Abstract
Introduction
The aim of the study is to report the preliminary clinical and functional outcomes of a modular cementless tumor resection prosthesis system (Megasystem-C®, Waldemar Link GmbH&Co. KG, Hamburg, Germany) in patients undergoing limb salvage surgery with wide resection in a lower extremity primary or metastatic malignant bone tumor.
Material and methods
Fifty-two consecutive patients (33 male and 19 female; mean age 37.1 years (range, 16 to 79) with primary or metastatic lower extremity malignant bone tumor who underwent wide resection and reconstruction with cementless Megasystem-C® system were included in the study. Patients were analyzed for age at diagnosis, gender, type and localization of the tumor, time of follow-up, patient and prosthesis survival, complications, oncological and functional outcomes.
Results
Mean follow-up time was 43.2 months (range, 8 to 66). Cumulative patient survival rate was 92.3 percent and cumulative prosthetic survival rate was 65.4 percent. 18 complications were recorded and 9 of them required revision (17.3 percent). Mean overall Musculoskeletal Tumor Society score score was 72.7 percent (range, 52 to 86). Subgroup analyzes demonstrated no difference in complication rates, overall patient or prosthetic survivals. Functional scores according to age, diagnosis and location of the reconstruction also were not significantly different.
Conclusion
The preliminary short-term follow-up results revealed that, the new generation modular cementless endoprosthetic system offers promising clinical and functional outcomes with reasonable complication rates.
Level of evidence
Level IV, Therapeutic study
Keywords: Bone tumor, Limb salvage, Tumor prosthesis, Modular, Cementless
Introduction
The life expectancy of patients with malignant bone tumors increased after improvements in the knowledge for tumor biology, advances in diagnostic abilities, adjuvant treatment modalities and surgical techniques.1, 2 Because of this, functional status of the patient becomes a major issue in the treatment. Limb-salvage surgery offers better functional outcomes and quality of life without a reduction in survival or an increase in morbidity when compared to amputation.3, 4, 5, 6 Selection of the type of limb-salvage procedure is based on the tumor location and the patient characteristics with aiming a durable reconstruction and favourable functional outcomes.7, 8, 9
Endoprosthetic reconstruction is a reliable option in periarticular tumor resections. It provides component modularity, improved fixation, near anatomic appearance and good-to-excellent functional results.10, 11, 12, 13 Modular endoprosthetic systems have either cemented and cementless stem fixation options. Early reports stated that, cemented modular systems were associated with intermediate to long-term problems of aseptic loosening, mechanical breakage and infection with high failure rates.14, 15, 16 Thereof, cementless stems have gained acceptance in limb sparing surgery to minimize the risk of failure. Recent studies demonstrated that, cementless prosthetic systems have favourable outcomes in terms of infection and aseptic loosening.17, 18, 19
The aim of this prospective case series is to report the preliminary clinical and functional outcomes of a modular cementless tumor resection prosthesis system with titanium tapered stem that has splines for cementless fixation.
Patients and methods
After obtaining institutional review board approval, we prospectively followed our patients who underwent limb reconstruction with the Megasystem-C® modular prosthesis system (Waldemar Link GmbH&Co. KG, Hamburg, Germany) after wide resection of a lower extremity malignant tumor, starting in 2008 up until 2012, in our institution. Inclusion criteria were to have malignant primary or metastatic tumor in femur and tibia which was histologically proven by biopsy, reconstruction with cementless stem fixation and prosthetic replacement as a primary reconstructive procedure. Patients with pelvic tumor, reconstruction with cemented stem fixation, reconstruction with a different modular prosthetic system and prosthetic replacement after a previously failed reconstructive surgery were excluded.
