Abstract
Background
Most heart failure with preserved ejection fraction (HFpEF) patients, at some point, present to an emergency department with typical symptoms of volume overload. Clinically, most respond well to standard diuretic therapy, sometimes at the cost of renal function. The study sought to define the prognostic significance of fluid status versus renal function in patients with HFpEF.
Methods
One hundred sixty-two consecutive patients with HFpEF were enrolled in our prospective registry. Twelve patients with clinically overt decompensation were excluded. Fluid status at baseline was determined by bioelectrical impedance spectroscopy. The primary outcome measure was a combined end point consisting of hospitalization for heart failure and/or death for cardiac reason.
Results
Mean age was 74.4 ± 8.4 years. Ninety-one (61%) patients were hypo- or normovolemic (relative fluid overload [Rel. FO] − 0.7 ± 5.7%) while 59 (39%) patients presented with fluid overload (Rel. FO 11.5 ± 2.7%). During a median follow-up of 24.3 months (interquartile range: 19.8–33.2), 34% of patients reached the combined end point. Multivariate Cox hazard analysis identified fluid overload (hazard ratio: 3.09; 95% confidence interval: 1.68–5.68; p < 0.001) as an independent predictor of adverse outcome. Patients with fluid overload and normal renal function showed a worse event-free survival compared to the subgroup with normohydration and impaired renal function (log-rank: p = 0.042).
Conclusion
HFpEF patients with measurable fluid overload face a dismal prognosis as compared to euvolemic patients. Our data, while preliminary, suggest that patients with fluid overload may face a better outcome under continued fluid removal irrespective of changes in eGFR.
Keywords: Heart failure with preserved ejection fraction Heart failure, Volume overload Congestion, Renal function, Bioelectrical impedance analysis
1. Background
Abnormal fluid distribution and volume overload are hallmarks of acute and chronic heart failure (HF) [1]. At some point in their disease, most HF patients present acutely to an emergency department with typical symptoms of progressive volume overload [2]. During the following hospitalization, most patients clinically respond well to standard diuretic therapy, usually at the cost of renal function. Based on the assumption that overt fluid overload is the result of progressive fluid accumulation [3], current international practice guidelines recommend a correction of volume status using diuretics to reduce the total fluid volume [4,5].
However, hospitalizations due to fluid overload remain frequent in HF patients, with a plethora of explanations seem to be applicable. First, while the dynamics and clinical significance of the heterogeneity in volume overload and fluid distribution are yet to be evaluated [6,7], clinicians may simply fail to adequately assess fluid status in the outpatient setting, due to a lack of objective methods of measurement [7]. Second, physicians are faced with the quandary to choose between guideline-recommended use of loop diuretics and strategies aiming at a long-term preservation of renal function by discontinuing diuretic treatment. Surrogate markers, such as the presence or absence of elevated jugular venous pressure, dyspnea, peripheral edema, third heart sound, or hepatojugular reflux, are commonly considered the mainstays of volume status evaluation [6]. However, these markers lack sensitivity and reliability, especially because affected patients often suffer from concomitant conditions that may mask or modulate fluid status, such as obesity, chronic obstructive pulmonary disease, chronic kidney disease (CKD), or diabetes mellitus (DM) [8]. While elevated serum levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) show a direct relationship with adverse outcome and higher New York Heart Association functional classes [2] in HF patients, its exact role in the estimation of volume overload is controversial. Despite a strong correlation between serum NT-proBNP and total body water [9], elevated NT-proBNP may also have other causes, such as atrial fibrillation (AF), pulmonary embolism, renal failure, advanced age, anemia, or bacterial sepsis [4,5].
Even invasively measured hemodynamic parameters, such as the pulmonary arterial wedge pressure (PAWP) failed to show a tight correlation with gold standard measurements [10], such as tracer techniques, e.g. iodinated131I human serum albumin [2].
Bioelectrical impedance spectroscopy (BIS) is a simple, non-invasive, and relatively inexpensive technique that allows an accurate assessment of fluid status. In the present study, we assessed fluid status and renal function of consecutively registered heart failure with preserved ejection fraction (HFpEF) patients without overt signs of decompensation and followed their clinical course. Specifically, we determined the prognostic significance of fluid status versus renal function, with the ultimate goal of perhaps judging the clinical practice of withdrawing fluid at the cost of impaired renal function.
2. Methods
2.1. Study design
Consecutive patients with a confirmed diagnosis of HFpEF were recruited in this prospective, observational, non-interventional registry performed at the Department of Cardiology of the Medical University of Vienna, Austria. The local Ethics Committee approved the study (EK #796/2010) and all patients gave their written informed consent prior to any study-related procedure.
Baseline data were collected on the day of enrollment and consisted of physical examination, 12-lead electrocardiogram, BIS, 6-min walk test (6-MWT) with Borg Dyspnea Score (BDS), and laboratory tests. Right heart catheter (RHC), coronary angiography, and transthoracic echocardiography (TTE) were performed within a maximum of 1 month. Patients with clinically overt decompensation and requirement for intravenous diuretic treatment were excluded from the protocol. Patients with significant valvular or congenital heart disease, significant coronary artery disease as diagnosed by coronary angiography or regional wall motion abnormalities of the left ventricle (LV) were also excluded.
