Abstract
Objective
In our study, we aimed to evaluate the influence of training on compliance and persistence with bisphosphonate treatment given on a weekly vs. monthly basis in postmenopausal osteoporosis patients.
Methods
A total of 979 patients with postmenopausal osteoporosis (mean age: 63.2 ± 7.2 years) were included in this national, multicenter, prospective non-interventional observational cohort registry study. Patients were randomized into training (n = 492, 50.3%, mean age: 63.4 ± 7.2 years) and control (n = 487, 49.7%, mean age: 63.0 ± 7.1 years) groups. Patients in each intervention group were given weekly (44.9% and 44.6% for training and control subjects, respectively) or monthly (55.1% and 55.4%, respectively) bisphosphonate regimens. After the initial visit, patients were followed up at three-month intervals throughout 12 months of treatment for evaluation of persistence, compliance and adverse events.
Results
On average, 79.4% of the patients were persistent with the treatment with a mean of 350.4 days of duration during the 12-month follow-up period. The mean compliance in the compliant and fully compliant group remained at an average of 86.6%. No significant difference was detected between the training and control groups in terms of compliance and persistence. Significantly longer persistence (360.0 ± 89.0 vs. 345.0 ± 108.0 days; p = 0.035), higher percentage of persistent patients (83.4% vs. 74.2%; p = 0.012) and higher compliance rates (88.8% vs. 83.3%; p = 0.002) were noted in monthly regimen patients in comparison to those given weekly regimen.
Conclusion
Our findings revealed remarkably high rates for persistence and compliance with bisphosphonate treatment in postmenopausal osteoporosis, with no impact of training on compliance and persistence rates. Longer persistence and better compliance rates were achieved with the monthly bisphosphonate regimen when compared to the weekly regimen.
Keywords: Osteoporosis, Bisphosphonate, Training, Dosing regimen, Compliance, Persistence
Introduction
Due to their efficacy in preserving bone mass and preventing fractures,1 bisphosphonates are commonly prescribed treatments for osteoporosis.2 As with other chronic conditions that are initially asymptomatic,3 failure of osteoporotic patients to follow the treatment prescribed (compliance) for the recommended duration (persistence) has been consistently reported.4, 5, 6
Improved adherence to antiresorptive therapy is more likely to significantly reduce osteoporosis-related fracture risk, use of physicians' services, and hospitalization rates.7 Therefore, several interventions for increasing patients persistence and compliance with bisphosphonate treatments have been developed in recent years, including reducing dosing frequency via new therapeutic options with longer dosing regimens, educational programs, and patient counseling by nurses.1, 4
The present national multicenter prospective non-interventional observational cohort registry study was designed to evaluate the influence of patient training on compliance and persistence with bisphosphonate treatment prescribed on a weekly vs monthly basis in patients with postmenopausal osteoporosis in Turkey.
Patients and methods
A total of 979 patients with postmenopausal osteoporosis (mean [SD] age: 63.2 [7.2] years) were included in this national multicenter prospective non-interventional observational cohort registry study conducted from February 2010 to September 2012 at 34 centers across Turkey. These clinics were selected on the basis of presence of an osteoporosis outpatient clinic and applicable local Standard Operating Procedure for selecting potential investigators and study sites. This study was conducted in 16 cities and 6 geographic regions throughout Turkey and included 22 community based hospitals, 12 university hospitals, and 3 private practice clinics. Of 1010 patients initially screened from 37 centers, 979 patients from 34 centers who met eligibility criteria were enrolled in the study and included in the final analysis. After enrollment, patients were randomized through an Interactive Voice Response system into training (n = 492, 50.3%, mean [SD] age: 63.4 [7.2] years) and control (n = 487, 49.7%, mean [SD] age: 63.0 [7.1] years) groups. Following medical evaluation and at the discretion of the physicians, patients in the training and control groups were prescribed weekly (44.9% and 44.6%, respectively) or monthly (55.1% and 55.4%, respectively) bisphosphonate regimens. Patients were followed up after the initial visit (visit 1, day 0) and then at 3-month intervals over the 12-month course of treatment.
Female patients aged ≥45 and ≤ 75 years who were clinically diagnosed by the investigator with postmenopausal osteoporosis according to WHO criteria and were appropriate for bisphosphonate therapy as defined clinically by the investigator were included in the present study. Patients enrolled in this registry study either were currently being treated for osteoporosis with bisphosphonates or were treatment-naïve at baseline. Patients receiving other osteoporosis treatments such as selective estrogen receptor modulators, hormone replacement therapy, calcitonin, or strontium ranelate, and patients with secondary osteoporosis were excluded from the study.
Written informed consent was obtained from each subject following a detailed explanation of the objectives and protocol of the study, which was conducted in accordance with the ethical principles stated in the Declaration of Helsinki and approved by the Turkish Ministry of Health according to local regulations.
Data on socio-demographic and clinical- and osteoporosis-related history (fractures, bone density measurements, dorsal kyphosis, and previous anti-osteoporosis treatments) were recorded for each patient at baseline. Data on persistence and compliance with treatment as well as study withdrawal were recorded during follow-up visits at months 3, 6, 9, and 12. Adverse event (AE) information was collected upon spontaneous reporting of the physicians. The primary endpoint of the study was the persistence and compliance with treatment based on the information given to the investigator by the patient. The secondary endpoint of the study was the effect of bisphosphonate treatment on withdrawals from the study due to AEs.
At the baseline visit, patients randomized to the training group were supplied with a “Training Kit,” including 4 training booklets (General Information on Osteoporosis, Osteoporosis and Exercise, Osteoporosis and Nutrition, Osteoporosis and Patient Rights) containing information on the disease, prepared by the Osteoporosis Patient Society of Turkey. During 12-month follow-up, 4 telephone calls (at months 2, 5, 8, and 11 of treatment) and 4 individual face-to-face interactive/educational meetings (at months 3, 6, 9, and 12 of treatment, covering awareness about osteoporosis, risk factors of osteoporosis, fractures, prevention, and treatment of osteoporosis) were conducted. During the telephone calls, patients were reminded to read the booklets, informed of the topic to be covered in the next educational meeting, and invited to the meeting. Patient education in the control group was implemented by physicians, as per routine clinical practice, without supplying training booklets.
