Abstract
Objectives
This study sought to compare the prognostic power of left ventricular end-diastolic pressure (LVEDP) and pulmonary arterial wedge pressure (PAWP) in heart failure with preserved ejection fraction (HFpEF).
Background
It is broadly accepted that direct measurement of LVEDP in HFpEF more robustly reflects left ventricular hemodynamics than PAWP.
Methods
A total of 173 consecutive HFpEF patients were prospectively enrolled. Of these, 152 patients fulfilled registry inclusion criteria. Study participants underwent clinical evaluation, lung function tests, echocardiography, cardiac magnetic resonance, coronary angiography, and invasive hemodynamic assessments with PAWP and LVEDP measurements in 1 procedure. The study endpoint was defined as hospitalization for heart failure or cardiac death.
Results
A modest pressure difference (2.0 ± 4.4 mm Hg) was observed between PAWP (21.5 ± 5.6 mm Hg) and LVEDP (19.5 ± 5.2 mm Hg) at baseline. After a mean follow-up of 23.5 ± 21.3 months, PAWP was predictive of outcome (p = 0.010), whereas LVEDP was not (p = 0.261) by Kaplan-Meier curves. By multivariate regression analysis, diffusion capacity of carbon monoxide (DLCO) was the only parameter that was independently related to the pressure difference between PAWP and LVEDP. When patients were stratified according to DLCO between ≤45% and >45%, those in the low DLCO group were found to have a more pronounced pressure drop between PAWP and LVEDP (3.1 ± 4.8 mm Hg vs. 0.8 ± 3.8 mm Hg, respectively; p = 0.031) and to be in more advanced disease stages.
Conclusions
Our data indicate that PAWP but not LVEDP is associated with outcome in HFpEF. A more pronounced difference between PAWP and LVEDP and more advanced disease is found in patients with low DLCO.
Keywords: diffusing capacity of carbon monoxide, filling pressures, heart failure with preserved ejection fraction, outcome
Left ventricular (LV) pressure is the main parameter that characterizes LV filling properties. In clinical practice, pulmonary artery wedge pressure (PAWP) by right heart catheter has been established as surrogate measurement and has largely replaced direct measurement of left ventricular end-diastolic pressure (LVEDP).
However, recent patient series have demonstrated a poor agreement between the 2 methods (1–3). Apart from potential technical limitations, particularly having to do with wedge measurements, that are usually put forward as an explanation for such differences, the disagreement between PAWP and LVEDP may well have pathobiological substrates. Hemodynamic studies dating back to the 1960s uncovered a relevant pressure drop between PAWP and the left atrium in patients with elevated left atrial pressure (4). This gradient was attributed to an elevation in pulmonary venous resistance caused by remodeling processes in the capillary and post-capillary vasculature.
Increased LVEDP or left atrial pressure is 1 of the hallmarks of heart failure with preserved ejection fraction (HFpEF). Although the pathoanatomical substrate for increased pulmonary venous resistance (i.e., remodeling and obliteration of the capillary and post-capillary pulmonary vascular bed) has recently been shown histologically in HFpEF cases, the relationship between PAWP and LVEDP remains unclear. In the present study, we compared PAWP and LVEDP pressure measurements and examined prognostic abilities of PAWP versus those of LVEDP. In a further step, we investigated the relationship between the PAWP and LVEDP pressure difference and diffusion capacity of carbon monoxide (DLCO) as a marker of pulmonary capillary function (5).
Methods
Study Population
We prospectively enrolled 173 consecutive patients with HFpEF in our observational, noninterventional registry. Patients had been referred to the Department of Cardiology of the Medical University of Vienna between December 2010 and April 2016. The Medical University of Vienna is a tertiary care center with a high-volume cardiac catheterization unit. The study protocol complied with tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Medical University of Vienna (EK #796/2010). Written informed consent was collected before study enrollment from all patients.