Fifty-two consecutive patients with primary or metastatic lower extremity malignant bone tumor who underwent wide resection and reconstruction with cementless Megasystem-C® system were included in the study. Mean age at diagnosis was 37.1 years (range, 16 to 79). There were 33 male and 19 female patients. Twenty nine of the patients had a right-sided and twenty-three of them had a left-sided tumor. Diagnosis was osteosarcoma in 17, metastatic disease in 11, Ewing sarcoma in 7 patients, giant-cell tumor in 5 patients, chondrosarcoma in 5 patients, multiple myeloma in 3 and liposarcoma, leiomyosarcoma, synovial sarcoma and neurofibrosarcoma in one patient each. Average follow-up time was 43.2 months (range, 8 to 66).
The reconstruction was in proximal femur in 18 patients, distal femur in 18 patients, proximal tibia in 10 patients and total femoral replacement in 6 patients. Detailed description of patient information including demographics is summarized in Table 2.
Table 2.
Patient demographics and tumor characteristics.
| Diagnosis | Number | Mean age | Gender F/M |
R/L | Tumor localization |
|||
|---|---|---|---|---|---|---|---|---|
| Proximal femur | Diaphysis femur | Distal femur | Proximal tibia | |||||
| Osteosarcoma | 17 | 20.1 | 5/12 | 10/7 | 3 | 2 | 7 | 5 |
| Ewing sarcoma | 7 | 17.1 | 3/4 | 3/4 | – | 2 | 3 | 2 |
| Metastases | 11 | 60.8 | 4/7 | 7/4 | 6 | 2 | 3 | – |
| Giant cell tumor | 5 | 32.6 | 3/2 | 2/3 | – | – | 3 | 2 |
| Chondrosarcoma | 5 | 55.4 | 2/3 | 4/1 | 3 | 1 | 1 | – |
| Othersa | 7 | 51.4 | 2/5 | 3/4 | 5 | 2 | – | – |
| Overall | 52 | 37.1 | 19/33 | 29/23 | 17 | 9 | 17 | 9 |
Including multiple myeloma, liposarcoma, leiomyosarcoma, synovial sarcoma, neurofibrosarcoma.
Patients were analyzed for age at diagnosis, gender, type and location of tumor, follow-up time, patient and prosthesis survivals, complications such as infection, dislocation, implant failure, aseptic loosening and soft-tissue related problems, oncological and functional outcomes.
Functional outcomes were determined with the revised Musculoskeletal Tumor Society (MSTS) rating scale.20 Failure of reconstruction was classified as described by Henderson et al (Table 1).21 According to this, failed reconstruction was defined as a reconstruction that required revision of the complete or failed portion of prosthesis, fixation of a periprosthetic fracture, soft-tissue reconstruction to restore joint stability such as instability, tendon rupture or aseptic wound dehiscence or endoprosthetic removal without revision and amputation.
Table 1.
Classification of the mode of failure in tumor resection prosthesis reconstruction as described by Henderson et al.
| Type of failure | Description | |
|---|---|---|
| Mechanical | 1 | Instability, tendon rupture, aseptic wound dehiscence |
| 2 | Clinical and radiographic evidence of aseptic loosening | |
| 3 | Periprosthetic or prosthetic fracture | |
| Non-mechanical | 4 | Infection requiring removal of prosthesis |
| 5 | Recurrence or progression of tumor | |
Statistical analyzes were performed with the SPSS statistical software package (version 20.0; SPSS, Chicago, Illinois). Kaplan–Meier analyzes were used to determine the patient and prosthesis survivals. Chi-square tests were used to determine a significant difference between overall, region, age or diagnosis-specific complication rates. Kruskal–Wallis test was also used to analyze overall and region-specific functional scores. For all analyzes, a p score less than 0.05 was seeked for a statistical significance.
