Abstract
Objective
This study examined the effects of HIV infection, methamphetamine dependence and their interaction on cortical thickness, area and volume, as well as the potential interactive effects on cortical morphometry of HIV and methamphetamine with age.
Method
T1-weighted structural images were obtained on a 3.0T General Electric MR750 scanner. Freesurfer v5.3.0 was used to derive cortical thickness, area and volume measures in thirty-four regions based on Desikan-Killiany atlas labels.
Results
Following correction for multiple statistical tests, HIV diagnosis was not significantly related to cortical thickness or area in any ROI, although smaller global cortical area and volume were seen in those with lower nadir CD4 count. HIV diagnosis, nevertheless, was associated with smaller mean cortical volumes in rostral middle frontal gyrus and in the inferior and superior parietal lobes. Methamphetamine dependence was significantly associated with thinner cortex especially in posterior cingulate gyrus, but was not associated with cortical area or volume following correction for multiple statistical tests. We found little evidence that methamphetamine dependence moderated differences in cortical area, volume or thickness for any ROI in the HIV seropositive group. Interactions with age revealed that HIV diagnosis attenuated the degree of age-related cortical thinning seen in non-infected individuals; intercepts indicated that young HIV seropositive individuals had thinner cortex than non-infected peers.
Conclusions
Methamphetamine dependence does not appear to potentiate a reduction of cortical area, volume or thickness in HIV seropositive individuals. The finding of thinner cortex in young HIV seropositive individuals and the association between CD4 nadir and global cortical area and volume argue for prioritizing early antiretroviral treatment.
Keywords: HIV infection, Methamphetamine dependence, Aging, Cortical thickness, Cortical area, Cortical volume
Highlights
-
•
HIV was associated with smaller cortical volumes in frontal and parietal regions.
-
•
Nadir (but not current) CD4 was associated with smaller global cortical area in HIV.
-
•
Methamphetamine-dependent subjects had thinner cortex in posterior cingulate.
-
•
Methamphetamine dependence did not exacerbate the cortical effect of HIV infection.
-
•
Young HIV adults showed cortical thinning similar to middle-aged uninfected adults.
1. Introduction
Methamphetamine dependence and HIV infection have been termed a “double epidemic” (Halkitis et al., 2001). A decade ago, populations at risk for HIV or infected with HIV used methamphetamine at a rate approximately 10 times higher than the general population (Colfax and Shoptaw, 2005). While current estimates show a decline in both methamphetamine use (National Institute of Drug Abuse, 2013) and new HIV infections (Hess et al., 2017), the joint occurrence is still a concern due to the reduced effectiveness of antiretroviral therapy among HIV infected people who are actively using methamphetamine (Ellis et al., 2003). In addition, methamphetamine consumption is associated with increased engagement in high-risk sexual behaviors, which can lead to the acquisition of HIV and other sexually transmitted infections. This relationship between methamphetamine use and sexual risk behavior has been found across genders and sexual orientations (Colfax and Shoptaw, 2005; Hittner, 2016).
The effects of concurrent HIV infection and methamphetamine dependence on brain morphometry and function could be hypothesized to relate in several ways: they could be additive (non-interactive), or multiplicative (interactive). An interactive effect could be adversely synergistic, meaning that the effects exacerbate one another (e.g., leading to a greater reduction in brain volume) or antagonistic, meaning that one effect counters the other. Evidence for a purely additive, non-interactive effect of HIV and methamphetamine dependence has been found at different levels of analysis, including such outcomes as brain metabolites (Chang, Ernst, Speck, & Grob, 2005), cerebral blood flow (Ances, Vaida, Cherner, & Yeh, 2011), and neuropsychological functioning (Carey et al., 2006; Rippeth et al., 2004; Scott, Woods, Matt, & Meyer, 2007).
Mechanistically, however, an adversely synergistic effect is also plausible. Methamphetamine dependence may potentiate the neurotoxic effects of the HIV pathogen via a variety of mechanisms, including alterations to the blood-brain-barrier (Cadet and Krasnova, 2007; Langford et al., 2003; Liang et al., 2008; Mahajan et al., 2008; Potula and Persidsky, 2008; Turchan, Anderson, & Hauser, 2001). However, (to our knowledge) the only existing morphometric study reported opposing influence of the two variables on volume in both distinct and overlapping brain regions (Jernigan et al., 2005). Specifically, HIV was associated with reduced frontal, temporal, limbic and striatal volume, whereas methamphetamine dependence was associated with increased volume in the basal ganglia and parietal cortex (Jernigan et al., 2005).
1.1. Independent Effects of HIV
HIV infection and methamphetamine dependence have each been associated with changes in brain morphometry, although results have been inconsistent. In some studies, HIV infection has been associated with reduced cortical volume (Stout et al., 1998; Behrman-Lay et al., 2016) or with regional cortical thinning (du Plessis et al., 2016; Shin et al., 2017; Sanford et al., 2018b; Sanford et al., 2017; Thompson et al., 2005). Other studies have found no difference in either cortical volume or thickness associated with HIV infection (Cole et al., 2018; Sanford et al., 2018a; Corrêa et al., 2016a; Seilhean et al., 1993). A recent meta-analysis found that total gray matter volume was significantly smaller among HIV infected individuals than among non-infected controls, although the standardized mean difference was small (−0.28; O'Connor et al., 2018). Factors that may explain the inconsistency across studies include viral load at the time of the study (Kallianpur et al., 2012; du Plessis et al., 2016; Thompson et al., 2005), retroviral treatment status (Corrêa et al., 2016b; Ellis et al., 2003), and age of the sample (Ances et al., 2010; Jernigan et al., 2005).
1.2. Independent effects of methamphetamine dependence
Methamphetamine users have been reported to have smaller cortical volumes compared with non-using control participants, however the lobes involved vary from study to study (Berman et al., 2008; Daumann et al., 2011; Hall et al., 2015a; Koester et al., 2012; Mackey and Paulus, 2013; Makris et al., 2008; Nakama et al., 2011; Thompson et al., 2004). Larger volumes have also been reported to be associated with methamphetamine use in the parietal and right superior frontal cortex (Jernigan et al., 2005; Kogachi et al., 2017)– the latter finding was observed only among males. Despite these findings of regional volume changes associated with methamphetamine use, a study of cortical thickness among amphetamine users found no regional differences between healthy controls and non-alcohol consuming amphetamine users (Lawyer et al., 2010). Factors that likely contribute to the variability across studies include gender (Kogachi et al., 2017), age at first use (Jernigan et al., 2005; Makris et al., 2008), total amount of drug exposure and potency (Koester et al., 2012; Mackey and Paulus, 2013; Nakama et al., 2011), current use vs. abstinence (Chang et al., 2005a; Kim et al., 2006), and current age (Nakama et al., 2011).
1.3. Cortical volume, thickness and area considered separately
As evident from this brief review, there is conflicting evidence about the presence, direction and localization of brain changes associated with HIV and methamphetamine dependence. In addition to the demographic and disorder-specific variables described above, previous inconsistencies may be partially due to heterogeneity in morphometric outcome variables. Early investigations of brain structure used available methods to estimate cortical volume, which captures both thickness and folding in a given area (Jernigan et al., 2005). Advances in segmentation software (Dale et al., 1999; Fischl, 2012; Fischl and Dale, 2000) now permit researchers to distinguish cortical thickness and surface area from their product—cortical volume—which tends to correlate more strongly with surface area than with thickness (Winkler et al., 2010). Cortical thickness and surface area show different patterns of change with age (Lemaitre et al., 2012), and twin studies have revealed distinct genetic contributions to each, supporting the importance of considering them as separate outcome measures in studies of brain morphometry (Joshi et al., 2011; Panizzon et al., 2009; Winkler et al., 2010).
