Abstract
Objective
The purpose of this study was to investigate the effects of the omentum, peritoneum, paratenon and skeletal muscle on the proliferation of the cartilage tissue using rabbit model as an in vivo culture medium.
Methods
6 months old forty-five New Zealand rabbits were randomized into omentum, peritoneum, muscle, and Achilles paratenon groups. Standard sized osteochondral grafts were harvested from right knees and immediately placed into the specified tissues. Control group was fresh cartilage at the end of follow-up. After five months, samples were collected and evaluated macroscopically by measuring their dimensions (vertical = D1, horizontal = D2, and depth = D3) and volumes, and histologically by counting the chondrocyte number using camera lucida method.
Results
Macroscopically, increase in mean values for D1 and D2 dimensions of specimens from paratenon and omentum compared to pretransplant dimensions was statistically significant (p < 0.05). Although, volume measurements were higher in omentum and peritoneum group compared to pretransplant dimensions, increase was not significant (p > 0.05). Histologically, mean chondrocyte count was 14.0 ± 0.6 in fresh articular cartilage. Mean chondrocyte counts were 14.4 ± 0.9 in omentum group, 15.4 ± 1.0 in peritoneum group, 9.7 ± 1.3 in muscle group and 9.2 ± 0.4 in Achilles paratenon group respectively. However, mean chondrocyte counts were higher in samples of omentum and peritoneum group compared to fresh articular cartilage, increase was not statistically significant (p > 0.05).
Discussion
Transplantation of the cartilage grafts into mesothelium enhanced the chondrocyte counts and volumes compared with the pretransplant measurements. Mesothelium may have the potential to be used as an in vivo culture medium for osteochondral tissue growth.
Keywords: Cartilage preservation, Mesothelium, Chondrocyte, Knee
Introduction
Adult articular cartilage defects have limited intrinsic healing capacity, because of being avascular, aneural, alymphatic, and having low cellularity. Traditional cartilage repair techniques lead to formation of fibrocartilaginous tissue with variable clinical outcomes.1, 2 Shortcomings of these techniques have led to development of different strategies such as ACI and MACI. In published histological reports, hyalinelike cartilage was found in variable percentage of cases ranging between 21% and 28%.3, 4 Although many studies suggest good clinical results and reliability of these techniques potential to recreate hyaline-like cartilage seemed to be more limited. Clinical results could be independent of factors such as preimplantation cell phenotype and histological repair progression, but enhancing these variables may help optimize the nature of regeneration following surgery.
From a biological point of view, maintenance of the chondrocytic phenotype is important for maturation of hyaline matrix and regenerative success.5 As well as phenotype maintenance, functional integrity depends on bioactive signals coming from matrix. Matrix proteins bind soluble growth factors and regulate their distribution, activation, and presentation to cells.6 Recently, there has been increased interest in creating biological scaffolds composed of extracellular matrix (ECM) derived from the decellularization of tissues.7 Similarity in the composition, microstructure and biomechanical properties of the decellularized scaffolds to those of the native tissues maximizes the promotion effect in the regeneration of both structural and functional tissues and organs.8 However, cartilage is a compact tissue, which is difficult to remove its cellular component. In addition, even though cells could be completely removed from cartilage by multiple decellularization processes, re-seeding of scaffold with chondrocytes can be problematic and original structure and mechanical property of the matrix may be destroyed.9 Hence there is a need for new techniques and new bioreactors to promote cartilage growth as well as preserving native ECM.