Clinical Definitions
Diagnosis of HFpEF was defined according to the current consensus statement of the European Society of Cardiology (6) and the American College of Cardiology Foundation/American Heart Association task force (7). For the diagnosis of HFpEF, all of the following diagnostic criteria had to be fulfilled: 1) clinical symptoms of heart failure (New York Heart Association [NYHA] functional class ≥II); 2) an echocardiographic LV ejection fraction ≥50% and an LV end-diastolic volume index ≤97 ml/m2; 3) evidence of abnormal LV relaxation, filling, or diastolic stiffness; and 4) serum N-terminal pro–B-type natriuretic peptide (NT-proBNP) concentrations ≥220 pg/ml. The presence of hypertension and diabetes mellitus was recorded according to the respective guidelines (8,9). DLCO was measured according to current standards (10).
Exclusion criteria were moderate and severe valvular heart disease, including mitral regurgitation, congenital heart disease, significant coronary artery disease requiring percutaneous coronary intervention or aortocoronary bypass surgery, and severe congenital abnormalities of the lungs, thorax, or diaphragm, as previously described (11). In addition, patients with chronic obstructive pulmonary disease with a forced expiratory volume in 1 s (FEV1) <50% were excluded (12).
Registry Endpoints and Follow-up
Patients were prospectively surveyed at 6-month intervals by outpatient visits or telephone contact in cases of immobility. The primary registry endpoint was a combined measurement consisting of hospitalization for heart failure and/or death for cardiac reasons. Local and external medical records, as well as telephone interviews with relatives, were used to ascertain causes of hospitalization and modes of death. A detailed report was created for every event and death that was reviewed by 2 independent physicians (D.B., S.A.).
Diagnostic Modalities
All patients underwent conventional transthoracic echocardiography (Vivid 5 and 7 units; General Electric Inc., Hanover, Maryland) according to the guidelines of the American Society of Echocardiography (13). All chamber sizes were quantified by echocardiography. Patients without contraindications (use of a pacemaker, and so forth) also underwent cardiac magnetic resonance for the assessment of left and right ventricular functions. Right heart catheter with LVEDP measurement and coronary angiography were performed as 1 procedure. LVEDP measurements were performed with a 5-F pigtail catheter (Cordis, Milpitas, California). No contrast medium was injected between PAWP and LVEDP measurements. Right heart measurements were performed using a 7-F Swan-Ganz catheter (Edwards Lifesciences GmbH, Vienna, Austria) by femoral access with fluoroscopic guidance.
Pressures were documented as a digitized mean over the whole respiratory cycle including at least 8 consecutive heart cycles, using CathCorLX (Siemens AG, Berlin, Germany). In addition to mean PAWP, the systolic pulmonary artery pressure (sPAP), diastolic (dPAP), and mean (mPAP) pressures, as well as right atrial pressure (RAP), were documented. LVEDP as manually checked in each patient. Because patients in atrial fibrillation have more complete equilibration of dPAP and LVEDP during longer R-R intervals, 8 consecutive beats with the longest R-R intervals were chosen to provide the best estimate of LVEDP.
Cardiac output was measured by thermodilution. Furthermore, the trans-pulmonary gradient (TPG) and diastolic pulmonary vascular pressure gradient (DPG) were calculated as previously described (14). The TPG was computed by subtracting PAWP from mPAP; DPG was calculated as the difference between dPAP and PAWP during a pull-back; pulmonary vascular resistance was calculated by dividing TPG by cardiac output; pulmonary pulse pressure was calculated as the difference between sPAP and dPAP; pulmonary arterial compliance as the stroke volume (cardiac output/heart rate)-to-pulmonary pulse pressure ratio.
Lung function tests including DLCO were performed using the Master Screen Body Jaeger spirometer (BD, Franklin Lakes, New Jersey). DLCO measurements were normalized for standard hemoglobin, assuming a value of 14.6 g/dl for men and 13.4 g/dl for women.
