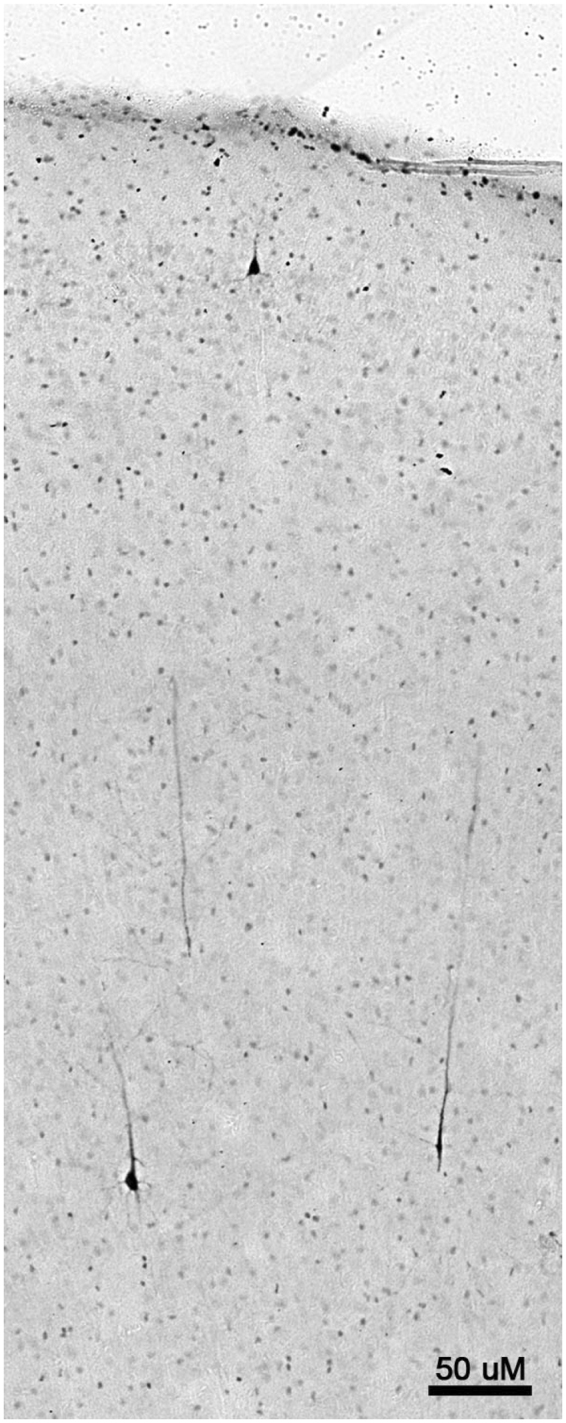
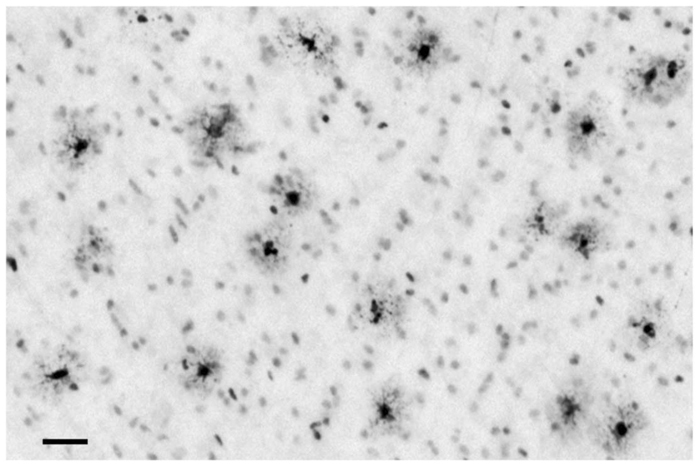
Histological appearance of dying neurones visualized by activated caspase-3 (AC3) immunohistochemistry (marker for apoptosis) following exposure to isoflurane (ISO). AC3-positive neurones are found in a randomly scattered and sparse distribution in control brains, and no specific brain regions are selectively affected. In contrast, following ISO exposure, neurones in deep layers (V and VI) and in a superficial layer (II) of the temporal, somatosensory and primary visual cortices are preferentially affected. As these neurones commit to apoptotic cell death they become filled with AC3 throughout their cell bodies and dendrites so that their entire morphology can be readily visualized. Shown here is an image from the temporal cortex of an infant non-human primate (NHP) brain exposed to ISO for 3 h. There are several AC3-positive pyramidal-shaped (presumed to be glutamatergic) neurones in layers V and VI that extend long apical dendrites towards the cortical surface along with a single positive neurone in layer II. Affected neurones in both the superficial and deep layers of the somatosensory cortex have exactly the same appearance. In the primary visual cortex, affected neurones in layer II have a similar appearance but those in deep layers are different (see Fig. 2). Bar=50 µm.