1.4. Current approach
Using current segmentation techniques, we examined cortical thickness, area and volume separately in a cross-sectional sample of individuals who varied in HIV status and methamphetamine dependence. In our first aim, we investigated quantitative brain differences in 137 participants across four groups in a 2 × 2 design: HIV seropositive and methamphetamine-dependent participants (HIV+/METH+), HIV seropositive with no methamphetamine dependence (HIV+/METH-), HIV seronegative with methamphetamine dependence (HIV-/METH+) and HIV seronegative with no methamphetamine dependence (HIV-/METH-). We also investigated the impact of disorder-specific variables on cortical morphometry. Given the inconsistent findings in the literature, we tested non-directional hypotheses in regions of interest (ROIs) that parcellated the cortex. This whole-cortex, ROI approach was taken with the goal of clarifying discrepant results in the literature by examining effects of HIV infection and methamphetamine dependence and their interaction on cortical volume and its separate component parts—thickness and area.
The second aim was to investigate separately the interactive effects of HIV infection with age and methamphetamine dependence with age on thickness, area and volume. HIV patients are living longer than a decade ago and individuals who began using methamphetamine in the United States during the 1980s and 1990s are also aging (Hunt et al., 2006; Miller, 2004). Thus, in addition to investigating the possible additive, adversely synergistic, or antagonistic effects of HIV infection and methamphetamine use, it is clinically important to also examine how the effects of these disorders on brain morphometry may differ by age. Findings in the literature have been mixed, supporting HIV-related accelerated (Pathai et al., 2013), premature (Chang et al., 2011; Thomas et al., 2013), or parallel aging (Cole et al., 2018), and methamphetamine-related accelerated aging (Nakama et al., 2011). To examine possible aging effects in our sample, we investigated the interactions of HIV diagnosis or methamphetamine dependence with age. We also looked for interactions between our clinical variables of interest and participant age; we acknowledge, however, that our cross-sectional design limits the conclusions we can draw about age-related trajectories.
2. Methods
2.1. Participants
One hundred and thirty-seven individuals were recruited into a 2 (HIV seropositive vs. seronegative) by 2 (METH dependent vs. non-dependent) design at the University of California, San Diego Translational Methamphetamine AIDS Research Center (TMARC). Sixty-nine HIV seropositive participants were recruited from HIV treatment centers and community organizations in San Diego County. Thirty-one of the seropositive participants were also methamphetamine dependent (HIV+/METH+). Twenty-eight individuals who were methamphetamine dependent but HIV seronegative (HIV-/METH+) were recruited from substance abuse outreach and rehabilitation programs in San Diego County. Forty individuals seronegative for HIV who were also not methamphetamine dependent were recruited from community advertisements and word-of-mouth from the same communities as the patient groups (HIV-/METH-). Groups were not explicitly matched on demographic variables at the time of recruitment nor were demographic characteristics used to select individuals for imaging. Thus, any group differences on demographic variables found in the analysis likely reflected population differences among the groups within the communities from which they were recruited. Although recruitment was not stratified by age, participants were classified as less than or equal to 50 years of age or greater when recruited.
Potential participants met general inclusion criteria if they were at least 18 years old, had the capacity to provide informed consent, and spoke and understood English sufficiently to take validly a battery of neuropsychological tests and to complete self-report scales. Individuals were excluded from the study if they had histories of medical, neurological or severe psychiatric conditions unrelated to HIV disease or to methamphetamine use. These exclusionary conditions included seizure disorders, closed head injuries with loss of consciousness >30 min, central nervous system neoplasms, opportunistic infections, Hepatitis C and schizophrenia. Additionally, participants were excluded from the study if they met criteria for alcohol dependence in the past year, drug abuse (including cocaine) in the past year or drug dependence in the past five years (with the exception of methamphetamine dependence in the methamphetamine group). Given its prevalence in our populations, participants with histories of marijuana abuse or dependence were included. Participants were excluded if they had Wide Range Achievement Test (WRAT) Reading Scale Score < 80, a history of learning disability, or were ineligible for magnetic resonance scans (e.g., metal contraindication, claustrophobia).
HIV status was determined by the Med Mira Rapid HIV antibody test. Among HIV+ participants, CD4+ T cell counts were measured by flow cytometry at a Clinical Laboratory Improvement Amendments-certified medical center laboratory. HIV RNA levels were measured in plasma by reverse transcriptase PCR (Roche Amplicor, v. 1.5, lower limit of quantification 50 copies/mL). For each individual testing HIV positive, a consensus case conference met to determine if the Frascati criteria for HIV-associated neurocognitive disorder (HAND) were met (Antinori et al., 2007). CD4 nadir was obtained by self-report, with confirmation by documented prior measurements in a subset of individuals. Date of nadir was also obtained by self-report, as was estimated duration of HIV diagnosis.
We interviewed potential participants for substance use in order to determine whether they met the criteria for methamphetamine dependence as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994). The interview provided information about age of first methamphetamine use, days since last use, lifetime quantity used, total days used, use density (lifetime grams per day of use), intravenous drug use, drug treatment history and other categories of drug use (Byrd et al., 2011).
The study was approved by the University of California, San Diego Institutional Review Board and informed consent was obtained from each participant prior to enrollment.
2.2. Imaging
T1-weighted structural images were obtained on a 3.0 T General Electric MR750 scanner with GE DV25 Software. Image acquisition used an Array Spatial Sensitivity Encoding Technique (ASSET) for parallel and accelerated imaging with an 8-channel head coil and acquired as an Inversion Recovery-Spoiled Gradient Recalled (IR-SPGR) echo sequence (TE = 3.2 ms, TR = 8.2 ms, TI = 450 ms, flip angle = 12°, NEX = 1, matrix = 256 × 256, FOV = 24 cm, in-plane resolution = 0.9375 mm × 0.9375 mm, slice thickness = 1.2 mm, 172 slices). Using Freesurfer v5.3.0, we removed extracranial signal; segregated gray matter, white matter, and cerebrospinal fluid compartments; corrected the B1 field bias; and reconstructed the cortical surface (Dale et al., 1999; Fischl, 2012; Fischl et al., 1999). The results of the cortical surface reconstruction were visually inspected and manually edited as needed. We next used Freesurfer to label each gyral region of interest (ROI) on the cortex using the Desikan-Killiany atlas (Desikan et al., 2006). Mean cortical area, volume and thickness were estimated for each ROI (Fischl and Dale, 2000; Fischl et al., 2004). Global measures for each of these three morphometric values were computed by averaging over all 68 ROIs across the two hemispheres. Global cortical volume and area were strongly correlated (r = 0.86, p < .001) in our sample, whereas volume and thickness were less strongly related (r = 0.55, p < .001). Global area and thickness were not significantly correlated (r = 0.10, p = .14). An estimate of intracranial volume was obtained from the Freesurfer processing (based on Buckner et al., 2004). Participants were not scanned if they tested positive for methamphetamine or other drugs (excluding marijuana) on the day of the scan. Participants reported abstaining from marijuana the day they were imaged.
