Abstract
Synthetic organic strategies that enable the catalytic and rapid assembly of a large array of organic compounds possessing multiple stereocenters in acyclic systems are somewhat rare, especially when it comes to reaching today’s high standards of efficiency and selectivity. In particular, the catalytic preparation of a three-dimensional molecular layout of a simple acyclic hydrocarbon skeleton possessing several stereocenters from simple and readily available reagents still represents a vastly uncharted domain. Here, we report a rapid, modular, stereodivergent and diversity-oriented unified strategy to construct acyclic molecular frameworks bearing up to four contiguous and congested stereogenic elements, with remarkably high levels of stereocontrol and in only three catalytic steps from commercially available alkynes. A regio- and diastereoselective catalytic Heck migratory insertion reaction of alkenylcyclopropyl carbinols merging selective C–C bond cleavage of a cyclopropane represents the key step.
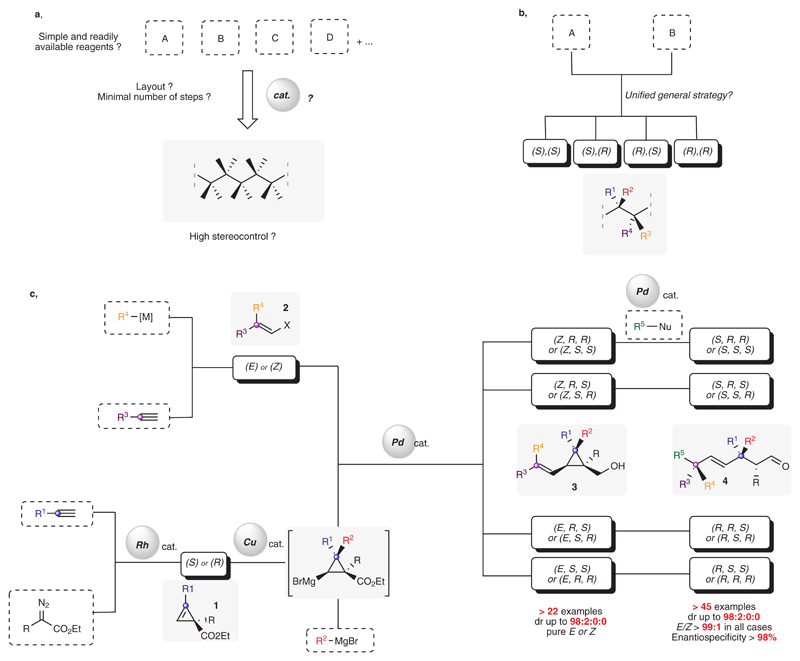
In light of many transformative advances, organic synthesis is expected to allow a chemist to create, prepare and investigate any organic molecule at will, regardless of its complexity. Despite the ever-increasing synthetic toolbox available to practitioners, the perfect “atomic scale manipulation of matter for the synthesis of anything and everything”1 represents a never-ending and yet unmet challenge. Growing expectations to provide faster, more cost-efficient and highly selective transformations, however, sets a high bar for modern chemical synthesis2–6. Tending towards these ideals, research efforts have not only been focusing on the development of newly diverse and more efficient methodologies, but also on expanding the realm of new possibilities through original conceptual advances. For instance, introducing the notion of chemical space in organic synthesis has spurred chemists to explore more efficiently all the potential structural and geometric isomers of a given scaffold7. Accordingly, the catalytic preparation of a three-dimensional molecular layout of a simple acyclic hydrocarbon skeleton possessing several stereocenters from simple and readily available reagents still represents a vastly uncharted domain (Fig. 1a).
Figure 1. Challenges for easily diversifiable and stereoselective preparation of acyclic hydrocarbon motifs.
a, General synthetic challenges related to access stereodefined acyclic hydrocarbon fragments. b, Stereodivergent coupling of A and B to create 1,2-stereocenters. c, Our proposed synthetic approach consisting in three sequential catalytic steps to prepare aldehydes featuring up to four contiguous stereogenic elements, from readily available reagents.
Molecular complexity rapidly rises with the number of stereogenic elements, their degree of substitution and the additional presence of neighboring functional groups8. Although considerable progress has been made these last decades in stereoselective synthesis9,10, general catalytic examples implemented for non-cyclic systems are still scarce, inherently due to the difficulty to discriminate between the different possible conformers of these flexible chains. Furthermore, the elaboration of congested systems adds another level of complexity as exemplified by the difficulty to selectively construct quaternary carbon stereocenters11–15. One ambitious task would entail selectively preparation of all possible isomers of a simple acyclic hydrocarbon motif featuring adjacent and congested stereogenic elements16, by means of a unified assembly-line17 approach concomitantly, allowing stereodivergency18 and diverse functionalization19–21. An emerging approach to address concurrently the aforementioned issues involves taking advantage of the exergonic ring-opening reactions of strained cycles22–26. Cyclopropanes, diversely and readily accessible with high levels of enantio- and diastereocontrol22,23,27,28, have been successfully utilized as precursors to stereodefined acyclic systems. Nonetheless, to ensure a selective outcome for such an approach concomitantly requires: (i) to control the selectivity of the C-C bond cleavage29–35 and (ii) to prevent epimerization at the resulting released stereocenters during the transformation. On the basis of the utility of this latter strategy, we have previously developed sequential protocols, based on various metal-assisted and controlled ring-opening modes of strained carbocycles to access stereodefined acyclic hydrocarbon fragments that contain sp2 and/or sp3 carbon stereogenic element(s)36–44.