Fig 2.
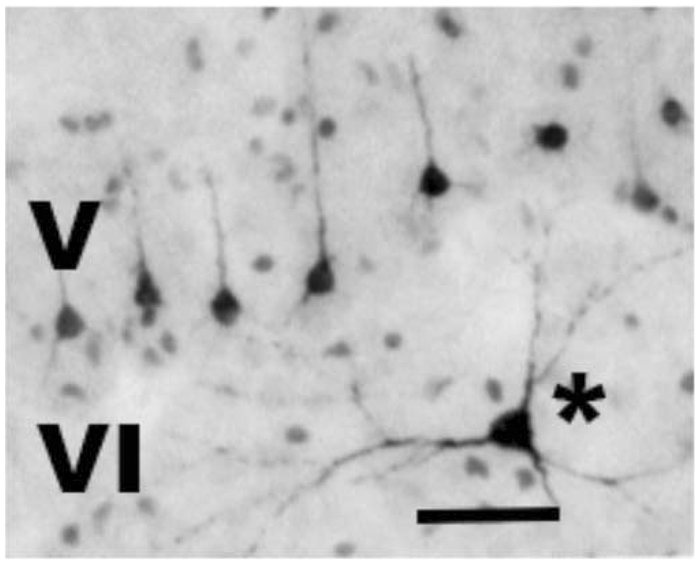
Typical appearance of apoptotic [activated caspase-3 (AC3)-positive] neuronal profiles in layers V and VI of the primary visual cortex following exposure to 3 h isoflurane (ISO) anaesthesia. The affected neurones in layer V are relatively small pyramidal neurones, presumed to be glutamatergic, that send axonal projections to the contralateral visual cortex.6 Their apical dendrites do not extend far beyond layer V. A second neuronal cell type typically affected (asterisk) has a multipolar shape and is located in layer VI or sometimes in a border zone between layers V and VI. This cell has morphological characteristics of a Martinotti cell, which is considered an inhibitory interneurone.6 Bar=50 µm.
Fig 3.
Typical appearance of apoptotic [activated caspase-3 (AC3)-positive] oligodendrocytes (oligos). Shown here are dying oligos in the corpus callosum following a 3 h exposure of the infant non-human primate (NHP) to isoflurane (ISO). These cells have many processes that emanate from the cell body in a radial pattern. Each process has an expanded thin sheet-like foot that is specialized to wrap around axons. As these sheet-like processes degenerate they cause the neuropil surrounding the cell body to have a dark smudgy appearance. Oligo degeneration following ISO exposure occurs diffusely and in an evenly distributed pattern throughout white matter regions of the brain. Bar=25 µm.
Fig 4.