Omentum and peritoneum are parts of mesothelium and essentially a serous membrane structure like synovium, pleura and pericardium.10 Omentum is a double sheet of peritoneum that extends from greater curvature of stomach overlying most abdominal organs. Omentum is highly vascularized and its matrix is rich with glycosaminoglycans and adhesive proteins.11 The overall regenerative capacity of the omentum, to maintain progenitor cell viability to limit formation of scar tissue at injury site, has long been demonstrated.12, 13 Besides recent studies demonstrated that omentum is a novel bioreactor suitable for autologous re-cellularization for tissue cultures.14, 15
In this preliminary study, our aim was to evaluate extra-articular tissue cultures, in particular mesothelium as chondrogenetic culture mediums comparatively, in terms of macroscopic and histological evaluations. Achilles paratenon is thought to contribute to healing process earlier than epitenon or internal fibroblasts.16 In mouse's Achilles Tendon, cells isolated from paratenon were also exhibited multipotency and when treated in chondrogenic media, cells reacted positively for collagen II comparable to articular cartilage.17 Chondrocytes cultured with muscle cells exhibited elevated expression of collagen II and while non-muscle cells do not promote cartilage matrix production, converting them into muscle cells enhanced their pro-chondrogenic activity.18
Our hypothesis was that mesothelium can be used as an in vivo culture medium for chondrocyte growth. During many clinical procedures the omentum is removed from patients without unhealthy events and parts of this tissue can be exposed by relatively simple microsurgery techniques.14, 19, 20 These tissues can be further processed to serve as autologous sites for engineering personalized cartilage tissue. To our knowledge, this is the first study in literature that investigates quantitatively and objectively the ‘‘in vivo’’ effect of mesothelium, Achilles paratenon and muscle tissue on chondrocyte growth by using ‘‘camera lucida’’ method for chondrocyte count.
Materials and methods
Study design
After local ethical committee approval a cross sectional in vivo study was performed. Independent variables were groups (control, omentum, peritoneum, skeletal muscle, Achilles paratenon) and dependent variables were chondrocyte counts and approximate volume of samples. Power analysis revealed that at least 5 rabbits needed to be allocated in each group to detect 20% increase in chondrocyte counts with a power of 95%. Forty-five skeletally mature male New Zealand rabbits, weighing on average 2.500 g (range 2.000–3.000 g), were randomly assigned to five groups: Group I omentum (n = 10), Group II peritoneum (n = 10), Group III Achilles paratenon (n = 10) and Group IV skeletal muscle (n = 10). To obtain a baseline measurement of chondrocyte count, fresh distal femoral condylar cartilage of five rabbits (control group, n = 5) were harvested and processed promptly for histological examination at the end of follow-up. Standard sizes of osteochondral grafts (5 × 5 × 10 mm) were harvested from right lateral femoral condyles and process of gathering is considered important to guarantee standard size of samples. Graft samples were kept in isoosmolar and isotonic saline solution while placed immediately into omentum, peritoneum, muscle and paratenon. They were provided with fresh water and rabbit chow ad libitum and housed individually in postoperative period. The rabbits were euthanized at fifth month and osteochondral autografts were allocated for analysis.
Surgical technique
Anesthesia was induced with intramuscular injection of 35 mg/kg ketamine hydrochloride and 5 mg/kg xylazin mixture. Under aseptic and sterile conditions medial parapatellar arthrotomy was performed in right knees. Following lateral subluxation of patella femoral condyles were exposed. Osteochondral grafts of standard dimensions (D1, vertical = 5 mm; D2, horizontal = 5 mm; D3, depth = 10 mm) were harvested from lateral femoral condyles. Capsule, subcutaneous tissue and skin closed appropriately. Grafts were stored isoosmolar and isotonic saline solution until implantation during the same session (approximately 20 min).
In order to implant grafts into mesothelium, animals were placed in a supine position, and abdomen was opened through 4-cm-long midline incision under sterile conditions. After opening peritoneum, grafts were fixed with colored Prolene 3/0 sutures into pocket formed in peritoneum. For omentum group, following omentum exploration with the same midline incision, a pocket was created without disrupting omentum vascularization and grafts were fixed with colored sutures in order to guide recollection of them for evaluations. Laparotomy incisions were closed in three layers.
For paratenon group a midline incision was made dorsally 1 cm proximal to ankle, and the Achilles tendon complex was dissected free from surrounding tissues without disrupting paratenon. Paratenon was then split longitudinally and carefully loosened from the tendon complex. Grafts were fixed into paratenon with colored non-absorbable sutures and incision closed appropriately.