As an example, we have been investigating the Pd-catalyzed Mizoroki–Heck intermolecular arylation of terminal olefins as a versatile and selective trigger for a remote cyclopropane unfolding event44. Despite the numerous chemical events that transpire, including critical long distance Pd-walk and selective Pd-assisted β-carbon fragmentation, the overall transformation was remarkably selective. Spurred by these central features, we set out to devise a strategy to address the highly challenging selective and stereodivergent preparation of a three-dimensional molecular layout with adjacent and congested stereogenic elements; importantly, this would be achieved in just three catalytic steps and involve readily available reagents (Fig. 1c). Herein, we disclose a unified, robust and modular approach for efficient synthesis of acyclic aldehydes, which are structurally complex and feature up to four contiguous congested stereogenic elements.
Results and Discussion
Straightforward and stereodivergent strategy for the synthesis of alkenylcyclopropyl carbinols
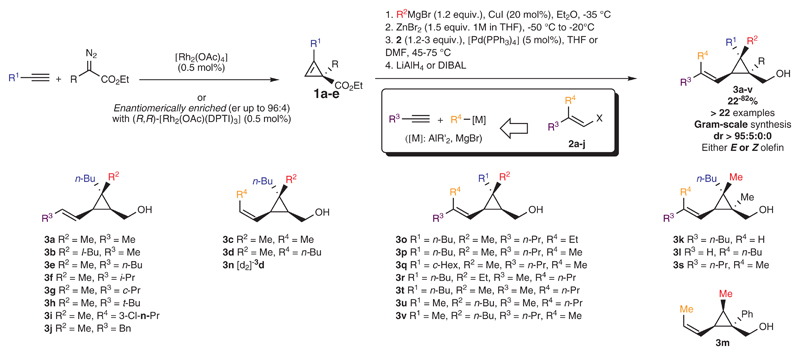
A unique attribute of our strategy is that the vinylcyclopropyl carbinol moiety is made to serve as temporal auxiliary for promoting high levels of stereochemical control45. Accordingly, we devised a step-economical, modular and highly stereoselective pathway for synthesis of compounds represented by 3 from simple and readily accessible alkynes by means of two catalytic processes. Alkenylcyclopropane motifs are found in various natural products and other biologically active compounds46, and have been used in transition-metal catalyzed transformations leading to various desirable scaffolds47. Yet, there are just a limited number of diastereo- and enantioselective protocols for generating alkenylcyclopropanes with a quaternary carbon in a non-fused system48. Based on our recently acquired experience on the catalytic enantioselective 1,2-bisalkylation of cyclopropenes49, we surmised that polysubstituted alkenylcyclopropyl carbinols 3 could be synthesized diastereo- and enantioselectively starting from a common cyclopropene precursor. Indeed, cyclopropenyl esters 1 may be accessed through Rh-catalyzed decomposition of diazoesters in the presence of terminal alkynes. Key to the success of our plan was that enantioselective versions of this [2+1] cycloaddition process have been reported (Fig. 2)50. Compounds of the general structure 1 were thus engaged in regio- and diastereoselective copper-catalyzed carbomagnesiation (with R2MgBr), leading to stereodefined cyclopropyl Grignard reagents51. Subsequent to transmetalation, the in situ generated organozinc compounds were coupled with stereodefined alkenyl halides 2 (stereospecifically generated from their corresponding terminal alkynes either by syn hydrometalation or by carbometalation and halogenolysis; see the Supplementary Information) catalyzed by a phosphine–Pd complex in a medium of increased polarity (through addition of THF or DMF). Importantly, the sp3-sp2 Negishi-type cross-coupling involving two congested and stereodefined systems did not cause scrambling at the stereogenic carbon centers. Reduction of the ester moiety then provided the desired diastereomerically enriched alkenylcyclopropyl carbinols 3a-3v in good overall yields (either pure E or Z olefins, dr ≥ 95:5:0:0 in all cases). Therefore, through this unified strategy, an assortment of bench-stable polysubstituted alkenylcyclopropanes 3 was prepared expeditiously, by a readily modifiable route, in high stereoisomeric purity, and on a multi-gram scale (see Figure 2).
Figure 2. Stereoselective preparation of polysubstituted alkenylcyclopropyl carbinols 3a-v.
Catalytic carbenoid insertion into terminal alkynes enabled the synthesis of cyclopropenyl esters 1a-e, either racemic or enantiomerically enriched for specific examples.50 Then, a diastereoselective copper-catalyzed carbomagnesiation afforded diastereomerically pure cyclopropylmagnesium halide compounds, which could be subjected to catalytic cross-coupling with alkenyl halides 2 (generated from terminal alkynes) to provide, after ester reduction, stereoisomerically enriched alkenylcylopropyl carbinol derivatives 3.