Statistical Analysis
Continuous data are presented as mean ± SD and were compared by using the Student t test. Categorical variables are expressed as counts and percentages and were compared using the chi-square test or Fisher exact test. Normality was checked visually using boxplots and normality tests such as the Kolmogorov-Smirnov test and Shapiro-Wilk test. Median values were compared using the Mann-Whitney U test. Kaplan-Meier plot (log-rank test) was applied to verify the time-dependent discriminative power of a respective variable. The influence of relevant parameters on the putative transvenous gradient, calculated by subtracting LVEDP from PAWP, was investigated first by univariate linear regression. All parameters with a significant influence in the univariate model entered the multivariate model by a stepwise procedure. The influence of PAWP, LVEDP, and the gradient between the 2 parameters on event-free survival was tested by univariate and multivariate regression analyses. The significance limit for a predictor to enter the respective model was 0.05, and the limit to stay in the model was 0.1. Pearson correlation coefficients were used to report the relationship between the gradient and respective parameters analyzed in this study. A 2-sided p value of <0.05 was used to indicate statistical significance. SPSS Statistics version 19.0 (IBM, Armonk, New York) was used for all analyses.
Results
Baseline Characteristics and Follow-up
Of the 173 patients referred, a total of 152 were enrolled and completed follow-up. Twenty-one patients were excluded because of alternative diagnoses, such as cardiac amyloidosis or sarcoidosis (n = 10), significant coronary artery disease (n = 7), and serum NT-proBNP levels <220 pg/ml (n = 4). A total of 106 participants (69.7%) were female at 71.1 years of age. Almost 70% of patients were in advanced NYHA functional classes (III and IV); 144 patients (94.7%) had a history of arterial hypertension, 60 study participants (39.5%) had diabetes, and 93 patients (61.2%) had atrial fibrillation.
Patients were followed for an average of 23.5 ± 21.3 months. Within this time frame, 51 cardiac events (33.6%) including cardiac death (n = 6) occurred. Three patients died due to noncardiac reasons (glioblastoma, pancreatic cancer, stroke) and were censored for the analysis.
PAWP Versus LVEDP
Mean PAWP (21.5 ± 5.6 mm Hg) was slightly higher than the LVEDP (19.5 ± 5.2 mm Hg) with a mean difference of 2.0 ± 4.4 mm Hg.
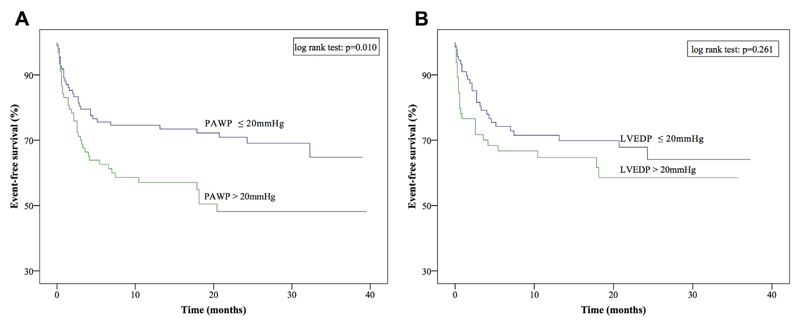
To clarify whether PAWP, as a measurement of LV filling pressures, or rather the gold standard parameter, LVEDP, was associated with event-free survival; Kaplan-Meier curves were plotted. To that end, patients were stratified according to the median PAWP (20 mm Hg) and the median LVEDP (20 mm Hg). Although PAWP was predictive of outcome (p = 0.010), LVEDP was not (p = 0.261) (Figure 1). In a multivariate regression model including the parameters PAWP, LVEDP, and pressure drop between the two, only PAWP with a hazard ratio of 1.055 (95% confidence interval: 1.003 to 1.110; p = 0.039) remained independently associated with outcome.
Figure 1. Kaplan-Meier Survival Curves According to Median PAWP and Median LVEDP.
(A) Kaplan-Meier survival curves according to the median PAWP and (B) according to median LVEDP. LVEDP = left ventricular end-diastolic pressure; PAWP = pulmonary artery wedge pressure.