2.3. Statistical Analysis prior to analyzing group differences in regional cortical thickness, area or volume, we used
Linear regression to investigate in the entire sample the association between potential covariates found in the literature to be related to these three cortical measurements. The regression model – using age, gender, and intracranial volume as predictors – revealed significant associations between age and global cortical area, volume and thickness, a significant association between intracranial volume and cortical area and volume but not thickness, and no significant association between gender and any of the three cortical values. In the analyses below, age was used as a covariate in the ANCOVAs involving area, volume or thickness, whereas intracranial volume was combined with age as covariates in the analysis of cortical area and cortical volume.
For each ROI, we performed a 2 × 2 analysis of covariance (ANCOVA) to investigate the association between HIV status (seropositive/seronegative), methamphetamine dependence (dependent/not) and their interaction with cortical area, volume and thickness. We calculated partial eta-squared (ηp2: small = 0.01; medium = 0.06; large = 0.14, based on definitions in Cohen, 1988, pp. 284–288) to estimate standardized effect sizes. To investigate the relationship between cortical thickness (area, volume), age and the age interactions with HIV status, we regressed cortical thickness onto HIV status (effects coded), age (mean centered), and their multiplicative interaction. We performed a similar analysis involving age and methamphetamine dependence. For these analyses, we used the False Discovery Rate to adjust for multiple statistical tests across ROIs (Benjamini and Hochberg, 1995) with q = 0.10. Given 34 ROIs and our total sample size, for any member of a set of trending p-values (single test p < .05) to be considered significant after correction for multiple statistical tests, at least one member of the set needed to have an η2 > 0.065, a medium effect size.
In exploratory analyses, we examined the associations of each of the three cortical measurements with disorder-related variables (HIV: viral load at the time of the study, CD4 nadir, and duration of infection; Methamphetamine: age at first use, total amount of drug exposure, duration of use, and log transformed density of use.)
To explore further possible multiplicative effects on brain variables of HIV infection with methamphetamine dependence, we performed regression analyses involving interactions of HIV diagnosis with the methamphetamine disorder-related variables and interactions of methamphetamine diagnosis with HIV disorder-related variables. For example, among individuals with HIV diagnoses (HIV+/METH- and HIV+/METH+), we regressed global cortical area onto methamphetamine dependence (HIV+/METH- vs. HIV+/METH+, effects coded), CD4 nadir (mean centered) and the multiplicative interaction of methamphetamine dependence with CD4 nadir. Similar analyses were performed among individuals with methamphetamine dependence. When performing these regression analyses, we were primarily interested in testing potential interaction effects. So, only the results of these interactions are reported below.
3. Results
3.1. Demographic and disorder related variables
The study groups did not differ significantly on gender or handedness (Table 1). There was no main effect of HIV diagnosis or a main effect of methamphetamine dependence or their interaction on age. Although there was no significant main effect of HIV diagnosis or the interaction of HIV diagnosis with METH dependence on education, methamphetamine-dependent participants (HIV-/METH+ and HIV+/METH+) had significantly less education than non-dependent participants, F(1, 129) = 10.575, p = .001, ηp2 = 0.08. Although there was no main effect of HIV diagnosis or methamphetamine dependence on WRAT reading (HIV: p = .841, ηp2 < 0.001; METH: p = .100, ηp2 = 0.021), the interaction of HIV with METH was significant for WRAT reading, F(1, 129) = 4.929, p = .028, ηp2 = 0.04, due to the low score of the METH dependent group among HIV seronegative participants.
Table 1.
Demographic characteristics of participants.
| Group | N | Age | Education | WRAT Reading | %Male | %Right Handed |
|---|---|---|---|---|---|---|
| Control HIV-/METH- |
40 | 40.35 (12.62) | 14.25 (2.10) |
106.98 (13.55) |
85.00 | 85.00 |
| HIV only HIV+/METH- |
38 |
44.79 (14.02) |
14.49 (2.50) |
102.16 (11.30) |
92.10 |
84.21 |
| METH only HIV-/METH+ |
28 |
39.93 (10.34) |
12.41 (2.37) |
98.00 (11.97) |
89.28 |
89.28 |
| Dual HIV+/METH+ |
31 |
41.00 (8.23) |
13.89 (2.04) |
103.26 (13.64) |
100.00 |
83.87 |
Note. Values in parentheses are standard deviations.
The HIV+/METH- and HIV+/METH+ groups did not significantly differ on current CD4 count (ηp2 < 0.01), reported CD4 nadir (ηp2 = 0.02), or estimated duration of infection (ηp2 = 0.01).
Moreover, the HIV+/METH- and HIV+/HIV+ groups were not significantly different in percentage with AIDS, HAND, or detectable plasma HIV RNA, or on ART (Table 2A). The HIV-/METH+ and HIV+/METH+ groups did not differ significantly on age methamphetamine was first used (ηp2 < 0.01), total days used (ηp2 < 0.01), total amount of methamphetamine used (ηp2 = 0.01), log10 density of use, (ηp2 < 0.01), or days since last use (ηp2 = 0.04). Similarly, the HIV-/METH+ and HIV+/METH+ groups did not differ on the percentage having been treated for methamphetamine dependence nor on history of intravenous drug use.
Table 2.
Clinical Characteristics of Patient Groups.
| Clinical Variable | HIV Infection | Methamphetamine Dependence | Dual |
|---|---|---|---|
| |||
| Duration of HIV Infection (Years) |
9.77 (9.21) |
8.10 (7.11) |
|
|
AIDS (Y/N) |
51.4% |
37.5% |
|
|
Current CD4 |
520.47 (257.84) |
562.34 (240.25) |
|
|
CD4 Nadir |
247.56 (180.79) |
296.67 (185.24) |
|
|
Combination ART1 |
83.8% |
93.8% |
|
|
Undetectable Plasma RNA |
62.2% |
56.7% |
|
|
HAND2 |
45.9% |
53.1% |
|
| |||
|
Age at First Use (Years) |
24.52 (10.04) |
25.07 (8.82) |
|
|
Days Since Last Use |
119.23 (145.52) |
183.21 (168.05) |
|
|
Total Lifetime Days Used |
2317.44 (1513.51) |
2338.24 (2513.84) |
|
|
Log10 (Total Lifetime Quantity) |
3.12 (0.66) |
2.93 (0.85) |
|
|
Use Density |
1.16 (1.17) |
1.34 (1.77) |
|
|
Intravenous Drug Use (Y/N) |
50.0% |
41.94% |
|
|
Methamphetamine Treatment – Ever (Y/N) |
80.8% |
78.6% |
|
All treated HIV patients but one were on highly active ART (antiretroviral therapy).
HAND: Percent of group diagnosed with HIV Associated Neurocognitive Disorder.
3.2. Imaging results
The literature does not provide clear support for a lateralized effect of HIV infection or methamphetamine dependence on brain volume, area, or thickness. Moreover, when we examined each ROI for interactions of brain laterality with HIV infection or methamphetamine dependence, none of the few significant single-test results observed across the three study variables survived multiple statistical test correction. Therefore, the results reported below are averaged across hemisphere for each ROI.