To implant grafts into the vastus lateralis muscle, mid belly of the vastus lateralis muscle was identified by palpation and skin was incised longitudinally over mid thigh. The fascia was opened and after blunt dissection, grafts were fixed into pocket created in the belly of muscle with non-absorbable colored sutures and incisions were closed appropriately. Weight bearing activities were ad libitum postoperatively. Subcutaneous meloxicam injection of 5 mg/kg was used for postoperative analgesia for 2 days.
Histological analysis
Animals were allocated for histological analysis euthanized at fifth months postsurgery. Grafts were harvested by the help of colored sutures. Tissue samples were fixed in 10% formalin solution. After fixation samples were dehydrated and embedded in paraffin subsequently. The samples within paraffin blocks were cut into 5-μm slices and stained with hematoxylin and eosin (H&E). After staining, the slides were evaluated qualitatively under Nikon Optiphot light microscopy (Nikon Corporation, Tokyo, Japan) at 40× and 100× magnifications for the assessment of their histological properties. Quantitative analysis was performed using a compound light microscope equipped with ‘‘camera lucida’’ device, and chondrocytes were counted in every slide in 10 different fields each of which included 0.01 mm2 area at 200× magnification. This method allowed to perform cell count objectively without need for being interrupted to move between different image planes which also minimized possibility to double count cells.21
Macroscopic evaluation
Macroscopic graft dimensions were meticulously measured using a ruler at day 0 before transplantation to confirm the standard size of all the grafts (5 × 5 × 10 mm). Second measurement was performed at 5 months after sample collection to assess change in dimensions.
Statistics
The statistical analysis aimed to assess; 1) comparison of mean chondrocyte numbers in groups at the end of follow-up, 2) comparison of macroscopic dimensions and approximate volumes of samples before and after transplantation. Analysis was assessed by using Kruskal–Wallis test. The power of being able to show the difference between the groups was 98.7%. SPSS®v21 software (SPSS Inc, Chicago, IL, USA) was used for analysis. Significance level was set as 0.05 (p < 0.05).
Results
All animals had returned their usual activities within 48 h postoperatively. One animal in paratenon and muscle groups suffered surgical site infection during post-operative period and excluded from the study. 1 animal in omentum group died because of respiratory tract infection. Remaining animals survived to end of follow up without unhealthy events.
Macroscopic evaluation
Grafts in omentum and peritoneum groups were surrounded by healthy minimally vascularized mesothelial tissue. Grafts appeared well incorporated to surrounding native tissue and appearance of hyaline cartilage was well preserved. However, grafts in muscle and paratenon groups were surrounded with a white, dense fibrous tissue making it difficult to remove from grafts. Macroscopically, dimensions and approximate volumes of each sample have been measured for all samples. Average values of vertical (D1), horizontal (D2) and depth (D3) measurements and approximate volumes of samples are given in Table 1. Average values of the D1, D2 and volume measurements for omentum and peritoneum samples were significantly higher than paratenon and muscle groups (p < 0.05). Average values of D3 in paratenon and muscle group were significantly lower than pretransplant dimensions (p < 0.05). Another finding was that the increase in mean values for D1 and D2 dimensions of specimens from peritoneum and omentum compared to pretransplant dimensions was statistically significant (p < 0.05). Although, volume measurements were higher in omentum and peritoneum groups compared to pretransplant dimensions, increase was not statistically significant (p > 0.05).
Table 1.
Average values (±SD) of vertical (D1), horizontal (D2), and depth (D3) diameters, and approximate volumes measured for specimens in experimental groups and for pretransplant specimens.
