Abstract
Objective
To explore the indications and efficacy of augmentative locking compression plate (LCP) or less invasive stabilization system (LISS)with autogenous bone grafting (BG) in treating distal femoral nonunion subsequent to failed retrograde intramedullary nailing (RIN).
Methods
A retrospective study was performed for 21 patients with distal femoral nonunion subsequent to failed RIN, who received therapy with either augmentative LCP (n = 11) or LISS with autogenous BG (n = 13). Operation time, time to union, union rate, time to renonunion, complication rate and SF-36 scores a year after hardware removal were compared between the two groups.
Results
The bone union occurred in 13/13 (100%) cases in augmentative LISS group versus 9/11 (81.8%) cases in augmentative LCP group [odds ratio (OR) = 3.21, 95% confidence interval (CI) 0.7–13]. Time to union, time to renonunion, complication rate of the augmentative LCP group were significantly more than that of the augmentative LISS with autogenous BG group (p = 0.023, p = 0.021 and p = 0.033). No significant difference was found in the average operation time of two groups (p = 0.121). At the follow-up a year after hardware removal, statistically significant HRQOL improvement in the augmentive LISS group was measured at the level of pain (p = 0.003) and general health perception (p = 0.011), as compared to the augmentive LCP group.
Conclusions
We suggest augmentative LCP, for distal femoral nonunios after RIN, may be optimal for that of typeAO33A fractures, whereas augmentative LISS for that of typeAO33C fractures more.
Keywords: Retrograde intramedullary nailing, Distal femoral nonunion, Augmentative locking plate, Autologous bone grafting
Distal femoral nonunion subsequent to failed retrograde intramedullary nailing (RIN) are often complicated with a stiff knee since the initial RIN surgery is accessed through the knee, together with the factors like near-joint injury, long-term immobility, and pain.1 So it is still a challenge for treatment of distal femoral nonunion subsequent to failed RIN. Removal of the hardware would be extremely difficult and massively invasive if this nonunion is treated by exchanging reamed nailing (ERN) or internal fixation after hardware removal. Moreover, ERN is not proper in treating distal femoral nonunion after RIN due to lack of a tight fit between the new larger nail and femoral cortices.1, 2, 3 It was reported that augmentative plate with leaving nail in situ were a ideal choice for the management of isthmal and nonisthmal (the femoral shaft was divided into isthmal and nonisthmal section which includes supraisthmal and infraisthmal section2, 4)femoral shaft nonunion after intramedullary nailing.4, 5, 6, 7 However, there are very few reports available on augmentative LCP or LISS with autogenous BG for aseptic femoral nonunion after RIN. In our study, the aim is to explore the indications and efficacy of augmentative LCP or LISS with autogenous BG in treating distal femoral nonunion subsequent to failed RIN.
Materials and methods
Study design
Between 2006 and 2013, 24 patients with distal femoral nonunion after RIN received therapy with either augmentative LCP (n = 11) or LISS (n = 13) with autogenous BG at two medical units (Changhai Hospital Affiliated to Second Military Medical University and Yangzhou No 1 People's hospital Affiliated to The Second Clinical School of Yangzhou University) (Table 1, Table 2). Patients were identified by queries of computerized records databases whose initial surgeries were performed at other hospitals. A nonunion was defined as a radiolucent line without signs of callus formation around femoral shaft fracture treated by interlocking intramedullary nailing (IMN) for at least six months. It was characterized as persistent pain at the fracture site which might get worse by mobilization or weight-loading. X-ray films of all patients displayed sclerotic margins without continuous callus spanning the fracture site or no callus at least three cortices.8 Radiographically, nonunions were considered either hypertrophic or atrophic. Hypertrophic nonunions present with abundant callus and persistent radiolucent line at the fracture site and atrophic nonunions are characterized by the absence of callus, resorption of the bone ends, and a significant fracture gap.8 The surgery method of augmentative LCP or LISS with autogenous BG was chosen randomly. In the present study, only patients aged between 20 and 60 years, or with aseptic nonunion were included. Patients were excluded with open fractures at the initial injury, pathologic fracture, suspected latent infection, leg length discrepancy of more than 1.5 cm, severe cardiovascular disease or a recent administration history of corticosteroids and immunosuppressive drugs. This was a retrospective study, the sample size of which was calculated and which was approved by the institutional review board at two medical centres. All patients signed informed consent forms before surgery.
Table 1.