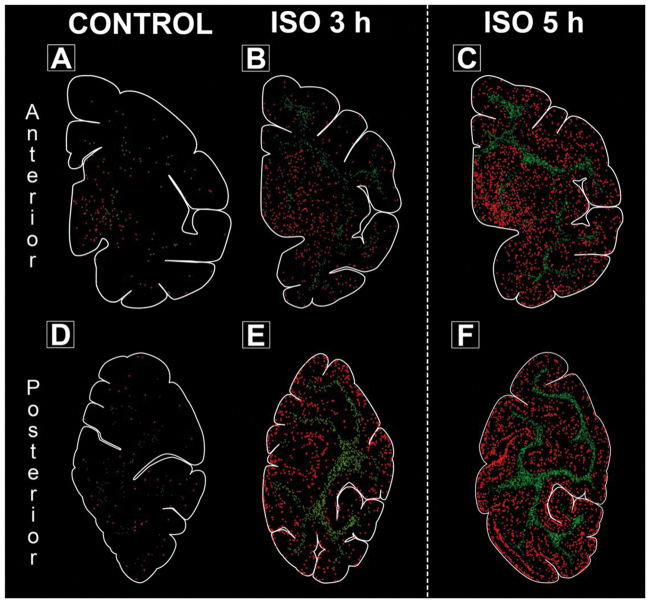
Computer plots based on the number and location of each activated caspase-3 (AC3)-stained neuronal profile show the distribution of apoptotic (AC3-positive) cells in anterior and posterior brain regions from representative animals after no anaesthesia exposure (panels A, D; control condition), after isoflurane (ISO) for 3 h (panels B, E) or after ISO for 5 h (panels C, F). The images for 5 h ISO are produced from a previously published paper6 but are shown here for comparison purposes. The anterior regions (panels A–C) include the temporal, cingulate, insular and entorhinal cortices along with the basal ganglia (caudate, putamen and internal capsule). The posterior regions (panels D–F) include visual cortical areas, cingulate cortex and Brodman area 7. Each apoptotic neurone is marked as a red circle whereas each apoptotic oligodendroglia is marked as a green X. The difference in toxicity between groups appears to be a general effect occurring in all regions rather than certain regions being selectively affected. There is a pattern of laminar distribution of apoptotic cells in cortical areas after 3 h ISO anaesthesia, which is particularly pronounced after the 5 h exposures and in the posterior portion of the neonatal non-human primate (NHP) brain.6
Statistical evaluation
Data were analysed by Student's t-test using GraphPad Prism software, version 4.0a (GraphPad Software, Inc., La Jolla, CA, USA).
Results
On the day of the experiment, the 3 h ISO and drug-naive controls were of comparable age [mean (sd): 6.3 (0.5) and 5.6 (1.1) days, respectively (range 4–7 days)] and body weight [mean (sd): 0.54 (0.05) and 0.51 (0.05) kg, respectively (range 0.43–0.63 kg)]. ISO anaesthesia was well tolerated by all infant animals and each was weaned from the ventilator and extubated within minutes following anaesthesia exposure. Physiological variables (end-tidal CO2, peripheral O2 saturation, electrocardiographic measures, non-invasive blood pressure, temperature, blood gases and metabolic profile, including pH, base excess, blood urea nitrogen, haematocrit, haemoglobin, serum Na+, Cl–, K+, and glucose and lactate levels) were monitored, and remained within normal species-specific ranges throughout the entire experimental period. Animals receiving ISO anaesthesia had moderately lower haemoglobin levels compared with control animals, reflecting the few additional blood draws and fluid support with i.v. balanced electrolyte solution.
To avoid using additional NHPs to re-establish that drug-naive animals display very low levels of physiological apoptosis, adjacent brain sections were quantified from control animals that also had been used in a previous study.6 Concerns that this tissue may have lost antigenicity over time were addressed by repeat unbiased stereology on the originally stained control tissue6 and comparison to the newly stained tissue. In direct comparison, the banked tissue from control animals had an average of 11 (4.8) [mean (sd)] profiles mm−3 (neurones + oligos) and the newly stained tissue from the same animals had 14 (5.2) [mean (sd)] profiles mm−3. A t-test revealed no statistically significant difference (t8=0.8423, P>0.05) indicating antigenicity did not change with longer storage times.