| Groups | D1 (mm) ± SD | D2 (mm) ± SD | D3 (mm) ± SD | Volume (mm3) ± SD |
|---|---|---|---|---|
| Omentum (n = 9) | 6.7 ± 0.7 | 6.3 ± 0.4 | 9.2 ± 0.8 | 403.7 ± 96.6 |
| Peritoneum (n = 10) | 6.4 ± 0.6 | 6.6 ± 0.6 | 8.5 ± 0.8 | 364.5 ± 61.4 |
| Achilles tendon (n = 9) | 4.5 ± 0.4 | 4.7 ± 0.8 | 6.5 ± 0.5 | 141.1 ± 41.1 |
| Muscle (n = 9) | 4.5 ± 0.8 | 4.6 ± 0.5 | 6.5 ± 0.7 | 143.2 ± 54.4 |
| Pretransplant | 5.0 | 5.0 | 10.0 | 250.0 |
Histological analysis
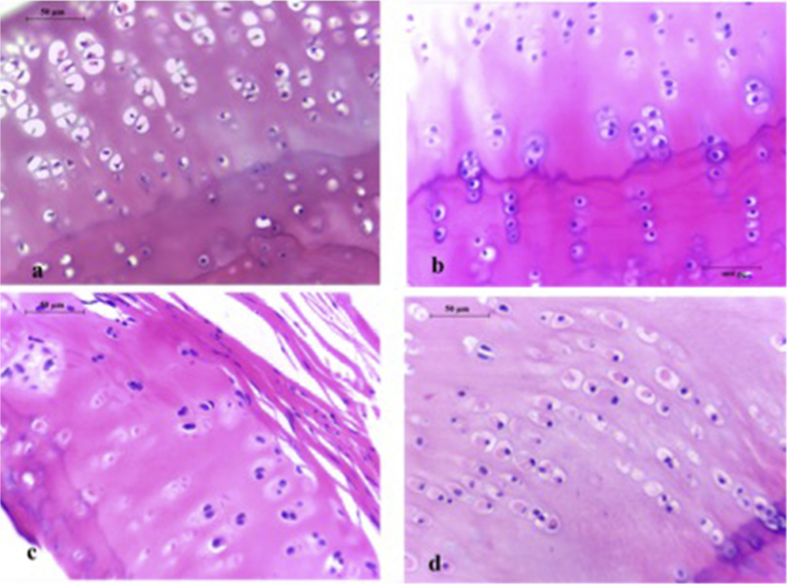
In two of samples in muscle and paratenon groups and one sample of omentum and paratenon groups, healthy hyalin cartilage was not observed and excluded from study. In remaining samples, chondrocytes in omentum and peritoneum groups were more evident and occasionally observed to be clustered in isogenous groups (Fig. 1). Such isogenous groups reflect the mitotic division of a chondrocyte whose daughter cells have not secreted much more matrix. Constantly double nuclei containing chondrocytes were observed. Basophilic matrix immediately adjacent to many of lacunae were well preserved in samples from omentum and peritoneum groups (Fig. 2). The predominately basophilic staining reflects preservation of the negatively charged aggrecan molecules in the matrix.
Fig. 1.
Histological images of the osteochondral samples at the end of follow up under ×400 magnification. (a) Native hyaline cartilage, some of the cells can be seen to have shrunk a bit because of fixation. (b) Sample from the rabbit number 6 in the peritoneum group. Note the isogenous groups and columnar distribution of cells under and above the tidemark. Lacunar density is well preserved. (c) Sample from the rabbit number 2 in the muscle group, occasionally lacunae are small and lack of euchromatic ovoid nucleus. (d) Sample from the rabbit number 3 in the paratenon group. Note the lack of basophilia and eosinophilia in the ground matrix. Cells are arranged in columnar formation but lack of isogenous groups.
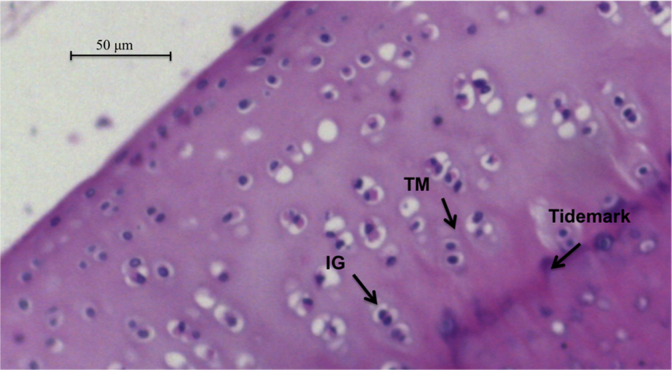
Fig. 2.