Results
Survival analysis
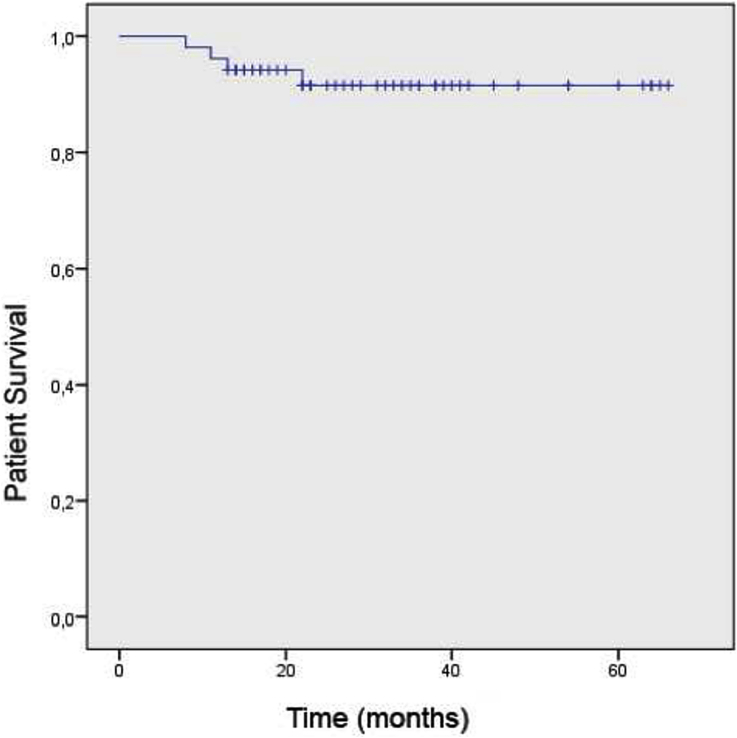
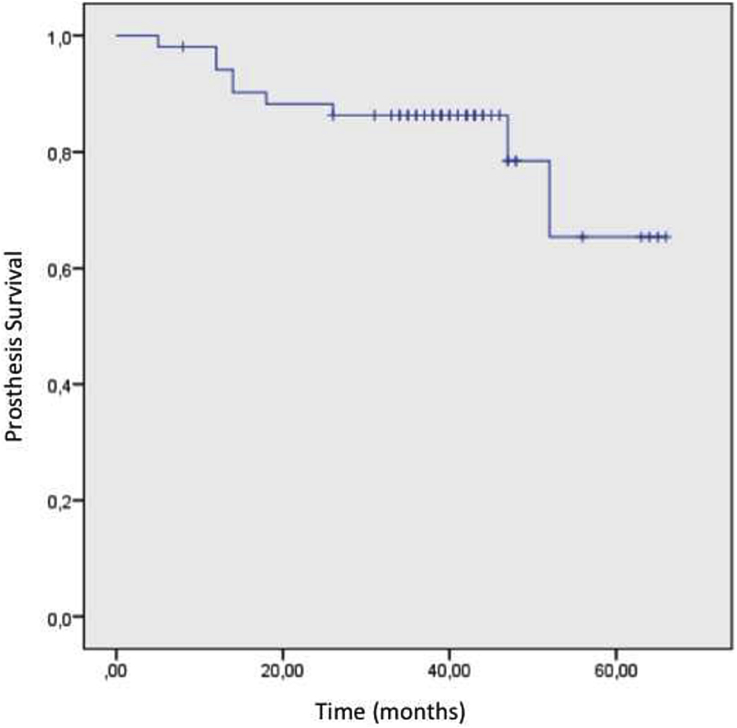
At the final follow-up, 48 patients were alive (92.3%) and implants were free of problems in 43 patients (82.3%). Kaplan–Meier survival analyzes revealed that, 5-year cumulative patient survival rate was 91.5% (Fig. 1). With allowing prosthetic removal as an end-point, 5-year cumulative implant survival rate was 65.4% (Fig. 2). There was no significant difference in the region specific prosthetic survivals (p = 0.332).
Fig. 1.
Kaplan–Meier analysis diagram demonstrating the overall patient survival.
Fig. 2.
Kaplan–Meier analysis diagram demonstrating the implant survival with using prosthesis removal as an end-point.