The primary outcome measure was a combined end point consisting of hospitalization due to HF and/or cardiac death. Patients were followed in 6-month intervals by outpatient visits or telephone calls in case of immobility. The predefined primary end point was ascertained through blind adjudication by a designated team of cardiologists.
2.2. Diagnosis of HFpEF
HFpEF was diagnosed according to the current consensus statement of the European Society of Cardiology (ESC) [11] and the guidelines of the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [5]. The following criteria had to be fulfilled: 1. signs or symptoms of HF, 2. left ventricular ejection fraction (LVEF) >50%, 3. Serum NT-proBNP >220 pg/ml on the day of enrollment, 4. evidence of LV diastolic dysfunction by TTE. For confirmation of diagnosis, RHC was performed in all but seven patients. HFpEF was confirmed if PAWP exceeded 12 mmHg.
2.3. Bioelectrical impedance spectroscopy
Patients underwent standardized evaluation of their fluid status using a portable whole-body BIS device, the Body Composition Monitor (BCM, Fresenius Medical Care, Bad Homburg, Germany). Patients were placed in supine position for at least 5 min before the evaluation of their fluid status. Electrodes were attached to the non-dominant hand and the ipsilateral foot. Measurements were conducted according to the manufacturer's manual. For each patient, only one bioelectrical impedance analysis was performed, as this method has an adequate reproducibility [12]. Fluid overload assessed by BCM is expressed as an absolute value in liters or as a relative value in %, calculated as the ratio between fluid overload (FO) and the content of extracellular water (ECW) and multiplied by 100 (Rel. FO = FO/ECW × 100%).
In this study, fluid overload was defined as Rel. FO ≥ 7% corresponding to the value of the 90th percentile for the reference cohort obtained from an age- and sex-matched healthy population when fluid status was measured with the same technology [13] and as it was used in a previous study in patients with CKD [14]. After baseline evaluation and inclusion in the present study, patients continued treatment at our outpatient clinic. Care-taking physicians at the outpatient clinic were independent from the study team and blinded to the results of the BIS measurement and RHC. Any decisions to adapt diuretic therapy were based on clinical assessment and according to recent guidelines on the management of HFpEF [4,5].
2.4. Transthoracic echocardiography
Patients received a TTE by board-certified physicians using high-quality scanners, such as GE Vivid 5, GE Vivid 7 (General Electric Medical System, Milwaukee, WI, USA), and Siemens Acuson Sequoia (Siemens Healthcare GmbH, Erlangen, Germany). The TTE was performed according to the guidelines of the American Society of Echocardiography [15]. Simpson's biplane method of discs was used to measure LVEF. The peak velocity of the tricuspid regurgitation jet assessed by continuous-wave Doppler together with right atrial pressure was used to measure systolic pulmonary arterial pressure (sPAP) [16].
2.5. Right heart catheter and coronary angiography
RHC was performed via a jugular or femoral access. A 7F Swan-Ganz catheter (Edwards, Irvine, CA, USA) was used for the assessment of hemodynamic parameters. The average of the filling pressures recorded over eight heart cycles were documented using CathCorLX (Siemens AG, Berlin and Munich, Germany). Cardiac output (CO) was assessed by thermodilution and by the Fick method and was expressed in liters/min. Pulmonary pulse pressure (PPP) was calculated as the difference between sPAP and diastolic pulmonary arterial pressure (dPAP). Transpulmonary pressure gradient (TPG) was calculated by subtracting PAWP from mean pulmonary arterial pressure (mPAP). Diastolic pressure gradient (DPG) was calculated as the difference between dPAP and PAWP. Pulmonary vascular resistance (PVR) was calculated by dividing TPG by CO and was expressed in dynes·s·cm−5. Pulmonary arterial compliance (PAC) was calculated as the ratio of stroke volume to PPP.
In the same session, patients underwent coronary angiography and those with at least one visual stenosis over 50% in one of the main vessels and/or over 70% in one of the distal vessels were excluded.
2.6. Other baseline tests
The 6-MWT was performed according to the American Thoracic Society guidelines on a corridor with a 50-m track [17]. The walking distance was measured after 6 min and patients had to grade their dyspnea on the basis of BDS between 0 and 10 [18]. Venous blood was used to measure NT-proBNP with an immunological test (Elecsys® Systems, Roche Diagnostics) and serum creatinine. The estimated glomerular filtration rate (eGFR) was derived from the Modification of Diet in Renal Disease equation. Impaired renal function was defined as an eGFR < 60 ml/min/1.73 m2 which is equivalent to CKD stage 3 or worse [19].
2.7. Statistical analysis
Data were analyzed with SPSS Statistics (version 23, IBM, for Macintosh). P values from two-sided tests ≤0.05 were considered statistically significant. Data were expressed as mean ± standard deviation or frequency and percent. Student' t test or Wilcoxon rank-sum test was used to compare continuous variables, as appropriate. χ2 test was used to assess group differences in categorical variables. Spearman rank correlation coefficient was utilized to measure the dependence between Rel. FO and non-normally distributed variables. For the association analysis between Rel. FO and values with Gaussian distribution, Pearson's correlation coefficient was applied. Cox proportional hazards analyses were done to determine the association of fluid overload and impaired renal function (run as categorical variables) with the predefined combined end point, adjusted for fluid overload, impaired renal function, 6-min walk distance (6-MWD), NT-proBNP, AF, and sPAP. The presence of DM and AF was entered as a categorical variable. Observation times for patients who died from a non-cardiac reason were censored. Results are expressed as hazard ratio (HR) with 95% confidence interval (CI). Crude survival curves were generated by the Kaplan—Meier method and compared with the log-rank test to verify the time-dependent discriminative power of the respective variable.