Parameters Associated with the Pressure Drop Between PAWP and LVEDP
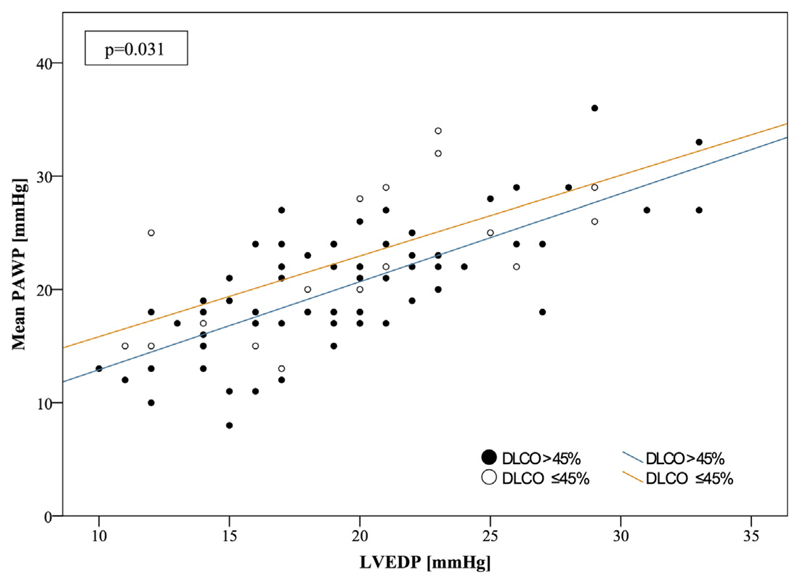
A significant correlation was found between the putative gradient and LVEDP (p < 0.001) as well as between the gradient and PAWP (p < 0.001). No correlations with other hemodynamic parameter were identified (sPAP: p = 0.406; dPAP: p = 0.077; mPAP: p = 0.263; RAP: p = 0.057; TPG: p = 0.080; DPG: p = 0.097; pulmonary vascular resistance: p = 0.319; pulmonary pulse pressure: p = 0.917; and pulmonary arterial compliance: p = 0.917). From a series of parameters obtained from pulmonary function tests, only DLCO was independently associated with the hypothetical gradient (p = 0.011) (Table 1). According to a recent study (15), patients were stratified into groups with DLCO ≤45% and >45%. In fact, patients with a DLCO ≤45% had a significantly higher transvenous pressure gradient (3.1 ± 4.8 mm Hg) than the remainder of the group (0.8 ± 3.8 mm Hg; p = 0.031) (Figure 2, Table 2). Interestingly, patients in the low DLCO group had higher PA pressures, shorter 6-min walk distances, higher NT-proBNP serum concentrations, worse renal function, and lower hematocrit concentrations (Table 2).
Table 1. Parameters Associated With the Gradient Between Pulmonary Artery Wedge Pressure and Left Ventricular End-Diastolic Pressure in Patients With Heart Failure and Preserved Ejection Fraction.
| R Value | Regression Slope (95% CI) | p Value | |
|---|---|---|---|
| Simple regression | |||
| Age, yrs | 0.070 | 0.037 (−0.048 to 0.121) | 0.390 |
| Gender | – | −0.711 (−2.351 to 0.929) | 0.393 |
| Body mass index, kg/m2 | 0.374 | −0.049 (−0.157 to 0.059) | 0.374 |
| Capillary partial pressure of oxygen, mm Hg | 0.204 | 0.072 (0.013 to 0.131) | 0.017 |
| Capillary partial pressure of carbon dioxide, mm Hg | −0.200 | −0.180 (−0.332 to −0.028) | 0.020 |
| Vital capacity, % | 0.026 | 0.005 (−0.026 to 0.036) | 0.763 |
| Forced expiratory volume in 1 s, % | −0.006 | −0.001 (−0.03 to 0.030) | 0.942 |
| Tiffeneau index, % | −0.048 | −0.016 (−0.040 to 0.710) | 0.581 |
| Diffusion capacity of carbon monoxide, % | −0.265 | −0.060 (−0.106 to −0.014) | 0.011 |
| Systolic pulmonary arterial pressure, mm Hg | 0.068 | 0.018 (−0.025 to 0.061) | 0.406 |
| Diastolic pulmonary arterial pressure, mm Hg | 0.144 | −0.085 (−0.009 to 0.179) | 0.077 |
| Mean pulmonary arterial pressure, mm Hg | 0.091 | 0.041 (−0.031 to 0.114) | 0.263 |
| Mean right atrial pressure, mm Hg | 0.155 | 0.133 (−0.004 to 0.270) | 0.057 |
| Cardiac output, l/min | −0.084 | −0.293 (−0.860 to 0.273) | 0.308 |
| Multiple regression | |||
| Diffusion capacity of carbon monoxide, % | −0.060 (−0.106 to −0.014) | 0.011 | |
Values are %, unless otherwise indicated. Bold indicates p < 0.05.