Oncological outcomes
Histopathologic evaluation have revealed tumor free surgical margins in all of the patients. Despite this, local recurrence or distant metastasis occurred in four patients. There were three patients with local recurrence; an 18 years-old male with osteosarcoma in the distal femur, a 60 years-old male with a lung carcinoma metastasis in the proximal femur and a 61 years-old male with a rectum carcinoma metastases in the distal femur. These patients were dead at the 22nd, 13th and 8th month, respectively, due to heavy metastasis. The fourth patient was a 16 years-old male with Ewing sarcoma in the proximal tibia with lung metastasis. The patient was dead 11 months after surgery due to pulmonary failure.
Complications
There was a total number of 18 complications (34.6 percent). The most common complications were infection (13 percent) and soft-tissue related problems (11 percent). Six patients had soft-tissue related problems such as simple skin necrosis and aseptic wound dehiscence at the early postoperative period, which were all treated with debridement and skin grafting, so did not require revision of the prosthesis. Nine patients (17.3 percent) had failure of reconstruction requiring revision. There was no soft-tissue problem (Type 1 failure) requiring revision. There was no aseptic loosening (Type 2 failure) in any patients. The segmental failure of the prosthesis due to design which was classified as structural (Type 3) failure occured in two patients. One of them had proximal femoral and the other had total femoral reconstruction. These two patients were revised at the first and third months, respectively. No periprosthetic fracture or dislocation occurred in any patients. Seven patients (3 distal femur, 3 proximal tibia and 1 proximal femur) had periprosthetic infection (Type 4 failure) which was successfully managed with two-staged revision surgery. First, prosthesis removed, antibiotic-loaded spacers implemented and proper parenteral antibiotic administrated. After eradication of infection. second stage was performed with modular endoprosthesis. All of these seven patients were infection free at the latest follow up. Local tumor recurrence was observed in three patients (Type 5 failure). Distribution of complications according to the type of reconstruction and diagnosis are summarized in Table 3, Table 4.
Table 3.
Distribution of complications according to type of reconstruction.
| Number | Complications (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Soft-tissue complication | Aseptic loosening | Structural failure | Infection | Tumor progression | Total | |||
| Reconstruction | Proximal femur | 18 | – | – | 1 | 1 | 1 | 3 |
| Total femur | 6 | 1 | – | – | – | – | 1 | |
| Distal femur | 18 | 3 | – | 1 | 3 | 2 | 9 | |
| Proximal tibia | 10 | 2 | – | – | 3 | – | 5 | |
| Overall | 52 | 6 | – | 2 | 7 | 3 | 18 | |
Table 4.
Distribution of the complications according to diagnosis.
| Number | Complications (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Soft-tissue complication | Aseptic loosening | Structural failure | Infection | Tumor progression | Total | |||
| Diagnosis | Osteosarcoma | 17 | 5 | – | – | 4 | 1 | 10 |
| Ewing sarcoma | 7 | – | – | – | 1 | – | 1 | |
| Metastasis | 11 | – | – | 1 | – | 2 | 3 | |
| Giant cell tumor | 5 | – | – | 1 | 1 | 2 | ||
| Chondrosarcoma | 5 | 1 | – | – | 1 | – | 2 | |
| Othersa | 7 | – | – | – | – | – | – | |
| Overall | 52 | 6 | – | 2 | 7 | 3 | 18 | |
Including multiple myeloma, liposarcoma, leiomyosarcoma, synovial sarcoma, neurofibrosarcoma.