3.2.1. Main effect of HIV Diagnosis
There were six ROIs where cortical area was trending smaller in the HIV seropositive group than in the seronegative group (rostral middle frontal gyrus, ηp2 = 0.03; caudal anterior cingulate, ηp2 = 0.04; fusiform gyrus, ηp2 = 0.04; inferior parietal lobe, ηp2 = 0.04; superior parietal lobe, ηp2 = 0.05 temporal pole, ηp2 = 0.06). However, the effect sizes were only small to medium, and none survived correction for multiple statistical tests. Five regions for which cortical area trended smaller among HIV infected participants also trended towards smaller cortical volumes (rostral middle frontal gyrus, ηp2 = 0.06; fusiform gyrus, ηp2 = 0.04; inferior parietal lobe, ηp2 = 0.08; superior parietal lobe, ηp2 = 0.07, temporal pole, ηp2 = 0.04). Additionally, cortical volumes trended smaller in the postcentral gyrus, ηp2 = 0.04 and in the precuneus, ηp2 = 0.04. The differences in cortical volumes remained significant following correction for multiple statistical tests in the rostral middle frontal gyrus and in the inferior and superior parietal lobes. Cortical thickness in the HIV seropositive and seronegative groups did not significantly differ for any ROI (all ηp2 < 0.03).
Among HIV seropositive participants, smaller global cortical area and volume were associated with lower CD4 nadir (area r = 0.35, p = .004; volume, r = 0.29, p = .02). Longer duration of infection was significantly associated with smaller global cortical volume (r = −0.33, p = .008). None of the HIV variables was significantly correlated with global cortical thickness.
3.2.2. Main effect of Methamphetamine Dependence
Cortical area did not differ between the methamphetamine-dependent and nondependent groups in any ROI (all ηp2 < 0.03). There was a trend for cortical volume to be smaller in the inferior parietal lobe (ηp2 = 0.04) and in the posterior cingulate gyrus (ηp2 = .03). However, the effect sizes were modest and did not survive multiple test correction. Cortical thickness trended smaller in the methamphetamine-dependent group in posterior cingulate (ηp2 = 0.07), middle temporal (ηp2 = 0.05), and inferior parietal lobe (ηp2 = 0.05), precuneus (ηp2 = 0.05), and caudal middle frontal (ηp2 = 0.03) and superior parietal cortex (ηp2 = 0.03). However, only the finding in the posterior cingulate gyrus remained significant after adjustment for multiple statistical tests. We continued to observe a significant effect of methamphetamine dependence on posterior cingulate thickness when the difference in education between the methamphetamine-dependent and non-dependent groups was statistically controlled.
Among methamphetamine-dependent participants (HIV+/METH+ and HIV-/METH+), individuals who were older at their first use of methamphetamine had thinner cortex globally, r = −0.29, p = .029. Thickness was not significantly related to duration of drug use, total drug use or recency of drug use. Global cortical area was negatively related to total days used, r = −0.29, p = .030, but was not significantly associated with age at first use, total drug used, and time since last use. The association of smaller global cortical area with more total days used remained significant after intracranial volume was added to the regression model. Global cortical volume was not significantly correlated with any methamphetamine use variable.
3.2.3. Interaction of HIV and Methamphetamine
In no ROI was the interaction of HIV infection with methamphetamine dependence significant for cortical thickness measures (all ηp2 < 0.03). There were six ROIs where the interaction of HIV diagnosis with methamphetamine dependence trended significant (p < .05, uncorrected) for cortical area (caudal anterior cingulate, ηp2 = 0.03; caudal middle frontal cortex, ηp2 = 0.03; cuneus, ηp2 = 0.05; isthmus of the cingulate gyrus, ηp2 = 0.04; lateral orbital frontal cortex, ηp2 = 0.03; precentral gyrus, ηp2 = 0.05), although none of these effects were large enough to survive multiple statistical test correction. Nonetheless, the pattern of the interactive effect of HIV diagnosis with methamphetamine dependence on cortical area was consistent across all of the ROIs. In the HIV seronegative groups, mean cortical area was smaller among methamphetamine-dependent participants, whereas in the HIV seropositive groups, mean cortical area was larger among methamphetamine-dependent participants. We observed trends for this same interactive pattern involving cortical volume in the precentral (ηp2 = 0.04) and paracentral (ηp2 = 0.03) gyri.
Among individuals with methamphetamine dependence, the presence or absence of HIV infection did not significantly interact with age at first methamphetamine use, days since last use, total days of use, total quantity used or density of use when predicting cortical thickness, area or volume. Moreover, among individuals with HIV infection, the presence or absence of methamphetamine dependence did not interact with current CD4 level, CD4 nadir, or duration of HIV infection when predicting cortical thickness, area, or volume.
3.3. Interactions with Age
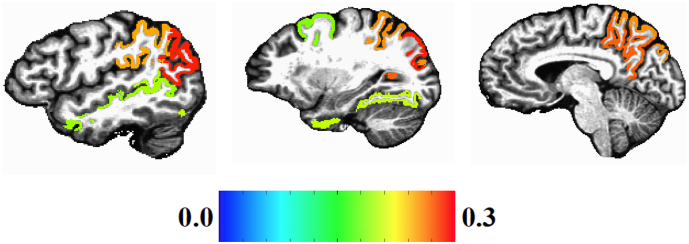
In no ROI was cortical area or volume significantly related to the interaction of age with HIV status. For cortical thickness, trend interactions of HIV status with age were found in 11 brain regions, although only eight of the ROIs survived multiple statistical test correction (caudal middle frontal cortex, fusiform gyrus, inferior parietal cortex, superior parietal cortex, superior temporal sulcus area, supramarginal gyrus, middle temporal gyrus, precuneus). Fig. 1 presents a brain map of the standardized regression weights for regions where a significant interaction of HIV status with age was observed for cortical thickness. The largest interaction effects were in posterior brain regions.
Fig. 1.
Standardized beta maps of Freesurfer regions with significant HIV by Age interaction effects on cortical thickness (multiple statistical test correction).
In the eight ROIs with a significant interaction of age with HIV diagnosis, the pattern of the interaction was the same. To summarize the interaction pattern, we averaged over cortical.
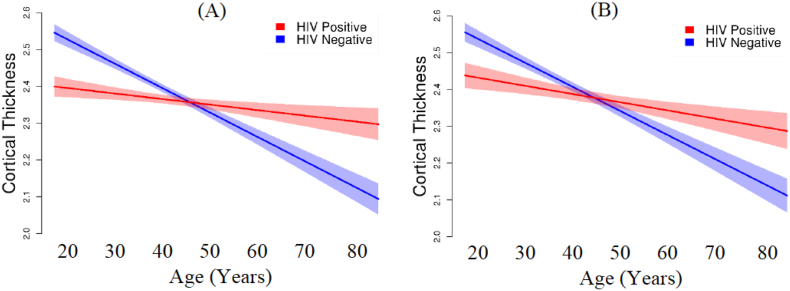
thickness associated with the eight ROIs. Not surprisingly, this linear regression model was also significant, F(3,133) = 18.705, p < .001, with HIV status significantly interacting with age: β = 0.281, p < 001 (Fig. 2). Moreover, although age and duration of HIV infection based on patient report were correlated, r = 0.54, p < .001, duration of HIV seropositive status was not significantly related to cortical thickness in regions where HIV interacted with age r2 = 0.048, p = .078. As shown in Fig. 2, age is associated with a more gradual slope among HIV seropositive individuals than among HIV seronegative individuals. Moreover, the Y-intercept for the HIV seropositive group was less than in the HIV seronegative group with no overlap in the 95%. confidence intervals (HIV seropositive intercept = 2.427 mm [95% CI: 2.339 to 2.512]; HIV seronegative intercept = 2.667 mm [95% CI: 2.590 to 2.743]). As seen in Fig. 2A, individuals with HIV infection who are in their early 20s have, on the average, global cortical thickness values similar to typical seronegative individuals in their early 40s. When we restricted the regression analysis to the 78 participants without methamphetamine dependence, the model remained significant, F(3, 74) = 13.988, p < .001, with HIV status significantly interacting with age (β = 0.267, p = 005), and showed the same pattern of results as in the full sample (Fig. 2B).