Histological images of the postoperative osteochondral sample from the rabbit number 4 in the omentum group after 5 months of follow up under ×400 magnification. Note the preservation of isogenous groups (IG) and columnar distribution of cells. Territorial matrix (TM), which is rich in sulfated glycosaminoglycans, is well preserved.
Mean values (with±SD) of chondrocyte numbers in all groups were demonstrated in Table 2. When groups were compared with the control group, significantly lower mean chondrocyte counts were found in muscle and paratenon groups (p < 0.05). Although mean chondrocyte counts were higher in samples of omentum and peritoneum group compared to fresh articular cartilage, increase was not statistically significant (p > 0.05).
Table 2.
The mean values (with ±SD) of the chondrocyte numbers from specimens and fresh hyalin cartilage.
| Groups | Mean chondrocyte numbers (±SD) |
|---|---|
| Control (n = 5) | 14.0 ± 0.6 |
| Omentum (n = 8) | 14.4 ± 0.9 |
| Paratenon (n = 9) | 15.4 ± 1.0 |
| Muscle (n = 7) | 9.7 ± 1.3 |
| Achilles paratenon (n = 7) | 9.2 ± 0.4 |
The mean numbers of chondrocytes were found to be significantly higher in omentum and paratenon samples than that of specimens of muscle and Achilles paratenon groups (p < 0.05). There was no statistical significance between peritoneum and omentum groups (p > 0.05).
Discussion
In vivo culture experiments have gained interest, because physiologic conditions can be provided readily.22, 23 In this preliminary study, we have most importantly found that, mean chondrocyte counts and volumes of grafts transplanted in omentum and peritoneum increased after five months. However, grafts in paratenon and skeletal muscle failed to show the same success. From clinical perspective, results of this study would possibly suggest the use of the omentum and peritoneum as a new, and native chondrogenic medium – like previously described media, e.g. the synovium,22 when the in vivo growth of the osteochondral tissue is required for any therapeutic purpose.
The chemical and ultrastructural characteristics of the matrix may have great influence on the behavior of chondrocytes in culture.24 ECM provides cells with a lot of instructive cues for inducing functional integrity.14 Matrix associated proteins cooperate to assemble matrices and bind to cells through matrix receptors. In this manner, collagen-based scaffolds may be insufficient to provide chondrocytes these valuable instructions to survive and mimic physiologic conditions.
The results of this preliminary study showed that, although statistically not significant, the mean chondrocyte counts and volumes of grafts transplanted in omentum and peritoneum increased after five months. This finding can be possibly explained by the theory that mesothelium may act as an in vivo culture medium to promote growth of chondrocytes in an osteochondral tissue. It should be noted that mesothelium has a mesenchymal origin and essentially a serous membrane structure like synovium, pleura and pericardium.10 The viability of chondrocytes in these regions can be attributable to the bioactive molecules of mesothelium and secretion of its own interstitial fluid like synovial tissue. Mesothelial cells have antiinflammatory and immune modulatory effects and secrete various growth factors.25 Additionally, mesothelial cells are endowed with such a degree of plasticity that, if placed in the appropriate microenvironment, they have a remarkable potential to generate other mesenchymal-derived cell lines.26 Besides, cartilaginous differentiation of the peritoneum without intra-abdominal malignancy has been already detected.27 After splenic tissue and pancreatic islet transplantation into the omentum, tissues manage to survive and continue their functions.28, 29 Also it has been found that omentum has provided a suitable in vivo site for hepatocytes to expand over scaffolds with abundant vessels which have perfused 3D structures.30 In these cases omentum provided autologous support for the growth of the seeded cells, functioning as an in vivo bioreactor. In our preliminary study, we have found that extracellular matrix and cell viability was well preserved in grafts transplanted in mesothelium. Hence we demonstrated the possible use of mesothelium as an in vivo and autologous bioreactor as opposed to the muscle and paratenon tissues. To our knowledge, there is no previous in vivo study in literature that demonstrated the effects of mesothelium on the growth of cartilage both macroscopically and histologically by using camera lucida method.