Functional outcomes
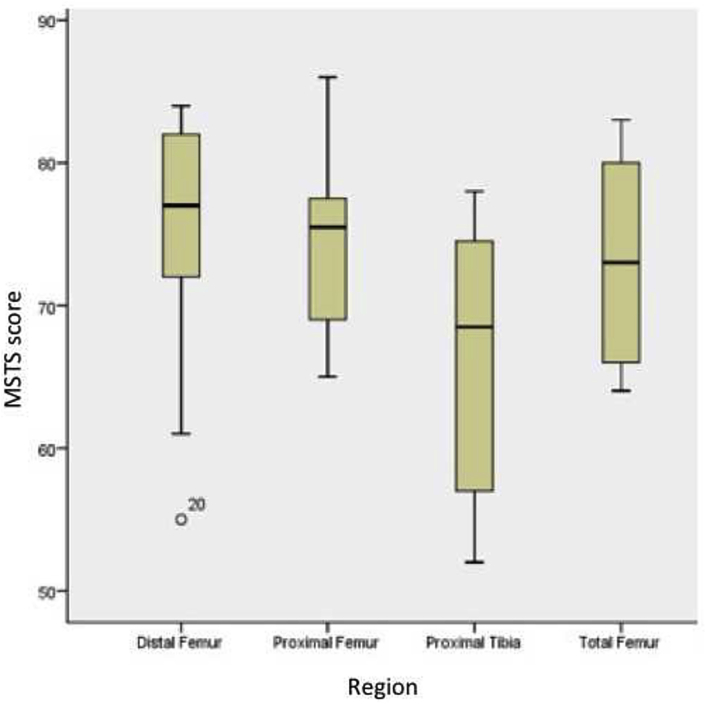
Mean overall MSTS score of the study cohort was 72.7 percent (range, 52 to 86). Functional scores of patients with different anatomical regions are summarized in Fig. 3.
Fig. 3.
MSTS scores according to different anatomical regions. Please note that, average MSTS score in proximal tibial reconstructions is lower than the other anatomical regions.
Discussion
There are several reconstruction options for limb salvage after a tumor resection such as arthrodesis, allografts, reimplanting the autoclaved tumor and endoprosthetic reconstruction.22 Metallic endoprosthesis is one of the most preferred methods, since it does not require healing of the reconstructed segment as in biological reconstructions. Thus, prosthetic replacement promotes a faster return to function.23, 24 Furthermore, component modularity allows reconstruction of massive defects such as total femoral resection.
Previous studies demonstrated favourable outcomes with cementless stem fixation in terms of aseptic loosening, infection and overall prosthesis survival.17, 19, 25 In our series, we also observed the success of cementless stem fixation. At the last follow-up, in 43 of 52 patients, implants were problem-free. Again we did not observe any reconstruction failure associated with aseptic loosening. The new titanium tapered stem with splines may be the cause for success, since this stem has a proven track record in femoral revision of total hip arthroplasty.26
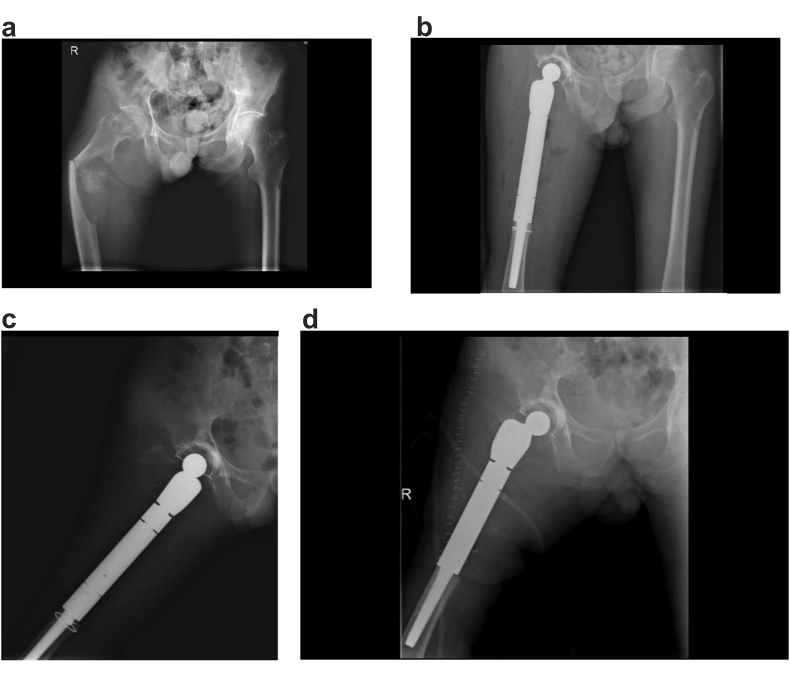
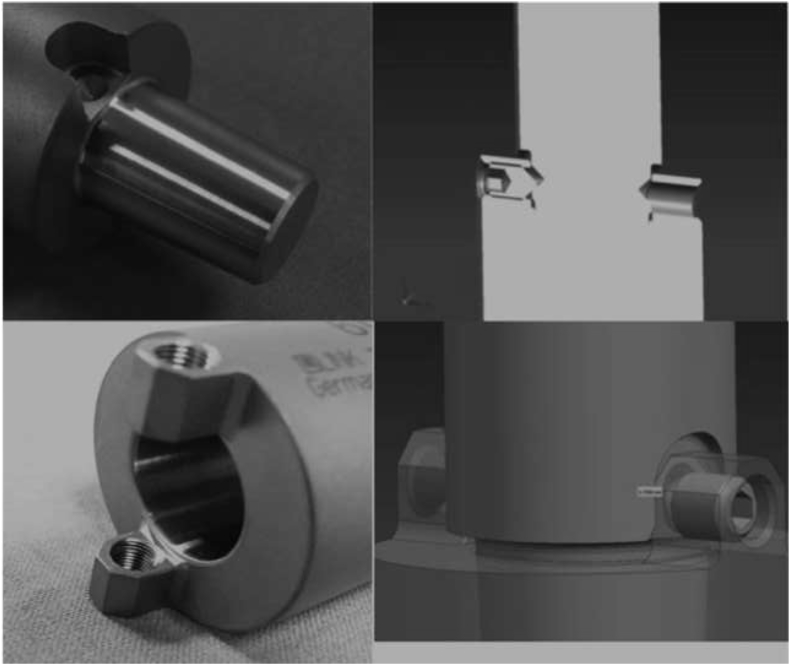
In the early applications of this system, modular segment dislodgement problem occurred in two patients at postoperative first and third months, respectively. In revision surgery, we observed that the problem was in the locking mechanism of the modular stem segments. With ours and others feedback, the locking mechanism and its surgical application technique was modified by the manufacturer.27 In the final version, which is in clinical use now, body segments or the stem are impacted together with a specially designed impaction instrumentation and then using two screws opposite, the joined two segments are locked rigidly (Fig. 4). Since then no another dislodgement in any patients was seen after this modification. This will perhaps improve the outcome in future.