Patients' demographics.
| Augmentative LCP (n = 11) | Augmentative LISS (n = 13) | P | |
|---|---|---|---|
| Age (yrs), mean ± SDb | 43.5 ± 8.6 | 46.7 ± 9.2 | 0.102 |
| Gender (%, male)c | 63.6 (7/11) | 53.8 (7/13) | 0.093 |
| Smoking, n (%)c | |||
| Yes | 3 (27.3%) | 4 (30.8%) | 0.121 |
| No | 8 (72.7%) | 9 (69.2%) | |
| Side, n (%)c | |||
| Left | 6 (54.5%) | 8 (61.5%) | 0.109 |
| Right | 5 (45.5%) | 5 (38.5%) | |
| Exposure of the first RIN surgery, n (%)c | |||
| Open | 8 (72.7%) | 8 (61.5%) | 0.112 |
| Closed | 3 (27.3%) | 5 (38.5%) | |
| Reaming of the first RIN surgery, n (%)c | |||
| Reamed | 4 (36.4%) | 5 (38.5%) | 0.105 |
| Non-reamed | 7 (63.6%) | 8 (61.5%) | |
| Numbers of distal locking screw in the first RIN surgery, median (range)b | 1.5 (1–3) | 1.5 (1–3) | 0.132 |
| Cortical bone defect, median (cm, range)b | 1 (0–3.5) | 1.5 (0–4.5) | 0.081 |
| Interlocking mode of nail, n (%)c | |||
| Static | 7 (63.6%) | 9 (69.2%) | 0.097 |
| Dynamic | 4 (36.4%) | 4 (30.8%) | |
| Previous number of operations, median (range)b | 1 (0–2) | 1.5 (0–3) | 0.090 |
| Nonunion type, n (%)a,c | |||
| Hypertrophic | 2 (18.2%) | 3 (23.1%) | 0.104 |
| Atrophic | 9 (81.8%) | 10 (76.9%) | |
| Interval from injury, median (yrs, range)b | 1.5 (0–2.5) | 2 (0–3.5) | 0.088 |
LCP/LISS, locking compression plate/liss invasive stabilization systerm.
RIN, retrograde intramedullary nailing.
Weber–Cech classification.
Mann–Whitney U test.
Fisher's exact chi-square test.
Table 2.
Patients' backgrounds.
| Case | Sex | Age | Fracture type (AO/ASIF) | Injury type | Mode of energy | Nonunion time (mons) | Nonunion cause | With a stiff knee | AP system | Follow-up time (mons) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 39 | 33-A2 | Accident | High | 12 | Distal locking screw loosening | Yes | LCP (9 holes) | 24 |
| 2 | F | 45 | 33-C1 | Fall | Low | 16 | Bone resorption | Yes | LCP (7 holes) | 16 |
| 3 | M | 33 | 33-A2 | Accident | High | 10 | Excessive shortness of IN | Yes | LCP (9 holes) | 18 |
| 4 | M | 48 | 33-C1 | Accident | High | 23 | Bone resorption | No | LCP (9 holes) | 21 |
| 5 | F | 45 | 33-C2 | Fall | Low | 11 | Excessive thinness of IN | No | LCP (7 holes) | 34 |
| 6 | M | 19 | 33-A2 | Accident | High | 19 | Bone resorption | Yes | LCP (9 holes) | 32 |
| 7 | F | 31 | 33-C1 | Accident | Low | 21 | Distal locking screw breakage | Yes | LCP (9 holes) | 12 |
| 8 | M | 46 | 33-A2 | Fall | High | 32 | Bone defect | No | LCP (9 holes) | 36 |
| 9 | F | 51 | 33-C1 | Fall | High | 15 | Distal locking screw loosening | Yes | LCP (7 holes) | 23 |
| 10 | M | 53 | 33-C2 | Crash | High | 18 | Bone defect | No | LCP (9 holes) | 28 |
| 11 | M | 38 | 33-A2 | Accident | Low | 17 | Inefficacy of distal locking screw | Yes | LCP (7 holes) | 20 |
| 12 | M | 48 | 33-A2 | Accident | Low | 12 | Excessive thinness of IN | No | LISS (7 holes) | 23 |
| 13 | F | 61 | 33-A2 | Fall | High | 23 | Bone defect | Yes | LISS (5 holes) | 18 |
| 14 | M | 32 | 33-C1 | Accident | High | 11 | Bone defect | No | LISS (7 holes) | 14 |
| 15 | M | 44 | 33-C1 | Fall | High | 14 | Excessive thinness of IN | Yes | LISS (7 holes) | 16 |
| 16 | F | 52 | 33-C2 | Crash | High | 28 | Distal locking screw loosening | Yes | LISS (5 holes) | 34 |
| 17 | M | 48 | 33-A2 | Accident | Low | 17 | Excessive shortness of IN | Yes | LISS (7 holes) | 28 |
| 18 | F | 49 | 33-C2 | Accident | High | 14 | Excessive shortness of IN | No | LISS (5 holes) | 24 |
| 19 | M | 36 | 33-C1 | Accident | High | 19 | Bone resorption | Yes | LISS (5 holes) | 12 |
| 20 | F | 50 | 33-C2 | Accident | High | 29 | Distal locking screw loosening | No | LISS (7 holes) | 25 |
| 21 | M | 29 | 33-A2 | Fall | Low | 21 | Distal locking screw loosening | Yes | LISS (7 holes) | 31 |
| 22 | F | 39 | 33-C2 | Accident | High | 12 | Bone defect | Yes | LISS (5 holes) | 36 |
| 23 | M | 44 | 33-A2 | Accident | Low | 10 | Distal locking screw loosening | No | LISS (7 holes) | 21 |
| 24 | F | 48 | 33-C2 | Crash | Low | 13 | Distal locking screw loosening | Yes | LISS (7 holes) | 30 |
F/M, female/male.