Stereoselective Pd-catalyzed Heck arylation of alkenylcyclopropyl carbinols with aryl boronic acids
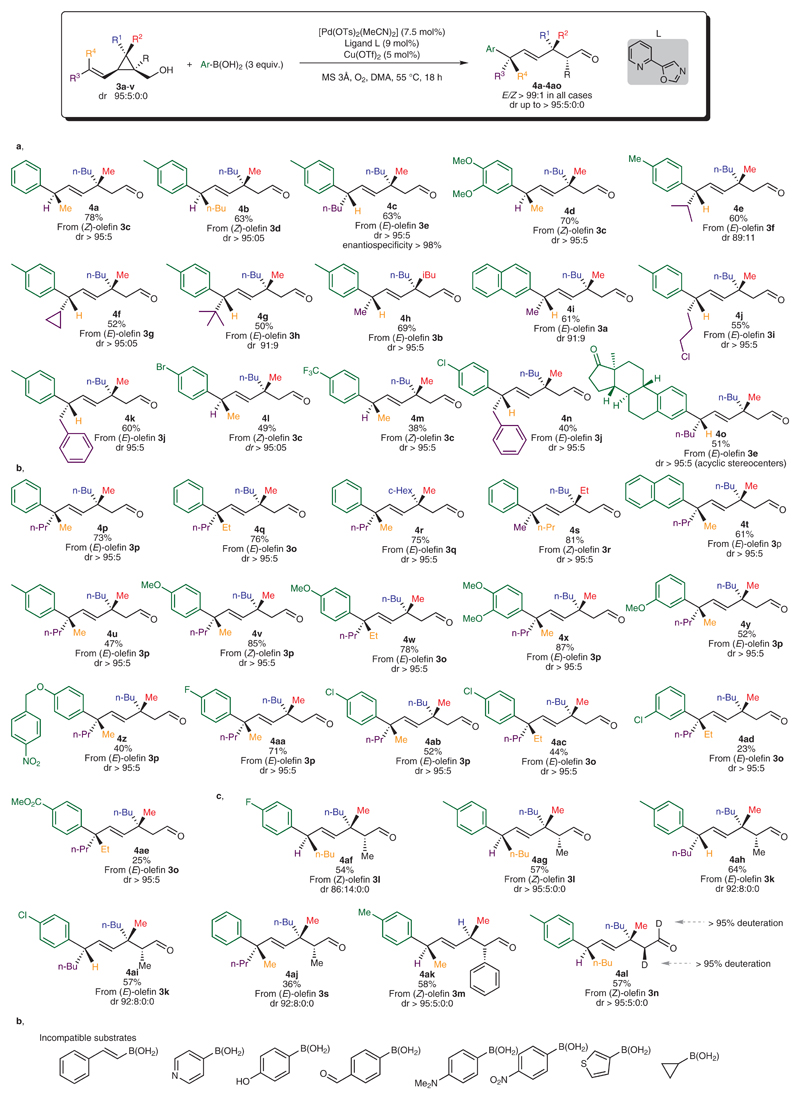
With a relatively straightforward and general method for preparation of 3 in hand, we focused on probing the feasibility of an ensuing Heck-type arylation process. Following a thorough optimization, we discovered that, similar to the findings reported by Sigman et al.52,53, with an aerobic Pd(II)-based catalyst (derived from [Pd(OTs)2(MeCN)2] and Cu(OTf)2) and 5-(2-pyridyl)-1,3-oxazole as the ligand (L), arylboronic acids may be coupled to 3, affording aldehydes 4 with excellent selectivity (3Å molecular sieves, DMA, 55 °C; Fig. 3). The presence of this particular ligand was necessary for achieving higher yields as well as more selective aryl migratory insertion. Under these optimal conditions, (Z)-3c could be converted to (3R*,6R*)-4a in 78% yield, as a single alkene isomer (E/Z >99:1), and with exceptional diastereoselectivity (d.r.>95:5); this latter transformation was diastereospecific as well, because (3S*,6R*)-4b and (3S*,6S*)-4c could be accessed with an nearly identical stereoisomeric purity by simply permuting the stereochemistry of the alkene in the corresponding starting materials (3d and 3e, respectively). The relative 1,4-configuration of 4d was determined by X-ray analysis of its 2,4-dinitrophenylhydrazone derivative 6a (see the Supplementary Information). Other relative configurations were assigned by analogy. It is especially noteworthy that the transformation of 3g was chemoselective, as the unsubstituted cyclopropane was preserved in product 4f. Substituting the olefin with bulkier alkyl chains (secondary or tertiary) led to a slight decrease in diastereoselectivity (4e) and regioselectivity (4g). However, to our delight, substrates containing more electron-rich and bulkier trisubstituted olefins (3o-v) could be similarly converted – in a single chemical step and with remarkably high levels of 1,4-diastereocontrol – to products bearing a second acyclic quaternary stereocenter (4p-ae, Fig. 3c). Varying substrate substituent patterns (3o, 3p, 3q and 3s) did not hamper yields and selectivities. The use of electron-rich arylboronic acids did not dramatically influence yields nor selectivities. Reactions with relatively electron-deficient arylboronic acids (4l-n, 4ab-4ae) were typically sluggish, and those involving substrates bearing an ortho-substituent were inefficient (i.e., low conversion to 3).