Persistence was defined as the time from initiation to discontinuation of bisphosphonate treatment and measured as the number of days from the date of the index prescription to the theoretical end of the last prescription issued during the follow-up period. Women were considered to have discontinued treatment if the gap between the end of one prescription and the start of another was >30 days. The proportion of women who persisted with treatment for 12 months was also determined.
Rate of compliance was defined as the proportion of days within the follow-up period for which patients were given a prescription for bisphosphonate. Patients were classified by their treating doctors at each visit as non-compliant (0–50% drug intake), partially compliant (50% drug intake), compliant (75% drug intake) and fully compliant (100% drug intake).
Assuming adherence to be improved via patient training and a treatment persistence rate of 70% in the trained patient group and 60% in the control group, with a power of [1 – beta] 90% and a 2-way error (alpha) estimated at 5%, sample size calculation revealed at least 476 patients were required for each group. Considering possible patient loss to follow-up, 500 patients were sought for each arm.
Data analyses were performed using Stata 10 software (StataCorp LP, College Station, TX, USA). Chi-square (χ2) test was used for the comparison of categorical data, and Mann–Whitney U test and Student's t-test were used for numerical data. Additionally, Kaplan–Meier curves were plotted for treatment persistence, and the effects of training on treatment compliance and persistence were evaluated by multivariate analysis. Due to the fact that this was a non-interventional disease registry study, patients lost to follow-up for any reason were considered as discontinued from the study. Missing data for these patients on the corresponding visits were not analyzed. Data were expressed as mean (SD), minimum–maximum, and percent (%) where appropriate. Statistical significance was considered as p < 0.05.
Results
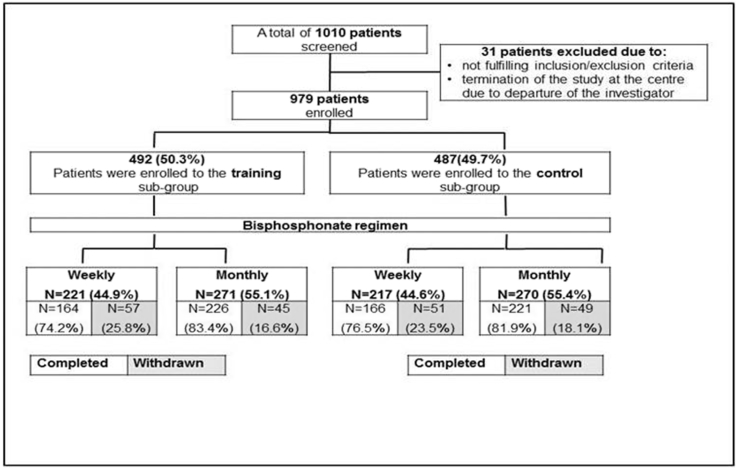
In the training group, 164 of 221 patients (74.2%) on a weekly regimen and 226 of 271 patients (83.4%) on a monthly regimen completed the study. In the control group, 166 of 217 patients (76.5%) on a weekly regimen and 221 of 270 patients (81.0%) on a monthly regimen completed the study (Fig. 1).
Fig. 1.
Patient disposition and rates for study completion and withdrawal.
Baseline general and osteoporosis-related characteristics are presented in Table 1 and Table 2, respectively.
Table 1.
Socio-demographic and baseline characteristics.
| Training (n = 492) |
Control (n = 487) |
Total (n = 979) |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age (years) | 63.4 (7.2) | 63.0 (7.1) | 63.2 (7.2) | |||
| Age for menopause | 46.4 (5.5) | 46.0 (5.9) | 46.2 (5.7) | |||
| Duration of menopause | 17.0 (8.2) | 17.1 (8.5) | 17.0 (8.4) | |||
| Age groups (years) | N (%) |
N (%) |
N (%) |
|||
| 45–55 | 81 (16.5) | 84 (17.2) | 165 (16.9) | |||
| 56–65 | 202 (41.1) | 208 (42.7) | 410 (41.9) | |||
| 66–75 | 209 (42.5) | 195 (40.0) | 404 (41.3) | |||
| Educational level | N (%) |
N (%) |
N (%) |
|||
| Illiterate | 113 (23.0) | 129 (26.5) | 242 (24.7) | |||
| Primary | 297 (60.4) | 269 (55.2) | 566 (57.8) | |||
| High school | 52 (10.6) | 44 (9.0) | 96 (9.8) | |||
| University | 30 (6.1) | 45 (9.2) | 75 (7.7) | |||