Figure 2. Correlation Curves of Mean PAWP and LVEDP.
Correlation curves are shown of mean PAWP and LVEDP stratified for DLCO. Patients with DLCO ≤45% had significantly higher gradients between PAWP and LVEDP than patients with DLCO >45% (3.1 ± 4.8 mm Hg vs. 0.8 ± 3.8 mm Hg, respectively; p = 0.031). PAWP and LVEDP were significantly correlated (p < 0.001). DLCO = diffusion capacity of carbon monoxide; other abbreviations as in Figure 1.
Table 2. Baseline Characteristics According to Diffusion Capacity of Carbon Monoxide.
| All Patients (N = 152) |
Patients With DLCO >45% (n = 122) |
Patients With DLCO ≤45% (n = 30) |
p Value | |
|---|---|---|---|---|
| Age, yrs | 70.0 ± 8.1 | 69.3 ± 8.4 | 72.5 ± 6.6 | 0.068 |
| Females | 106 (69.7) | 87 (71.3) | 19 (63.3) | 0.441 |
| Cardiac events | 51 (33.6) | 37 (30.3) | 14 (46.6) | 0.089 |
| Body mass index, kg/m2 | 30.9 ± 6.4 | 31.1 ± 5.9 | 29.9 ± 6.3 | 0.406 |
| 6-MWD, m | 329.5 ± 108.9 | 343.5 ± 102.0 | 275.8 ± 120.1 | 0.005 |
| Tiffeneau index | 74.1 ± 12.1 | 75.4 ± 12.2 | 70.7 ± 10.0 | 0.112 |
| History of smoking | 46 (30.3) | 39 (32.0) | 7 (23.3) | 0.277 |
| LA diameter, mm | 61.8 ± 7.5 | 61.4 ± 7.1 | 63.6 ± 8.3 | 0.205 |
| LV diameter, mm | 43.8 ± 5.3 | 43.8 ± 4.7 | 43.5 ± 7.3 | 0.793 |
| LV function, EF, %* | 64.9 ± 10.8 | 65.4 ± 10.8 | 63.5 ± 10.7 | 0.476 |
| RA diameter, mm | 62.1 ± 8.4 | 61.8 ± 7.7 | 63.1 ± 10.9 | 0.539 |
| RV diameter, mm | 36.6 ± 8.1 | 35.8 ± 7.9 | 40.1 ± 8.1 | 0.022 |
| RV function, EF, %* | 53.7 ± 11.6 | 54.1 ± 11.1 | 52.5 ± 13.5 | 0.577 |
| eGFR, ml/min/1.73 m2 | 60.2 ± 17.5 | 62.0 ± 17.5 | 52.8 ± 15.6 | 0.017 |
| Hematocrit, g/dl | 38.1 ± 4.7 | 38.5 ± 4.7 | 36.2 ± 4.5 | 0.027 |
| NT-proBNP, pg/ml | 1,068 (412–1,727) | 971 (366–1,613) | 1,477 (766–2,447) | 0.005 |
| Systolic PAP, mm Hg | 54.9 ± 17.7 | 52.5 ± 15.9 | 65.5 ± 21.1 | 0.008 |
| Diastolic PAP, mm Hg | 22.4 ± 7.4 | 21.6 ± 6.5 | 25.6 ± 9.8 | 0.017 |
| Mean PAP, mm Hg | 34.9 ± 10.0 | 33.6 ± 8.9 | 40.9 ± 12.6 | 0.020 |
| PAWP, mm Hg | 20.3 ± 5.3 | 20.2 ± 5.3 | 22.7 ± 5.9 | 0.072 |
| LVEDP, mm Hg | 19.8 ± 5.3 | 19.4 ± 5.0 | 19.6 ± 5.3 | 0.830 |
| PAWP-LVEDP, mm Hg | 1.3 ± 4.1 | 0.8 ± 3.8 | 3.1 ± 4.8 | 0.031 |
| RAP, mm Hg | 13.0 ± 5.5 | 12.1 ± 5.4 | 14.5 ± 5.6 | 0.050 |
| Cardiac output, l/min | 5.3 ± 1.4 | 5.4 ± 1.4 | 5.0 ± 1.3 | 0.126 |
| SvO2, % | 64.6 ± 9.0 | 65.8 ± 7.6 | 59.7 ± 12.7 | 0.036 |
| PVR, dyn⋅s⋅cm−5 | 231.6 ± 131.4 | 211.5 ± 95.2 | 318.8 ± 209.4 | 0.028 |
| PAC, ml/mm Hg | 2.7 ± 1.3 | 2.8 ± 1.2 | 2.0 ± 0.9 | 0.005 |
