Abstract
Objectives
The aim of this study was to determine long term follow up of the patients who had femoral head osteonecrosis and had been treated with free vascularized fibular grafting.
Patients and methods
We retrospectively reviewed 28 hips of 21 patients who had undergone free vascularized fibular grafting for the treatment of osteonecrosis of femoral head. There were 16 male and 5 female patients. The mean age of the patients at the time of surgery was 30.7 years (between 15 and 53 years). The mean follow-up time was 7.6 years (between 5 years and 9.2 years).
Results
During follow-up, one patient died because of leukemia, and one patient was lost. The remaining 26 hips of 19 patients were evaluated. According to the Ficat classification, at the time of surgery, 17 hips were in grade 2 and 9 hips were in grade 3. The post-operative Harris hip scores in grade II disease were excellent in 12 patients, good in 3 patients, and fair in 1 patient. In grade III disease, 1 patient was excellent, 5 patients were good, and 1 patient was fair. There was a significant increase in HHS scores (61 ± 9.7 vs 84 ± 17.8, p < 0.001).
Conclusion
Free vascularized fibular grafting yields extremely good results, particularly in pre-collapse stages of disease in young patients. The operation time does not mark increased if the surgical team is “familiar” with the procedure, and the residual fibular defect of the donor site does not impair the functions of daily living.
Level of Evidence
Level IV, Therapeutic study.
Keywords: Osteonecrosis of the femoral head, Free vascularized fibular grafting
Introduction
Osteonecrosis of the femoral head is a disabling disease that frequently affects adults aged 20–50 years.1 Whatever the etiology, the disease often progresses to femoral head collapse and hip joint arthritis if left untreated.2 Treatment options for the disease mainly depend upon the stage and age of the patient. According to many studies, the etiology of the disease is not a determinant for a particular technique.3, 4 Older age, severely collapsed femoral head, and arthritic patients are accepted as candidates for arthroplasty. Treatment options before collapse of the femoral head aim for regeneration of the subchondral necrotic bone to prevent collapse and preserve sphericity of the femoral head. Core decompression with or without cancellous bone grafting, non-vascularized strut grafts, tantalum rods, electrode insertions, different types of osteotomies, and free or muscle pedicled viable bone grafts have been presented for this purpose.5, 6, 7 Among these techniques, core decompression with vascularized fibular grafting technique removes all necrotic bone and fills the remaining cavity with vascularized bone graft and spongious graft that contain osteoinductive and osteoconductive properties together. The procedure is not new to our knowledge, but most reports in the literature are from a small number of pioneering clinics utilizing the free vascularized fibular grafting (FVFG) technique. In this study, we presented our experience in treatment with FVFG in pre- and post-collapse stages of disease. We report medium-term (mean: 7.6 years) follow-up results.
Patients and methods
In this study, 28 hips of 21 patients (16 male, 5 female) who underwent FVFG for the treatment of osteonecrosis of femoral head in the years between 2005 and 2009 were retrospectively reviewed. Mean age of patients at the time of surgery was 30.7 years (range: 15–53 years). Seven patients who had bilateral hip involvement were operated in 5- to 8-month intervals. The etiologies were collum femoris fracture in 2 hips (7.1%), steroid use in 18 hips (64.3%), and idiopathic in 8 hips (28.5%). The osteonecrosis of patients was diagnosed and staged according to Ficat classification by evaluation of plain roentgenograms and magnetic resonance imaging findings. Mean follow-up duration of patients was 7.6 years (range: 5–9.2 years). Due to the follow-up period of less than 10 years, conversion to total hip arthroplasty (THA) was accepted as failure of the procedure. Radiologic progress of treatment was followed with plain roentgenograms taken in 3-month intervals for the first year, and then once yearly. The radiographs of patients were evaluated for trabecular bone appearance at the tip of the vascularized fibular graft, indicating new bone formation. Preservation of spherical femoral head and loss of subchondral cystic lesions and crescent sign were also accepted as part of the healing of the lesion. Loss of sphericity and decrease in the joint space indicated the progress of the disease. Functional outcomes of survived hips were evaluated using Harris Hip Score (HHS) system. Scores greater than 90 points were accepted as excellent, scores between 80 and 89 points were good, scores between 70 and 79 points were fair, and scores less than 70 points were poor.