Fig. 2.
Effect of the interaction of age and HIV status on global cortical thickness (mm). (A) All participants (N = 137). (B) Participants without methamphetamine dependence (N = 78).
In no ROI was cortical area, volume or thickness related to the interaction of methamphetamine dependence with age after correction for multiple statistical tests. There was a trend, however, for the methamphetamine-dependent groups to be associated with smaller area of the isthmus of the cingulate gyrus with increasing age compared with the methamphetamine negative groups (p = .04).
4. Discussion
4.1. Effects of HIV and methamphetamine dependence
We observed a trend for HIV positive individuals to have smaller cortical area in six regions of interest, although these effects were small and did not survive multiple statistical test correction. In three of these six regions (rostral middle frontal gyrus and the inferior and superior parietal lobes), we observed smaller cortical volumes at a medium effect size, which were significant after multiple-test correction. Associations between HIV status and cortical thickness were small and non-significant in all brain areas. A recent meta-analysis found significant effects of HIV status on brain volumes, with smaller effects appearing in more recently published papers (O'Connor et al., 2018). The authors attributed the trend of diminishing effect sizes to the increased use of combined antiretroviral treatment among HIV infected patients. In our sample, 88.3% of individuals with HIV infection were on cART with average CD4 counts within the normal range. Even with this high treatment rate, we found associations between HIV clinical variables and structural brain measurements. In particular, CD4 nadir was positively associated with both global cortical area and volume, whereas duration of HIV infection was associated with smaller global cortical volume. Other groups have similarly reported that the severity of the initial immune compromise in HIV has long-term effects on brain area and volume, even when patients are adequately treated (Guha et al., 2016; Hua et al., 2013; Sanford et al., 2017).
We found only small and statistically non-significant effects of methamphetamine dependence on regional cortical area and only small trends towards reduced cortical volume in the inferior parietal lobe and in the posterior cingulate cortex. Methamphetamine dependence trended towards an association with smaller cortical thickness in some frontal and temporal regions and in the inferior parietal lobe and posterior cingulate gyrus, with the effect in the posterior cingulate gyrus surviving multiple statistical test correction. Because we found, as have others, that cortical volume is more strongly related to cortical area than cortical thickness (Winkler et al., 2010), our finding that methamphetamine dependence was associated with thickness rather than area suggests that methamphetamine's impact on brain morphometry might be too subtle for cortical volume measures to detect reliably – except in more chronically exposed samples where total days of use has sufficiently accumulated to affect cortical area. The subtlety of the methamphetamine effect and variation in accumulated methamphetamine use across studies likely contribute to the inconsistent brain volume findings reported in the methamphetamine literature.
4.2. No interaction between HIV status and methamphetamine dependence
All trends for an interactive effect of HIV status and methamphetamine dependence on regional cortical volume, area, or thickness were small and did not survive correction for multiple statistical tests. Moreover, the diagnosis of methamphetamine dependence did not interact with any HIV disease variables, nor did HIV status interact with methamphetamine variables. The small effect sizes of the interaction of HIV diagnosis with methamphetamine dependence agrees with previous findings for brain metabolites (Chang et al., 2005a, Chang et al., 2005b) and cerebral blood flow (Ances et al., 2011). We did find six regions where an interaction of HIV infection with methamphetamine dependence was significant for cortical area prior to correction for multiple statistical tests. In all six areas, the pattern of the effect was inconsistent with the hypothesis that methamphetamine dependence would exacerbate the adverse effect of HIV infection on the brain. Rather, the pattern was compatible with an antagonistic effect – mean area was smaller in the methamphetamine positive group than in the methamphetamine negative group when participants were seronegative for HIV, but larger in the methamphetamine positive group when participants were seropositive for HIV. There were trends towards this same interactive pattern for cortical volume in precentral and paracentral gyri.
In previous work, investigators have speculated that volumetric increases associated with methamphetamine use are due to enhanced neuroplasticity or neuroinflammation (Chang et al., 2005a; Jernigan et al., 2005). That increased cortical area was associated with methamphetamine dependence only among HIV infected individuals, if replicated, would support the neuroinflammatory hypothesis. Factors that could contribute to enhanced neuroinflammation in individuals positive for both HIV infection and methamphetamine use include reduced effectiveness of antiretrovirals, initiation of methamphetamine use after seroconversion, and reduced adherence to antiretroviral treatment (Ellis et al., 2003; Montoya et al., 2016; Moore et al., 2012). Several neuropathological mechanisms associated with neuroinflammation that could increase cortical area or volume include astrocytic hypertrophy, cytotoxic edema, and vasogenic edema caused by a breakdown of the blood-brain barrier (Harukuni et al., 2002; Fukuda and Badaut, 2012). The possibility that cytotoxic edema, in particular, might occur during HIV-related neuroinflammation is supported by the finding that the aquaporin AQP4, which contributes to cytoplasmic edema, is significantly elevated in brain homogenates of non-demented HIV-infected individuals (Benga and Huber, 2012; St Hillaire et al., 2005). The hypothesis that aquaporins contribute to volumetric and area changes in HIV and methamphetamine merits further exploration.
4.3. Interactions with age
4.3.1. Methamphetamine dependence
There were no trend interactions of methamphetamine by age for cortical thickness, area, or volume that survived multiple statistical test correction. Our negative finding differs from the steeper age-related slope in methamphetamine users compared with non-users reported for cortical volume by Nakama et al. (2011). However, the average number of days of methamphetamine use in our study (~77 months) was considerably less than in the Nakama study (120 months). Given that in the present study total days of methamphetamine use was negatively correlated with cortical area, the less accumulated use observed in our sample might have placed our participants at less risk for accelerated, age-related decline. Taken together, the results from our study and prior work imply that very long-term methamphetamine use (perhaps >10 years) places users at risk for accelerated, age-related brain tissue loss, but that early abstinence might mitigate this risk.
4.3.2. HIV status
Although there was no significant HIV diagnosis by age interaction for regional cortical area or volume, there were significant HIV by age interactions associated with regional thickness in eight ROIs. Across these ROIs, we observed a pattern in which HIV seropositive participants showed a more gradual decline in cortical thickness with older age than did HIV seronegative participants (Fig. 2). These results imply that the cortex is thinner among young HIV seropositive individuals compared to their seronegative age peers, and is an age-related replication of cortical thinning in other recently reported studies of HIV infection (du Plessis et al., 2016; Kallianpur et al., 2012; Sanford et al., 2017; Shin et al., 2017; Sanford et al., 2018b).
