Abstract
Objective
Failed Back Syndrome (FBS) is unacceptable relief of pain or recurrence of symptoms in patients after spinal surgery, such as laminectomy. One possible cause of FBS is peridural fibrosis (PF). PF is the overproduction of scar tissue adjacent to the dura mater. Bleeding can cause PF after laminectomy. Ostene is an alkylene oxide copolymer material used to stop bleeding from bony surfaces. Floseal is a gelatin thrombin matrix sealant used to assist fibrin formation and to promote coagulation.
Methods
Total of 32 female Sprague–Dawley rats were evenly allotted to 4 experimental groups: laminectomy only, laminectomy + Ostene (Baxter International, Inc., Deerfield, IL, USA), laminectomy + Floseal (Baxter International, Inc., Deerfield, IL, USA), and laminectomy + Adcon-L (aap Implantate AG, Berlin, Germany). After performing total laminectomy, agents were placed over dura mater. Spinal column of test subjects was harvested 6 weeks after laminectomy. Histopathological examination of samples was based on Masson's trichrome and hematoxylin and eosin staining. PF observed in the groups was graded using system previously described by He et al. Statistically significant p value was defined as p < 0.005.
Results
Present study revealed that Adcon-L, Ostene, and Floseal groups had reduced PF compared with laminectomy only group (p = 0.001). Comparison of Ostene and Floseal groups with Adcon-L group yielded no significant difference.
Conclusion
Reoperation as result of FBS has greater risk and often has poor outcome; surgeons must take precautions to avoid FBS, such as careful selection of appropriate patient and operation technique. Ostene and Floseal may be applied and left in the operation field safely during laminectomy to reduce occurrence of PF after procedure.
Keywords: Failed back syndrome, Peridural fibrosis, Spinal surgery, Hemostatic matrix, Alkylene oxide copolymer
Failed Back Syndrome (FBS) is described as long-term unacceptable relief from pain or recurrence of symptoms in patients who have undergone spinal surgery, such as laminectomy.1, 2 FBS is a complex combination of circumstances. Possible causes include errors in preoperative assessment and surgical indication (the most important and preventable), surgical complications (e.g., dural tears, nerve root injury, infection, or inadequate hemostasis), or development of new pathology (e.g., adjacent segment degeneration, instability, or fibrosis).3
One of the primary causes of FBS is peridural fibrosis (PF), which is characterized by overproduction of scar tissue between dura mater and surrounding tissue.4 PF was first described in 1948 by Key and Ford.5, 6
Hemostasis is the arrest of bleeding through natural means, such as vasoconstriction, or surgical technique. Bleeding is a principal cause of preoperative and postoperative complications; therefore, achieving adequate hemostasis is a critical surgical step. Numerous devices (e.g., electrocautery, thermocoagulation) and agents (e.g., oxidized cellulose, gelatin products) may be used to establish hemostasis; however, excessive PF that develops as result of insufficient hemostasis is a common complication of laminectomy.
The most widely used example of physical barriers used to discontinue bleeding from the bone during surgery is bone wax, which is composed of paraffin and honey bee cera alba (beeswax). Bone wax was used during the US Civil War to stop bleeding after amputations. It is an efficient homeostasis agent, but also has some unwanted effects, such as poor bone healing or osteogenesis.7, 8 Ostene (Baxter International, Inc., Deerfield, IL, USA) is a relatively new, water-soluble, alkylene oxide copolymer material often used to block the blood oozing from bony surfaces.9
Gelatin thrombin matrix sealants, composed of gelatin matrix and human thrombin, were also developed to assist in fibrin formation and promote coagulation.10 These agents have dual mechanism of action in the coagulation cascade: providing contact activation and activated thrombin.11 Floseal (Baxter International, Inc., Deerfield, IL, USA) is commonly used in spinal surgery to stop bleeding and reduce operation time. It is a well-known biocompatible hemostatic matrix composed of bovine gelatin matrix and human derived thrombin. Floseal is often used to control bleeding during surgical procedures when other procedures are ineffective.
Adcon-L (aap Implantate AG, Berlin, Germany) gel is mechanical barrier composed of gelatin and dextran sulfate and has the approval of the US Food and Drug Administration (FDA) for the use against fibrosis.12 The purpose of using mechanical barriers is to reduce PF by separating the dura mater from the surrounding healing tissues.