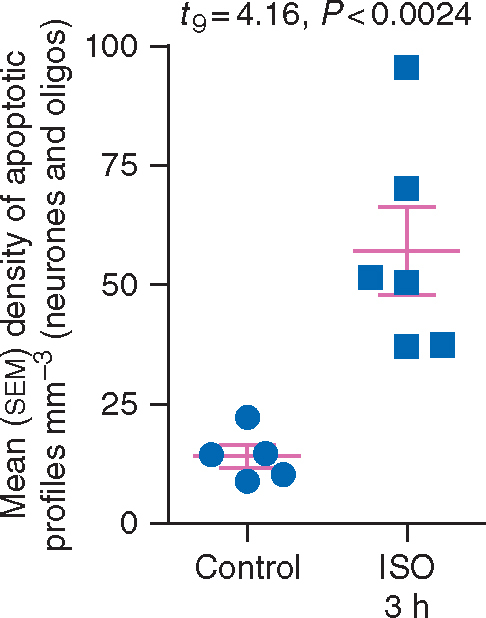
Next, we compared counts from the newly stained controls and the 3 h ISO group. There was a large increase (over four-fold) in apoptotic profiles in the 3 h ISO group compared with the physiological apoptosis in controls (Fig. 5). The mean (sd) density of apoptotic profiles (neurones + oligos) for controls was 14 (5.2) mm−3 (n=5) whereas it was 57 (22) mm−3 for the 3 h ISO group (n=6). A t-test revealed that 3 h ISO treatment significantly increased apoptosis compared with control (t9=4.160, P<0.01). Apoptotic profiles were further compared between animals sacrificed 5 h after ISO exposure and those sacrificed 3 h after ISO exposure. Apoptotic cells densities of the two animals observed for 5 h after the end of the ISO anaesthetic were close to the group mean and did not differ from that of the four ISO exposed animals that were observed for 3 h. Data are displayed as a scatter plot in Fig. 5 and highlight that all six animals exposed to ISO had apoptosis scores higher than any of the five animals in the control group. Thus, although the apoptosis response was more extreme in some animals than others, no animal exposed to 3 h ISO escaped the apoptogenic action of the ISO exposure.
Fig 5.
Density of apoptotic profiles in infant macaque brains following exposure to isoflurane (ISO) for 3 h or no anaesthesia (Control). The mean (sem) density of apoptotic profiles (neurones + oligos) mm−3 for the 3 h group was significantly (P<0.001) increased compared with control. All six of the brains exposed to 3 h ISO had higher density counts than any of the five control brains.
In order to confirm the identity of brain cells undergoing apoptosis and to clarify whether some cell populations are more vulnerable than others, we applied additional histological staining procedures, including IHC staining with reagents specific for various central nervous system (CNS) cell types or cell death processes. Antibodies to AC3 detected all cells dying by apoptosis in either grey or white matter regions of the brain (Fig 1, Fig 2, Fig 3, Fig 4, Fig 6).
Fig 6.
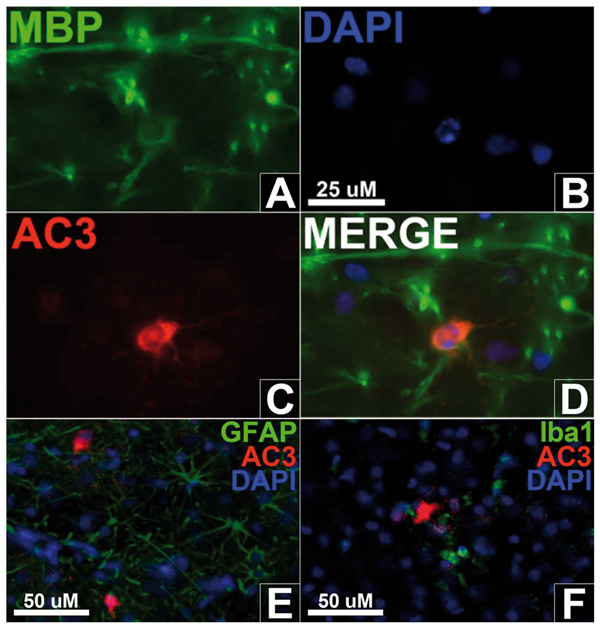
Triple-fluorescent labelling marks oligodendrocytes with myelin-basic protein (A; MBP), cell nuclei with DAPI (B) and apoptosis with activated caspase-3 (C; AC3) in the white matter after 3 h ISO exposure. A merged image (D) shows a cell with a pyknotic nucleus co-labelled with AC3 and MBP, indicating the apoptotic cell is an oligodendrocyte. Triple-fluorescent labelling with glial fibrillary acidic protein (GFAP) to mark astrocytes (E) or Iba1 to mark microglia (F) display no co-labelling with AC3, suggesting neither cell type undergoes apoptosis.