AP, augmentive plating.
LCP/LISS, locking compression plate/liss invasive stabilization systerm.
Surgery
Subperiosteal dissection was performed to expose the lateral or 1/3 to 1/2 of anterolateral fracture ends along the original incision. Periosteum or muscle dissection was minimized to avoid blood supply damage. The dense fibrous soft tissue and sclerotic bone around the fracture site was cleared completely. Of all patients, 11 cases were treated with augmentative LCP (Synthes, USA)with autogenous BG including 4 cases of 7-hole LCP and 7 cases of 9-hole LCP, and 13 cases augmentative LISS (Synthes, USA)with autogenous BG including 5 cases of 5-hole LISS and 8 cases of 7-hole LISS. The choice of locking plating of different sizes was based on the length of fracture line and size of bone defect. The 3–3.5 mm Kirschner wire was used to enable the bicortical screw to travel through the cortical bones completely. The unicortical locking screw may be used to avoid RIN baffle. Three to four locking screws were fixed on distal and proximal ends of the plate and compression with LCP was not applied. Autologous iliac grafting with an average of 9.86 ± 0.22 g (range 6.5–13 g) was applied to all patients regardless of nonunion type. Aerobic and anaerobic cultures were collected from the nonunion sites in all cases to rule out insidious infections.
Of all patients, 15 cases with a stiff knee underwent an open soft-tissue arthrolysis under general anesthesis while hardware was removed. The procedure is performed with a tourniquet. The original scar is re-opened and the incision deepened to the capsule. The knee is opened laterally and the thickened capsule excised from the joint. This capsular scar tissue can be up to 13 mm thick. The hardware was firstly exposed and removed successfully. Adhesions under the suprapatellar pouch are released. A lateral release is performed to free the extensor mechanism and to allow access to the scar tissue in the lateral gutter and beneath the patellar tendon. The scar tissue is removed from the medial gutter. The scar tissue which tethers the patellar tendon may cause patella infera, and patellar height must be recovered to maximise recovery. After this release, the patella can be everted without the need for a quadriceps snip, turn down, or osteotomy of the tibial tuberosity. The PCL, the popliteus tendon, and/or posterior capsule may require release in order to correct the fixed-flexion deformity.
After nonunion revision surgery, the drainage tubes were placed for 1–2 days depending on the drainage volume. The patients started to mobilize hip and knee joints with assistance of Continuous Passive Motion (CPM) machine to avoid extension knee apparatus adhesion. Meanwhile, the patients were encouraged to take isometric and isotonic functional training of quadriceps actively. Eight weeks after, the patients could gradually have weight-bearing mobilization on crutches followed by full weight bearing walk once obvious continuous callus appeared in X-ray films.