| DPG, mm Hg | 2.1 ± 5.8 | 1.8 ± 5.1 | 3.5 ± 7.9 | 0.198 |
| TPG, mm Hg | 14.7 ± 7.2 | 13.8 ± 6.0 | 18.1 ± 10.3 | 0.059 |
Values are mean ± SD, n (%). All values, except for NT-proBNP (p < 0.001), were distributed normally; therefore medians (interquartile ranges) for NT-pro BNP are presented. Bold indicates p < 0.05. *Measurements were derived from cardiac magnetic resonance imaging (n = 96).
6-MWD = 6-min walk distance; DPG = diastolic pressure gradient; EF = ejection fraction; eGFR = estimated glomerular filtration rate; LA = left atrium; LV = left ventricle; LVEDP = left ventricular end-diastolic pressure; PAC = pulmonary arterial compliance; PAP = pulmonary arterial pressure; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance; RA = right atrium; RAP = right atrial pressure; RV = right ventricle; SvO2 = mixed venous oxygen saturation; TPG = transpulmonary pressure gradient.
Discussion
The present study was undertaken to clarify the relationship between LVEDP and PAWP with respect to outcomes in a cohort of well-defined HFpEF patients. By contrast with the general assumption that LVEDP in this condition is the more robust parameter, we found PAWP but not LVEDP to be associated with event-free survival. Based upon the observation that PAWP was slightly higher than respective LVEDP measurements, we postulated the presence of a transvenous pressure gradient in at least a subset of HFpEF patients. This gradient would be a putative one because PAWP is time-averaged pressure, whereas LVEDP is a single time point measurement. PAWP therefore is affected not only by LV filling pressure but also by mitral regurgitation, atrial volume, and stiffness (16), as well as by residual transvenous pressure gradient between capillaries and the left atrium (17).
According to multivariate regression analysis, DLCO was the only parameter that was independently related with the putative pressure gradient. When patients were stratified according to DLCO, those in the low DLCO group were found to have a more pronounced pressure drop between PAWP and LVEDP than patients in the high DLCO group (Figure 2, Table 2). When we analyzed data according to DLCO further, we found that patients in the low DLCO group were characterized by higher PA pressures and were in more advanced stages of disease with regard to 6-min walk test, renal function, serum NT-proBNP and hematocrit concentrations than the high DLCO group (Table 2).