The original technique described by Urbaniak and Aldridge et al was used in all cases.8 All patients were operated under general anesthesia in lateral decubitus position by the same surgical team, who were experienced in microsurgery. To reduce operation time, the preparation of hip and harvesting of fibular graft were performed concurrently by 2 teams (Fig. 1). Preoperatively, the extent and spatial localization of the lesion was studied on magnetic resonance images. Intraoperative C-arm fluoroscope was used for anteroposterior and frog leg lateral views (Fig. 2). Prior to placement of the fibular graft, bone graft mixture composed of cancellous autografts harvested from the greater trochanter and 5–10 cc demineralized bone matrix (DBM) allograft (Osteoplant Activagen Injectable Paste [OGS-ACI5], Bioteck S.p.A., Arcugnano, Italy) was used in all patients. The ascending branch of the lateral femoral circumflex artery with accompanying veins was used as recipient vessels (Fig. 3).
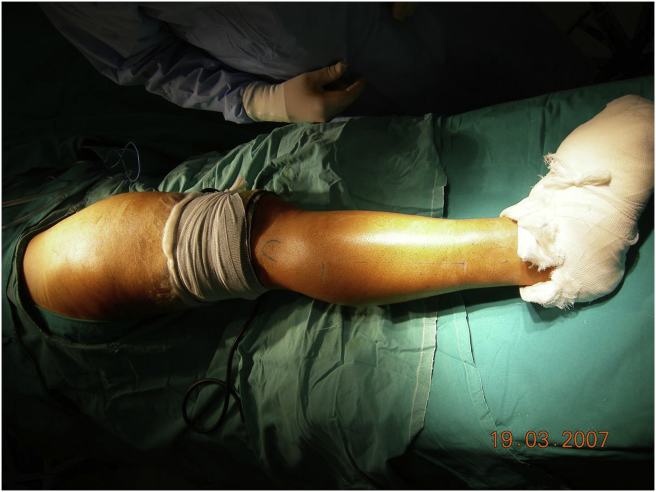
Fig. 1.
Completely scrubbed lower extremity, sterile tourniquet used.
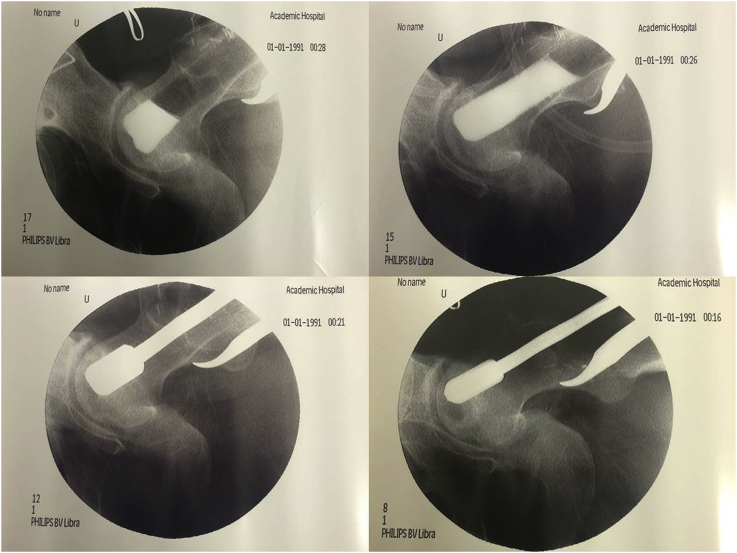
Fig. 2.
Debridement of osteonecrosis field and evaluation of cavity formed by contrast agent.
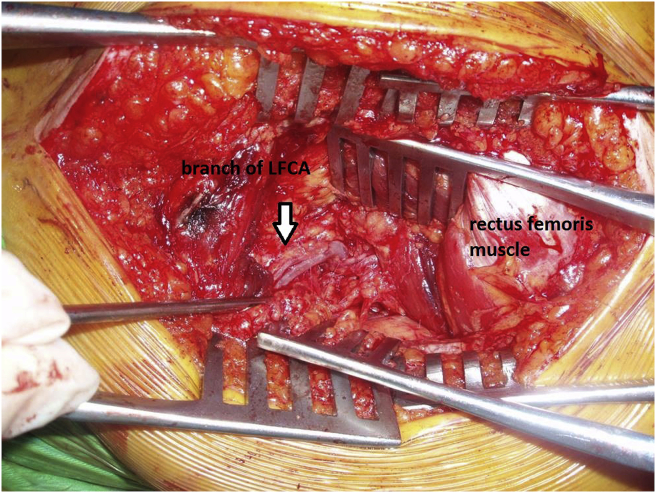
Fig. 3.