Fig. 4.
A 54 years-old male admitted to our department with a pathological fracture in the right proximal femur due to lung carcinoma metastasis (a). Wide resection and reconstruction with modular cementless tumor resection prosthesis (b). Five months later, he admitted to the emergency department with significant thigh pain. Radiograph demonstrates the rotational instability between prosthetic modules caused by module dislodgement (c). The patient underwent revision surgery (d).
In our series, infection was the most common cause of failure. Infection is a major concern in prosthetic reconstruction after tumoral resections.28, 29, 30 Our infection rate of 13% was comparable with the literature. In a comprehensive study of Henderson et al, an overall infection rate of 8.4 percent in 2174 patients was reported for various anatomical regions.21 The two-staged revision approach was successful in the treatment of infection in all six patients (Fig. 5).
Fig. 5.
In the final version which is in clinical use now, body segments or the stem are impacted together with a specially designed impaction instrumentation and then using two screws opposite, the joined two segments are locked rigidly. After the modification, no segment dislodgement occurred (Courtesy of Waldemar Link GmbH&Co. KG, Hamburg, Germany).
With modern treatment modalities, life expectancy has increased in patients with malignant musculoskeletal tumors. Thus, functional outcomes became more important to provide a better quality of life for the patients in their remaining lifetime. Modular prosthetic replacement after tumor resection offers good functional outcomes.31, 32 In our study, an average MSTS score of 72.7% indicates that, the new generation cementless modular prosthetic system also has favourable functional outcomes. Average MSTS score in proximal tibial reconstructions was lower than the other anatomical regions. In proximal tibial resections, reattachment of patellar tendon probably has negative impacts on the extensor mechanism function. New generation prosthesis systems as the one used in our study have the advantage of better attachment options for the patellar tendon.