Our finding that nadir—but not current—CD4 count was inversely correlated with global cortical area and volume is consistent with prior working showing stronger associations of disease history factors (nadir CD4 and duration of infection) than current disease factors with overall cortical volume (Cohen et al., 2010; Guha et al., 2016; Hua et al., 2013). Our findings contribute to the growing body of evidence that morphometric changes associated with HIV diagnosis might be due to brain injury that occurs after infection but before antiretroviral treatment begins. In a longitudinal study, the duration of untreated HIV infection was associated with cortical thinning in the frontal, temporal, and cingulate cortex in newly-infected individuals (Sanford et al., 2018a). Moreover, patients who underwent proton spectroscopy soon after HIV infection showed evidence of neuroinflammation in the basal ganglia and occipital gray matter which reversed after antiretroviral treatment (Sailasuta et al., 2012). These results support the importance of beginning antiretroviral treatment as soon after HIV infection as possible in order to prevent neuroinflammation and neuronal damage. The average time between HIV infection and diagnosis is 5.6 years in the United States (Hall et al., 2015b). Moreover, youthful age is one of the risk factors for poor treatment adherence (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2017; Lall et al., 2015). Together, delays in beginning ARV treatment and poor treatment adherence likely account for the lower intercept among HIV positive individuals when cortical thickness was regressed onto age.
Alternative factors could explain both the lower intercept and attenuated slope observed for cortical thickness in the HIV seropositive group. The observed cortical thinness associated with HIV status could have been present premorbidly. Demographic variables hypothesized to contribute to brain reserve, such as education and general intellectual functioning, have been associated with thinner cortex or with the trajectory of cortical change over time among HIV-negative individuals (Liu et al., 2012; Menary et al., 2013; Shaw et al., 2006). However, in our study, there was no main effect of HIV status on education or premorbid verbal intelligence, which argues against the notion that any premorbid difference was related to cognitive reserve. It is possible that the attenuated slope observed in our HIV seropositive participants reflected an age-related, neuroprotective effect of HAART. In a recent study, treatment duration in recently-initiated patients was associated with small increases in cortical thickness in the right and temporal lobes (Sanford et al., 2018a). Yet, evidence is mounting that chronic anti-retroviral treatment potentiates neuronal damage rather than protecting neurons from aging, despite their acute benefits (Ciavatta et al., 2017; Underwood et al., 2015). Sorting out the favorable versus unfavorable effects of HAART on long-term brain health is a fertile topic for future HIV research.
4.3.3. Limitations
This study has a number of limitations. The cross-sectional design precludes drawing causal conclusions and is susceptible to cohort effects, such as differential morbidity that might have biased who survived to the older ages. Most of our older participants fell into the young-old range, limiting the conclusions we could draw about the effects of HIV infection and methamphetamine dependence in more advanced age groups. Worth noting are three recent longitudinal studies that have shown no differences in brain morphometry (Corrêa et al., 2016b) or no difference in morphometric trajectory between HIV seropositive and control participants across a 2-year period of middle age (Cole et al., 2018; Sanford et al., 2018b). These findings suggest that the age-related trajectories we observed cross-sectionally may not reflect true patterns of individual-level change. Finally, the methamphetamine positive and negative groups were not matched on education – potentially complicating the attribution of methamphetamine dependence as a cause of the cortical thinning we observed in the posterior cingulate gyrus. To address this potential limitation, we entered education into an analysis as a covariate and found that the posterior cingulate gyrus remained significantly thinner in the METH positive group, mitigating somewhat the interpretive concerns associated with the education mismatch.
5. Conclusion
In summary, we generally found small statistical effects of HIV infection on regional cortical thickness or area with no effect surviving multiple statistical test correction. Mean cortical volume was significantly smaller among individuals living with HIV infection in the rostral middle frontal gyrus and in the inferior and superior parietal lobes. Methamphetamine dependence was associated with reduced regional thickness only in the posterior cingulate. Exploratory analyses of disorder-related variables revealed that lower CD4 nadir was associated with smaller global cortical area and volume, and total days of methamphetamine use was associated with smaller global cortical area.
We did not find robust evidence that methamphetamine status potentiates an adverse effect of HIV infection on cortical area, volume or thickness. In several cortical regions, we did observe interaction trends where methamphetamine dependence was associated with larger cortical area, but only in the HIV seropositive group. In general, however, the most robust effects of HIV infection and methamphetamine dependence on cortical structure appear to be additive.
Examining age-related changes in morphometry in relation to our clinical variables, we found that younger HIV seropositive individuals had thinner cortex compared to age peers, and the HIV seropositive group showed an attenuated age-related decline in cortical thickness. The apparent impact of HIV infection on cortical thickness at a young age coupled with the finding that lower CD4 nadir was associated with smaller cortical area highlights the need for initiating combined antiretroviral therapy as soon as possible after HIV diagnosis in order to prevent acute neural injury and its long-term consequences.
Acknowledgments
Acknowledgements
The research was supported by the National Institute on Drug Abuse P50 DA026306 to the University of California San Diego (PI: Igor Grant). Prior to funding, NIDA provided scientific review of the Translational Methamphetamine AIDS Research Center and performs annual review of the Center's progress. Otherwise, the funding source did not contribute to the study design; the collection, analysis or interpretation of data; writing the manuscript; or the decision to submit the article for publication.
Declarations of Interest and Bias
Katherine E. MacDuffie None
Gregory G. Brown None
Benjamin S. McKenna None
Thomas T. Liu None
MJ Meloy None
Brianna Tawa None
Sarah Archibald None
Christine Fennema-Notestine None
J. Hampton Atkinson, Jr. None
Ronald J. Ellis None
Scott L. Letendre None
John Hesselink None
Mariana Cherner None
Igor Grant None
References
- American Psychiatric Association . American Psychiatric Association.DSM-IV (4thed.); Washington, DC: American Psychiatric Association: 1994. Diagnostic and statistical manual of mental disorders: DSM-IV (4thed.). Washington, DC. [Google Scholar]