View of lateral femoral circumflex artery in the fatty tissue on vastus intermedius.
Patients were treated with low-molecular-weight heparin (20 mg enoxaparin/day subcutaneously) for 3 weeks and intravenous antibiotic prophylaxis during hospitalization. All patients were maintained in absolute bed rest for 5 days and mobilized with crutches without weight-bearing on the affected side on the sixth postoperative day. Weight-bearing was prohibited until the third postoperative month. Then partial weight-bearing (20–25 kg) was initiated using a single Canadian-type crutch for the affected side for 45 days and gradually increased until full weight-bearing was achieved in the sixth postoperative month. Patients were informed about thumb flexion contractures and encouraged to stretch to extension.
Categorical variables were presented in a number of cases (percentage), with continuous variables as mean ± standard deviation. Normal distribution was tested with skewness and kurtosis. Paired t-test was used to compare the changes in patients' pre- and post-operative HHS scores. A p-value of <0.05 was considered significant for all tests. SPSS software (version 11.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
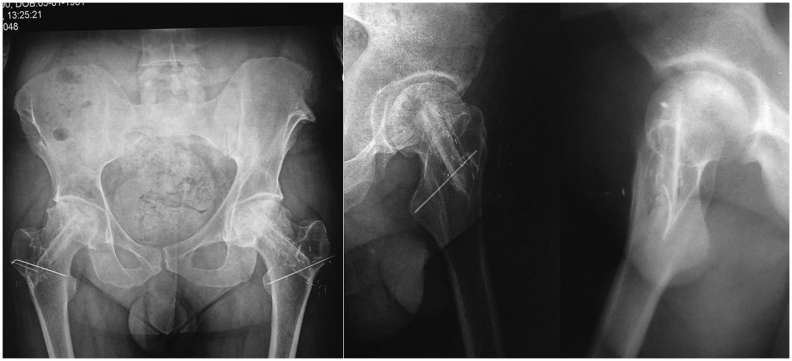
During the follow-up period, 1 patient died because of leukemia, and 1 patient was lost. The remaining 26 hips of 19 patients were evaluated. According to Ficat classification, 17 hips were grade 2, and 9 hips were grade 3 at the time of surgery. At final follow-up, in 3 patients, the disease had progressed and eventually had been treated with THA. No complications related to the surgery—such as infection, deep venous thrombosis, femoral neck fracture, subtrochanteric fracture, peroneal nerve palsy, or severe flexion contracture of the great toe—were observed. Postoperative HHS in grade 2 disease was excellent in 12 patients, good in 3 patients, and fair in 1 patient. In grade 3 disease, 1 patient was excellent, 5 patients were good, and 1 patient was fair. There was a significant increase in HHS scores (61 ± 9.7 vs 84 ± 17.8, p < 0.001). Radiographic trabecular bone formation at the tip of the fibular graft was detected in all patients with stage 2 and stage 3 disease. There was no sign of collapse or joint narrowing in patients with HHS greater than 80 points (Fig. 4). In 6 patients, the progress of collapse and joint narrowing was observed on plain X-rays (Fig. 5). The degree of collapse and joint narrowing were directly correlated with HHS.
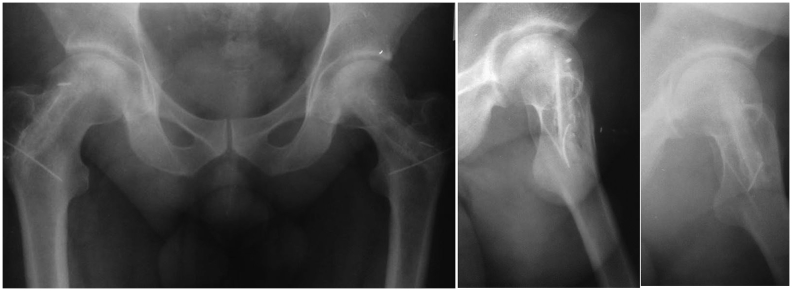
Fig. 4.