Figure 3. Oxidative Pd-catalyzed Heck coupling of aryl boronic acids with alkenylcyclopropyl carbinols.
a, Reactions were carried out on a 0.15 – 0.5 mmol scale. Yields were determined after silica gel chromatography. Diastereoisomeric and E/Z ratios were determined by analysis of 1H NMR spectra of unpurified product mixtures. b, Heck arylation of alkenylcyclopropanes bearing 1,2-disubstituted olefin moieties; these reactions provide access to stereodiads that contain a tertiary and a quaternary stereogenic center at a distance of four methylene units. c, Heck arylation of alkenylcyclopropanes bearing 1,1,2-trisubstituted olefin moieties; such processes allow access to stereodiads with two quaternary stereogenic centers at a distance of four methylene units (we obtained 4t by using 1.5 equiv. of the corresponding aryl boronic acid). d, When R ≠ H, the transformation selectively afforded 1,4,5-stereotriads, without any observable epimerization at the tertiary stereocenter adjacent to the aldehyde moiety. e, Unsuccessful boronic acids tested. Tf: triflyl, n-Pr: n-propyl, n-Bu: n-butyl, c-Hex: cyclohexyl, i-Bu: iso-butyl, Ts: tosyl, DMA: N,N-dimethylacetamide.
Next, we probed the reactivity of 1,1,2,2,3-pentasubstituted vinylcyclopropyl carbinols (R ≠ H), presenting an additional stereocenter (3k-n and 3s). The presence of adjacent cyclopropyl quaternary stereocenters did not impact fragmentation selectivity, and, what is more, the same conditions led to the formation of the corresponding aldehydes without epimerization of the somewhat sensitive Cα stereogenic center (Fig. 3d). As before, aryl migratory insertion was diastereospecific (4ag, 4ah). The relative stereochemistry of such 1,4,5-stereochemical triad was determined by X-ray crystallographic analysis of the 2,4-dinitrophenylhydrazone derivative (6b) of 4ah. By engaging 3m, we were able to access 4ak, which features three tertiary stereocenters, including one that is rather labile (benzylic and α-to a carbonyl group), further illustrating the notably mild conditions and the excellent selectivity of the Pd-assisted carbon-carbon bond cleavage. Our findings clearly illustrate that these reaction conditions tolerate several potentially sensitive functional groups such as ethers (4d, 4v, 4w–z), halides (4j, 4l–n, 4aa–4ad), nitro (4z), ketone (4o) and ester moieties (4ae). Notably, this approach enabled us to fuse alkenylcyclopropyl carbinol 3e with a steroid framework (derived from estrone), affording 4o as a single alkene isomer (E) and in high diastereomeric ratio.
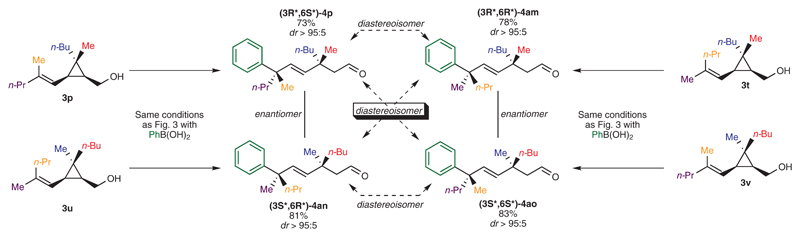
To assess further the stereodivergency of our approach, we decided to synthesize all possible stereoisomers of a three-dimensional molecular scaffold possessing adjacent and congested stereogenic centers by permuting the substitution pattern at the quaternary stereocenter and at the olefin (3p, 3t, 3u, 3v). We were pleased to note that the exact similar protocol led us to access the corresponding four stereoisomers (respectively, 4p, 4am, 4an, 4ao) with remarkably comparable isolated yields and high stereoselectivities (Fig. 4). This set of experiments confirmed that the diastereoselective outcome was essentially dictated by the geometry of the original olefin moiety in 3 (either E or Z), independently of the nature at the other alkyl substituents (R1, R2, R3 and R4). Such results explicitly showed the exceptional diastereodivergence and robustness of our strategy, as the different substituent groups could easily be permutated without affecting the stereoselective outcome of the key Pd-catalyzed aryl migratory insertion coupled with the selective carbon-carbon bond cleavage nor the E/Z ratio (see Fig. 4).
Figure 4. Stereodivergent and selective construction of four stereoisomers of a similar scaffold.