- Ances B.M., Vaida F., Yeh M.J., Liang C.L., Buxton R.B., Letendre S. HIV Infection and Aging Independently Affect Brain Function as Measured by Functional Magnetic Resonance Imaging. The Journal of Infectious Diseases. 2010;201(3):336–340. doi: 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances B.M., Vaida F., Cherner M., Yeh M.J., Liang C.L., Gardner C., HIV Neurobehavioral Research Center (HNRC) Group HIV and chronic methamphetamine dependence affect cerebral blood flow. Journal of Neuroimmune Pharmacology. 2011;6(3):409–419. doi: 10.1007/s11481-011-9270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A., Arendt G., Becker J.T., Brew B.J., Byrd D.A., Cherner M., Clifford D.B., Cinque P., Epstein L.G., Goodkin K., Gisslén M., Grant I., Heaton R.K., Joseph J., Marder K., Marra C.M., McArthur J.C., Nunn M., Price R.W., Pulliam L., Robertson K.R., Sacktor N., Valcour V., Wojna V.E. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman-Lay A.M., Paul R.H., Heaps-Woodruff J., Baker L.M., Usher C., Ances B.M. Human immunodeficiency virus has similar effects on brain volumetrics and cognition in males and females. Journal of neurovirology. 2016;22(1):93–103. doi: 10.1007/s13365-015-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benga O., Huber V.J. Brain water channel proteins in health and disease. Molecular aspects of medicine. 2012;33(5):562–578. doi: 10.1016/j.mam.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple statistical testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Berman S., O'Neill J., Fears S., Bartzokis G., London E.D. Abuse of Amphetamines and Structural Abnormalities in the Brain. Ann. N. Y. Acad. Sci. 2008;1141(1):195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C., Snyder A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Byrd D.A., Fellows R.P., Morgello S., Franklin D., Heaton R.K., Deutsch R., Grant I., CHARTER Group Neurocognitive impact of substance use in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2011;58:154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J.L., Krasnova I.N. Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotoxicity research. 2007;12(3):181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- Carey C.L., Woods S.P., Rippeth J.D., Gonzalez R., Heaton R.K., Grant I., The HIV Neurobehavioral Research Center (HNRC) Group Additive Deleterious Effects of Methamphetamine Dependence and Immunosuppression on Neuropsychological Functioning in HIV Infection. AIDS and Behavior. 2006;10(2):185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Chang L., Cloak C., Patterson K., Grob C., Miller E.N., Ernst T. Enlarged striatum in abstinent methamphetamine abusers: A possible compensatory response. Biological Psychiatry. 2005;57(9):967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Ernst T., Speck O., Grob C.S. Additive Effects of HIV and Chronic Methamphetamine Use on Brain Metabolite Abnormalities. Am J Psychiatry. 2005;162(2):361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Andres M., Sadino J., Jiang C.S., Nakama H., Miller E., Ernst T. Impact of apolipoprotein E ε4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage. 2011;58(4):1017–1027. doi: 10.1016/j.neuroimage.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavatta V.T., Bichler E.K., Speigel I.A., Elder C.C., Teng S.L., Tyor W.R., García P.S. In vitro and Ex vivo Neurotoxic Effects of Efavirenz are Greater than Those of Other Common Antiretrovirals. Neurochemical Research. 2017:1–13. doi: 10.1007/s11064-017-2358-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Cohen R.A., Harezlak J., Schifitto G., Hana G. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. Journal of neurovirology. 2010;16(1):25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.H., Caan M.W., Underwood J., Francesco D., Zoest R.A., Wit F.W.…Majoie C.B. No evidence for accelerated aging-related brain pathology in treated human immunodeficiency virus: longitudinal neuroimaging results from the comorbidity in relation to AIDS (COBRA) project. Clin. Infect. Dis. 2018;66(12):1899–1909. doi: 10.1093/cid/cix1124. [DOI] [PubMed] [Google Scholar]
- Colfax G., Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Current HIV/AIDS Reports. 2005;2(4):194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- Corrêa D.G., Zimmermann N., Netto T.M., Tukamoto G., Ventura N., de Castro Bellini Leite S. Regional Cerebral Gray Matter Volume in HIV-Positive Patients with Executive Function Deficits. Journal of Neuroimaging. 2016;26(4):450–457. doi: 10.1111/jon.12327. [DOI] [PubMed] [Google Scholar]
- Corrêa D.G., Zimmermann N., Tukamoto G., Doring T., Ventura N., Leite S.C.B., Gasparetto E.L. Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. Journal of Magnetic Resonance Imaging. 2016;44(5):1262–1269. doi: 10.1002/jmri.25263. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Daumann J., Koester P., Becker B., Wagner D., Imperati D., Gouzoulis-Mayfrank E., Tittgemeyer M. Medial prefrontal gray matter volume reductions in users of amphetamine-type stimulants revealed by combined tract-based spatial statistics and voxel-based morphometry. Neuroimage. 2011;54(2):794–801. doi: 10.1016/j.neuroimage.2010.08.065. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- du Plessis S., Vink M., Joska J.A., Koutsilieri E., Bagadia A., Stein D.J., Emsley R. Prefrontal cortical thinning in HIV infection is associated with impaired striatal functioning. Journal of Neural Transmission. 2016;123:643–651. doi: 10.1007/s00702-016-1571-0. [DOI] [PubMed] [Google Scholar]
- Ellis R.J., Childers M.E., Cherner M. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. Journal of Infectious Diseases. 2003;188(12):1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- Fischl B. Freesurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11044–11049. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical Surface-Based Analysis II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fukuda A.M., Badaut J. Aquaporin 4: a player in cerebral edema and neuroinflammation. Journal of neuroinflammation. 2012;9(1):279. doi: 10.1186/1742-2094-9-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Brier M.R., Ortega M., Westerhaus E., Nelson B., Ances B.M. Topographies of cortical and subcortical volume loss in HIV and aging in the cART era. Journal of Acquired Immune Deficiency Syndromes. 2016;73:374–383. doi: 10.1097/QAI.0000000000001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis P.N., Parsons J.T., Stirratt M.J. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission. Journal of Homosexuality. 2001;41(2):17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- Hall M.G., Alhassoon O.M., Stern M.J., Wollman S.C., Kimmel C.L., Perez-Figueroa A., Radua J. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. The American Journal of Drug and Alcohol Abuse. 2015;41(4):290–299. doi: 10.3109/00952990.2015.1044607. [DOI] [PubMed] [Google Scholar]
- Hall H.I., Song R., Landmann Szwarcwald C., Green T. Time from infection with the Human Immunodeficiency Virus to diagnosis, United States. Journal of Acquired Immune Deficiency Syndrome. 2015;69:248–251. doi: 10.1097/QAI.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harukuni I., Kirsch J.R., Bhardwaj A. Cerebral resuscitation: role of osmotherapy. Journal of anesthesia. 2002;16(3):229–237. doi: 10.1007/s005400200030. [DOI] [PubMed] [Google Scholar]
- Hess K.L., Johnson A.S., Hu X., Li J., Wu B., Yu C., Morgan M.S. Diagnoses of HIV infection in the United States and dependent areas. 2017:2016. [Google Scholar]
- Hittner J.B. Meta-analysis of the association between methamphetamine use and high-risk sexual behavior among heterosexuals. Psychology of Addictive Behaviors. 2016;30(2):147–157. doi: 10.1037/adb0000162. [DOI] [PubMed] [Google Scholar]
- Hua X., Boyle C.P., Harezlak J., Tate D.F., Yiannoutsos C.T., Cohen R., Taylor M.J. Disrupted cerebral metabolite levels and lower nadir CD4+ counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatmentpatients on stable treatment. NeuroImage: clinical. 2013;3:132–142. doi: 10.1016/j.nicl.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D., Kuck S., Truitt L. Abt. Associates Inc.; Bethesda, MD: 2006. Methamphetamine use: Lessons learned. (U. S. Department of Justice Document No. 209730) [Google Scholar]
- Jernigan T.L., Gamst A.C., Archibald S.L., Fennema-Notestine C., Mindt M.R., Marcotte T.L. Effects of Methamphetamine Dependence and HIV Infection on Cerebral Morphology. American Journal of Psychiatry. 2005;162(8):1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Joshi A.A., Leporé N., Joshi S.H., Lee A.D., Barysheva M., Stein J.L. The contribution of genes to cortical thickness and volume. Neuroreport. 2011;22(3):101–105. doi: 10.1097/WNR.0b013e3283424c84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur K.J., Kirk G.R., Sailasuta N., Valcour V. Regional cortical thinning associated with detectable levels of HIV DNA. Cerebral Cortex. 2012;22:2065–2075. doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Lyoo I.K., Hwang J., Chung A., Sung Y.H., Kim J. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. The International Journal of Neuropsychopharmacology. 2006;9(2):221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Koester P., Tittgemeyer M., Wagner D., Becker B., Gouzoulis-Mayfrank E., Daumann J. Cortical thinning in amphetamine-type stimulant users. Neuroscience. 2012;221:182–192. doi: 10.1016/j.neuroscience.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Kogachi S., Chang L., Alicata D., Cunningham E., Ernst T. Sex differences in impulsivity and brain morphometry in methamphetamine users. Brain Structure and Function. 