3. Results
3.1. Patient characteristics
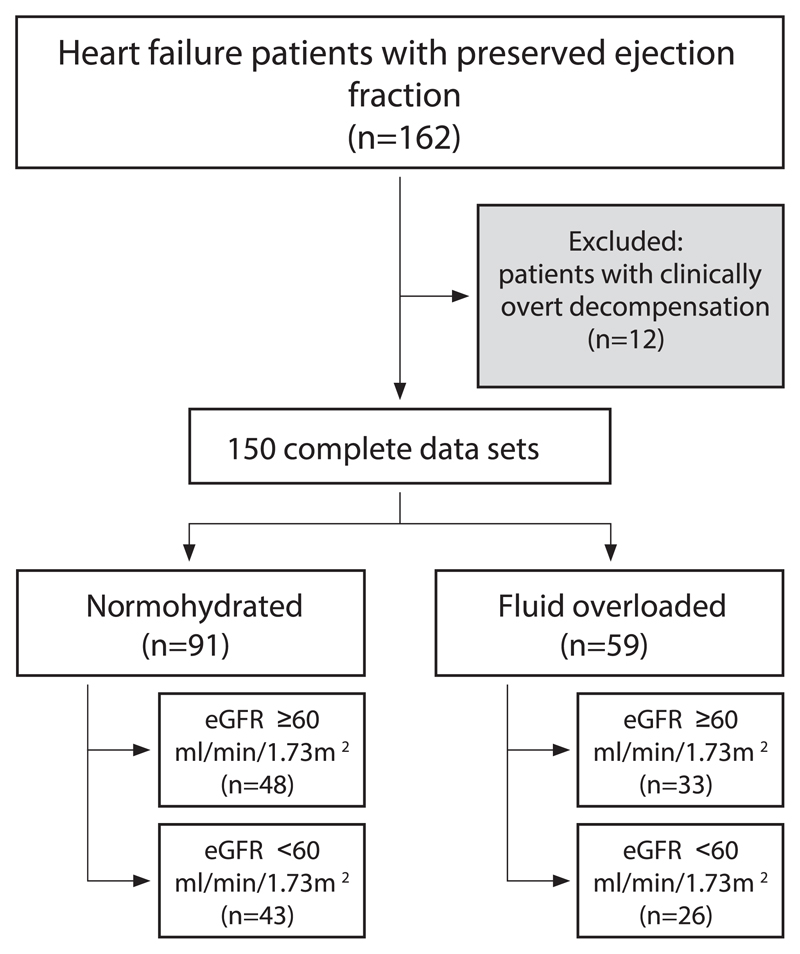
Between December 2010 and July 2015, 162 consecutive patients with HFpEF were enrolled in our prospective, observational, non-interventional registry. Twelve patients were overtly decompensated at the baseline examination with the requirement of immediate therapy and were therefore excluded from further analyses. A detailed patient disposition of the remaining patients, according to fluid status and eGFR, is displayed in Fig. 1.
Fig. 1.
Patient disposition Of the 162 consecutive patients with heart failure and preserved ejection fraction, 12 patients were excluded due clinically overt decompensation (requirement of intravenous diuretic treatment), 91 (61%) patients were hypo- or normovolemic; 59 (39%) patients presented with fluid overload. eGFR indicates estimated glomerular filtration rate.
Patient baseline characteristics are displayed in Table 1. One hundred four (69%) study participants were female. Mean age was 74.4 ± 8.4 years. Ninety-one (61%) patients were hypo- or normovolemic (Rel. FO −0.7 ± 5.7%), while 59 (39%) patients presented with fluid overload (Rel. FO 11.5 ± 2.7%). Patients with fluid overload were older than normohydrated patients (76.2 ± 8.9 vs. 73.2 ± 7.9 years; p = 0.036) and walked shorter in the 6-MWT (290.0 ± 127.6 vs. 363.0 ± 99.3 m; p < 0.001). eGFR did not differ significantly between normohydrated and fluid overloaded patients (65.0 ± 22.9 vs. 63.2 ± 22.1 ml/min/1.73 m2; p = 0.639). When comparing sex and BMI between normohydrated and fluid overloaded patients, we observed no significant differences (p = 0.204 for sex and p = 0.669 for BMI). The frequency of DM was higher in the fluid overload group (50% vs. 28.9%; p = 0.014). Use of diuretics was similar (67.2% of fluid overloaded vs. 74.4% of normohydrated patients; p = 0.343). Patients with fluid overload had significantly larger left atrial diameters (63.3 ± 5.4 vs. 60.7 ± 6.3 mm; p = 0.013) as well as right atrial diameters (63.2 ± 6.5 vs. 60.8 ± 7.2 mm; p = 0.044) compared to normohydrated patients. The ratio of the early diastolic transmitral flow velocity (E) to the mitral annular velocity (e′) was significantly higher in the fluid overload group (19.7 vs. 12.9; p = 0.021); the relationship between E/e′ and Rel. FO was linear (r = 0.419; p < 0.001).