Discrepancies and poor correlations between PAWP and LVEDP measurements have been previously described and ascribed to patient age and comorbidities (2,3). Therefore, LVEDP measurement has been propagated as the gold standard assessment of LV filling pressures in patients who are referred for the evaluation of pulmonary hypertension. In a recent study, Hoeper et al. (15) investigated 108 patients with HFpEF and pulmonary hypertension. As an explanation for the adverse outcome in the low DLCO group, the authors put forward a pathological remodeling process of the capillary and post-capillary pulmonary vasculature in patients with a DLCO below 45% (15). In fact, such disease processes have been described in histopathological studies of HFpEF patients (14). An important limitation of aforementioned studies is the lack of direct measurements of LVEDP for comparison with PAWP. In the present study, LVEDP and PAWP were obtained in 1 single procedure in a prospective manner. A pressure gradient was identified in the subgroup of patients with a low DLCO. We speculate that both phenomena reduced DLCO and that the presence of a transvenous gradient resulted from remodeling processes in the capillary and/or post-capillary bed. Similar to findings by Hoeper et al. (15), lung function parameters in the low DLCO group were comparable to those in the high DLCO group (Table 2). However, from a hemodynamic point of view, patients in the low DLCO group were significantly more compromised, which was also mirrored by a worse clinical status. In parallel with the higher transvenous pressure gradient and higher PAWP in patients with low DLCO, the gradient between dPAP and LVEDP was also elevated compared to the that in the high DLCO group (Table 2). These findings are in line with those from previous experiments by Lee (18), who published an electron microscopy study of the alveolocapillary barrier of chronically congested heart failure patients. He found substantial proliferation of type II granular pneumocytes and irregular thickening of alveolar epithelial and capillary basement membrane. These ultrastructural changes correlated with the duration of heart failure and mean PAWP. In another study performed in a canine model of heart failure with induction of high filling pressures, morphometric analysis of the alveolocapillary barrier showed that endothelial, interstitial, and epithelial thicknesses were increased compared with those in healthy control animals (19).
Previous studies described a systematic underestimation of LVEDP by digitized mean PAWP measurement (2,20). However, patient populations in these studies were markedly different from our cohort. Halpern et al. (2) studied more than 4,000 patients without reporting referral diagnosis. It remains unclear, whether HFpEF patients were part of the cohort (2). Ryan et al. (20) reported data for 61 pulmonary hypertension patients of whom only 16 had post-capillary disease (20). From a patho-mechanistic point of view, rather patients with chronic postcapillary pressure elevation are prone to develop thickening of the alveolocapillary barrier compared to those with pre-capillary pulmonary vascular disease or other conditions.
Study Limitations
Due to the single-center setting of our study, a center-specific bias cannot be excluded. A further drawback is the relatively small number of events. Large-scale multicenter clinical studies will be necessary for further confirmation. However, limiting data collection to 1 center brings several advantages: 1) adherence to a constant clinical routine; 2) constant quality of work-up; and 3) constant follow-up.
LVEDP and PAWP were not measured simultaneously, and the positions of the PAWP measurements were not confirmed by oxygen saturation. It is possible that these factors affected the accuracy of the measurements, although we do not believe they significantly influenced our results. These practices are not routinely performed at most centers, and our protocols reflect common hemodynamic practice. In our experience, PAWP measurements guided by fluoroscopy and characteristic waveform patterns can be used to consistently and accurately position catheters for measuring PAWP. Reproducibility of measurements was not specifically tested. One important limitation of our findings was that the impact of technical issues, such as response of measuring instruments to rapidly changing quantifies and the natural frequency of the measuring system, might have affected all given measurements. Future studies using high-fidelity LVEDP measurements should be considered in order to support our hypothesis. Another limitation of the present study is that the exact pathology underlying reduced DLCO remains speculative, and only approximately 20% of the entire cohort had a DLCO ≤45%. Because patients did not undergo exercise testing by spiroergometry, it remains speculative whether the exercise limitation was rather caused by cardiovascular or pulmonary impairment. Furthermore, no corrections for multiplicity of influence factor testing have been performed due to the limited sample size, which may increase the risk of false positive selection of influence parameters. However, results found have a high medical plausibility and confirm previous findings (15).