In the present study, histopathological examination was conducted to compare effect on PF of Ostene and Floseal with Adcon-L in an experimental rat model.
Materials and methods
This study was approved by the ethics committee of Kobay DHL, A.S. Adult female Sprague–Dawley rats (weighing between 250 g and 300 g each; n = 32) were randomly assigned to 4 equal groups. All groups were kept in room that was climate controlled at constant 23 °C with 12-h light and dark cycles. All subjects had free access to water and food. Surgeries were carried out between 8:00 and 12:00 a.m.
Surgical procedure
Under aseptic circumstances, animals were anesthetized with intramuscular injection mixture of ketamine (Ketalar; Pfizer, Inc., NY, NY, USA) 90 mg/kg and xylazine (Rompun; Bayer, A.G., Leverkusen, Germany) 10 mg/kg. All animals' vital parameters, such as arterial saturation, cardiac rate, and rectal temperature, were monitored. Body temperature of the subjects was maintained at 37 °C±5 °C with heating pad.
After shaving and cleansing of the skin, animals were placed in stereotaxic apparatus and posterior midline incision was performed on thoracolumbar region. Paraspinal muscles were retracted and total laminectomy was performed at L2-L4 levels using microscope. Ostene, Floseal, and Adcon-L were placed over dura mater and laminectomy bone borders in thin layer of approximately 1 mm thickness. Site of laminectomy was marked with nylon suture (Ethilon; Ethicon Inc., Somerville, NJ, USA) in neighboring tissue. Following laminectomy, paraspinal fascia and muscles were closed with 4-0 absorbable sutures. Skin incisions were closed with staples. Subjects were placed in warming chamber, and body temperature was maintained at approximately 37 °C. All subjects were kept in cages with 1 rat per cage. Postoperative care included regular bladder excretion. All test groups were kept in 23 °C temperature room with free access to water and food for 6 weeks before spines were harvested. Only 3 subjects had slight monoparesia after surgical procedure and there was no premature loss of any subject.
Experimental groups
The rats were randomly divided into 4 groups of 8 (n = 32), as summarized in Table 1.
Table 1.
Experimental group details.
| Groups | Procedure |
|---|---|
| Group 1 Ostene (O) | Laminectomy and Ostene |
| Group 2 Floseal (F) | Laminectomy and Floseal |
| Group 3 Adcon-L (A) | Laminectomy and Adcon-L |
| Group 4 Sham-operated (negative control) (L) | Only laminectomy |
Group 1: Laminectomy + Ostene: (n = 8)
After laminectomy was performed, Ostene was placed on lamina surface and over dura.
Group 2: Laminectomy + Floseal: (n = 8)
After performing laminectomy, Floseal was placed over dura.
Group 3: Laminectomy + Adcon-L (A): (n = 8)
After laminectomy, Adcon-L was placed over dura.
Group 4: Laminectomy (L): Sham-operated animals (negative control group): (n = 8)
In this group, lumbar skin incision was made, para-vertebral muscles were dissected, and laminae were exposed. Laminectomies were carried out at L2-4 level as described above. Group results are summarized in Table 1.
Histopathological evaluation
Histopathological examinations were based on Masson's trichrome and hematoxylin eosin staining. Blind observer evaluated histological sections microscopically. All samples were prepared after fixation with formalin, decalcification, and rehydration. Paravertebral muscles, bones, spinal cord and peridural space were enbloc sectioned. Paraffin-fixed samples were prepared and sections were obtained. Area of fibrosis was graded at 40× magnification.
Level of PF was graded by microscopic evaluation using principles previously described by He et al13 and is provided in Table 2.
Table 2.
Grades of fibrosis.
| Grade 0 | No scar tissue was observed over dura mater. |
| Grade 1 | Only thin fibrous bands were observed between scar tissue and dura mater. |
| Grade 2 | Continuous adherence of fibrous bands was observed in two-thirds of laminectomy defect. |
| Grade 3 | Fibrous scar tissue adherence to peridural space was larger, affecting more than two-thirds of laminectomy defect, or adherence extended to the nerve roots. |
Hematoxylin has blue-purple color and stains nucleic acids, and eosin has pink color, which stains proteins nonspecifically. In a sample, nuclei are stained blue, whereas cytoplasm and extracellular matrix have different degrees of pink coloration (Fig. 1). In Masson's trichrome staining, muscle fibers are observed as red, collagen tissues and bone are blue or green, cytoplasm is seen as red or pink, and cell nuclei are observed as dark brown to black (Fig. 2). The results of experimental group staining are summarized in Table 3.