As AC3 fully engulfs soma and neurites (Figs 1 and 2), neurones were easily distinguished morphologically. Consistent with previous research,6 neurones made up the vast majority of cells in grey matter regions. Neuronal cell death was most heavily concentrated in populations engaged in sensory information processing, especially those located in the somatosensory, temporal and primary visual cortices. Figure 4 shows density plots of AC3-positive profiles in anterior brain regions (including temporal, cingulate, insular and entorhinal cortices and basal ganglia; upper panel) and in posterior brain regions [including parietal cortex (Brodman area 7), cingulate cortex (area 31), occipital lobe cortex (area 19), visual cortex (areas 17, 18); lower panels]. For comparison purposes only, Fig. 4 also shows images for 5 h ISO exposure from an animal used in a previous study.6 Whereas plots from control animals (no ISO anaesthesia; Fig. 4A and D) show sparse and random distribution of apoptotic cells across different brain regions, plots from animals after 3 h ISO anaesthesia (Fig. 4B and E) show many more AC3-positive cells in both grey and white matter. Direct visual comparison suggests that 5 h ISO anaesthesia is even more injurious (Fig. 4C and F).
ISO exposure at this early age affected both cortical and subcortical areas. Cortical grey matter, particularly in posterior regions, showed a laminar distribution of dying cells in grey matter after 3 h ISO exposure, similar to findings following longer ISO exposures.6 Further histological detail of dying neurones visualized by AC3 immunohistochemistry (Figs 1 and 2) demonstrates that neurones in layers II, V and VI of the NHP brain were particularly sensitive to 3 h ISO exposure during the first week of life. Apoptotic cell death in white matter after 3 h ISO anaesthesia predominantly affected young oligos as demonstrated by co-labelling for AC3 and myelin basic protein (Fig. 6A–D), a specific marker for oligos. Myelin basic protein is particularly densely expressed in young mature oligos just prior to or in the early stages of myelin formation. The same AC3-positive profiles did not stain positive for antibodies specific for astrocytes or microglia (Fig. 6E and F, respectively) consistent with previous research.15 Apoptotic oligo profiles were distributed diffusely and in a relatively even pattern throughout all white matter regions (Fig. 3).
Discussion
We found that exposure to a surgical plane of ISO anaesthesia for 3 h is sufficient to cause widespread apoptosis of neurones and oligos in the infant rhesus macaque brain. ISO is a member of the halogenated ether family (isoflurane, sevoflurane, desflurane) of volatile inhalational agents used frequently in paediatric anaesthesia. There are no prior studies that show exposure to one of these inhalational anaesthetics for a period as brief as 3 h can cause apoptotic cell death in the infant NHP brain. Slikker and colleagues12 reported that exposure of the infant NHP brain to ketamine for 24 h induced an apoptotic reaction that was restricted to one cell type (neurones) and one brain region (frontal cortex), and that exposure to ketamine for 3 h at the same dose was not sufficient to induce this reaction. While this might signify a difference in toxic threshold between ISO and ketamine, it more likely reflects a difference in methodology. We have previously shown that exposure to a surgical plane of ketamine anaesthesia for 5 h in both infant and foetal NHPs triggers a significant and robust apoptotic reaction that is not restricted to frontal cortex, and that ketamine, like ISO6 7 15 and propofol,8 affects both neurones9 and oligos.16 Therefore, we cannot rule out the possibility that by our methods, exposure to ketamine for 3 h would induce a significant apoptotic reaction affecting both neurones and oligos.
It is important to compare our results with those from previous investigations that tested the effects of a 5 h ISO exposure in the same NHP model.6 First, according to the data, the amount of apoptosis increases with increasing length of exposure, suggesting that severity of the toxic action of ISO is proportional to duration of exposure, at least within the time frames of these experiments. Second, both 5 and 3 h ISO exposures produced evenly dispersed oligoapoptosis throughout the white matter with the 5 h exposure producing denser apoptosis in the corona radiata and internal capsule. Third, both exposures produced neuronal apoptosis throughout all cortical regions, including frontal, temporal, parietal, motor, somatosensory, auditory, insular and entorhinal cortices. However, 5 h ISO neurotoxicity was more laminar and dense in cortical layers II and V of the visual cortex. Finally, neuronal apoptosis was also seen in subcortical regions including the caudate, putamen and thalamus, but to a much higher extent following 5 h ISO. Additional testing is needed to ascertain whether the relationship of increased neurotoxicity with longer exposure is maintained at intervals both shorter and longer than those already tested.