Conclusions
In HFpEF patients, PAWP measurements are more closely related to outcome than those of LVEDP. We speculate here that both low DLCO and a pressure gradient between PAWP and LVEDP reflect thickening of the alveolocapillary membrane due to chronic congestion. Both parameters are associated with disease severity and should be addressed in future largescale studies.
Perspectives.
Competency in Medical Knowledge
It is generally accepted that LVEDP is the clinical gold standard for hemodynamic evaluation of the left ventricle. Pulmonary arterial wedge pressure is broadly used in every day practice as a surrogate of LVEDP. In the present study, we compared the 2 parameters with respect to their prognostic power in patients with heart failure and preserved ejection fraction and could show that PAWP measurements were more closely related with outcome than LVEDP.
Translational Outlook
We speculate here that both low DLCO and a pressure gradient between PAWP and LVEDP reflect thickening of the alveolo-capillary membrane due to chronic congestion. Both parameters are associated with disease severity and should be addressed in future large-scale studies.
Acknowledgments
Dr. Mascherbauer has received support from the Austrian Society of Cardiology, the Österreichischer Herzfonds, and Austrian fellowship grant KLI 245. Dr. Bonderman has received support from Austrian fellowship grant KLI 246. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- DLCO
diffusion capacity of carbon monoxide
- HFpEF
heart failure with preserved ejection fraction
- LVEDP
end-diastolic filling pressure
- PAWP
pulmonary artery wedge pressure
References
- 1.Bitar A, Selej M, Bolad I, Lahm T. Poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. PloS One. 2014;9:e87304. doi: 10.1371/journal.pone.0087304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpern SD, Taichman DB, Bitar A, Selej M, Bolad I, Lahm T. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. Chest. 2009;136:37–43. doi: 10.1378/chest.08-2784. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira RK, Ferreira EV, Ramos RP, et al. Usefulness of pulmonary capillary wedge pressure as a correlate of left ventricular filling pressures in pulmonary arterial hypertension. J Heart Lung Transplant. 2014;33:157–62. doi: 10.1016/j.healun.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger PC, Seipp HWJr, Patel DJ. Relationship of pulmonary artery-wedge pressure to left atrial pressure in man. Circulation Res. 1962;11:315–8. doi: 10.1161/01.res.11.2.315. [DOI] [PubMed] [Google Scholar]
- 5.Allanore Y, Borderie D, Avouac J, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Semin Arthritis Rheum. 2008;58:284–91. doi: 10.1002/art.23187. [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 9.Authors/Task Force M. Ryden L, Grant PJ, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 10.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 11.Mascherbauer J, Marzluf BA, Tufaro C, et al. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056–65. doi: 10.1161/CIRCIMAGING.113.000633. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–91. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143:758–66. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 15.Hoeper MM, Meyer K, Rademacher J, Fuge J, Welte T, Olsson KM. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. J Am Coll Cardiol HF. 2016;4:441–9. doi: 10.1016/j.jchf.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Melenovsky V, Hwang S-J, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 17.Melenovsky V, Andersen MJ, Andress K, Reddy YN, Borlaug BA. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail. 2015;17:1161–71. doi: 10.1002/ejhf.417. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS. Electron microscopic studies on the alveolar-capillary barrier in the patients of chronic pulmonary edema. Jpn Circ J. 1979;43:945–54. doi: 10.1253/jcj.43.945. [DOI] [PubMed] [Google Scholar]
- 19.Townsley MI, Fu Z, Mathieu-Costello O, West JB. Pulmonary microvascular permeability. Responses to high vascular pressure after induction of pacing-induced heart failure in dogs. Circulation Res. 1995;77:317–25. doi: 10.1161/01.res.77.2.317. [DOI] [PubMed] [Google Scholar]
- 20.Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012;163:589–94. doi: 10.1016/j.ahj.2012.01.024. [DOI] [PubMed] [Google Scholar]