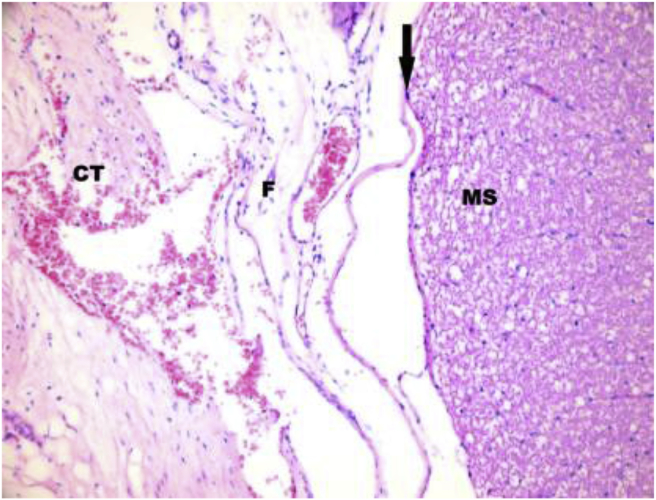
Fig. 1.
Hematoxylin-eosin stain ×200 sample of grade 1 fibrosis. CT: Connective Tissue; F: Fibrosis; MS: Medulla spinalis. Arrow indicates dura mater.
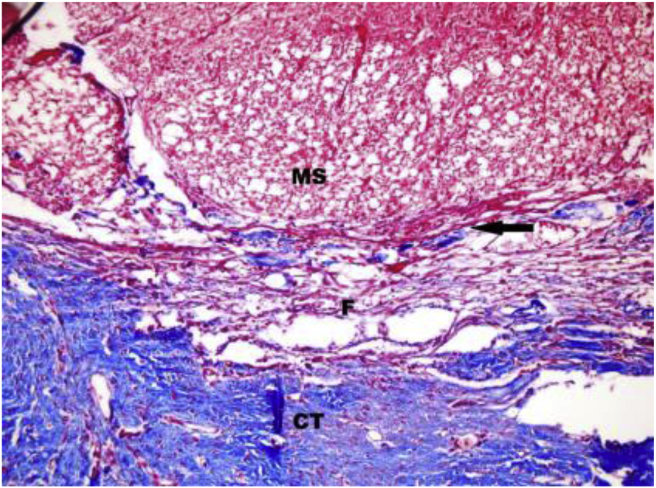
Fig. 2.
Masson's trichrome stain ×200 sample of grade 3 fibrosis. CT: Connective Tissue; F: Fibrosis; MS: Medulla spinalis. Arrow indicates dura mater.
Table 3.
Peridural fibrosis grades of experimental groups.
| Ostene | Floseal | Adcon-L | Laminectomy |
|---|---|---|---|
| 1 | 1 | 0 | 2 |
| 1 | 1 | 0 | 3 |
| 1 | 0 | 1 | 2 |
| 1 | 0 | 1 | 3 |
| 0 | 0 | 0 | 3 |
| 1 | 0 | 0 | 1 |
| 1 | 1 | 0 | 2 |
| 0 | 0 | 0 | 2 |
Statistical analysis
Data analysis was performed using SPSS for Windows version 15.0 (IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) test was performed to examine difference between 4 experimental groups, and groups were compared individually using Tukey's post hoc analysis test. Statistically significant p value was defined as p < 0.005.
Results
Analysis of the difference between 4 experimental groups with ANOVA test revealed significant difference (p = 0.001). Post hoc analysis (Tukey's test) was used to evaluate significant differences between groups.
Antifibrosis effect in Adcon-L group was compared with laminectomy only group using post hoc analysis, and significant difference was found (p = 0.001).
Post hoc analysis comparison of antifibrosis effect of Ostene group and laminectomy only also yielded significant difference (p = 0.001).
Furthermore, significant difference was found in post hoc analysis comparison of Floseal group and laminectomy only group (p = 0.001).