Reaction conditions employed were similar as in Fig. 3. Reactions were carried out on a 0.25 mmol scale. Yields were determined after silica gel chromatography. Diastereoisomeric and E/Z (>99:1) ratios were determined by analysis of 1H NMR spectra of unpurified product mixtures. Ph: phenyl.
Stereoselective Pd-catalyzed Heck arylation of alkenylcyclopropyl carbinols with different nucleophiles
The present method is applicable to coupling reagents other than aryl boronic acids. For instance, by a similar protocol, the union of cyclohexenyl triflate or furanylboronic acid and 3c was accomplished, affording 4ap and 4aq54. As demonstrated previously by the use of alkenyl alcohols55, Fujiwara-Moritani (or dehydrogenative Heck)56 reactions in the presence of N-methylindole could be performed to generate the desired heterocyclic compounds (e.g., 4ar and 4as) with exceptional selectivity (Fig. 5a). Few others heterocycles were tested in the dehydrogenative Heck reaction such as N-methyl pyrrole, 2-methylfurane and thiophene without any success.
Figure 5. Oxidative Pd-catalyzed Heck coupling of other nucleophiles with alkenylcyclopropyl carbinols and proposed rationale regarding stereoselective migratory insertion.
a, Extension to other nucleophiles, reactions were carried out on 0.25 mmol scale. Yields were determined after silica gel chromatography. Diastereoisomeric and E/Z ratios (>99:1 in all cases) were determined by analysis of 1H NMR spectra of unpurified product mixtures. b, Proposed mechanistic model for Pd-catalyzed Heck regio- and diastereoselective aryl migratory insertion / selective β-carbon elimination. Ar: aryl, Ln: ligand, X: couterion.
Issues of mechanism
To account for the remarkably selective catalytic transformation of 3 into 4, the following mechanism might be suggested (Fig. 5b). Based on the available experimental data, we postulate that the high diastereoselectivity with which 4 is generated might be due to the following factors: (i) The alkenylcyclopropyl carbinol adopting a conformation to minimize steric repulsion involving a sizeable alkyl chain (s-trans preferred to s-cis conformation in 3). (ii) Chelation of LnPd(Ar)+X- species (I) with the hydroxyl and the alkenyl moieties, leading to a diastereoselective aryl migratory insertion (II). Such remarkable diastereofacial discrimination offers a plausible rationale for the complete diastereospecificity when various R1, R2, R3 and R4 units are involved (Fig. 4); in line with our hypothesis, selectivity depended largely on the initial geometry of the olefin moiety in 3. The β-cyclopropyl organopalladium complex II would then undergo selective syn β-carbon elimination, furnishing III exclusively as an E-alkene isomer (cf. 4). It therefore appears that selectivity in C–C bond cleavage is directly dependent on the presence of a chelating alcohol moiety, rather than as a consequence of substitution pattern at C5 or C6 (e.g., 4ak). The final carbonyl complex VI is thus formed by subsequent chain walking within the organopalladium intermediate III (i.e., β-hydride elimination/re-insertion). Moreover, when R ≠ H, not only does the purported tertiary organopalladium compound seems to be configurationally stable, the hydride re-addition probably takes place on the same diastereoface (V); this is manifested by the finding that the overall process did not lead to epimerization of the tertiary stereocenter in 4af-al. Furthermore, by means of a labeling experiment (4al, Fig. 3d), we are able to rule out the possibility of direct extrusion via the corresponding enol form (IV)57–61. Release of the carbonyl-containing product (4), containing up to three stereochemically defined sp3–carbon sterogenic centers and an (E)-1,2-disubstituted alkene moiety, regenerates the Pd-based complex. There was no observable transformation when the corresponding methyl ether was used (vs. alcohol), underscoring the importance of rapid extrusion of the catalytically active Pd complex.
Conclusions
We have designed a strategy that entails a succession of highly stereoselective carbon–carbon forming reactions catalyzed by a transition metal complex. As such, complicated and otherwise difficult-to-access acyclic fragments may be prepared from simple and readily available reagents. We illustrate that various acyclic hydrocarbon motifs may be synthesized in a step-wise manner by the following chemo- and stereoselective C–C forming bond processes: Rh-catalyzed [2+1] cycloaddition, Cu-catalyzed carbomagnesiation/Pd-catalyzed cross-coupling followed by ester reduction, and regio- and diastereoselective Pd-catalyzed oxidative Heck migratory insertion to trigger selective unfolding of a cyclopropane intermediate (see Figs 2, 3 and 5). Notably, products bearing adjacent quaternary carbon stereogenic centers were synthesized from readily accessible terminal alkynes through just three catalytic reactions. The stereodivergence of this new approach was highlighted through preparation of four different stereoisomers of compounds that represent the same constitutional isomer (see Fig. 4). The investigations described above demonstrate that by judicious implementation of distinct transformations, carried out in a particular sequence, synthesis routes may be conceived that are of considerable utility, render streamlined access to previously unknown stereoisomeric motifs a realistic possibility.