| Comorbid diseases | 340 (69.1) | 341 (70.0) | 681 (69.6) | |||
| Diabetes Mellitus | 63 (18.5) | 71 (20.8) | 134 (19.7) | |||
| Hypertension | 241 (70.9) | 227 (66.6) | 468 (68.7) | |||
| Hyperlipidemia | 118 (34.7) | 121 (35.5) | 239 (35.1) | |||
| Heart failure | 20 (5.9) | 13 (3.8) | 33 (4.8) | |||
| COPD | 11 (3.2) | 9 (2.6) | 20 (2.9) | |||
| Bronchial Asthma | 26 (7.6) | 29 (8.5) | 55 (8.1) | |||
| Genetic Diseases | 1 (0.3) | 0 (0.0) | 1 (0.1) | |||
| Allergy | 10 (2.9) | 9 (2.6) | 19 (2.8) | |||
| Past history of surgical operation | 260 (52.9) | 273 (56.1) | 533 (54.4) | |||
| Duration of comorbidity (years) | N |
Mean (SD) |
N |
Mean (SD) |
N |
Mean (SD) |
| Hypertension | 162 | 8.0 (6.3) | 160 | 8.0 (7.0) | 322 | 8.0 (6.6) |
| Hyperlipidemia | 52 | 5.6 (4.9) | 58 | 5.4 (4.9) | 110 | 5.4 (4.9) |
| Diabetes mellitus | 41 | 7.8 (8.0) | 43 | 7.5 (5.3) | 84 | 7.6 (6.7) |
| Vital signs | N |
Mean (SD) |
N |
Mean (SD) |
N |
Mean (SD) |
| SBP (mmHg) | 459 | 128.0 (17.0) | 442 | 127 (17) | 127.9 (16.7) | |
| DBP(mmHg) | 459 | 80.0 (10.0) | 442 | 80 (10) | 79.8 (10.2) | |
| Pulse (bpm) | 450 | 77.0 (7.0) | 438 | 77 (7) | 76.9 (7.3) | |
| Height (cm) | 469 | 156.0 (6.0) | 471 | 156 (6) | 156.0 (6.3) | |
| Weight (kg) | 469 | 68.0 (11.4) | 471 | 67.9 (12.0) | 67.9 (11.7) | |
| Life style characteristics | N (%) |
N (%) |
N (%) |
|||
| Coffee consumption (>3 cups/day) | 16 (3.3) | 9 (1.8) | 25 (2.6) | |||
| Cola consumption | 7 (1.4) | 6 (1.2) | 13 (1.3) | |||
| (>3 glasses/day) Tea consumption (>3 glasses/day) | 257 (52.2) | 251 (51.5) | 508 (51.9) | |||
| Alcohol consumption | 16 (3.3) | 19 (3.9) | 35 (3.6) | |||
| Smoking | 68 (13.8) | 53 (10.9) | 121 (12.4) | |||
| <1 package/day | 37 (54.4) | 37 (69.8) | 74 (61.2) | |||
| 1 package/day | 29 (42.6) | 15 (28.3) | 44 (36.4) | |||
| 2 packages/day | 2 (2.9) | 1 (1.9) | 3 (2.5) | |||
| Regular consumption of milk | 119 (24.2) | 118 (24.2) | 237 (24.2) | |||
| Regular consumption of milk products | 328 (66.7) | 301 (61.8) | 629 (64.2) | |||
| Regular exercise | 121 (24.6) | 117 (24.0) | 238 (24.3) | |||
| Concomitant treatments | 313 (63.6) | 301 (61.8) | 614 (62.7) | |||
| Antihypertensive drugs | 218 (69.7) | 197 (65.5) | 415 (67.6) | |||
| NSAIDs | 30 (9.6) | 33 (11.0) | 63 (10.3) | |||
| Lipid lowering drugs | 77 (24.6) | 79 (26.3) | 156 (25.4) | |||
| Antidepressant drugs | 31 (9.9) | 29 (9.6) | 60 (9.8) | |||
| Other | 104 (33.2) | 93 (30.9) | 197 (32.1) | |||
Table 2.
Osteoporosis-related baseline characteristics and study treatment.
| Training (n = 492) |
Control (n = 487) |
Total (n = 979) |
||||
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||||
| Past history of osteoporosis related fracture | 99 (20.1) | 103 (21.1) | 202 (20.6) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Fracture number | 1.3 (0.9) | 1.4 (0.8) | 1.4 (0.8) | |||
| Time since fracture (years) | 5.8 (6.5) | 4.5 (5.2) | 5.1 (5.9) | |||
| n (%) |
n (%) |
n (%) |
||||
| Compression fracture by radiography | 74 (15.0) | 79 (16.2) | 153 (15.6) | |||
| D1-D6 | 7 (9.5) | 14 (17.7) | 21 (13.8) | |||
| D7 | 13 (17.6) | 12 (15.2) | 25 (16.3) | |||
| D8 | 11 (14.9) | 10 (12.7) | 21 (13.7) | |||
| D9 | 10 (13.5) | 13 (16.5) | 23 (15.0) | |||
| D10 | 14 (18.9) | 6 (7.6) | 20 (13.1) | |||
| D11 | 12 (16.2) | 22 (27.9) | 34 (22.2) | |||
| D12 | 11 (14.9) | 22 (27.9) | 33 (21.6) | |||
| L1 | 12 (16.2) | 16 (20.3) | 28 (18.3) | |||