Table 1.
Baseline characteristics of patients with heart failure with preserved ejection fraction classified according to fluid status. Data are presented as mean ± standard deviations or n (%).
| Variable | Normohydrated (n = 91) | Fluid overloaded (n = 59) | p value |
|---|---|---|---|
| Female sex, n (%) | 67 (73.6) | 37 (62.7) | 0.204 |
| Age, years | 73.2 ± 7.9 | 76.2 ± 8.9 | 0.036 |
| Body mass index, kg/m2 | 30.5 ± 6.0 | 31.0 ± 6.9 | 0.669 |
| Six-minute walk distance, m | 363.0 ± 99.3 | 290.0 ± 127.6 | < 0.001 |
| Borg dyspnea score | 3.3 ± 2.2 | 4.1 ± 2.8 | 0.047 |
| NYHA classes III & IV, n (%) | 62 (68.1) | 44 (74.6) | 0.465 |
| NT-pro BNP, pg/ml | 784.2 ± 599.6 | 1744.4 ± 1925.3 | < 0.001 |
| Hematocrit, % | 39.5 ± 4.8 | 37.1 ± 5.6 | 0.002 |
| eGFR, ml/min/1.73m2 | 65.0 ± 22.9 | 63.2 ± 22.1 | 0.639 |
| Urea, mg/dl - Comorbidities | 23.8 ± 11.8 | 24.5 ± 12.7 | 0.708 |
| Atrial fibrillation, n (%) | 53 (58.9) | 32 (55.2) | 0.734 |
| Diabetes mellitus, n (%) | 26 (28.9) | 29 (50.0) | 0.014 |
| Arterial hypertension, n (%) | 88 (97.8) | 57 (98.3) | 0.834 |
| Smoking, n (%) -Medication | 28 (31.8) | 21 (36.2) | 0.596 |
| Beta blocker, n (%) | 68 (75.6) | 43 (74.1) | 0.848 |
| Diuretic, n (%) | 67 (74.4) | 39 (67.2) | 0.343 |
| Statin, n (%) | 35 (38.9) | 27 (46.6) | 0.396 |
NYHA = New York Heart Association classification; NT-pro-BNP = N-terminal prohormone of brain natriuretic peptide; eGFR = estimated glomerular filtration rate.
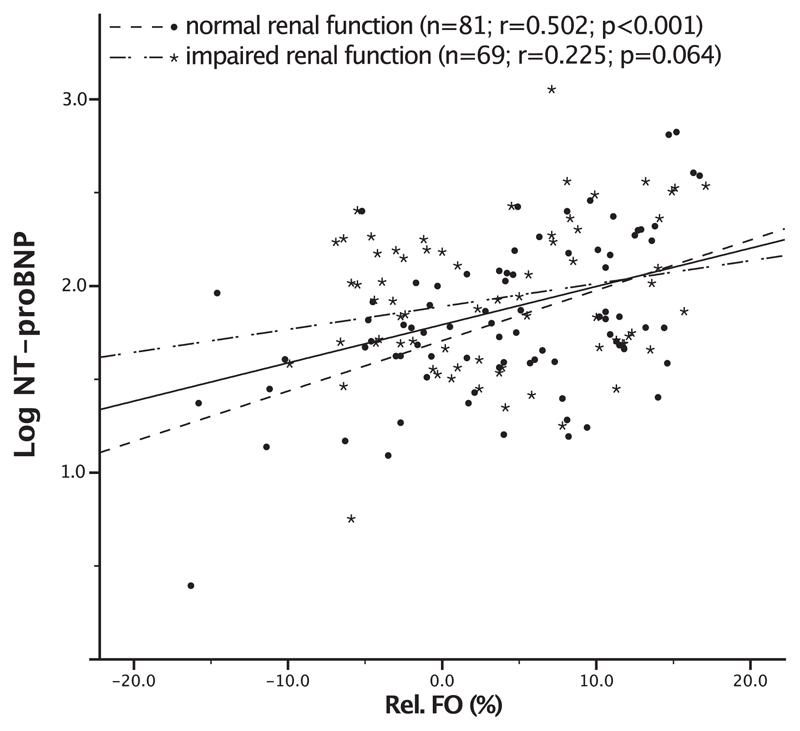
Plasma levels of NT-proBNP were higher in fluid overloaded than in normohydrated subjects (1744.4 ± 1925.3 vs. 784.2 ± 599.6 pg/ml; p < 0.001); the relationship between NT-proBNP levels and Rel. FO was linear (Pearson correlation coefficient r = 0.304; p < 0.001). In the subgroup of patients with normal renal function log NT-proBNP significantly correlated with Rel. FO (r = 0.502; p < 0.001), while in the subgroup with impaired renal function no correlation could be found (r = 0.225; p = 0.064). Fig. 2 depicts a scatterplot for log NT-proBNP and Rel. FO according to renal function.
Fig. 2.