Methods
General procedure for the Heck arylation of alkenylcyclopropyl carbinols with aryl boronic acids
In a dry one-neck flask under O2 (balloon, 1 atm), 5-(2-pyridyl)-1,3-oxazole (9 mol%), [Pd(MeCN)2(OTs)2] (7.5 mol%), Cu(OTf)2 (5 mol%), 3 Å molecular sieves (100% w/w substrate) and alkenylcyclopropyl carbinol 3 (1 equiv.) were dissolved in DMA (8 mL/mmol substrate). The arylboronic acid (3 equiv.) was then rapidly poured into the reactional mixture, which was allowed to stir for 18 h at 55 °C. After the reaction was judged to be complete, the mixture was diluted by the addition of brine and EtOAc. The phases were separated and the aqueous phase was successively washed three times with EtOAc. The combined organic phases were then dried over anhydrous MgSO4 and concentrated under reduced pressure. Silica gel chromatography (gradient eluent 100% hexane to 5% Et2O/hexane) afforded aldehyde 2.
Supplementary Material
Supplementary Information is available in the online version of the paper.
Acknowledgements
This research was supported by the Israel Science Foundation administrated by the Israel Academy of Sciences and Humanities (330/17) and by the European Research Council under the European Community’s Seventh Framework Program (ERC grant agreement no. 338912). I.M. is holder of the Sir Michael and Lady Sobell Academic Chair.
Footnotes
Data availability. The X-ray crystallographic coordinates for the structure of compound 6a (Accession code CCDC 1813305) and compound 6b (Accession code CCDC 1813312) reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC). This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
Author Contributions J.B., D.P. and I.M. planned the research. J.B. and D.P. conducted and analysed experiments. I.M. directed the project, and wrote the manuscript with contributions from J.B. and D.P. All authors discussed the results and commented on the manuscript.
The authors declare no competing financial interests.
Author Information Reprints and permissions information is available at www.nature.com/reprints. Readers are welcome to comment on the online version of the paper.
References
- 1.Burrows CJ. Holy grails in chemistry, part II. Acc Chem Res. 2017;50:445. doi: 10.1021/acs.accounts.7b00102. [DOI] [PubMed] [Google Scholar]
- 2.Gaich T, Baran PS. Aiming for the ideal synthesis. J Org Chem. 2010;75:4657–4673. doi: 10.1021/jo1006812. [DOI] [PubMed] [Google Scholar]
- 3.Burns NZ, Baran PS, Hoffmann RW. Redox economy in organic synthesis. Angew Chem Int Ed. 2009;48:2854–2867. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]
- 4.Wender PA, Verma VA, Paxton TJ, Pillow TH. Function-oriented synthesis, step economy, and drug design. Acc Chem Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 5.Baran PS, Maimone TS, Richter JM. Total synthesis of marine natural products without using protective groups. Nature. 2007;446:404–408. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- 6.Trost BM. The atom economy – a search for synthetic efficiency. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 7.Lovering F, Bikker J, Humblet C. Escape from the flatland: increasing saturation as an approach to improving clinical success. J Med Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 8.Böttcher T. An additive definition of molecular complexity. J Chem Inf Model. 2016;56:462–470. doi: 10.1021/acs.jcim.5b00723. [DOI] [PubMed] [Google Scholar]
- 9.de Vries JG, Molander GA, Evans PA, editors. Stereoselective Synthesis in Science of Synthesis. Thieme; 2011. [Google Scholar]
- 10.Carreira E, Kvaerno L. Classics in Stereoselective Synthesis. Wiley-VCH; Weiheim: 2009. [Google Scholar]
- 11.Burns M, et al. Assembly-line synthesis of organic molecules with tailored shapes. Nature. 2014;513:183–188. doi: 10.1038/nature13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science. 2013;340:1065–1068. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- 13.Quasdorf KW, Overman LE. Catalytic enantioselective synthesis of quaternary stereocentres. Nature. 2014;516:181–191. doi: 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das JP, Marek I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem Commun. 2011;47:4593–4623. doi: 10.1039/c0cc05222a. [DOI] [PubMed] [Google Scholar]
- 15.Trost BM, Jiang C. Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis. 2006:369–396. [Google Scholar]