The short term follow-up is the major limitation in this study. In addition, lack of homogenity in the study population, especially in terms of diagnosis, chemotherapy or radiotherapy exposure and concomitant soft-tissue reconstruction procedures might have an impact on the results. On the other hand, to our knowledge, this is one of the largest study in the literature of this device.
In conclusion, our preliminary results revealed that, the new generation modular cementless prosthetic system with titanium tapered stem design offers promising clinical and functional outcomes and reasonable complication rates. In addition, with cementless stem technology, the risk of failure associated with aseptic loosening seems to be reduced.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Bacci G., Picci P., Ferrari S. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer. 1993;72:3227–3238. doi: 10.1002/1097-0142(19931201)72:11<3227::aid-cncr2820721116>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Link M.P., Goorin A.M., Miser A.W. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 3.Rougraff B.T., Simon M.A., Greenberg D.B., Mankin H.J. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Jt Surg Am. 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Simon M.A., Aschliman M.A., Thomas N., Mankin H.J. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Jt Surg Am. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 5.Mavrogenis A.F., Abati C.N., Romagnoli C., Ruggieri P. Similar survival but better function for patients after limb salvage versus amputation for distal tibia osteosarcoma. Clin Orthop Relat Res. 2012 Jun;470(6):1735–1748. doi: 10.1007/s11999-011-2238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris J.E., Left A.R., Gitelis S., Simon M.A. Function after amputation, arthodesis or arthrplasty for tumors about the knee. J Bone Jt Surg. 1990;72A:1477–1485. [PubMed] [Google Scholar]
- 7.Horowitz S., Glasser D., Lane J., Healey J. Prosthetic and extremity survivorship in limb salvage: how long do the reconstructions last? Clin Orthop Relat Res. 1993;293:280. [PubMed] [Google Scholar]
- 8.Khattak M.J., Umer M., Haroon-ur-Rasheed, Umar M. Autoclaved tumor bone for reconstruction: an alternative in developing countries. Clin Orthop Relat Res. 2006;447:138–144. doi: 10.1097/01.blo.0000205876.05093.80. [DOI] [PubMed] [Google Scholar]
- 9.Farid Y., Lin P.P., Lewis V.O., Yasko A.W. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. doi: 10.1097/01.blo.0000181491.39048.fe. [DOI] [PubMed] [Google Scholar]
- 10.Malawer M.M., Chou L.B. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Jt Surg Am. 1995;77A:1154–1165. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Roberts P., Chan D., Gimer R.J., Sneath R.S., Scales J.T. Prosthetic replacement of the distal femur for primary bone tumours. J Bone Jt Surg Br. 1991;73B:762. doi: 10.1302/0301-620X.73B5.1894662. [DOI] [PubMed] [Google Scholar]
- 12.Zeegen E.N., Aponte-Tinao L.A., Hornicek F.J., Gebhardt M.C., Mankin H.J. Survivorship analysis of 141 modular metallic endoprostheses at early followup. Clin Orthop Relat Res. 2004;420:239–250. doi: 10.1097/00003086-200403000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Guo W., Ji T., Yang R., Tang X., Yang Y. Endoprosthetic replacement for primary tumours around the knee: experience from Peking University. J Bone Jt Surg Br. 2008;90:1084–1089. doi: 10.1302/0301-620X.90B8.20240. [DOI] [PubMed] [Google Scholar]
- 14.Ward W.G., Johnston K.S., Dorey F.J., Eckardt J.J. Loosening of massive proximal femoral cemented endoprostheses. Radiographic evidence of loosening mechanism. J Arthroplasty. 1997;12:741–750. doi: 10.1016/s0883-5403(97)90003-6. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz A.J., Kabo J.M., Eilber F.C., Eilber F.R., Eckardt J.J. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res. 2010;468(8):2198–2210. doi: 10.1007/s11999-009-1197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unwin P.S., Cannon S.R., Grimer R.J., Kemp H.B.S., Sneath R.S., Walker P.S. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Jt Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]