| L2 | 12 (16.2) | 10 (12.7) | 22 (14.4) | |||
| L3 | 8 (10.8) | 10 (12.7) | 18 (11.8) | |||
| L4 | 6 (8.1) | 12 (15.2) | 18 (11.8) | |||
| L5 | 7 (9.5) | 12 (15.2) | 19 (12.4) | |||
| Increase in dorsal kyphosis | 64 (16.1) | 62 (15.5) | 126 (15.8) | |||
| Bone density scan (DEXA) | 469 (95.3) | 471 (96.7) | 940 (96.0) | |||
| Hologic | 230 (49.0) | 200 (42.5) | 430 (45.7) | |||
| Lunar | 162 (34.5) | 181 (38.4) | 343 (36.5) | |||
| Other | 77 (16.4) | 90 (19.1) | 167 (17.8) | |||
| Previous anti-osteoporosis treatment | 341 (69.3) | 331 (68.0) | 672 (68.6) | |||
| Anti-osteoporotic agent (AOA) | 288 (84.5) | 286 (86.4) | 574 (85.4) | |||
| Exercise + Diet + AOA | 24 (7.0) | 17 (5.1) | 41 (6.1) | |||
| Exercise + AOA | 12 (3.5) | 11 (3.3) | 23 (3.4) | |||
| Diet + AOA | 13 (3.8) | 9 (2.7) | 22 (3.3) | |||
| Exercise + Diet | 1 (0.3) | 5 (1.5) | 6 (0.9) | |||
| Diet only | 2 (0.6) | 2 (0.6) | 4 (0.6) | |||
| Exercise only | 1 (0.3) | 1 (0.3) | 2 (0.3) | |||
| Anti-osteoporotic agents | n (%) |
n (%) |
n (%) |
|||
| Alendronat | 162 (27.6) | 155 (28.5) | 317 (28.0) | |||
| Calcium + Vitamin D | 167 (28.4) | 142 (26.1) | 309 (27.3) | |||
| Risedronat | 107 (18.2) | 95 (17.5) | 202 (17.8) | |||
| Calcitonin | 57 (9.7) | 58 (10.7) | 115 (10.2) | |||
| Ibandronat | 34 (5.8) | 35 (6.4) | 69 (6.1) | |||
| Strontium Ranelate | 28 (4.8) | 25 (4.6) | 53 (4.7) | |||
| SERM | 24 (4.1) | 23 (4.3) | 47 (4.2) | |||
| Others | 9 (1.5) | 11 (2.1) | 20 (1.8) | |||
| Total | 588 (100.0) | 544 (100.0) | 1.132 (100.0) | |||
| Duration of anti-osteoporotic treatment (years) | N |
Mean (SD) |
N |
Mean (SD) |
N |
Mean (SD) |
| Alendronat | 158 | 2.5 (2.4) | 151 | 2.3 (2.0) | 309 | 2.4 (0.2) |
| Calcium + Vitamin D | 160 | 2.9 (3.6) | 135 | 2.8 (2.3) | 295 | 2.8 (3.1) |
| Risedronat | 107 | 2.1 (1.7) | 93 | 2.0 (1.5) | 200 | 2.0 (1.6) |
| Calcitonin | 57 | 1.8 (1.2) | 56 | 1.7 (1.6) | 113 | 1.7 (0.4) |
| Ibandronat | 33 | 1.2 (0.8) | 34 | 1.5 (1.3) | 67 | 1.4 (1.1) |
| Strontium Ranelate | 27 | 1.6 (1.6) | 24 | 1.4 (0.9) | 51 | 1.5 (1.3) |
| SERM | 17 | 2.1 (1.4) | 20 | 1.5 (1.1) | 37 | 1.8 (1.3) |
| Study treatment regimen | N (%) |
N (%) |
N (%) |
|||
| Weekly biphosphonate | 221 (44.9) | 217 (44.6) | 438 (44.7) | |||
| Alendronate 70 mg | 124 (56.1) | 122 (56.2) | 246 (56.2) | |||
| Risedronate 35 mg | 97 (43.9) | 95 (43.8) | 192 (43.8) | |||
| Monthly biphosphonate | 271 (55.1) | 270 (55.4) | 541 (55.3) | |||
| Ibandronate 150 mg | 106 (39.1) | 104 (38.5) | 210 (38.8) | |||
| Risedronate 75 mg | 116 (42.8) | 101 (37.4) | 217 (40.1) | |||
| Risedronate 150 mg | 49 (18.1) | 65 (24.1) | 114 (21.1) | |||
For their bisphosphonate regimen, 56.2% of patients received 70 mg alendronate, and 43.8% received 35 mg risedronate on a weekly basis; 38.8% of patients received 150 mg ibandronate, 40.1% received 75 mg risedronate, and 21.1% received 150 mg risedronate on a monthly basis (Table 2).
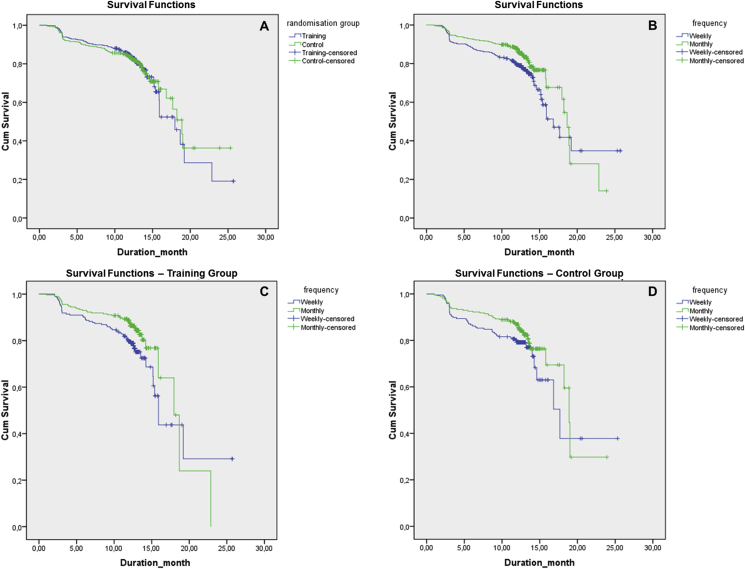
The percentage of persistent patients was 79.3% for the training group and 79.5% for the control group. Overall duration of treatment persistence was 353 (97.8) days, with no significant difference between training and control groups (Table 3), as also shown via Kaplan–Meier analysis (p = 0.971, Log-rank Mantel–Cox evaluation) (Fig. 2a).
Table 3.
Treatment persistence for training and control groups in relation to weekly or monthly regimen.