Correlation plot between NT-proBNP and relative fluid overload Scatterplot for log N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and relative fluid overload (Rel. FO) according to renal function. The solid line is the regression line for all subjects (r = 0.304; p < 0.001). The dashed line indicates the regression line for patients with normal renal function (r = 0.502; p < 0.001). Patients with impaired renal function showed no significant correlation between NT-proBNP and Rel. FO (dash-dotted line; r = 0.225; p = 0.064).
3.2. Hemodynamic characteristics
Seven patients refused to undergo RHC. Table 2 illustrates hemodynamic characteristics according to fluid status. Patients with fluid overload showed significantly higher filling pressures, including sPAP (57.4 ± 18.6 vs. 49.3 ± 17.2 mmHg; p = 0.009), dPAP (23.4 ± 7.4 vs. 20.9 ± 6.4 mmHg; p = 0.030), and mPAP (35.8 ± 9.6 vs. 32.3 ± 9.2 mmHg; p = 0.031). Significant between-group differences were also encountered in the majority of calculated hemodynamic parameters. While PPP (33.9 ± 14.6 vs. 28.4 ± 12.7 mmHg; p = 0.019) as well as PVR (284.7 ± 182.0 vs. 209.9 ± 106.8 dynes·s·cm−5; p = 0.002) were significantly higher in the fluid overloaded subgroup, PAC (2.3 ± 1.5 vs. 3.3 ± 1.9 ml/mmHg; p = 0.003) was notably reduced compared to the normohydrated subgroup. While the mean TPG was higher in patients with fluid overload (15.5 ± 7.0 vs. 13.3 ± 6.3 mmHg; p = 0.052), no statistically significant difference could be detected. No significant differences could be shown for DPG (p = 0.120) or PAWP (p = 0.214).
Table 2.
Hemodynamic characteristics of patients with heart failure with preserved ejection fraction classified according to fluid status. Data are presented as mean ± standard deviations.
| Variable | Normohydrated (n = 86) |
Fluid overloaded (n = 57) |
P value |
|---|---|---|---|
| – directly measured parameters | |||
| Systolic pulmonary arterial pressure, mmHg | 49.3 ± 17.2 | 57.4 ± 18.6 | 0.009 |
| Diastolic pulmonary arterial pressure, mmHg | 20.9 ± 6.4 | 23.4 ± 7.4 | 0.030 |
| Mean pulmonary arterial pressure, mmHg | 32.3 ± 9.2 | 35.8 ± 9.6 | 0.031 |
| Pulmonary artery wedge pressure, mmHg | 19.0 ± 5.8 | 20.2 ± 6.1 | 0.214 |
| Cardiac Output, l/min | 5.3 ± 1.5 | 4.7 ± 1.4 | 0.027 |
| – calculated parameters | |||
| Pulmonary pulse pressure, mmHg | 28.4 ± 12.7 | 33.9 ± 14.6 | 0.019 |
| Transpulmonary pressure gradient, mmHg | 13.3 ± 6.3 | 15.5 ± 7.0 | 0.052 |
| Diastolic pressure gradient, mmHg | 1.9 ± 4.6 | 3.2 ± 5.1 | 0.120 |
| Pulmonary vascular resistance, dynes·s·cm−5 | 209.9 ± 106.8 | 284.7 ± 182.0 | 0.002 |
| Pulmonary arterial compliance, ml/mmHg | 3.3 ± 1.9 | 2.3 ± 1.5 | 0.003 |
| Stroke volume, ml | 79.1 ± 24.5 | 67.0 ± 24.1 | 0.004 |
3.3. Outcome
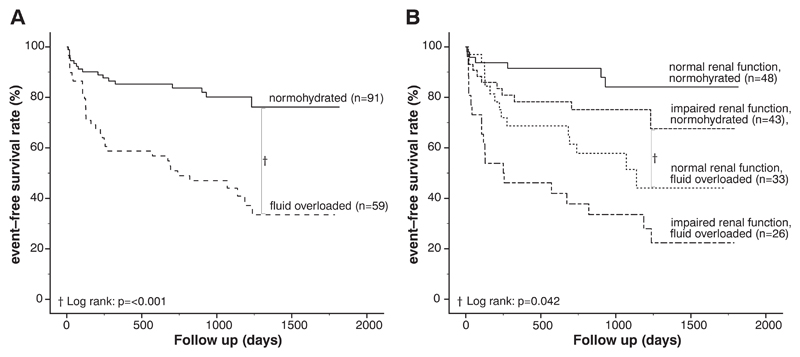
During a median follow-up of 24.3 months (interquartile range: 19.8–33.2), 51 (34%) patients reached the combined end point. Forty (78%) patients were hospitalized for HF, 11 (22%) patients died due to cardiac reasons. One patient died due to a non-cardiac reason (stroke). No patients were lost to follow-up. Of 59 patients with detectable fluid overload, 34 (58%) reached the combined end point, while only 17 (19%) of 91 normohydrated patients experienced hospitalization for HF or cardiac death (p < 0.001). Kaplan–Meier survival curves confirmed worse survival for fluid overloaded versus normohydrated patients (Fig. 3A, p < 0.001 by log-rank test). A Cox proportional hazards regression model was used to identify variables associated with risk of the combined end point. As shown in Table 3, the multivariate Cox regression model adjusted for age, gender, BMI, 6-MWD, NT-proBNP, AF, and DM identified fluid overload as an independent predictor of worse outcome in patients with HFpEF (HR 3.09; 95% CI 1.68–5.68; p < 0.001). Likewise, patients with impaired renal function had an elevated risk of hospitalization for HF and/or death for cardiac reasons (HR 1.92; 95% CI 1.07–3.47; p = 0.027). In addition, higher sPAP values (HR 1.02; 95% CI 1.01–1.04; p = 0.001) and the presence of DM (HR 3.29; 95% CI 1.76–6.15; p < 0.001) predicted adverse outcome.