Data collection and outcome measurement
Data collected included demographics (ages, gender, smoking, fracture type, side, injury type, mode of energy, exposure and reaming of the first RIN surgery, numbers of distal locking screw in the first RIN surgery, interlocking mode of nail, previous number of operations, nonunion type and time and cause, cortical bone defect, interval from injury, with or without a stiff knee), operation time, time to union, union rate and postoperative and related complications (Table 1, Table 2, Table 3). Outpatient follow-ups were carried out at 1, 2, 3, 4, 6, and 12 months after surgery and then once every year. The clinical evaluation of all patients was performed by an independent examiner. Radiological examinations included femoral plain radiographs in 2 views (anteroposterior view and lateral view) to monitor callus growth. Malalignment was defined as >5°angulations, >15°rotation and >2 cm length discrepancy as measured by radiography.8 Follow-ups at interval of a month was carried out for those without obvious progression of healing four months after surgery. Operative time and intraoperative blood loss, along with any related complications during the study, were recorded. The data were extracted through patient chart review and computerized records that are linked to patient records in the community and other hospitals.
Table 3.
Comparison of outcomes between the two groups.
| Augmentative LCP (n = 11) | Augmentative LISS (n = 13) | p | |
|---|---|---|---|
| Mean operation time, mins | |||
| mean ± SDa | 109.3 ± 20.2 | 112.8 ± 24.3 | 0.121 |
| Mean time to union, mons | |||
| mean ± SDa | 9.7 ± 1.8 | 5.1 ± 0.6 | 0.023* |
| Union rate, n (%)b | 9 (81.8%) | 13 (100%) | 0.039* |
| Mean time to renonunion, mons | |||
| mean ± SDa | 8.0 ± 0.8 | 0 | 0.021* |
| Complication rate postoperatively, n (%)b | 0.033* | ||
| Infection | 0 (0) | 1 (7.7%) | |
| Renonunion | 2 (18.2%) | 0 (0) | |
*p < 0.05.
Mann–Whitney U test.
Fisher's exact chi-square test.
In this study, the Medical Outcomes Study 36-Item Short Form (SF-36)9 is accepted for assessment of health related quality of life (HRQOL) of all patients 1 year after hardware removal (Table 4). The questionnaires had been either self-administered by the patients or by in-person interviewers, and the data were provided by them to an interviewer either during an office visit or by telephone. The questionnaire typically takes 15–20 min to complete. The SF-36 is a multi-purpose, short-form health survey that consists of 36 question measures comprising three aspects of health: functional ability, well-being and overall health. In an attempt to quantify these aspects, the SF-36 assesses eight domains of quality of life: physical function, role limitations due to physical problems, role limitations due to emotional problems, social function, mental health, energy or vitality, pain and general health perception. A single item also assesses the patient's perception of changes in health. The total result is most often shown in the form of the profile defined with eight points that represent the measure of individual aspects of health transformed into a unique scale whose theoretical minimum is a score of 0 and the maximum a score 100. On all scales, higher results indicate better subjective health.9
Table 4.
Comparison of SF-36 scores 1 year after hardware removal between the two groups.
| Augmentative LCP (n = 11) | Augmentative LISS (n = 13) | p | |
|---|---|---|---|
| Physical functioning, median (interquartile range)a | 58.0 (55.0–60.0) | 60.0 (58.0–65.0) | 0.108 |
| Role limitation due to physical problems, median (interquartile range)a | 100.0 (75.0–100) | 100.0 (75.0–100) | 0.238 |
| Role limitation due to emotional problems, median (interquartile range)a | 100.0 (100–100) | 100.0 (100–100) | 0.089 |
| Social functioning, median (interquartile range)a | 72.7 (66.4–77.8) | 73.8 (68.8–79.9) | 0.063 |
| Mental health, median (interquartilerange)a | 72.4 (69.7–84.0) | 73.3 (70.4–85.5) | 0.077 |
| Energy vitality, median (interquartilerange)a | 70.0 (65.0–70.0) | 70.0 (65.0–70.0) | 0.662 |
| Pain, median (interquartilerange)a | 77.8 (66.7–77.8) | 84.4 (78.9–94) | 0.003* |
| General health perception, median (interquartile range)a | 62.0 (57.0–67.0) | 67.0 (62.0–72.0) | 0.011* |
*p < 0.05.
Mann–Whitney U test.
Statistical analysis
Data were analyzed with SPSS version 18.0 statistical software (SPSS Inc, Chicago, Illinois). Descriptive frequencies and percentages were tabulated. Pearson's chi-square test or Fisher's exact chi-square test, as appropriate, was used to detect differences in nonparametric variables, Unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) are presented. Continuous variables were compared using the t-test or the Mann–Whitney U-test, as appropriate. Statistical significance was set at p < 0.05 (power 80%).
Results
A total of 24 patients with distal femoral nonunion subsequent to failed RIN were identified, who received therapy with either augmentive LCP (n = 11) or LISS (n = 13) with autologous BG. No significant difference in demographics of patients was showed between the two groups (p > 0.05) (Table 1).