| Treatment persistence | Training (n = 492) | Control (n = 487) | Total (979) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Duration (days) | N | Mean (SD) | p value | N | Mean (SD) | p value | N | Mean (SD) | p value1 |
| Overall | 492 | 355.5 (94.6) | – | 487 | 350.4 (101.1) | – | 979 | 353.0 (97.8) | 0.363 |
| Weekly regimen | 221 | 350.0 (106.0) | 0.111 | 217 | 340 (109.0) | 0.172 | 438 | 345.0 (108.0) | 0.035 |
| Monthly regimen |
271 |
360.0 (84.0) |
270 |
359 (94.0) |
541 |
360.0 (89.0) |
|||
| Left the study |
Ongoing/Completed |
p value |
Left the study |
Ongoing/Completed |
p value |
Left the study |
Ongoing/Completed |
p value |
|
| 3rd month | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Overall | 64 (13.0) | 428 (87.0) | – | 58 (11.9) | 429 (88.1) | – | 122 (12.5) | 857 (87.5) | 0.565 |
| Weekly regimen | 38 (17.2) | 183 (82.8) | 0.016 | 27 (12.4) | 190 (87.6) | 0.836 | 65 (14.8) | 373 (85.2) | 0.058 |
| Monthly regimen | 17 (9.6) | 245 (90.4) | 31 (11.4) | 239 (88.5) | 57 (10.6) | 484 (89.4) | |||
| 6th month | |||||||||
| Overall | 93 (18.9) | 399 (81.1) | – | 90 (18.5) | 397 (81.5) | – | 183 (18.7) | 796 (81.3) | 0.744 |
| Weekly regimen | 50 (22.6) | 171 (77.4) | 0.082 | 39 (18.0) | 178 (82.0) | 0.647 | 89 (20.3) | 349 (79.7) | 0.351 |
| Monthly regimen | 43 (15.9) | 228 (84.1) | 51 (18.9) | 219 (81.1) | 94 (17.4) | 447 (82.6) | |||
| 9th month | |||||||||
| Overall | 109 (22.2) | 383 (77.8) | – | 116 (23.8) | 371 (76.2) | – | 225 (23.0) | 754 (77.0) | 0.583 |
| Weekly regimen | 51 (23.1) | 170 (76.9) | 0.957 | 54 (24.9) | 163 (75.1) | 0.929 | 105 (23.9) | 333 (76.1) | 0.921 |
| Monthly regimen | 58 (21.4) | 213 (78.6) | 62 (23.0) | 208 (77.0) | 120 (22.2) | 421 (77.8) | |||
| 12th month | 102 (20.7) | 390 (79.3) | – | 100 (20.5) | 387 (79.5) | – | 202 (20.6) | 777 (79.4) | 0.939 |
| Weekly regimen | 57 (25.8) | 164 (74.2) | 0.012 | 51 (23.5) | 166 (76.5) | 0.146 | 108 (24.7) | 330 (75.3) | 0.005 |
| Monthly regimen | 45 (16.6) | 226 (83.4) | 49 (18.1) | 221 (81.9) | 94 (17.4) | 447 (82.6) | |||
Mann–Whitney U test 1 training vs. control.
Values in bold indicate statistical significance (p < 0.05).
Fig. 2.
Treatment persistence: a) in training vs control groups, b) in weekly vs monthly treatment regimen in the overall population, c) in weekly vs monthly treatment regimen in the training group, d) in weekly vs monthly treatment regimen in the control group. Log-rank Mantel–Cox test of equality of survival distributions for the different levels of frequency: = 0.001, df = 1, p = 0.971; = 7.267, df = 1, p = 0.007; = 4.695, df = 1, p = 0.030; = 2.697, df = 1, p = 0.101.
Duration of treatment persistence was significantly longer in patients on a monthly rather than weekly bisphosphonate regimen in the overall study population (360.0 [89.0] vs 345.0 [108.0] days, respectively; p = 0.035) (Table 3), as also confirmed by Kaplan–Meier analysis (18.7 [0.4] vs 16.8 [1.0] months, respectively; p = 0.007, Log-rank Mantel–Cox) (Fig. 2b).
Persistence rate was significantly higher in patients on a monthly rather than weekly bisphosphonate regimen at 12 months post-enrollment (82.6 vs 75.3%, p = 0.005) in the overall population, at 3-month follow-up (90.4 vs 82.8%, p = 0016) and 12-month follow-up (83.4 vs 74.2%, p = 0.012) in the training group, while similar rates for persistence were noted with respect to frequency of treatment regimen in the control group (Table 3). Kaplan–Meier analysis also indicated higher persistence with a monthly rather than weekly regimen (weekly: 15.9 [0.4]; monthly: 17.9 [1.2]; p = 0.030, Log-rank Mantel–Cox) in the training group (Fig. 2c), while no difference was found in the control group (Fig. 2d).
Training group patients were significantly more compliant with their treatments than controls at 3-month (47.2% vs 40%, p = 0.021) and 6-month (47.3% vs 37.6%, p = 0.003) follow-up visits, while no significance was shown at later visits. During the course of the study, the pooled compliant and fully compliant groups constituted an average 86.6% in the overall population, with no significant difference between training (87.3%) and control (86.0%) groups. Overall, a monthly regimen was associated with higher compliance rates at 6-month (p = 0.038), 9-month (p = 0.012), and 12-month (p = 0.002) follow-up visits. Patients with monthly treatment regimens, allocated to either the training or control groups, were more compliant when compared to weekly regimens only at 12-month follow-up (p = 0.020 and p = 0.038, respectively) (Table 4).
Table 4.
Compliance with treatment in training and control groups and with respect to weekly or monthly bisphosphonate regimen.