Fig. 3.
Kaplan–Meier curves Panel A: Kaplan–Meier curves according to the two defined groups: [1] Normohydrated and [2] Fluid overloaded. Patients with fluid overload showed an adverse outcome in comparison with normohydrated patients. (p < 0.001). Panel B: Kaplan–Meier curves depicting HFpEF patients stratified by fluid status and renal function. Patients with fluid overload and normal renal function showed a significantly worse event-free survival compared to normohydrated patients with impaired renal function (log-rank: p = 0.042).
Table 3.
Clinical predictors of outcome in patients with heart failure and preserved ejection fraction. Values are presented as mean ± standard deviations or n (%). *P values were derived from simple and multiple Cox regression analysis.
| Variable | No event (n = 99) | Event (n = 51) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| Crude hazard ratio (95% CI) | P value* | adjusted hazard ratio (95% CI) | P value* | |||
| Sex female/male, n | 71/28 | 33/18 | 0.80 (0.54–1.42) | 0.458 | ||
| Age, years | 74.2 ± 8.6 | 74.8 ± 8.0 | 1.00 (0.97–1.03) | 0.768 | ||
| Body mass index, kg/m2 | 29.9 ± 5.4 | 31.3 ± 6.6 | 1.07 (1.02–1.13) | 0.004 | ||
| Fluid overload, n (%) | 25 (25.3) | 34 (66.7) | 3.64 (2.03–6.52) | < 0.001 | 3.09 (1.68–5.68) | < 0.001 |
| Six-minute walk distance, m | 352.9 ± 115.3 | 297.7 ± 112.6 | 0.99 (0.99–1.00) | 0.005 | ||
| NT-pro-BNP, pg/ml | 977.1 ± 1058.8 | 1520.6 ± 1792.1 | 1.00 (1.00–1.01) | 0.002 | ||
| Impaired renal function, n (%) | 39 (39.4) | 30 (58.8) | 1.85 (1.06–3.23) | 0.031 | 1.92 (1.07–3.47) | 0.027 |
| –Comorbidities | ||||||
| Atrial fibrillation, n (%) | 54 (63.5) | 31 (36.5) | 1.26 (0.71–2.23) | 0.422 | ||
| Diabetes mellitus, n (%) | 23 (41.8) | 32 (58.2) | 4.43 (2.46–7.98) | < 0.001 | 3.29 (1.76–6.15) | < 0.001 |
| - Hemodynamic characteristics | ||||||
| Systolic pulmonary arterial pressure, mmHg | 48.0 ± 15.9 | 61.3 ± 19.0 | 1.03 (1.01–1.04) | < 0.001 | 1.02 (1.01–1.04) | 0.001 |
| Diastolic pulmonary arterial pressure, mmHg | 20.3 ± 6.4 | 24.9 ± 6.8 | 1.08 (1.03–1.12) | < 0.001 | ||
| Mean pulmonary arterial pressure, mmHg | 31.4 ± 8.5 | 38.2 ± 9.6 | 1.06 (1.03–1.08) | < 0.001 | ||
| Pulmonary artery wedge pressure, mmHg | 18.4 ± 5.9 | 21.4 ± 5.6 | 1.07 (1.02–1.12) | 0.002 | ||
CI = confidence interval; NT-pro-BNP = N-terminal prohormone of brain natriuretic peptide.
In a further analysis, where the cohort was stratified according to fluid status and renal function, the subgroup with fluid overload combined with impaired renal function faced a significantly worse outcome as compared to all other subgroups (Fig. 3B). Kaplan–Meier survival analysis showed a significantly better event-free survival for the subgroup with normohydration and impaired renal function compared to the subgroup with fluid overload and normal renal function (p = 0.042 by log-rank test).
4. Discussion
A major goal in the management of patients with HFpEF is to prevent or relieve chronic fluid overload, thereby allowing them to maintain low intra-cardiac pressures and low NT-proBNP levels. If left untreated, fluid accumulation usually leads to the clinical picture of overt decompensation and urgent hospital admission may become inevitable. In particular, patients with impaired right ventricular function [20] and high left ventricular filling pressures [21] face a dismal prognosis with recurrent hospitalizations.
In the present study, we demonstrate that BIS, a non-invasive and easy-to-use technique, was useful for the detection of fluid overload in consecutive HFpEF patients without clinically overt peripheral edema. In fact, more than one third of our patients were overhydrated. In this group, 34 (58%) patients experienced hospitalization for HF or cardiac death within the observation period. By contrast, only 17 (19%) normohydrated patients were hospitalized or died during the same time period (p < 0.001). Thus, BIS was able to identify a subgroup of patients at high risk for cardiac events or death. It remains speculative, whether early therapeutic intervention with volume-targeted diuretic treatment would have improved outcome, and further studies are warranted. A growing body of evidence, however, suggests that device-guided diuretic treatment may reduce hospital admissions for decompensated HF. The CHAMPION study [22] tested the hypothesis that HF patients with persistently increased PAP were at high risk of hospital admission due to decompensation [23]. Therefore, all participants received an implantable pressure sensor and continuous PAP monitoring allowed early therapeutic intervention, once PAP increases were registered. While patients in the treatment group had PAP measurements transmitted daily, which were used to guide medication adaptation, patients in the control group received drug and device treatment according to standard care. The treatment group showed a 33% reduction in HF-related hospital admissions and a 16% reduction in all-cause admissions [22].