The apoptotic response to either 3 or 5 h of ISO6 is particularly pronounced in neuronal populations within the parietal, temporal and occipital cortices, which have important roles in somatosensory, auditory and visual information processing. The ISO-induced apoptotic response in oligos was widely distributed throughout white matter regions of the brain at all levels. Oligos are responsible for myelinating axons and are thus essential for normal neuronal function. They become sensitive to anaesthesia-induced apoptosis when they are just beginning to engage in the myelination process.6, 7, 8 15 31 Therefore, loss of oligos and associated disruption of myelinogenesis could contribute, together with loss of neurones, to long-term NDI that recent human research [see below] has documented as a potential outcome of anaesthetic exposure in early infancy.
Several independent research groups have recently reported16, 17, 18, 19, 20, 21, 22, 23, 24, 25 32, 33, 34, 35, 36 that exposure of human infants to anaesthesia and surgery is associated with increased risk for long-term NDI. Currently, this evidence is interpreted as inconclusive with respect to the question of human susceptibility because of incomplete control over potentially important variables (a weakness of all retrospective human epidemiological research). An important issue requiring further clarification is whether a single exposure to anaesthesia can cause long-term NDI. Based on retrospective analysis, one group reported16,18 19 that significant NDI in children was observed only after exposure to anaesthesia two or more times, but their findings17 also show that, regardless of the number of exposures, a total exposure time equal to or greater than 120 min is associated with a significant increase in NDI. Evidence from other studies21, 22, 23, 24 is consistent with the interpretation that single brief exposures may be associated with a significant increase in NDI whereas other retrospective studies found no such association.24 25 Importantly, two recent reports from prospective clinical trials suggest that infant exposure to an anaesthetic for about one hour or less in the context of minor surgery (herniotomy) may not increase the risk of NDI during later child development.27 28 However, there are still questions about the predictive power of behavioural testing in children at such a young age. Indeed, a recent retrospective study of infants exposed to sedative and analgesic drugs found no behavioural deficits at around two years of age37 only to discover significant results at four years in the same cohort.34 Based on these contradictory clinical results, the association between anaesthetic duration and NDI remains an open question that deserves further study.
The methods used to demonstrate apoptotic brain injury in animals are invasive and cannot be used in human research. Thus, we have no current means of directly proving or disproving susceptibility of the developing human brain to this type of injury. Here, we demonstrated that the primate brain of infant macaques, which has many fundamental features in common with the primate brain of infant humans, is highly sensitive to anaesthesia-induced apoptotic injury. Moreover, as we showed in previous research,6 each anaesthetic-exposed infant macaque in this study had higher apoptosis counts than any of the non-exposed controls, suggesting that a single ISO exposure at clinically relevant doses (surgical tolerance) and duration (3 h) causes structural changes in the infant primate brain. These findings are relevant to current clinical practice, as a number of surgical interventions and diagnostic procedures in paediatric medicine require total anaesthesia durations comparable with those tested here. Future research using NHPs is needed to identify the long-term behavioural and cognitive consequences of the observed damage following 3 h ISO exposure as compared with the previously studied effects of 5 h ISO exposure.29 Furthermore, additional experiments are necessary to determine potential dosing thresholds for neuroapoptosis induced by ISO and other drugs frequently used in paediatric anaesthesia.
Authors' contributions
Study design/planning: K.K.N., G.A.D., J.W.O., A.M.B.
Study conduct: K.K.N., S.A.J., F.M.M., G.A.D., L.D.M., A.M.B.
Data analysis: K.K.N., J.W.O., A.M.B.
Writing paper: K.K.N., J.W.O., K.J.S., A.M.B.
Revising paper: All Authors.
Acknowledgments
The authors are grateful to the late John W. Olney for his unlimited support and his deeply dedicated mentoring over many years. We admire the tremendous legacy that he leaves behind. We thank the clinical staff at the Oregon National Primate Research Center, Beaverton, OR, USA, for their technical expertise.
Declaration of interest
The authors declare no conflicts of interest.
Funding
(K.K.N.) US National Institute of Child Health and Human Development (grants R01-HD052664, R01-HD052664S and U54-HD087011, which supports the Intellectual and Developmental Disabilities Research Center at Washington University) to K.K.N. IARS Frontiers in Anesthesia Research Award 2012 and the Office of the Director, US National Institutes of Health (P51-OD011092, which supports the operation of the Oregon National Primate Research Center) to A.M.B.
Handling editor: Hugh C. Hemmings Jr
Contributor Information
K.K. Noguchi, Email: noguchik@psychiatry.wustl.edu.