| According to randomization, n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compliance with treatment | 3rd month | 6th month | 9th month | 12th month | ||||||||
| Training (n = 434) | Control (n = 435) | Total (n = 869) | Training (n = 402) | Control (n = 402) | Total (n = 804) | Training (n = 388) | Control (n = 375) | Total (n = 763) | Training (n = 401) | Control (n = 392) | Total (n = 793) | |
| Non-compliant | 7 (1.6) | 13 (3.0) | 20 (2.3) | 5 (1.2) | 9 (2.2) | 14 (1.7) | 12 (3.1) | 6 (1.6) | 18 (2.4) | 12 (3.0) | 5 (1.3) | 17 (2.1) |
| Partially compliant | 50 (11.5) | 60 (13.8) | 110 (12.7) | 41 (10.2) | 56 (13.9) | 97 (12.1) | 34 (8.8) | 44 (11.7) | 78 (10.2) | 39 (9.7) | 50 (12.8) | 89 (11.2) |
| Compliant | 172 (39.6) | 188 (43.2) | 360 (41.4) | 166 (41.3) | 186 (46.3) | 352 (43.8) | 163 (42.0) | 167 (44.5) | 330 (43.3) | 156 (38.9) | 169 (43.1) | 325 (41.0) |
| Fully compliant | 205 (47.2) | 174 (40.0) | 379 (43.6) | 190 (47.3) | 151 (37.6) | 341 (42.4) | 179 (46.1) | 158 (42.1) | 337 (44.2) | 194 (48.4) | 168 (42.9) | 362 (45.6) |
| p valuea |
0.021 |
0.003 |
0.283 |
0.162 |
||||||||
| According to bisphosphonate regimen, n (%) | ||||||||||||
| Compliance with treatment | 3rd month | 6th month | 9th month | 12th month | ||||||||
| Weekly (n = 187) | Monthly (n = 247) | Total (n = 434) | Weekly (n = 172) | Monthly (n = 230) | Total (n = 402) | Weekly (n = 174) | Monthly (n = 214) | Total (n = 388) | Weekly (n = 170) | Monthly (n = 231) | Total (n = 401) | |
| Training group | ||||||||||||
| Non-compliant | 4 (2.1) | 3 (1.2) | 7 (1.6) | 3 (1.7) | 2 (0.9) | 5 (1.2) | 7 (4.0) | 5 (2.3) | 12 (3.1) | 6 (3.5) | 6 (2.6) | 12 (3.0) |
| Partially compliant | 25 (13.4) | 25 (10.1) | 50 (11.5) | 21 (12.2) | 20 (8.7) | 41 (10.2) | 22 (12.6) | 12 (5.6) | 34 (8.8) | 21 (12.4) | 18 (7.8) | 39 (9.7) |
| Compliant | 75 (40.1) | 97 (39.3) | 172 (39.6) | 72 (41.9) | 94 (40.9) | 166 (41.3) | 71 (40.8) | 92 (43.0) | 163 (42.0) | 70 (41.2) | 86 (37.2) | 156 (38.9) |
| Fully compliant | 83 (44.4) | 122 (49.4) | 205 (47.2) | 76 (44.2) | 114 (49.6) | 190 (47.3) | 74 (42.5) | 105 (49.1) | 179 (46.1) | 73 (42.9) | 121 (52.4) | 194 (48.4) |
| p valuea | 0.196 | 0.178 | 0.053 | 0.038 | ||||||||
| Control group | Weekly (n = 194) | Monthly (n = 241) | Total (n = 435) | Weekly (n = 178) | Monthly (n = 224) | Total (n = 402) | Weekly (n = 167) | Monthly (n = 208) | Total (n = 375) | Weekly (n = 168) | Monthly (n = 224) | Total (n = 392) |
| Non-compliant | 7 (3.6) | 6 (2.5) | 13 (3.0) | 4 (2.2) | 5 (2.2) | 9 (2.2) | 3 (1.8) | 3 (1.4) | 6 (1.6) | 3 (1.8) | 2 (0.9) | 5 (1.3) |
| Partially compliant | 22 (11.3) | 38 (15.8) | 60 (13.8) | 27 (15.2) | 29 (12.9) | 56 (13.9) | 22 (13.2) | 22 (10.6) | 44 (11.7) | 25 (14.9) | 25 (11.2) | 50 (12.8) |
| Compliant | 93 (47.9) | 95 (39.4) | 188 (43.2) | 88 (49.4) | 98 (43.8) | 186 (46.3) | 79 (47.3) | 88 (42.3) | 167 (44.5) | 79 (47.0) | 90 (40.2) | 169 (43.1) |
| Fully compliant | 72 (37.1) | 102 (42.3) | 174 (40.0) | 59 (33.1) | 92 (41.1) | 151 (37.6) | 63 (37.7) | 95 (45.7) | 158 (42.1) | 61 (36.3) | 107 (47.8) | 168 (42.9) |
| p valuea | 0.608 | 0.134 | 0.117 | 0.020 | ||||||||
| Overall | Weekly (n = 381) | Monthly (n = 488) | Total (n = 869) | Weekly (n = 350) | Monthly (n = 454) | Total (n = 804) | Weekly (n = 341) | Monthly (n = 442) | Total (n = 763) | Weekly (n = 338) | Monthly (n = 455) | Total (n = 793) |
| Non-compliant | 11 (2.9) | 9 (1.8) | 20 (2.3) | 7 (2.0) | 7 (1.5) | 14 (1.7) | 10 (2.9) | 8 (1.9) | 18 (2.4) | 9 (2.7) | 8 (1.8) | 17 (2.1) |
| Partially compliant | 47 (12.3) | 63 (12.9) | 110 (12.7) | 48 (13.7) | 49 (10.8) | 97 (12.1) | 44 (12.9) | 34 (8.1) | 78 (10.2) | 46 (13.6) | 43 (9.5) | 89 (11.2) |
| Compliant | 168 (44.1) | 192 (39.3) | 360 (41.4) | 160 (45.7) | 192 (42.3) | 352 (43.8) | 150 (44.0) | 180 (42.7) | 330 (43.3) | 149(44.1) | 176 (38.7) | 325 (41.0) |
| Fully compliant | 155 (40.7) | 224 (45.9) | 379 (43.6) | 135 (38.6) | 206 (45.4) | 341 (42.4) | 137 (40.2) | 200 (47.4) | 337 (44.2) | 134 (39.6) | 228 (50.1) | 362 (45.6) |
| p valuea | 0.181 | 0.038 | 0.012 | 0.002 | ||||||||
Non-compliant:0–50% drug intake, partially compliant: 50% drug intake, compliant:75% drug intake; Fully compliant:100% drug intake.
Values in bold indicate statistical significance (p < 0.05).
Mann–Whitney U test.
Among recorded withdrawals in the training (n = 30) and control (n = 26) groups, the most common cause was non-reimbursement of the cost of bisphosphonates by government health plan on the basis of regular DXA evaluations (18/102 training group, 10/100 control group). Patients who could not obtain their refills were not able to continue the study. In 12 cases, study discontinuation was related to AEs.
AEs in the training group (n = 7) were mostly gastrointestinal symptoms (nausea, n = 6). One death of a 62-year-old female who had cardiac insufficiency, diabetes mellitus, and hypertension was reported, but cause of death and causality with the study drug was not specified by the physician. In the control group, reported AEs (n = 5) were myocardial infarction (n = 1), nausea due to other medical conditions (n = 2), chest pain (n = 1), and death (n = 1, due to myocardial infarction unrelated to study medication).