The role of NT-proBNP in the assessment of congestion remains uncertain. While a strong correlation with fluid status could be demonstrated in patients with normal renal function, no relation between the two parameters could be found in patients with impaired renal function. As an alternative to an implantable device, home-based BIS monitoring or BIS use in the outpatient setting could provide an attractive, novel diagnostic option to guide treatment.
Removal of excess extracellular fluid with diuretics to treat or prevent peripheral and/or pulmonary edema is one of the mainstays of volume management and recommended by the latest ESC and ACCF/AHA guidelines [4,5]. The aim is to achieve and maintain euvolemia with the lowest possible dose. However, questions have been raised about the safety of prolonged use of loop diuretics. Long-term treatment may result in a significant decrease in glomerular filtration, presumably due to activation of the renin–angiotensin–aldosterone system as well as the sympathetic nervous system [24]. Depending on the aggressiveness of diuresis, a reduction in intravascular volume may cause hemoconcentration and renal dysfunction during treatment [25]. In line with a general clinical experience that impaired renal function is associated with adverse outcome in HF patients, several authors have provided clear evidence for this observation over the recent years [26–30]. One of the clinical interpretations of these findings is that mild overhydration may be an acceptable goal for diuretic treatment if renal impairment can be avoided. The ADHERE study, which was performed in patients with acutely decompensated HF, suggests that treatment strategies should also aim at long-term preservation of renal function [27]. Based on these findings, doses of diuretics are often reduced or even withheld, if renal function deteriorates. Although purely observational, our findings do not support a clinical approach where diuretic doses are driven by kidney function parameters instead of the actual volume status. By Kaplan–Meier analysis, patients with fluid overload and normal renal function as defined by eGFR ≥ 60 ml/min/1.73m2 showed a significantly worse event-free survival compared to the subgroup with normohydration and impaired renal function (log-rank: p = 0.042). These findings provide some support for the conceptual premise of a continued fluid removal independent from changes in eGFR.
4.1. Study limitations
This study was not designed to present correlations between BIS measurement and any clinical marker for congestion. We did not validate BIS results against established reference methods, such as dual-energy X-ray absorptiometry, isotope dilution, or total body potassium measurement. However, all reference methods have their limitations and act on the assumption of constant age and constant hydration status. In this respect, a mean age of 74.4 ± 8.4 years also makes an interpretation of BIS results challenging, since particularly in elderly subjects, changes in hydration status may represent a redistribution of body fat. Given a relatively high mean BMI (30.1 ± 6.1 kg/m2) in the investigated group, small changes in hydration status can easily be amplified by BIS [31]. Nevertheless, the differences between direct and indirect methods suggest that none of the analyzed methods served as a true “gold standard,” because indirect methods are almost equally precise compared to direct estimation methods [32]. Due to extensive experience with this method in large cohorts with renal failure and fluid overload [14,33], our results seem robust in identifying patients at high risk for cardiac events and/or death.
Because this study has been performed in a single center, a center-specific bias cannot be excluded. However, there are some major advantages in limiting data collection to a single center: (a) inclusion of a homogenous patient population, (b) adherence to a constant clinical routine, (c) consistent quality of echocardiographic and RHC workup, (d) constant follow-up of the patient cohort. Another limitation of the study is that we did not differentiate between various HFpEF phenotypes and respective biomarkers [34–36].
5. Conclusion
As a conclusion, BIS technology is a valuable tool to identify individuals with fluid overload and at risk for cardiac events among a series of prospectively registered HFpEF patients. Irrespective of a lack of device-guided interventional trials with euvolemia-targeted diuretic therapy, we believe that the incorporation of BIS in the management of HFpEF patients may improve risk assessment, treatment, and potentially outcome.
Footnotes
Conflict of interest
There are no conflicts of interest.