Sixth postoperative year roentgenogram of patient whose hips were operated at stage 2.
Fig. 5.
Seventh and eighth postoperative year roentgenograms of patient whose hips were operated at stage 3.
Discussion
If the osteonecrotic lesion at the femoral head is large and involves the lateral pillar, the progress to collapse is inevitable; this usually occurs in less than 3 years, according to the study of Ohzono, which observed the natural progress of 115 untreated hips.9 Many treatment modalities have been proposed for the treatment of this disease. Osteotomies of the proximal femur, including femoral neck and intertrochanteric region, have been recommended to replace the osteonecrotic lesion area under the stress of body weight with an unaffected viable healthy portion of the femoral head. Rotation, varus, flexion, extension and medializing-type osteotomies have been proposed for this purpose.2 The rotational osteotomy described by Sugioka is a very demanding procedure, with regards to both the planning required and the need to preserve the branches of a medial femoral circumflex artery during the procedure.5 Osteotomies also distort the anatomy and biomechanics of the hip joint. Analyzing the results of 115 osteotomies performed by Schneider et al, all osteotomies were associated with a high incidence of complications and low survival rate, and they provided only temporary benefits.10 Core decompression alone may be accepted, similar to the first generation of intralesional surgical interventions. Arlet and Ficat proposed the cause of osteonecrosis as the increase in intraosseous hypertension, intramedullary venous stasis, and edema due to distortion of the blood supply to the femoral head.11 It was believed that the procedure would diminish intraosseous pressure and allow restoration of blood flow in the hypoxic femoral head. According to the results of large series, core decompression may be effective in small lesions and early stages (stage 1) of disease, but without mechanical and osteogenetic support of bone grafts, large lesions and advanced stages of disease eventually result in failure.6 According to the meta-analysis by Castro et al, further surgical interventions have been found necessary in 16%, 37%, and 71% of cases after core decompression of osteonecrosis at Steinberg stages 1, 2, and 3, respectively.12 Combining core decompression with porous tantalum implants was proposed as structural support to the subchondral bone required for prevention of collapse without having donor-site morbidity of the fibular graft. According to the meta-analysis of 6 randomized controlled trials including 256 cases, this treatment option is recommended for small lesions in early stages of young patients. Large lesions and advanced stages of disease were associated with poor results.7 Vascularized iliac bone grafting was combined with tantalum rods to achieve better results in large lesions and advanced stages of disease.13 The FVFG technique described by Urbaniak and Aldridge contains unique steps for treatment of osteonecrosis of the femoral head. Adequate curettage of lesions shortens the healing period by reducing the time it would take to replace all dead bone with new bone formation if the lesion heals without treatment. Control scans of a contrast-filled cavity during the procedure ensure complete removal of necrotic bone.8 Filling the remaining cavity with cancellous autologous bone grafts with osteoinductive and osteoconductive properties provides the scaffold for new bone formation. We believe that grafting the cavity properly is important for new bone formation. In this study, specially designed instruments for implantation of grafts into the cavity were used (Fig. 6). DBM allograft was used as a bone graft extender and mixed