After a mean follow-up of 19.8 months (range 12–36 months), The bone union occurred in 13/13 (100%) cases in augmentative LISS group versus 9/11 (81.8%) cases in augmentative LCP group [odds ratio (OR) = 3.21, 95% confidence interval (CI) 0.7–13] (Table 3). Two patients with a stiff knee treated with augmentative LCP for distal femoral nonunion (type AO33C1 and C2 respectively) obtained secondary nonunion at 7 and 9 months postoperatively. They declined next internal revision operation for the reason of economy, who had final bone healing 3 and 5 months after autogenous iliac bone grafting (BG) with cast application. Time to union and time to renonunion of the augmentative LCP group were significantly more than that of the augmentative LISS with autogenous BG group (p = 0.023 and p = 0.021). No significant difference was found in the average operation time of two groups (p = 0.121) (Table 3).
One patient in augmentive LISS group had delayed wound infection 10 months after surgery, but had achieved the bone union. The wound was healed successfully 2 weeks after hardware removal. The complication rate in augmentive LCP group significantly was higher than the augmengtive LISS group (p = 0.033) (Table 3). Hardware removal was routinely performed approximately 1 year after bony union (Fig. 1, Fig. 2). Of all patients, 15 cases with a stiff knee underwent an open soft-tissue arthrolysis while hardware was removed. Range of motion of stiff knee improved obviously after arthrolysis. Mean extension was changed from 4.65°(0°–11°) preoperatively to 0.8°(−5°∼4°) postoperatively and mean flexion changed from 78.6°(60°–90°) preoperatively to 115.3°(100°–125°) postoperatively. At the follow-up 1 year after hardware removal, statistically significant HRQOL improvement in the augmentive LISS group was measured at the level of pain (p = 0.003) and general health perception (p = 0.011), as compared to the augmentive LCP group. At the level of physical problems, role limitations due to emotional problems, social function, mental health, energy or vitality assessment, there were no statistically significant differences between the two groups (p = 0.517) (Table 4). No patients had experienced failure of internal fixation, neurovascular injury, malalignmental union or other complications.
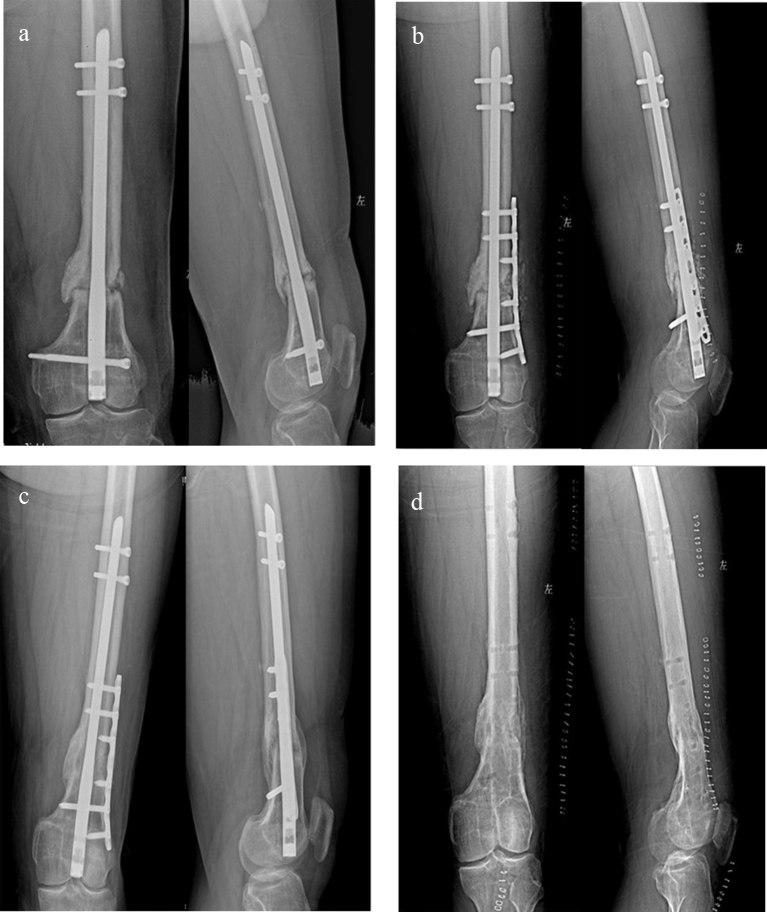
Fig. 1.
a Distal femoral nonunion after retrograde intramedullary nail of type AO33A2; b Instant x-ray after treatment by augmentative LCP with autogenous BG, only one distal locking screw of RIN was removed due to its baffle for augmentative LCP; c Bony union was achieved at 5 months after the surgery; d X-ray after hardware removal.