- 16.Douglas CJ, Overman LE. Catalytic asymmetric synthesis of all-carbon quaternary stereocenters. Proc Natl Acad Sci USA. 2004;101:5363–5367. doi: 10.1073/pnas.0307113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corey EJ, Guzman-Perez A. The catalytic enantioselective construction of molecules with quaternary stereocenters. Angew Chem Int Ed. 1998;37:388–401. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 18.Trabocchi A, editor. Diversity-Oriented Synthesis: Basics and Applications in Organic Synthesis and Chemical Biology. John Wiley and Sons,Inc; 2013. [Google Scholar]
- 19.O’Connor CJ, Beckmann HSG, Spring DR. Diversity-oriented synthesis: producing chemical tools for dissecting biology. Chem Soc Rev. 2012;41:4444–4456. doi: 10.1039/c2cs35023h. [DOI] [PubMed] [Google Scholar]
- 20.Burke M, Schreiber S. A planning strategy for diversely-oriented synthesis. Angew Chem Int Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 21.Marek I, et al. All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C–C bonds per chemical step. J Am Chem Soc. 2014;136:2682–2694. doi: 10.1021/ja410424g. [DOI] [PubMed] [Google Scholar]
- 22.Kulinkovich OG. Cyclopropanes in Organic Synthesis. John Wiley & Sons; Hoboken, NJ: 2015. [Google Scholar]
- 23.Schneider TF, Kaschel J, Werz DB. A new golden age for donor-acceptor cyclopropanes. Angew Chem Int Ed. 2014;53:5504–5523. doi: 10.1002/anie.201309886. [DOI] [PubMed] [Google Scholar]
- 24.Rubin M, Rubina M, Gevorgyan V. Transition metal chemistry of cyclopropenes and cyclopropanes. Chem Rev. 2007;107:3117–3179. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]
- 25.Reissig H-U, Zimmer R. Donor-acceptor-substituted cyclopropane derivatives and their applications in organic synthesis. Chem Rev. 2003;103:1151–1196. doi: 10.1021/cr010016n. [DOI] [PubMed] [Google Scholar]
- 26.Wong HNC, et al. Use of cyclopropanes and their derivatives in organic synthesis. Chem Rev. 1989;89:165–198. [Google Scholar]
- 27.Ebner C, Carreira EM. Cyclopropanation strategies in recent total syntheses. Chem Rev. 2017;117:11651–11679. doi: 10.1021/acs.chemrev.6b00798. [DOI] [PubMed] [Google Scholar]
- 28.Lebel H, Marcoux J-F, Molinaro C, Charette AB. Stereoselective cyclopropanation reactions. Chem Rev. 2003;103:977–1050. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- 29.Fumagalli G, Stanton S, Bower JF. Recent methodologies that exploit C–C single bond cleavage of strained ring systems by transition metals. Chem Rev. 2017;117:9404–9432. doi: 10.1021/acs.chemrev.6b00599. [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Chatani N, editors. Cleavage of Carbon-Carbon Single Bonds by Transition Metals. Wiley-VCH; Weiheim, Germany: 2016. [Google Scholar]
- 31.Souillart L, Cramer N. Catalytic C–C bond activation via oxidative addition to transition metals. Chem Rev. 2015;115:9410–9464. doi: 10.1021/acs.chemrev.5b00138. [DOI] [PubMed] [Google Scholar]
- 32.Marek I, Masarwa A, Delaye P-O, Leibeling M. Selective carbon–carbon bond cleavage for the stereoselective synthesis of acyclic systems. Angew Chem Int Ed. 2014;54:414–429. doi: 10.1002/anie.201405067. [DOI] [PubMed] [Google Scholar]
- 33.Dong G, editor. Topics in Current Chemistry. Springer-Verlag Berlin Heidelberg; 2014. C–C Bond Activation. [Google Scholar]
- 34.Ruhland K. Transition-metal-mediated cleavage and activation of C–C single bonds. Eur J Org Chem. 2012;2012:2683–2706. [Google Scholar]
- 35.Murakami M, Matsuda T. Metal-catalysed cleavage of carbon-carbon bonds. Chem Commun. 2011;47:1100–1105. doi: 10.1039/c0cc02566f. [DOI] [PubMed] [Google Scholar]
- 36.Roy SR, Didier D, Kleiner A, Marek I. Diastereodivergent combined carbometalation/zinc homologation/C–C fragmentation reaction as an efficient tool to prepare acyclic allylic quaternary carbon stereocenters. Chem Sci. 2016;7:5989–5994. doi: 10.1039/c6sc02191c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F-G, Eppe G, Marek I. Brook rearrangement as a trigger for the ring opening of strained carbocycles. Angew Chem Int Ed. 2016;55:714–718. doi: 10.1002/anie.201510094. [DOI] [PubMed] [Google Scholar]
- 38.Vasseur A, Perrin L, Eisenstein O, Marek I. Remote functionalization of hydrocarbons with reversibility enhanced stereocontrol. Chem Sci. 2015;6:2770–2776. doi: 10.1039/c5sc00445d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simaan M, Delaye P-O, Shi M, Marek I. Cyclopropene derivatives as precursors to enantioenriched cyclopropanols and n-butenals possessing quaternary carbon stereocenters. Angew Chem Int Ed. 2015;54:12345–12348. doi: 10.1002/anie.201412440. [DOI] [PubMed] [Google Scholar]
- 40.Masarwa A, et al. Merging allylic carbon–hydrogen and selective carbon–carbon bond activation. Nature. 2014;505:199–203. doi: 10.1038/nature12761. [DOI] [PubMed] [Google Scholar]
- 41.Delaye P-O, Didier D, Marek I. Diastereodivergent carbometalation / oxidation / selective ring opening: formation of all-carbon quaternary stereogenic centers in acyclic systems. Angew Chem Int Ed. 2013;52:5333–5337. doi: 10.1002/anie.201300664. [DOI] [PubMed] [Google Scholar]