Ostene and Adcon-L groups were compared using post hoc analysis and no significant difference was found (p = 0.282).
No significant difference was found in post hoc analysis comparison of Floseal and Adcon-L groups (p = 0.968).
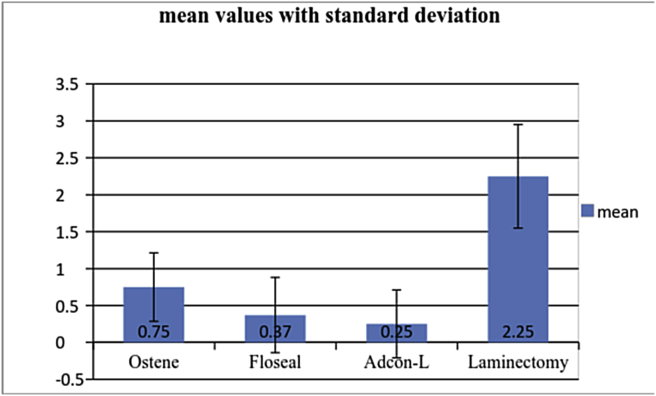
Complete results are provided in Table 4 and Graph 1.
Table 4.
Mean peridural fibrosis grade and standard deviation values of groups, with p values.
| Group | n | Mean | SD | P | P A-L | P O-L | P F-L | P O-A | P F-A |
|---|---|---|---|---|---|---|---|---|---|
| Ostene (O) | 8 | 0.75 | 0.46 | 0.001 | 0.001 | 0.001 | 0.001 | 0.282 | 0.968 |
| Floseal (F) | 8 | 0.37 | 0.51 | 0.001 | |||||
| Adcon-L (A) | 8 | 0.25 | 0.46 | 0.001 | |||||
| Laminectomy (L) | 8 | 2.25 | 0.7 | 0.001 |
Graph 1.
Peridural fibrosis grades of experimental groups with standard deviation.
Overall, study results revealed that Adcon-L, Ostene, and Floseal groups displayed less PF compared with laminectomy only group. Comparison of Ostene and Floseal groups with Adcon-L group produced no significant difference.
Discussion
To avoid occurrence of FBS, surgeons must first make careful selection of appropriate patient and operation technique. After lumbar disc operations, between 8% and 40% of patients continue to have inadequate pain relief.3 FBS frequently leads to reoperation. Reoperation has more potential risks, such as bleeding, dural laceration, nerve root damage and cerebrospinal fluid leakage.14, 15 Minimally invasive techniques and proper hemostasis are indispensable for prevention of PF.
Wound healing is a multi-stage process. When inflammation develops following laminectomy, lymphocytes, fibroblasts, and macrophages are seen. Pro-inflammatory cytokines are secreted and healing cascades are triggered. The redundant form of wound healing is PF. PF has multi-factorial pathophysiology. Examinations of PF have revealed that overexpressed scar tissue is composed primarily of extracellular matrix components, such as fibronectin and collagen fibers, and less of cellular components, such as fibroblasts.16 PF is generally observed to a certain degree after every laminectomy as a normal response of the tissues. On the other hand, excessive PF is a leading cause of FBS. Although we have discovered some clues about PF, basic pathophysiological mechanism has not been clarified. PF disturbs the normal tissue architecture and causes fibroblast accumulation and abnormal protein (such as fibronectin) deposition in the extracellular matrix.6 It has been postulated that fibroblasts transform into myoblasts. It is also thought that perhaps pro-inflammatory cytokines trigger PF. The most likely of these is transforming growth factor beta 1, which has a role in the accumulation of fibroblasts, myoblast transformation, and abnormal protein deposition in the extracellular matrix.2
Uncontrolled surgical bleeding is significantly related to increased morbidity and also leads to prolonged hospitalization and higher healthcare costs.10 Electrocautery, sutures, compression, and hemostatic agents (e.g. oxidized cellulose, gelatin products) are generally used to achieve hemostasis during spinal surgery. Operative techniques, agents, and mechanical barriers are used to reduce PF, including preservation of the ligamentum flavum during surgery, autologous fat grafts, polytetrafluorethylene barriers, tacrolimus, decorin, rosuvastatin, and Adcon-L.2, 6, 15, 17
Water-soluble Ostene, which is absorbed within 48 h, is a relatively new alkylene oxide copolymer hemostatic material used to stop bleeding from bony structures. Ostene has some advantages over bone wax. Magyar et al stated that Ostene does not have adverse effects on bone healing or osteogenesis. Lee et al reported that bone wax can cause adhesions.7, 9, 18 The current English-language literature does not offer any report on the effects of Ostene on PF. Our results revealed that Ostene has antifibrosis effect.