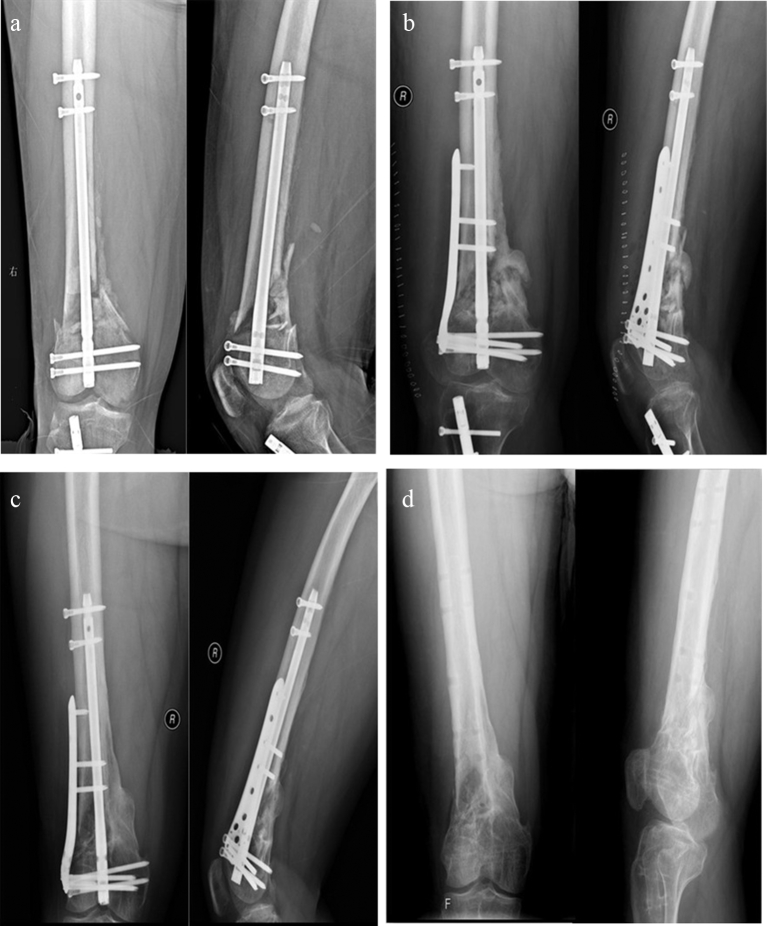
Fig. 2.
a Distal femoral nonunion after retrograde intramedullary nail of type AO33C2; b Instant x-ray after treatment by augmentative LISS with autogenous BG; c Bony union was achieved at 8 months after the surgery; d X-ray after hardware removal.
Discussion
It is believed that instability of rotation is the dominant reason for hypertrophic nonunion after intramedullary nails, whereas the mechanical instability mainly results from malpractice of surgeons such as inappropriate choice of the nails, non-standard operating technique, and inefficacy of the distal locking screws (<2 pieces of screws) etc.4, 5, 6, 7 Soft tissue injury subsequent to excessive periosteal and muscular stripping at the fracture site during open reduction is the main reason for atrophic nonunion after intramedullary nails.1, 2, 3 Instability of rotation can be corrected either by exchanging the larger intramedullary nails or by plate fixation after removing the hardware. The premise of ERN in correcting the mechanical instability, is the close touch between the larger intramedullary nails and marrow cavity at the nonunion site.10 Therefore, patients who suffered from isthmal femoral shaft fracture, without obvious bone defects, can achieve satisfactory results using ERN.4 Yet, for femoral nonunion after RIN of the infra-isthmal femoral shaft fracture and distal femoral fracture, the therapeutic efficacy often is dissatisfactory using ERN due to lack of a tight fit between the new larger nail and femoral cortices.1, 2, 3, 4 Moreover, removal of the intramedullary nails in this nonunion, which is often complicated with a stiff knee, would be extremely difficult and massively invasive. As it requires a surgical approach via knee joint, which will aggravate the dysfunction of the injured knee. Therefore, it will not be an ideal choice to apply ERN or perform plate fixation after removing the hardware for treating distal femoral nonunion subsequent to failed RIN.