with autologous cancellous grafts in large lesions. Adding DBM allografts increases the amount of grafts to properly fill the cavity. In large lesions particularly, the cancellous graft harvested from a trochanteric region may be insufficient, and emptying the trochanteric region may cause iatrogenic fracture. Furthermore, it was shown that DBM allograft extenders enhanced the consolidation of spinal fusions.14 Therefore, the use of DBM allografts in our patients may be related to high survival rates in large lesions. Following cancellous graft placement, introducing a strut graft is the theoretical approach to mechanically support an undermined subchondral cortex of the femoral head to prevent collapse. Nonvascularized strut cortical bone, autografts, or allografts have been used for this purpose.15 Besides mechanical support, the hypothesis governing the vascularization of a strut graft is to provide continuous osteoinductive support to impacted cancellous grafts for ossification. As described in the original technique, folding the periost at the tip of the fibula exposes the cambium layer to benefit from its osteogenic effect (Fig. 7).16 Supporting this theory, vascularized bone grafts yield better results than nonvascularized grafts.17, 18 Several authors reported very consistent and satisfactory results with this procedure.19, 20, 21 Brunelli reported good-to-excellent results (78%) with more than 5-year follow-up.19 Yoo et al reported a 91% success rate in a follow-up interval of 3–10 years.20 Urbaniak et al showed an 83% survival rate of the fibular graft in 646 procedures after follow-ups as long as 17 years.21 He also reported the results of a prospective study of 103 consecutive hips treated with FVFG for symptomatic osteonecrosis of the femoral head.1 Age, etiology, size, location, and stage of the osteonecrotic lesion have all been proposed as factors influencing the outcome of vascularized fibular grafting for osteonecrosis of the femoral head. However, a review of the literature produced conflicting results regarding the effect of these factors on clinical outcomes. Higher survival rates in younger patients are a common finding of most reports.22, 23 While some authors report steroid use as a poor prognostic factor, others associated idiopathic and alcohol-related osteonecrosis of the femoral head with worse prognoses.23, 24 On the other hand, some reports present no relationship between outcomes and etiology.1 The extent of osteonecrotic lesions may be a determining factor for prognosis of FVFG treatment. According to Kerboull, the necrotic angle, which shows the extent of the lesion, is calculated by adding the areas of osteonecrosis on the anteroposterior and frog lateral views.25 Lesions with an angle larger than 200° commonly produce poor results with femoral head-preserving procedures.23, 26 In contrast, in a long-term (mean: 14.4 years) survival analysis of 65 hips, Eward et al found no correlation between the size of lesion and conversion to THA; however, all patients were at the pre-collapse stage.24 In the present study, patients were not classified according to the lesion size.
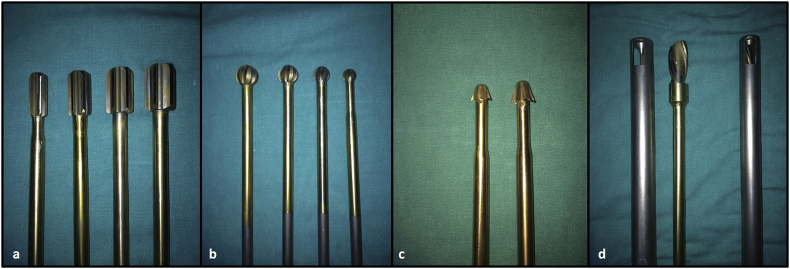
Fig. 6.
Straight reamers in increasing size provide a tunnel size matching with the fibula (a). Ball-tipped reamers (b) and reverse cutters (c) are used inside the lesion. Graft impactor composed of a drill with large helix angle inside the metal tube with distal openings. Grafts are placed in the tube and the drill is advanced through. Clockwise spinning of drill impacts grafts inside the cavity (d). Removal of the tube leaves a space to inset fibular graft.
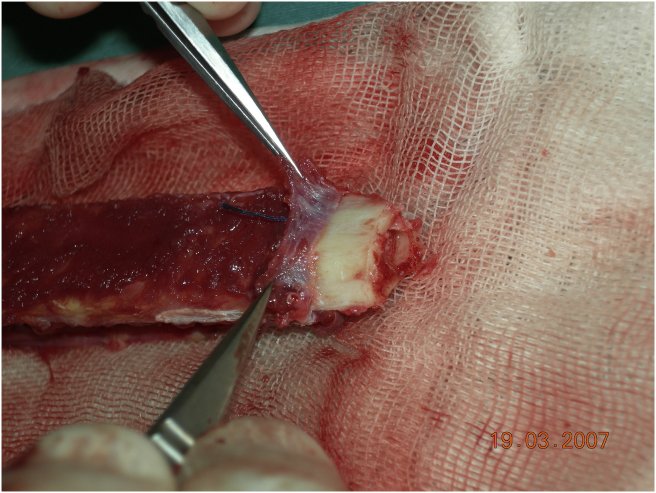
Fig. 7.
Periosteum stripped and folded at the end of the fibula and view of cambium layer.