Floseal is a biocompatible sealant compound composed of bovine gelatin matrix and human-derived thrombin that is absorbed within approximately 6–8 weeks. Floseal has maximum swell volume of 10%–20% after 10 min. It has been suggested that use of Floseal is an efficient way to achieve hemostasis quickly, reduce the amount of postoperative bleeding, and the duration of hospitalization.10, 19 Our results indicated that Floseal reduced PF in comparison with laminectomy only group (p = 0.001). Review of the literature revealed that Dogulu et al also found that Floseal decreased adhesion after laminectomy.20
Adcon-L is a well-known prototype of mechanical barrier to prevent fibrosis. Adcon-L is composed of gelatin and dextransulfate.12 It is bioresorbable material that has antifibrosis effect.21, 22 McKinley and Shaffer evaluated cost-effectiveness of Adcon-L in lumbar surgery and stated that Adcon-L patients experienced less pain, did not often seek additional pain medication, and were able to return to work earlier than control group.23 In our study, Adcon-L barrier was used for the positive control group. Although there are reports refuting antifibrosis effect of Adcon-L,24 when compared with laminectomy group, our results indicated that Adcon-L effectively prevented PF (p = 0.001).
Conclusion
In this study, histopathological examination and statistical comparison were used to investigate the effects of Ostene, Floseal and Adcon-L on PF. Results revealed that Ostene and Floseal, which are used to achieve hemostasis during spinal surgery when necessary, can be applied safely in laminectomy operations and do not cause PF. They also reduced PF experienced after laminectomy. In comparison of Ostene with Adcon-L and Floseal with Adcon-L no statically significant difference was observed between groups. We conclude that Adcon-L, which has US FDA approval, prevents PF more effectively, but these statistics may have been affected by the number of subjects in the study groups. The antifibrosis effects of Ostene and Floseal warrant more investigation.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Long D.M. Failed back surgery syndrome. Neurosurg Clin N Am. 1991;2:899–919. [PubMed] [Google Scholar]
- 2.Gurer B., Kahveci R., Gokce E.C., Ozevren H., Turkoglu E., Gokce A. Evaluation of topical application and systemic administration of rosuvastatin in preventing epidural fibrosis in rats. Spine J. 2015;15:522–529. doi: 10.1016/j.spinee.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Pereira P., Avelino A., Monteiro P., Vaz R., Castro-Lopes J.M. New insights from immunohistochemistry for the characterization of epidural scar tissue. Pain Physician. 2014;17:465–474. [PubMed] [Google Scholar]
- 4.Skaf G., Bouclaous C., Alaraj A., Chamoun R. Clinical outcome of surgical treatment of failed back surgery syndrome. Surg Neurol. 2005;64:483–489. doi: 10.1016/j.surneu.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Key J.A., Ford L.T. Experimental intervertebral disc lesions. J Bone Jt Surg Am. 1948;30:621–630. [PubMed] [Google Scholar]
- 6.Zhang C., Kong X., Ning G. All-trans retinoic acid prevents epidural fibrosis through NF-kB signaling pathway in post-laminectomy rats. Neuropharmacology. 2014;79:275–281. doi: 10.1016/j.neuropharm.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard R.F., Jensen H., Vind-Kezunovic S., Jakobsen T., Søballe K., Hasenkam J.M. Bone healing after median sternotomy: a comparison of two hemostatic devices. J Cardiothorac Surg. 2010;5:117. doi: 10.1186/1749-8090-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magyar C.E., Aghaloo T.L., Atti E., Tetradis S. Ostene, a new alkylene oxide copolymer bone hemostatic material, does not inhibit bone healing. Neurosurgery. 2008 October;63(4 suppl 2):373–378. doi: 10.1227/01.NEU.0000316859.03788.