- 42.Simaan S, Marek I. Hydroformylation reaction of alkylidenecyclopropane derivatives: a new pathway for the formation of acyclic aldehydes containing quaternary stereogenic carbons. J Am Chem Soc. 2010;132:4066–4067. doi: 10.1021/ja100544c. [DOI] [PubMed] [Google Scholar]
- 43.Simaan S, Goldberg AFG, Rosset S, Marek I. Metal-catalyzed ring-opening of alkylidenecyclopropanes: new access to building blocks with an acyclic quaternary stereogenic center. Chem Eur J. 2010;16:774–778. doi: 10.1002/chem.200902656. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Bruffaerts J, Vasseur A, Marek I. A unique Pd-catalysed Heck arylation as a remote trigger for cyclopropane selective ring-opening. Nat Commun. 2017;8:14200. doi: 10.1038/ncomms14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oestreich M. Directed Mizoroki–Heck reactions. Top Organomet Chem. 2007;24:169–192. [Google Scholar]
- 46.Katsuda Y. Progress and future in pyrethroids. Top Curr Chem. 2012;314:1–30. doi: 10.1007/128_2011_252. [DOI] [PubMed] [Google Scholar]
- 47.Jiao L, Yu X. Vinylcyclopropane derivatives in transition-metal-catalyzed cycloadditions for the synthesis of carbocyclic compounds. J Org Chem. 2013;78:6842–6848. doi: 10.1021/jo400609w. [DOI] [PubMed] [Google Scholar]
- 48.Müller DS, et al. Tandem hydroalumination/Cu-catalyzed asymmetric vinyl metalation as a new access to enantioenriched vinylcyclopropane derivatives. Org Lett. 2017;19:3970–3973. doi: 10.1021/acs.orglett.7b01661. [DOI] [PubMed] [Google Scholar]
- 49.Dian L, Müller DS, Marek I. Asymmetric copper-catalyzed carbomagnesiation of cyclopropenes. Angew Chem Int Ed. 2017;56:6783–6787. doi: 10.1002/anie.201701094. [DOI] [PubMed] [Google Scholar]
- 50.Lou M, et al. A new chiral Rh(II) catalyst for enantioselective [2+1]-cycloaddition. Mechanistic implications and applications. J Am Chem Soc. 2004;126:8916–8918. doi: 10.1021/ja047064k. [DOI] [PubMed] [Google Scholar]
- 51.Didier D, et al. Modulable and highly diastereoselective carbometalations of cyclopropenes. Chem Eur J. 2014;20:1038–1048. doi: 10.1002/chem.201303569. [DOI] [PubMed] [Google Scholar]
- 52.Mei T-S, Werner EW, Burckle AJ, Sigman MS. Enantioselective redox-relay oxidative Heck arylations of acyclic alkenyl alcohols using boronic acids. J Am Chem Soc. 2015;135:6830–6833. doi: 10.1021/ja402916z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mei T-S, Patel HH, Sigman MS. Enantioselective construction of remote quaternary stereocentres. Nature. 2014;508:340–344. doi: 10.1038/nature13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel HH, Sigman MS. Enantioselective palladium-catalyzed alkenylation of trisubstituted alkenols to form allylic quaternary centers. J Am Chem Soc. 2016;138:14226–14229. doi: 10.1021/jacs.6b09649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Santiago SB, Crawford JM, Sigman MS. Enantioselective dehydrogenative Heck arylations of trisubstituted alkenes with indoles to construct quaternary stereocenters. J Am Chem Soc. 2015;137:15668–15671. doi: 10.1021/jacs.5b11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Bras J, Muzart J. Intermolecular dehydrogenative Heck reactions. Chem Rev. 2011;111:1170–1214. doi: 10.1021/cr100209d. [DOI] [PubMed] [Google Scholar]
- 57.Larionov E, Lin L, Guénée L, Mazet C. Scope and mechanism in palladium-catalyzed isomerizations of highly substituted allylic, homoallylic, and alkenyl alcohols. J Am Chem Soc. 2014;136:16882–16894. doi: 10.1021/ja508736u. [DOI] [PubMed] [Google Scholar]
- 58.Dang Y, Qu S, Wang Z-X, Wang X. A computational mechanistic study of an unprecedented Heck-type relay reaction: insight into the origins of regio- and enantioslectivities. J Am Chem Soc. 2014;136:986–998. doi: 10.1021/ja410118m. [DOI] [PubMed] [Google Scholar]
- 59.Xu L, et al. Mechanism, reactivity, and selectivity in palladium-catalyzed redox relay Heck arylations of alkenyl alcohols. J Am Chem Soc. 2014;136:1960–1967. doi: 10.1021/ja4109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hilton MJ, et al. Investigating the nature of palladium chain-walking in the enantioselective redox-relay Heck reaction of alkenyl alcohols. J Org Chem. 2014;79:11841–11850. doi: 10.1021/jo501813d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin L, Romano C, Mazet C. Palladium-catalyzed long-range deconjugative isomerization of highly substituted α,β-unsaturated carbonyl compounds. J Am Chem Soc. 2016;138:10344–10350. doi: 10.1021/jacs.6b06390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.