The radiographic stage of disease is another point of interest for predicting the prognosis of treatment. According to many reports, the probability of THA increases with advanced stages of the Steinberg scheme, whereas it is 0%–10% in stage 2, 12%–23% in stage 3, 17%–30% in stage 4, and 27%–60% in stage 5.20, 27 Eward found no difference between Ficat stage 1 and stage 2 regarding conversion to THA.24 Similarly, Marciniak found no relationship between initial radiographic stage and final clinical outcome or overall rate of graft survival.28 In the present series, 3 THAs (10.7%) were applied to 2 bilaterally involved patients, both hips of one at 5.5 years, and one side of the other patient at the sixth postoperative year. Both hips of the bilateral THA patient were in stage 2 at the time of primary surgery. The other patient's hip was in stage 3 at the time of surgery.
The patency of all microvascular anastomoses in the postoperative period is the subject of a debate, particularly in cases where no monitor, such as skin paddle, is present. To improve the outcome, some authors recommend to inset fibular graft with a skin paddle.29 Although skin paddle shows the patency of anastomoses, it does not provide insight to revascularization inside the femoral head. According to our experience, localization of the perforator of the skin paddle is not consistent and may lift and kink the pedicle of the graft when sutured to the skin. Bone scintigraphy using technetium-99m methylene diphosphonate and single-photon emission computed tomography are effective methods in assessment of bone blood flow in the postoperative period. However, these 2 tests are usually performed within the first postoperative week of surgery (preferably within 72 h) to rule out any thrombus formation.30 According to Berggren and colleagues, a positive bone scan one week or more after surgery does not show patency of the vessels or bone viability, as a result of new bone formation at the surface of the graft due to creeping substitution. Thus, it has been recommended to perform bone scans at the week of surgery to avoid false positive uptake.31, 32 Dynamic contrast-enhanced magnetic resonance imaging is another method used to evaluate the degree of bone marrow perfusion of the fibular graft following injection of gadolinium contrast medium.33 In the literature, we were not able to find any study using these tests for long-term follow-up.
In this study, patients were postoperatively assessed with standing anteroposterior roentgenograms and evaluated in terms of joint space narrowing and shape of the femoral head. We believe that these criteria are sufficient to evaluate the radiologic outcomes of the FVFG technique. Furthermore, we believe that proper surgical technique is another important factor for success. Particularly, preventing the pedicle of the graft from being stranded inside the femoral tunnel and adjusting the pedicle length so as not to be kinked or stretched are the key technical points of the procedure.34 Vascularised fibular grafting provides the most consistently successful results of any joint-preserving methods, such as core decompression, conventional bone grafting, and osteotomy.35 Our results illustrate that FVFG yields extremely good results, particularly in pre-collapse stages of disease in young patients. In the present study, 15 of 17 (88%) Ficat stage 2 hips obtained good or excellent HHS results. The outcomes of less invasive techniques like core-decompression are unpredictable, and published results of these techniques are not superior to FVFG.36 Vascularization of fibular strut graft does not result in a marked increase in operation time if the surgical team is familiar with the procedure. The residual fibular defect of the donor site does not impair the functions of daily living. As mentioned above, theoretically, there are many factors present which may interfere with the expected results of FVFG, but we found that there was no clear evidence of contraindications for FVFG of avascular necrosis of the femoral head, except in very late stages and elderly patients.