A.M. Brambrink, Email: noguchik@psychiatry.wustl.edu, amb2457@cumc.columbia.edu.
References
- 1.Ikonomidou C, Bosch F, Miksa M. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 2.Jevtovic-Todorovic V, Hartman RE, Izumi Y. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straiko MMW, Young C, Cattano D. Lithium protects against anesthesia-induced developmental neuroapoptosis. Anesthesiology. 2009;110:862–868. doi: 10.1097/ALN.0b013e31819b5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creeley CE, Olney JW. The young: neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2010;110:442–448. doi: 10.1213/ANE.0b013e3181c6b9ca. [DOI] [PubMed] [Google Scholar]
- 5.Slikker W, Zou X, Hotchkiss CE. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 6.Brambrink AM, Evers AS, Avidan MS. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–638. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110(Suppl 1):i29–i38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brambrink AM, Evers AS, Avidan MS. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredriksson A, Pontén E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 11.Satomoto M, Satoh Y, Terui K. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 12.Paule MG, Li M, Allen RR. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman K, Robertson N, Martin L, Dissen G, Neuringer M, Brambrink A. Multiple exposures of general anesthetic during the first 2 weeks of life alter motor and behavioral development in rhesus macaques. J Neurosurg Anesthesiol. 2015;27:402–403. [Google Scholar]
- 15.Brambrink AM, Back SA, Riddle A. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brambrink AM, Evers AS, Avidan MS. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:524–531. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder RT, Flick RP, Sprung J. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bong CL, Allen JC, Kim JTS. The effects of exposure to general anesthesia in infancy on academic performance at age 12. Anesth Analg. 2013;117:1419–1428. doi: 10.1213/ANE.0b013e318299a7c2. [DOI] [PubMed] [Google Scholar]
- 19.Flick RP, Katusic SK, Colligan RC. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–e1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprung J, Flick RP, Katusic SK. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 22.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li GA. retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ing C, DiMaggio C, Whitehouse A. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 24.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen TG, Pedersen JK, Henneberg SW. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 26.Glatz P, Sandin RH, Pedersen NL, Edstedt Bonamy A, Eriksson LI, Granath FN. Academic performance after anesthesia and surgery during childhood: a large-scale nation-wide study. Int Anesth Res Soc. 2015 [Google Scholar]
- 27.Glatz P, Sandin RH, Pedersen NL, Bonamy A-K, Eriksson LI, Granath F. Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 2017;171:e163470. doi: 10.1001/jamapediatrics.2016.3470. [DOI] [PubMed] [Google Scholar]
- 28.Davidson AJ, Disma N, de Graaff JC. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet (London, England) 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman K, Robertson ND, Dissen GA. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2016 doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andropoulos DB, Greene MF. Anesthesia and developing brains—implications of the FDA warning. N Engl J Med. 2017 doi: 10.1056/NEJMp1700196. [DOI] [PubMed] [Google Scholar]
- 31.Sun LS, Li G, DiMaggio CJ. Feasibility and pilot study of the Pediatric Anesthesia NeuroDevelopment Assessment (PANDA) project. J Neurosurg Anesthesiol. 2012;24:382–388. doi: 10.1097/ANA.0b013e31826a0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creeley CE, Olney JW. Drug-induced apoptosis: mechanism by which alcohol and many other drugs can disrupt brain development. Brain Sci. 2013;3:1153–1181. doi: 10.3390/brainsci3031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andropoulos DB, Ahmad HB, Haq T. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth. 2014;24:266–274. doi: 10.1111/pan.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerra G, Robertson CMT, Alton GY. Neurotoxicity of sedative and analgesia drugs in young infants with congenital heart disease: 4-year follow-up. Paediatr Anaesth. 2014;24:257–265. doi: 10.1111/pan.12257. [DOI] [PubMed] [Google Scholar]
- 35.Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr. 2012;160:409–414. doi: 10.1016/j.jpeds.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Morriss FH, Saha S, Bell EF. Surgery and neurodevelopmental outcome of very low-birth-weight infants. JAMA Pediatr. 2014;168:746–754. doi: 10.1001/jamapediatrics.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerra GG, Robertson CMT, Alton GY. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatr Anaesth. 2011;21:932–941. doi: 10.1111/j.1460-9592.2011.03581.x. [DOI] [PubMed] [Google Scholar]