2017;222:215–227. doi: 10.1007/s00429-016-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall P., Lim S.H., Khairuddin N., Kamarulzaman A. Review: An urgent need for research on factors impacting adherence to and retention in care among HIV-positive youth and adolescents from key populations. Journal of the International AIDS Society. 2015;18(Suppl. 1):19393. doi: 10.7448/IAS.18.2.19393. (http://www.jiasociety.org/index.php/jias/article/view/19393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D., Adame A., Grigorian A., Grant I., McCutchan J.A., Ellis R.J., HIV Neurobehavioral Research Center Group Patterns of selective neuronal damage in methamphetamine-user AIDS patients. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2003;34(5):467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Lawyer G., Bjerkan P.S., Hammarberg A., Jayaram-Lindström N., Franck J., Agartz I. Amphetamine dependence and co-morbid alcohol abuse: associations to brain cortical thickness. BMC pharmacology. 2010;10(1):5. doi: 10.1186/1471-2210-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H., Goldman A.L., Sambataro F., Verchinski B.A., Meyer-Lindenberg A., Weinberger D.R., Mattay V.S. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of aging. 2012;33(3):617. doi: 10.1016/j.neurobiolaging.2010.07.013. (e1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Wang X., Chen H., Song L., Ye L., Wang S.-H. Methamphetamine Enhances HIV Infection of Macrophages. The American Journal of Pathology. 2008;172(6):1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Julkunen V., Paajanen T., Westman E., Wahlund L.O., Aitken A., Muehlboeck S. Education increases reserve against Alzheimer’s disease—evidence from structural MRI analysis. Neuroradiology. 2012;54(9):929–938. doi: 10.1007/s00234-012-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S., Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neuroscience & Biobehavioral Reviews. 2013;37(3):300–316. doi: 10.1016/j.neubiorev.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S.D., Aalinkeel R., Sykes D.E., Reynolds J.L., Bindukumar B., Adal A. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Research. 2008;1203:133–148. doi: 10.1016/j.brainres.2008.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Gasic G.P., Kennedy D.N., Hodge S.M., Kaiser J.R., Lee M.J., Iosifescu D.V. Cortical thickness abnormalities in cocaine addiction—a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron. 2008;60(1):174–188. doi: 10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menary K., Collins P.F., Porter J.N., Muetzel R., Olson E.A., Kumar V., Luciana M. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 2013;41:597–606. doi: 10.1016/j.intell.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. History and epidemiology of amphetamine abuse in the United States. In: Inciardi J., McElrath K., editors. The American drug scene. 4th Ed. Roxbury Publishing; Los Angeles, CA: 2004. [Google Scholar]
- Montoya J.L., Cattie J., Morgan E., Woods S.P., Cherner M., Moore D.J., Translational Methamphetamine AIDS Research Center (TMARC) Group, T The impact of age, HIV serostatus and seroconversion on methamphetamine use. The American journal of drug and alcohol abuse. 2016;42(2):168–177. doi: 10.3109/00952990.2015.1114625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.J., Posada C., Parikh M., Arce M., Vaida F., Riggs P.K., Atkinson J.H. HIV-infected individuals with co-occurring bipolar disorder evidence poor antiretroviral and psychiatric medication adherence. AIDS and Behavior. 2012;16(8):2257–2266. doi: 10.1007/s10461-011-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama H., Chang L., Fein G., Shimotsu R., Jiang C.S. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011;106:1474–1483. doi: 10.1111/j.1360-0443.2011.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Drug Abuse . 2013. What is the Scope of Methamphetamine use in the United States?https://www.drugabuse.gov/publications/research-reports/methamphetamine/what-scope-methamphetamine-abuse-in-united-states (September) [Google Scholar]
- O'Connor E.E., Zeffiro T.A., Zeffiro T.A. Brain Structural Changes following HIV Infection: Meta-Analysis. American Journal of Neuroradiology. 2018;39(1):54–62. doi: 10.3174/ajnr.A5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents . Department of Health and Human Services; 2017. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. (Available at http://www.aidsinfo.nih.gov/ContentFiles/ AdultandAdolescentGL.pdf. Accessed 2/27/2018) [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E., Neale M. Distinct Genetic Influences on Cortical Surface Area and Cortical Thickness. Cerebral Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S., Bajillan H., Landay A.L., High K.P. Is HIV a model of accelerated or accentuated aging? Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2013;69(7):833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potula R., Persidsky Y. Adding Fuel to the Fire: Methamphetamine Enhances HIV Infection. The American Journal of Pathology. 2008;172(6):1467–1470. doi: 10.2353/ajpath.2008.080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth J.D., Heaton R.K., Carey C.L., Marcotte T.D., Moore D.J., Gonzalez R., HNRC group Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(01):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Sailasuta N., Ross W., Ananworanich J., Chalermchai T., DeGruttola V., Lerdlum S., Spudich S. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PloS one. 2012;7(11) doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R., Cruz A.L.F., Scott S.C., Mayo N.E., Fellows L.K., Ances B.M., Collins D.L. Regionally specific brain volumetric and cortical thickness changes in HIV-infected Patients in the HAART era. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2017;74(5):563–570. doi: 10.1097/QAI.0000000000001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R., Ances B.M., Meyerhoff D.J., Price R.W., Fuchs D., Zetterberg H.…Collins D.L. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary HIV infection. Clin. Infect. Dis. 2018 doi: 10.1093/cid/ciy362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R., Fellows L.K., Ances B.M., Collins D.L. Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA neurology. 2018;75(1):72–79. doi: 10.1001/jamaneurol.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilhean D., Duyckaerts C., Vazeux R., Bolgert F., Brunet P., Katlama C. HIV-1-associated cognitive/motor complex: Absence of neuronal loss in the cerebral neocortex. Neurology. 1993;43(8):1492–1499. doi: 10.1212/wnl.43.8.1492. [DOI] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N.E.E.A., Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shin N.Y., Hong J., Choi J.Y., Lee S.K., Lim S.M., Yoon U. Retrosplenial cortical thinning as a possible major contributor for cognitive impairment in HIV patients. European Radiology. 2017:1–9. doi: 10.1007/s00330-017-4836-6. [DOI] [PubMed] [Google Scholar]
- St Hillaire C., Vargas D., Pardo C.A., Gincel D., Mann J., Rothstein J.D., McArthur J.C., Conant K. Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. Journal of Neurovirology. 2005;11:535–543. doi: 10.1080/13550280500385203. [DOI] [PubMed] [Google Scholar]
- Stout J.C., Ellis R.J., Jernigan T.L., Archibald S.L., Abramson I., Wolfson T., Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. Archives of neurology. 1998;55(2):161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Thomas J.B., Brier M.R., Snyder A.Z., Vaida F.F., Ances B.M. Pathways to neurodegeneration Effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80(13):1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Hayashi K.M., Simon S.L., Geaga J.A., Hong M.S., Sui Y., London E.D. Structural abnormalities in the brains of human subjects who use methamphetamine. Journal of Neuroscience. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Dutton R.A., Hayashi K.M., Toga A.W., Lopez O.L., Aizenstein H.J., Becker J.T. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences. 2005;102(43):15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J., Robertson K.R., Winston A. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS. 2015;29:253–261. doi: 10.1097/QAD.0000000000000538. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J., Almasy L., Zilles K., Fox P.T. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53(3):1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]