Conflict of interest
None declared.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Urbaniak J.R., Coogan P.G., Gunneson E.B., Nunley J.A. Treatment of osteonecrosis of the femoral head with FVFG. A long-term follow-up study of one hundred and three hips. J Bone Jt Surg Am. 1995;77:681–694. doi: 10.2106/00004623-199505000-00004. 1. [DOI] [PubMed] [Google Scholar]
- 2.Scher M.A., Jakim I. Intertrochanteric osteotomy and autogenous bone-grafting for avascular necrosis of the femoral head. J Bone Jt Surg Am. 1993;75-A:1119–1133. doi: 10.2106/00004623-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Zalavras C.G., Lieberman J.R. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014 Jul;22(7):455–464. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 4.Moya-Angeler J., Gianakos A.L., Villa J.C., Ni A., Lane J.M. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015 Sep 18;6(8):590–601. doi: 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugioka Y. Transtrochanteric anterior rotational osteotomy of the femoral head in the treatment of osteonecrosis affecting the hip: a new osteotomy operation. Clin Orthop. 1978;130:191–201. [PubMed] [Google Scholar]
- 6.Smith S.W., Fehning T.K., Griffin W.L., Beaver W. Core decompression of the osteonecrotic femoral head. J Bone Jt Surg Am. 1995;77:674. doi: 10.2106/00004623-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Li L., Shi Z.J., Wang J., Li Z.H. Porous tantalum rod implant is an effective and safe choice for early-stage femoral head necrosis: a meta-analysis of clinical trials. Eur J Orthop Surg Traumatol. 2013 Feb;23(2):211–217. doi: 10.1007/s00590-012-0962-7. [DOI] [PubMed] [Google Scholar]
- 8.Aldridge J.M., 3rd, Berend K.R., Gunneson E.E., Urbaniak J.R. FVFG for the treatment of postcollapse osteonecrosis of the femoral head. J Bone Jt Surg Am. 2004 Mar;86-A(suppl 1):87–101. doi: 10.2106/00004623-200403001-00012. Surgical technique. [DOI] [PubMed] [Google Scholar]
- 9.Ohzono K., Saito M., Takaoka K. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Jt Surg Br. 1991 Jan;73(1):68–72. doi: 10.1302/0301-620X.73B1.1991778. [DOI] [PubMed] [Google Scholar]
- 10.Schneider W., Aigner N., Pinggera O., Knahr K. Intertrochanteric osteotomy for avascular necrosis of the head of the femur. Survival probability of two different methods. J Bone Jt Surg Br. 2002 Aug;84(6):817–824. doi: 10.1302/0301-620x.84b6.12837. [DOI] [PubMed] [Google Scholar]
- 11.Schroer W.C. Current concepts on tile pathogenesis of osteonecrosis of the femoral head. Orthop Rev. 1994;23:487. [PubMed] [Google Scholar]
- 12.Castro F.P., Barrack R.L. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop. 2000;29:187–194. [PubMed] [Google Scholar]
- 13.Zhao D., Zhang Y., Wang W. Tantalum rod implantation and vascularized iliac grafting for osteonecrosis of the femoral head. Orthopedics. 2013 Jun;36(6):789–795. doi: 10.3928/01477447-20130523-26. [DOI] [PubMed] [Google Scholar]
- 14.Lee K.J., Roper J.G., Wang J.C. Demineralized bone matrix and spinal arthrodesis. Spine J. 2005 Nov-Dec;5(6 suppl):217S–223S. doi: 10.1016/j.spinee.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Keizer S.B., Kock N.B., Dijkstra P.D., Taminiau A.H., Nelissen R.G. Treatment of avascular necrosis of the hip by a non-vascularised cortical graft. J Bone Jt Surg Br. 2006 Apr;88(4):460–466. doi: 10.1302/0301-620X.88B4.16950. [DOI] [PubMed] [Google Scholar]
- 16.Augustin G., Antabak A., Davila S. The periosteum. Part 1: anatomy, histology and molecular biology. Injury. 2007 Oct;38(10):1115–1130. doi: 10.1016/j.injury.2007.05.017. Epub 2007 Sep 24. Review. Retraction in: Injury. 2008 Jul;39(7):824. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.Y., Kim Y.G., Kim P.T., Ihn J.C., Cho B.C., Koo K.H. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Jt Surg Am. 2005 Sep;87(9):2012–2018. doi: 10.2106/JBJS.D.02593. [DOI] [PubMed] [Google Scholar]
- 18.Tetik C., Başar H., Bezer M., Erol B., Ağir I., Esemenli T. Comparison of early results of vascularized and non-vascularized fibular grafting in the treatment of osteonecrosis of the femoral head. Acta Orthop Traumatol Turc. 2011;45(5):326–334. doi: 10.3944/AOTT.2011.2259. [DOI] [PubMed] [Google Scholar]