Discussion
Our findings revealed no difference between training and control groups in terms of treatment compliance and persistence rates in postmenopausal osteoporosis patients during a 12-month follow-up period. A monthly bisphosphonate regimen was associated with significantly longer persistence, higher percentage of persistent patients, and higher compliance when compared to a weekly regimen in the overall population. A significantly higher percentage of persistent patients in the training group was observed only with those on the monthly regimen. Both monthly and weekly treatment regimens were well tolerated, with completion of study by 74.2% of training and 76.5% of control patients on the weekly regimen, and 83.4% of training and 81.0% of control patients on the monthly regimen.
Although bisphosphonates are the preferred therapy for preventing and treating osteoporosis,8 their effectiveness is severely compromised by poor adherence,3, 9 with reported failure to comply with treatment or discontinuation of treatment in 50%–75% of patients within 12 months of commencement.1, 4
In a prior review of 14 databases in the USA, Canada, France, and the UK, compliance with bisphosphonates was reported to range from 59% to 81%, with persistence rates of 18%–78% at 1-year follow-up.10 A large American prescription database study reported 1-year rate of persistence with bisphosphonates to range from 30% to 51%.11
The percentage of persistent patients in the present cohort was 79.4% overall, 79.3% in the training, and 79.5% in the control groups, with an average of 353.0, 355.5, and 350.4 days, respectively, of duration during 12-month follow-up. During the course of the study, the pooled compliant and fully compliant group remained an average 86.3% of the study population, with compliance rates of 86.6% in the overall population, 87.3% in the training, and 86.0% in the control groups at 12-month follow-up.
Data from randomized clinical trials and analyses of large databases may be associated with falsely elevated persistence rates, and lower rates are thus expected in real-life clinical practice.12 Hence, the rate of adherence in the present study population seems promisingly high, despite the fact that our study was designed to reflect everyday clinical practice with limited follow-up visits, in accordance with the primary care setting.
Furthermore, given that 81% of patients in both training and control regimens were persistent at the 6-month follow-up visit in our study, rate of adherence with bisphosphonate regimens in our study population were also higher than rates reported in the PERSIST (PERsistence Study of Ibandronate verSus alendronaTe) study (56.6%),12 which was similarly designed.
However, while data from PERSIST showed that persistence was significantly higher in the antiosteoporotic treatment (monthly ibandronate) plus patient support program group compared with the antiosteoporotic treatment only (weekly alendronate) group,12 our findings revealed no significant impact of patient training on persistence and compliance rates, whereas significantly better persistence and compliance was found in the monthly regimen rather than weekly regimen bisphosphonate treatment groups.
Data from another education-related interventions also revealed that a patient support program with use of automated phone calls and letters to inform the patients increased the number of patients starting on osteoporosis medication, whereas adherence was not significantly different from the control group after 10 months of follow-up.13, 14 Similarly, distributing an educational leaflet about osteoporosis during initiation of treatment was reported not to improve adherence to therapy when compared with routine practice.15 Consistent with the concept that reducing the complexity and frequency of dosing regimens improves adherence and persistence with bisphosphonates in patients with osteoporosis,16, 17 our findings revealed that monthly bisphosphonate regimen was associated with significantly longer persistence, higher percentage of persistent patients, and higher compliance than weekly regimen in the overall population, along with significantly higher percentage of persistent patients in the training group.
Indeed, better adherence in our patients treated with a monthly rather than weekly regimen may also be associated with patient preferences for the more convenient dosing regimen and thereby the likelihood of physicians to focus more on monthly dosing and to provide appropriate counseling for this treatment modality.7
Improvement in compliance and attaining persistence for ≥6 months of bisphosphonate therapy were reported to be associated with significant reduction in fracture risk by 51% and 28%, respectively.9 Given that improving adherence may have a greater impact than improved drug efficacy on patient health outcomes,18 switching from a weekly to monthly bisphosphonate regimen seems to offer a useful strategy for improving adherence and thus long-term fracture prevention in Turkish patients receiving long-term bisphosphonate therapy who are having difficulty complying with daily or weekly dosage regimens.
It has been suggested that highly adherent patients engage in more health-oriented behaviors such as following lifestyle recommendations for avoiding fracture, including exercise, correction of eyesight, and organization of the home environment to minimize the risk of falls, which contribute to a reduction in fracture risk.9 Therefore, although no significant impact of training on compliance and persistence was observed with bisphosphonate regimens in our study population within the 12-month follow-up period, it would have been difficult to improve on the already remarkably high adherence rates, along with higher awareness, of trained patients on critical points related to osteoporosis. Training seems to be associated with longer-term benefits that extend beyond 12 months.
In the present study population, only 5.4% of the patients withdrew from the study due to AEs. Both regimens were well tolerated, with gastrointestinal disorders being the most commonly reported AEs, consistent with previous reports in the literature.6, 11, 12, 19, 20, 21 The foremost limitation of the present study is the observational design, which depended on voluntary patient information rather than use of strict monitoring devices to determine compliance. Nevertheless, despite the limitation of the observational design, our findings provide data in a large representative sample of Turkish individuals with osteoporosis. Collection of data based on follow-up visits every 3 months may be another limitation, given that it may have caused better adherence to treatment during the study period, causing the results to be confounded by the subjects' awareness of being monitored. Our study population consisted of both treatment-naïve patients and patients who were currently receiving the drug regimen. Although this may be associated with the likelihood of former training and compliance levels with previous prescriptions to impact study findings, inclusion criteria for the study population were decided to reflect the patient profile encountered in actual clinical practice, in which treatment-naïve patients are infrequent in the overall osteoporosis patient population. Furthermore, treatment-naïve and treated patients were equally allocated to study groups during randomization. Lack of adequate previous patient training is also a problem prevalent among patients who are currently receiving anti-osteoporosis treatment. Another limitation is the inability to determine treatment burden despite the fact that poor adherence has a significant impact on health economic assessment in osteoporosis.
In conclusion, the present study concerning the impact of patient training on persistence and compliance with bisphosphonate treatment on a weekly vs monthly basis in postmenopausal osteoporosis in Turkey revealed high rates of persistence and compliance regardless of the use of training. However, adherence was significantly improved with a monthly vs weekly bisphosphonate regimen.