Augmentative compression plate (ACP) fixation in treating femoral nonunion after intramedullary nails has be reported with satisfactory efficacy.4, 5, 6, 7 The indications of ACP have be expanded by several doctors. YJ et al11 reported that six patients with femoral or tibial nonunion after intramedullary nails were treated by augmentative LCP, X-ray imaging showed obvious bone callus formation at the broken ends of the fracture at mean 4.5 months (3–7) after surgery. Though ACP technique is less invasive and has higher union rate compared with exchanging nailing, its indication is still controversial due to the limitation of lack of prospective studies and less sample sizes.4 Recently, Park et al12 retrospectively reviewed 39 patients with femoral shaft nonunions after intramedullary nails treated by ACP with BG, and illustrated that the absolute indications of such technique maybe include non-isthmal femoral shaft nonunions, isthmal femoral shaft nonunions with bone defects, femoral nonunions in which the IN is hard to remove. In present study, fifteen (62.5%) patients with a stiff knee, treated by augmentive LCP or LISS with autologous BG obtained the bony union successfully with a mean time of 5.4 months (range 4–7 months). All patients with a stiff knee underwent arthrolysis under general anesthesis while hardware was removed, and a satisfactory function of knee joint eventually was achieved through full intraoperative release and active functional exercise on the affected knee postoperatively. Given the current nervous situation of the doctor-patient relationship in China, hardware removal was routinely performed for all patients after bone union. At the follow-up 1 year after hardware removal, statistically significant HRQOL improvement in the augmentive LISS group was measured at the level of pain (p = 0.003) and general health perception (p = 0.011), as compared to the augmentive LCP group. Augmentative LCP or LISS associated with BG maybe is an ideal choice for aseptic femoral nonunion after RIN with a stiff knee. Its advantages may lie in preservation of intramedullary nails, less invasions, shorter operation time, and higher healing rate.
Mechanical instability and destruction of biological environment are primarily responsible for bony nonunion and both sides often co-exist simultaneously.4, 5, 6, 7 In our study, nonunion cause of all patients were analyzed in detail including inefficacy of distal locking screw, bone resorption, excessive shortness of intramedullary nails, excessive thinness of intramedullary nails, bone defects, locking screw breakage or prolapse, and open injury or reduction etc which mainly are related to malpractice of surgeons. Theoretically speaking, BG need not be performed for patients with hypertrophic nonunions, but when augmentive LCP or LISS surgery was applied, it should also involve exposure of the fracture sites, and clearance of the fabric soft tissues and sclerotic bones, which would probably damage the local callus and blood supply even for patients with hypertrophic nonunions. Therefore, autogenous BG may be beneficial for bony healing regardless of nonunion type when treated by augmentive LCP or LISS. Given the current nervous situation of the doctor-patient relationship in China, autogenous BG, in order to lower renonunion rate, was applied for all patients regardless of nonunion type in the present study. Though previous studies showed that either augmentative DCP or LCP, in treating femoral nonunion after intramedullary nails, showed an excellent efficacy.6, 13, 14, 15 But the biggest advantage of LCP, compared with DCP, is that single cortex fixation can be performed by the locking between the screw and the plating when the intramedullary nails obstructed fixation of screws. Moreover, it can be implanted using the minimal invasive plating osteosynthesis (MIPO) technique, causing fewer damages to the blood supply of the local soft tissues.15 However, for nonunion of AO33C type distal femoral fracture after failed RIN, the premise of obtaining the mechanical stability may be to increase more screws fixation on distal fracture end due to its unique anatomical and mechanical characteristics. Augmentative LCP may not be suitable for this kind of nonunion, whereas LISS may be an ideal choice since more locking screws can be fixed on the distal fracture end. Furthermore, LISS have the advantages of minimal invasion, single or double cortex fixation and anatomical fixation for the femoral condyle. In this study, time to union and time to renonunion of the augmentative LCP group were significantly more than that of the augmentative LISS with autogenous BG group, two patients with a stiff knee treated with augmentative LCP for distal femoral nonunion (type AO33C1 and C2 respectively) obtained secondary nonunion at 7 and 9 months postoperatively. Other cases all achieved bony union successfully. The reason for nonunion in two patients maybe be related to decreased mechanical stability between the fracture ends. Therefore, for distal femoral nonunion after RIN, it is believed that augmentative LISS may be more optimal for that of typeAO33C fractures than augmentative LCP. One patient in augmentive LISS group had delayed wound infection 10 months after surgery whose wound was healed successfully 2 weeks after hardware removal. We think this may result from some factors such as the initial high-energy injury, poor condition of the soft tissues, a stiff knee, and a low level of systemic immunity.