There were several limitations of this study. Firstly, mechanical competence of grafts was not investigated. Secondly, histomorphometric assays were not performed so we could not evaluate the content or alterations of the protein organization of the matrix. However, in histological analysis there were no fibroblastic invasion and basophilic staining, columnar organization and glassy matrix were well preserved in grafts transplanted in mesothelium. Thirdly, we did not perform any intra- or interobserver reliability tests of these measurements. A senior investigator who was blinded to groups (M.S.) objectively and reliably quantified the chondrocytes in all specimens.
This study is a preliminary step of obtaining evidence on a new concept for engineering personalized cartilage tissue. Following the first definition of the “biological tropism” between the synovium and articular cartilage,22 the results of the present study may encourage musculoskeletal investigators to consider the second biological tropism between the mesothelium and cartilage in the near future. The use of mesothelium may provide nutrients, oxygen and bioactive molecules for the growth of tissues. Besides mesothelium is not a cell specific environment, incubation of tissues like cartilage can be better controlled and considered more realistically. Further basic investigations and prospective and randomized clinical studies are required to justify the use of mesothelium in clinical settings.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Steadman J.R., Briggs K.K., Rodrigo J.J., Kocher M.S., Gill T.J., Rodkey W.G. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 2.Hangody L., Dobos J., Balo E., Panics G., Hangody L.R., Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38(6):1125–1133. doi: 10.1177/0363546509360405. [DOI] [PubMed] [Google Scholar]
- 3.Henderson I., Lavigne P., Valenzuela H., Oakes B. Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res. 2007;455:253–261. doi: 10.1097/01.blo.0000238829.42563.56. [DOI] [PubMed] [Google Scholar]
- 4.Enea D., Cecconi S., Busilacchi A., Manzotti S., Gesuita R., Gigante A. Matrix-induced autologous chondrocyte implantation (MACI) in the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):862–869. doi: 10.1007/s00167-011-1639-1. [DOI] [PubMed] [Google Scholar]
- 5.Zheng M.H., Willers C., Kirilak L. Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue Eng. 2007;13(4):737–746. doi: 10.1089/ten.2006.0246. [DOI] [PubMed] [Google Scholar]
- 6.Hynes R.O., Naba A. Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4(1) doi: 10.1101/cshperspect.a004903. a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng C.W., Solorio L.D., Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32(2):462–484. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshiba T., Lu H., Kawazoe N., Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10(12):1717–1728. doi: 10.1517/14712598.2010.534079. [DOI] [PubMed] [Google Scholar]
- 9.Gong Y.Y., Xue J.X., Zhang W.J., Zhou G.D., Liu W., Cao Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32(9):2265–2273. doi: 10.1016/j.biomaterials.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 10.Dobbie J.W. Serositis: comparative analysis of histological findings and pathogenetic mechanisms in nonbacterial serosal inflammation. Perit Dial Int. 1993;13(4):256–269. [PubMed] [Google Scholar]
- 11.Platell C., Cooper D., Papadimitriou J.M., Hall J.C. The omentum. World J Gastroenterol. 2000;6(2):169–176. doi: 10.3748/wjg.v6.i2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrager J.B., Wain J.C., Wright C.D. Omentum is highly effective in the management of complex cardiothoracic surgical problems. J Thorac Cardiovasc Surg. 2003;125(3):526–532. doi: 10.1067/mtc.2003.12. [DOI] [PubMed] [Google Scholar]
- 13.Patel R.S., Gilbert R.W. Utility of the gastro-omental free flap in head and neck reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2009;17(4):258–262. doi: 10.1097/MOO.0b013e32832cba42. [DOI] [PubMed] [Google Scholar]