References
- [1].Hamilton RW, Buckalew VM. Sodium, water, and congestive heart failure. Ann Intern Med. 1984;100(6):902–904. doi: 10.7326/0003-4819-100-6-902. [DOI] [PubMed] [Google Scholar]
- [2].Tuy T, Peacock WF. Fluid overload assessment and management in heart failure patients. Semin Nephrol. 2012 Jan;32(1):112–120. doi: 10.1016/j.semnephrol.2011.11.014. [DOI] [PubMed] [Google Scholar]
- [3].Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure-re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008 Feb;10(2):165–169. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- [4].Ponikowski P, Voors AA, Anker SD, Bueno H, JGF C, AJS C, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016 May 20; doi: 10.1002/ejhf.592. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- [5].Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013 Oct 15;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- [6].Miller WL, Mullan BP. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. J Am Coll Cardiol HF. 2014 Jun;2(3):298–305. doi: 10.1016/j.jchf.2014.02.007. [DOI] [PubMed] [Google Scholar]
- [7].Parrinello G, Greene SJ, Torres D, et al. Water and sodium in heart failure: a spotlight on congestion. Heart Fail Rev. 2015 Jan;20(1):13–24. doi: 10.1007/s10741-014-9438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chakko S, Woska D, Martinez H, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med. 1991;90(1):353–359. doi: 10.1016/0002-9343(91)80016-f. [DOI] [PubMed] [Google Scholar]
- [9].Booth J, Pinney J, Davenport A. N-terminal proBNP - marker of cardiac dysfunction, fluid overload, or malnutrition in hemodialysis patients? Clin J Am Soc Nephrol. 2010 Jun;5(6):1036–1040. doi: 10.2215/CJN.09001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol. 2004 May 15;93(10):1254–1259. doi: 10.1016/j.amjcard.2004.01.070. [DOI] [PubMed] [Google Scholar]
- [11].Paulus WJ, Tschöpe C, Sanderson JE, et al. Eur Heart J. 20. Vol. 28. Oxf Univ Press; 2007. Oct, How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology; pp. 2539–2550. [DOI] [PubMed] [Google Scholar]
- [12].Moissl U, Wabel P, Chamney P, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006 Sep;27(9):921–933. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- [13].Wieskotten S, Heinke S, Wabel P, et al. Bioimpedance-based identification of malnutrition using fuzzy logic. Physiol Meas. 2008 May;29(5):639–654. doi: 10.1088/0967-3334/29/5/009. [DOI] [PubMed] [Google Scholar]
- [14].Hung S-C, Kuo K-L, Peng C-H, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014 Mar;85(3):703–709. doi: 10.1038/ki.2013.336. [DOI] [PubMed] [Google Scholar]
- [15].Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015 Mar;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- [16].Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009 Mar;10(2):165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- [17].ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- [18].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- [19].Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- [20].Aschauer S, Kammerlander AA, Zotter-Tufaro, et al. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J Heart Fail. 2015 Oct 9;18(1):71–80. doi: 10.1002/ejhf.418. [DOI] [PubMed] [Google Scholar]
- [21].Donal E, Lund LH, Oger E, et al. New echocardiographic predictors of clinical outcome in patients presenting with heart failure and a preserved left ventricular ejection fraction: a subanalysis of the Ka (Karolinska) Ren (Rennes) Study. Eur J Heart Fail. 2015 May 29;17(7):680–688. doi: 10.1002/ejhf.291. [DOI] [PubMed] [Google Scholar]
- [22].Abraham WT, Stevenson LW, Bourge RC, et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016 Jan 30;387(10017):453–461. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- [23].Zile MR, Adamson PB, Cho YK, et al. Hemodynamic factors associated with acute decompensated heart failure: part 1–insights into pathophysiology. J Card Fail. 2011 Apr;17(4):282–291. doi: 10.1016/j.cardfail.2011.01.010. [DOI] [PubMed] [Google Scholar]
- [24].Felker GM, O'Connor CM, Braunwald E. Heart Failure Clinical Research Network Investigators. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail. 2009 Jan;2(1):56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010 Jul 20;122(3):265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006 Feb 7;113(5):671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- [27].Heywood JT, Fonarow GC, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007 Aug;13(6):422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- [28].Unger ED, Dubin RF, Deo R, et al. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2015 Dec 3;18(1):103–112. doi: 10.1002/ejhf.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].ter Maaten JM, Damman K, Verhaar MC, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016 Feb 10;18(6):588–598. doi: 10.1002/ejhf.497. [DOI] [PubMed] [Google Scholar]
- [30].Damman K, Solomon SD, Pfeffer MA, et al. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM-study programme. Eur J Heart Fail. 2016 Jul 18; doi: 10.1002/ejhf.609. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [31].Weyer S, Zink MD, Wartzek T, et al. Bioelectrical impedance spectroscopy as a fluid management system in heart failure. Physiol Meas. 2014 Jun;35(6):917–930. doi: 10.1088/0967-3334/35/6/917. [DOI] [PubMed] [Google Scholar]
- [32].Raimann JG, Zhu F, Wang J, et al. Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int. 2014 Apr;85(4):898–908. doi: 10.1038/ki.2013.358. [DOI] [PubMed] [Google Scholar]
- [33].Antlanger M, Hecking M, Haidinger M, et al. Fluid overload in hemodialysis patients: a cross-sectional study to determine its association with cardiac biomarkers and nutritional status. BMC Nephrol. 2013 Dec 2;14(266) doi: 10.1186/1471-2369-14-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].D'Elia E, Vaduganathan M, Gori M, Gavazzi A, Butler J, Senni M. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur J Heart Fail. 2015 Oct 23;17(12):1231–1239. doi: 10.1002/ejhf.430. [DOI] [PubMed] [Google Scholar]
- [35].Kao DP, Lewsey JD, Anand IS, et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015 Aug 6;17(9):925–935. doi: 10.1002/ejhf.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sanders-van Wijk S, van Empel V, Davarzani N, et al. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail. 2015 Oct 16;17(10):1006–1014. doi: 10.1002/ejhf.414. [DOI] [PubMed] [Google Scholar]