In conclusion, the virtues of this study lies in that it should be a retrospective, cohort study. The results of this study suggested that augmentative LCP, for distal femoral nonunios after RIN, may be optimal for that of typeAO33A fractures, whereas augmentative LISS for that of typeAO33C fractures more. However, a prospective observational study with larger sample size is further needed.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Banaszkiewicz P.A., Sabboubeh A., McLeod I. Femoral exchange nailing for aseptic non-union: not the end to all problems. Injury. 2003;34:349–356. doi: 10.1016/s0020-1383(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 2.Yang K.H., Kim J.R., Park J. Nonisthmal femoral shaft nonunion as a risk factor for exchange nailing failure. J Trauma Acute Care Surg. 2012;72:E60–E64. doi: 10.1097/TA.0b013e318239caca. [DOI] [PubMed] [Google Scholar]
- 3.Said G.Z., Said H.G., el-Sharkawi M.M. Failed intramedullary nailing of femur: open reduction and plate augmentation with the nail in situ. Int Orthop. 2011;35:1089–1092. doi: 10.1007/s00264-010-1192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J., Kim S.G., Yoon H.K. The treatment of nonisthmal femoral shaft nonunions with im nail exchange versus augmentation plating. J Orthop Trauma. 2010;24:89–94. doi: 10.1097/BOT.0b013e3181b8dafd. [DOI] [PubMed] [Google Scholar]
- 5.Birjandinejad A., Ebrahimzadeh M.H., Ahmadzadeh-Chabock H. Augmentation plate fixation for the treatment of femoral and tibial nonunion after intramedullary nailing. Orthopedics. 2009;32:409. doi: 10.3928/01477447-20090511-12. [DOI] [PubMed] [Google Scholar]
- 6.Chen C.M., Su Y.P., Hung S.H. Dynamic compression plate and cancellous bone graft for aseptic nonunion after intramedullary nailing of femoral fracture. Orthopedics. 2010;33:393. doi: 10.3928/01477447-20100429-18. [DOI] [PubMed] [Google Scholar]
- 7.Hakeos W.M., Richards J.E., Obremskey W.T. Plate fixation of femoral nonunions over an intramedullary nail with autogenous bone grafting. J Orthop Trauma. 2011;25:84–89. doi: 10.1097/BOT.0b013e3181dfbb33. [DOI] [PubMed] [Google Scholar]
- 8.Frölke J.P., Patka P. Definition and classification of fracture non-unions. Injury. 2007;38(Suppl 2):S19–S22. doi: 10.1016/s0020-1383(07)80005-2. [DOI] [PubMed] [Google Scholar]
- 9.Keller S.D., Ware J.E., Jr., Bentler P.M. Use of structural equation modeling to test the construct validity of the SF-36 Health Survey in ten countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1179–1188. doi: 10.1016/s0895-4356(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 10.Wu C.C. Exchange nailing for aseptic nonunion of femoral shaft: a retrospective cohort study for effect of reaming size. J Trauma. 2007;63:859–865. doi: 10.1097/01.ta.0000233663.24838.76. [DOI] [PubMed] [Google Scholar]
- 11.Ye J., Zheng Q. Augmentative locking compression plate fixation for the management of long bone nonunion after intramedullary nailing. Arch Orthop Trauma Surg. 2012;132:937–940. doi: 10.1007/s00402-012-1497-4. [DOI] [PubMed] [Google Scholar]
- 12.Park J., Yang K.H. Indications and outcomes of augmentation plating with decortication and autogenous bone grafting for femoral shaft nonunions. Injury. 2013;44:1820–1825. doi: 10.1016/j.injury.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Ueng S.W., Chao E.K., Lee S.S. Augmentative plate fixation for the management of femoral nonunion after intramedullary nailing. J Trauma. 1997;43:640–644. doi: 10.1097/00005373-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Gao K.D., Huang J.H., Tao J. Management of femoral diaphyseal nonunion after nailing with augmentative locked plating and bone graft. Orthop Surg. 2011;3:83–87. doi: 10.1111/j.1757-7861.2011.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadkarni B., Srivastav S., Mittal V. Use of locking compression plates for long bone nonunions without removing existing intramedullary nail:review of literature and our experience. J Traum. 2008;65:482–486. doi: 10.1097/TA.0b013e31817c9905. [DOI] [PubMed] [Google Scholar]