- 14.Shevach M., Soffer-Tsur N., Fleischer S., Shapira A., Dvir T. Fabrication of omentum-based matrix for engineering vascularized cardiac tissues. Biofabrication. 2014;6(2) doi: 10.1088/1758-5082/6/2/024101. 024101. [DOI] [PubMed] [Google Scholar]
- 15.Porzionato A., Sfriso M., Macchi V. Decellularized omentum as novel biologic scaffold for reconstructive surgery and regenerative medicine. Eur J Histochem. 2013;57(1) doi: 10.4081/ejh.2013.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gott M., Ast M., Lane L.B. Tendon phenotype should dictate tissue engineering modality in tendon repair: a review. Discov Med. 2011;12(62):75–84. [PubMed] [Google Scholar]
- 17.Mienaltowski M.J., Adams S.M., Birk D.E. Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon. Tissue Eng Part A. 2013;19(1-2):199–210. doi: 10.1089/ten.tea.2012.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cairns D.M., Lee P.G., Uchimura T., Seufert C.R., Kwon H., Zeng L. The role of muscle cells in regulating cartilage matrix production. J Orthop Res. 2010;28(4):529–536. doi: 10.1002/jor.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe T., Kajiyama K., Harimoto N., Gion T., Nagaie T. Laparoscopic omentectomy for preoperative diagnosis of torsion of the greater omentum. Int J Surg Case Rep. 2012;3(3):100–102. doi: 10.1016/j.ijscr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milleo F.Q., Campos A.C., Santoro S. Metabolic effects of an entero-omentectomy in mildly obese type 2 diabetes mellitus patients after three years. Clinics (Sao Paulo) 2011;66(7):1227–1233. doi: 10.1590/S1807-59322011000700018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sargon M.F., Celik H.H., Aksit M.D., Karaagaoglu E. Quantitative analysis of myelinated axons of corpus callosum in the human brain. Int J Neurosci. 2007;117(6):749–755. doi: 10.1080/00207450600910119. [DOI] [PubMed] [Google Scholar]
- 22.Bilge O., Doral M.N., Atesok K. The effects of the synovium on chondrocyte growth: an experimental study. Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2011;19(7):1214–1223. doi: 10.1007/s00167-010-1391-y. [DOI] [PubMed] [Google Scholar]
- 23.Yang Q., Peng J., Guo Q. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29(15):2378–2387. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Gigante A., Bevilacqua C., Ricevuto A., Mattioli-Belmonte M., Greco F. Membrane-seeded autologous chondrocytes: cell viability and characterization at surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):88–92. doi: 10.1007/s00167-006-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura I., Sakamoto Y., Shibasaki M., Kobayashi Y., Matsuo H. Release of endothelins and platelet-activating factor by a rat pleural mesothelial cell line. Eur Respir J. 2000;15(1):170–176. doi: 10.1183/09031936.00.15117000. [DOI] [PubMed] [Google Scholar]
- 26.Herrick S.E., Mutsaers S.E. The potential of mesothelial cells in tissue engineering and regenerative medicine applications. Int J Artif Organs. 2007;30(6):527–540. doi: 10.1177/039139880703000611. [DOI] [PubMed] [Google Scholar]
- 27.Fadare O., Bifulco C., Carter D., Parkash V. Cartilaginous differentiation in peritoneal tissues: a report of two cases and a review of the literature. Mod Pathol. 2002;15(7):777–780. doi: 10.1097/01.MP.0000017565.19341.63. [DOI] [PubMed] [Google Scholar]
- 28.Marques R.G., Petroianu A., Coelho J.M., Portela M.C. Regeneration of splenic autotransplants. Ann Hematol. 2002;81(11):622–626. doi: 10.1007/s00277-002-0564-2. [DOI] [PubMed] [Google Scholar]
- 29.Kin T., Korbutt G.S., Rajotte R.V. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transpl. 2003;3(3):281–285. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee H., Cusick R.A., Utsunomiya H., Ma P.X., Langer R., Vacanti J.P. Effect of implantation site on hepatocytes heterotopically transplanted on biodegradable polymer scaffolds. Tissue Eng. 2003;9(6):1227–1232. doi: 10.1089/10763270360728134. [DOI] [PubMed] [Google Scholar]