- 19.Brunelli G., Brunelli G. Free microvascular fibular transfer for idiopathic femoral head necrosis: long term follow up. J Reconstr Microsurg. 1991;7:285–295. doi: 10.1055/s-2007-1006786. [DOI] [PubMed] [Google Scholar]
- 20.Yoo M.C., Kim K.I., Hahn C.S., Parvizi J. Long-term followup of vascularized fibular grafting for femoral head necrosis. Clin Orthop Relat Res. 2008 May;466(5):1133–1140. doi: 10.1007/s11999-008-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korompilias A.V., Lykissas M.G., Beris A.E., Urbaniak J.R., Soucacos P.N. Vascularised fibular graft in the management of femoral head osteonecrosis: twenty years later. J Bone Jt Surg Br. 2009 Mar;91(3):287–293. doi: 10.1302/0301-620X.91B3.21846. [DOI] [PubMed] [Google Scholar]
- 22.Berend K.R., Gunneson E.E., Urbaniak J.R. FVFG for the treatment of postcollapse osteonecrosis of the femoral head. J Bone Jt Surg Am. 2003 Jun;85A(6):987–993. doi: 10.2106/00004623-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Kawate K., Yajima H., Sugimoto K. Indications for FVFG for the treatment of osteonecrosis of the femoral head. BMC Musculoskelet Disord. 2007 Aug 8;8:78. doi: 10.1186/1471-2474-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eward W.C., Rineer C.A., Urbaniak J.R., Richard M.J., Ruch D.S. The vascularized fibular graft in precollapse osteonecrosis: is long-term hip preservation possible? Clin Orthop Relat Res. 2012 Oct;470(10):2819–2826. doi: 10.1007/s11999-012-2429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerboul M., Thomine J., Postel M., Merle d'Aubigne R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Jt Surg Br. 1974;56:291–296. [PubMed] [Google Scholar]
- 26.Mont M.A., Hungerford D.S. Nontraumatic avascular necrosis of the femoral head. J Bone Jt Surg Am. 1995;77:459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Chen C., Lin C., Chen W., Shih H., Wueng S.W.N., Lee M.S. Vascularized iliac bone grafting for osteonecrosis with segmental collapse of the femoral head. J Bone Jt Surg. 2009;91:2390–2394. doi: 10.2106/JBJS.H.01814. [DOI] [PubMed] [Google Scholar]
- 28.Marciniak D., Furey C., Shaffer J.W. Osteonecrosis of the femoral head. A study of 101 hips treated with vascularized fibular grafting. J Bone Jt Surg Am. 2005 Apr;87(4):742–747. doi: 10.2106/JBJS.D.02004. [DOI] [PubMed] [Google Scholar]
- 29.Cho B.C., Kim S.Y., Lee J.H., Ramasastry S.S., Weinzweig N., Baik B.S. Treatment of osteonecrosis of the femoral head with free vascularized fibular transfer. Ann Plast Surg. 1998 Jun;40(6):586–593. doi: 10.1097/00000637-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Schuepbach Jonas, Dassonville Olivier, Poissonnet Gilles, Demard Francois. Early postoperative bone scintigraphy in the evaluation of microvascular bone grafts in head and neck reconstruction. Head Face Med. 2007;3:20. doi: 10.1186/1746-160X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berggren A., Weiland A.J., Ostrup L.T. Bone scintigraphy in evaluating the viability of composite bone grafts revascularized by microvascular anastomoses, conventional autogenous bone grafts, and free nonvascularized periosteal grafts. J Bone Jt Surg Am. 1982;64:799–809. [PubMed] [Google Scholar]
- 32.Droll K.P., Prasad V., Ciorau A., Gray B.G., McKee M.D. The use of postoperative bone scintigraphy to predict graft retention. Can J Surg. 2007 Aug;50(4):261–265. [PMC free article] [PubMed] [Google Scholar]
- 33.Bey E., Paranque A., Pharaboz C., Cariou J.L. Postoperative monitoring of free fibular grafts by dynamic magnetic resonance imaging. Preliminary results in three cases of mandibular reconstruction. Ann Chir Plast Esthet. 2001 Feb;46(1):10–17. [PubMed] [Google Scholar]
- 34.Tan C.H., Sathappan S.S., Chew Y.C., Ong H.S. Technical challenges of advanced hip osteonecrosis managed using a vascularised fibular graft. Singap Med J. 2007 Nov;48(11):e299–e303. [PubMed] [Google Scholar]
- 35.Mont M.A., Jones L.C., Hungerford D.S. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Jt Surg Am. 2006;88-A:1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 36.Scully S.P., Aaron R.K., Urbaniak J.R. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 2000 Feb;82(2):290–291. [PubMed] [Google Scholar]