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wellisz T., An Y.H., Wen X., Kang Q., Hill C.M., Armstrong J.K. Infection rates and healing using bone wax and a soluble polymer material. Clin Orthop Relat Res. 2008 Feb;466(2):481–486. doi: 10.1007/s11999-007-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echave M., Oyagüez I., Casado M.A. Use of Floseal®, a human gelatine-thrombin matrix sealant, in surgery: a systematic review. BMC Surg. 2014 Dec;20(14):111. doi: 10.1186/1471-2482-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tackett S.M., Calcaterra D., Magee G., Lattouf O.M. Real-world outcomes of hemostatic matrices in cardiac surgery. J Cardiothorac Vasc Anesth. 2014 Dec;28(6):1558–1565. doi: 10.1053/j.jvca.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers K.E., Robertson J.T., Espinoza T. Reduction of epidural fibrosis in lumbar surgery with Oxiplex adhesion barriers of carboxymethylcellulose and polyethylene oxide. Spine J. 2003;3:277–284. doi: 10.1016/s1529-9430(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 13.He Y., Revel M., Loty B. A quantitative model of postlaminectomy scar formation. Effects of a non steroidal anti-inflammatory drug. Spine (Phila Pa 1976) 1995;20:557–563. doi: 10.1097/00007632-199503010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Benoist M., Ficat C., Baraf P., Cauchoix J. Postoperative lumbar epiduro- arachnoiditis. Diagnostic and therapeutic aspects. Spine (Phila Pa 1976) 1980;5:432–436. doi: 10.1097/00007632-198009000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Yan L., Li X., Wang J. Immunomodulatory effectiveness of tacrolimus in preventing epidural scar adhesion after laminectomy in rat model. Eur J Pharmacol. 2013;699:194–199. doi: 10.1016/j.ejphar.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Laurent G.J., Chambers R.C., Hill M.R., McAnulty R.J. Regulation of matrix turnover: fibroblasts, forces, factors and fibrosis. Biochem Soc Trans. 2007;35:647–651. doi: 10.1042/BST0350647. [DOI] [PubMed] [Google Scholar]
- 17.Li S., Xia H., Han C. Retrospective analysis on correlation factors of preserving the ligamentum flavum in microendoscopic discectomy. Clin Neurol Neurosurg. 2015 Dec;139:46–50. doi: 10.1016/j.clineuro.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Lee T.C., Chang N.K., Su F.W. Systemic and local reactions of a water-soluble copolymer bone on a bony defect of rabbit model. Surg Neurol. 2009 Dec;72(suppl 2):S75–S79. doi: 10.1016/j.surneu.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Yao H.H., Hong M.K., Drummond K.J. Haemostasis in neurosurgery: what is the evidence for gelatin-thrombin matrix sealant? J Clin Neurosci. 2013 Mar;20(3):349–356. doi: 10.1016/j.jocn.2012.09.005. Epub 2013 Feb 4. [DOI] [PubMed] [Google Scholar]
- 20.Dogulu F., Durdag E., Cemil B., Kurt G., Ozgun G. The role of FloSeal in reducing epidural fibrosis in a rat laminectomy model. Neurol Neurochir Pol. 2009 Jul–Aug;43(4):346–351. [PubMed] [Google Scholar]
- 21.Kasimcan M.O., Bakar B., Aktaş S., Alhan A., Yilmaz M. Effectiveness of the biophysical barriers on the peridural fibrosis of a postlaminectomy rat model: an experimental research. Injury. 2011 Aug;42(8):778–781. doi: 10.1016/j.injury.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Kurt G., Aytar M.H., Doğulu F. A comparison of the local effectiveness of mitomycin C, aprotinin, and Adcon-L in experimental peridural fibrosis. Surg Neurol. 2008 Dec;70(6):608–613. doi: 10.1016/j.surneu.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 23.McKinley D.S., Shaffer L.M. Cost effectiveness evaluation of Adcon-L adhesion control gel in lumbar surgery. Neurol Res. 1999;21(suppl 1):67–71. doi: 10.1080/01616412.1999.11741030. [DOI] [PubMed] [Google Scholar]
- 24.Richter H.P., Kast E., Tomczak R., Besenfelder W., Gaus W. Results of applying ADCON-L gel after lumbar discectomy: the German ADCON-L study. J Neurosurg. 2001 Oct;95(2 suppl):179–189. doi: 10.3171/spi.2001.95.2.0179. [DOI] [PubMed] [Google Scholar]