- 17.Pala E.M.A., Mavrogenis A.F., Angelini A., Henderson E.R., Douglas Letson G., Ruggieri P. Cemented versus cementless endoprostheses for lower limb salvage surgery. J Buon. 2013;18(2):496–503. [PubMed] [Google Scholar]
- 18.Bruns J., Delling G., Gruber H., Lohmann C.H., Habermann C.R. Cementless fixation of megaprostheses using a conical fluted stem in the treatment of bone tumours. J Bone Jt Surg Br. 2007;89(8):1084–1087. doi: 10.1302/0301-620X.89B8.19236. [DOI] [PubMed] [Google Scholar]
- 19.Abraham J.A., Weaver M.J., Ready J.E., Raskin K.A., O'Brien E., Hornicek F.J. Short-term outcomes of cementless modular endoprostheses in lower extremity reconstruction. Curr Orthop Pract. 2012;23:213–217. [Google Scholar]
- 20.Enneking W.F., Dunham W. A system for the functional evaluation of Reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–249. [PubMed] [Google Scholar]
- 21.Henderson E.R., Groundland J.S., Pala E. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Jt Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 22.Simon M.A. Limb salvage for osteosarcoma in the 1980s. Clin Orthop Relat Res. 1991;270:264–270. [PubMed] [Google Scholar]
- 23.Horowitz S.M., Lane J.M., Otis J.C., Healey J.H. Prosthetic arthroplasty of the knee after resection of a sarcoma in the proximal end of the tibia. A report of sixteen cases. J Bone Jt Surg Am. 1991;73:286–293. [PubMed] [Google Scholar]
- 24.Pala E., Trovarelli G., Calabrio T., Angelini A., Abati C.N., Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clin Orthop Relat Res. 2015;473(3):891–899. doi: 10.1007/s11999-014-3699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flint M.N., Griffin A.M., Bell R.S., Ferguson P.C., Wunder J.S. Aseptic loosening is uncommon with uncemented proximal tibia tumor prostheses. Clin Orthop Relat Res. 2006;450:52–59. doi: 10.1097/01.blo.0000229300.67394.77. [DOI] [PubMed] [Google Scholar]
- 26.Regis D., Sandri A., Bonetti I., Braggion M., Bartolozzi P. Femoral revision with the Wagner tapered stem: a ten-to 15-year follow-up study. J Bone Jt J Br. 2011;93(10):1320–1326. doi: 10.1302/0301-620X.93B10.25927. [DOI] [PubMed] [Google Scholar]
- 27.Berbari E.F., Hanssen A.D., Duffy M.C. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 28.Galasso O., Mariconda M., Brando A., Ianno B. Disassembly of a distal femur modular prosthesis after tumor resection. J Arthroplasty. 2010;25(2):334.e5-9. doi: 10.1016/j.arth.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 29.McDonald D.J., Capanna R., Gherlinzoni F. Influence of chemotherapy on perioperative complications in limb salvage surgery for bone tumors. Cancer. 1990;65:1509–1516. doi: 10.1002/1097-0142(19900401)65:7<1509::aid-cncr2820650710>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 30.Mavrogenis A.F., Pala E., Angelini A. Infected prostheses after lower-extremity bone tumor resection: clinical outcomes of 100 patients. Surg Infect (Larchmt) 2015;16(3):267–275. doi: 10.1089/sur.2014.085. [DOI] [PubMed] [Google Scholar]
- 31.Mavrogenis A.F., Mitsiokapa E.A., Sakellariou V.I., Tzanos G., Papagelopoulos P.J. Functional and radiographic outcome after tumor limb salvage surgery using STANMORE megaprostheses. J Buon. 2011 Apr-Jun;16(2):353–360. [PubMed] [Google Scholar]
- 32.Qadir I., Umer M., Baloch N. Functional outcome of limb salvage surgery with mega-endoprosthetic reconstruction for bone tumors. Arch Orthop Trauma Surg. 2012;132(9):1227–1232. doi: 10.1007/s00402-012-1542-3. [DOI] [PubMed] [Google Scholar]