Conflict of interest
None declared.
Acknowledgments
This study is funded by Sanofi Turkey. KAPPA Consultancy Training Research Ltd (Istanbul, Turkey) provided editorial service and Monitor Medical Research and Consulting (Istanbul, Turkey) statistical analysis funded by Sanofi Turkey.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Appendix. INSTRUCT Study Group (in descending order of number of patients enrolled by the centers):
-
1.
Hikmet Kocyigit, Izmir Ataturk Training and Research Hospital, Izmir
-
2.
Nurten Eskiyurt and Sina Esmaeilzadeh, Istanbul University, Istanbul Faculty of Medicine, Istanbul
-
3.
Omer Kuru, Ondokuz Mayis University, Faculty of Medicine, Samsun
-
4.
Ebru Yilmaz Yalcinkaya and Fatma Karaagac, Istanbul Physical Therapy and Rehabilitation Training and Research Hospital, Istanbul
-
5.
Ahmet Baybali, Cekirge State Hospital, Bursa
-
6.
Nese Erdogan, Derince Training and Research Hospital, Kocaeli
-
7.
Sibel Eyigor, Ege University, Faculty of Medicine, Izmir
-
8.
Hatice Bodur, Ankara Numune Hospital, Ankara
-
9.
Ulku Akarirmak, Istanbul University, Cerrahpasa Faculty of Medicine, Istanbul (Study coordinator)
-
10.
Banu Kuran, Sisli Etfal Training and Research Hospital, Istanbul
-
11.
Ferda Ozdemir, Trakya University, Faculty of Medicine, Edirne
-
12.
Rezzan Gunaydin, Izmir Training and Research Hospital, Izmir
-
13.
Ozlen Peker, Dokuz Eylul University, Faculty of Medicine, Izmir
-
14.
Ayse Ekim Aydemir, Eskisehir Training and Research Hospital, Eskisehir
-
15.
Jale Irdesel, Uludag University, School of Medicine, Bursa
-
16.
Filiz Meryem Sertpoyraz, Tepecik Training and Research Hospital, Izmir
-
17.
Cihan Jarar, Sakarya Training and Research Hospital, Sakarya
-
18.
Oya Topuz, Pamukkale University, School of Medicine, Denizli
-
19.
O. Faruk Sendur, Adnan Menderes University, School of Medicine, Aydin
-
20.
Pinar Borman, Ankara Training and Research Hospital, Ankara
-
21.
Rana Erdem, Ankara Training and Research Hospital, Ankara
-
22.
Mustafa Calis, Erciyes University, School of Medicine, Kayseri
-
23.
Ayse Turhanoglu, Mustafa Kemal University, School of Medicine, Hatay
-
24.
Cengiz Bahadir, Haydarpasa Training and Research Hospital, Istanbul
-
25.
Filiz Gengor, Bornova State Hospital, Izmir
-
26.
Nese Ozgirgin, Ankara Physical Therapy and Rehabilitation Hospital, Ankara
-
27.
Erdal Akgol, Fatih State Hospital, Trabzon
-
28.
Sumru Ozel, Ankara Physical Therapy and Rehabilitation Hospital, Ankara
-
29.
Aylin Rezvani, Bezm-i Alem University, Faculty of Medicine, Istanbul
-
30.
Alev Cevikol, Diskapi Yildirim Beyazit Training and Research Hospital, Ankara
-
31.
Celalettin Orzan, Private Ada Hospital, Giresun
-
32.
Müfit Akyuz, Ankara Physical Therapy and Rehabilitation Hospital, Ankara
-
33.
Remzi Cevik, Dicle University, Faculty of Medicine, Diyarbakir
-
34.
Halil Ucan, Ankara Physical Therapy and Rehabilitation Hospital, Ankara
References
- 1.Cramer J.A., Lynch N.O., Gaudin A.F., Walker M., Cowell W. The effect of dosing frequency on compliance and persistence with bisphosphonate therapy in postmenopausal women: a comparison of studies in the United States, the United Kingdom, and France. Clin Ther. 2006;28:1686–1694. doi: 10.1016/j.clinthera.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Moen M.D., Keam S.J. Denosumab: a review of its use in the treatment of postmenopausal osteoporosis. Drugs Aging. 2011;28:63–82. doi: 10.2165/11203300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Reginster J.Y., Rebenda V. Patient preference in the management of postmenopausal osteoporosis with bisphosphonates. Clin Interv Aging. 2006;1:415–423. doi: 10.2147/ciia.2006.1.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiligsmann M., McGowan B., Bennett K., Barry M., Reginster J.Y. The clinical and economic burden of poor adherence and persistence with osteoporosis medications in Ireland. Value Health. 2012;15:604–612. doi: 10.1016/j.jval.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Compston J.E., Seeman E. Compliance with osteoporosis therapy is the weakest link. Lancet. 2006;368:973–974. doi: 10.1016/S0140-6736(06)69394-X. [DOI] [PubMed] [Google Scholar]
- 6.Cortet B., Bénichou O. Adherence, persistence, concordance: do we provide optimal management to our patients with osteoporosis? Joint Bone Spine. 2006;73:e1–e7. doi: 10.1016/j.jbspin.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Vytrisalova M., Blazkova S., Palicka V. Self-reported compliance with osteoporosis medication-qualitative aspects and correlates. Maturitas. 2008;60:223–229. doi: 10.1016/j.maturitas.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Verron E., Bouler J.M. Is bisphosphonate therapy compromised by the emergence of adverse bone disorders? Drug Discov Today. 2014;19:312–319. doi: 10.1016/j.drudis.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Cotté F.E., Mercier F., De Pouvourville G. Relationship between compliance and persistence with osteoporosis medications and fracture risk in primary health care in France: a retrospective case-control analysis. Clin Ther. 2008;30:2410–2422. doi: 10.1016/j.clinthera.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Cramer J.A., Gold D.T., Silverman S.L., Lewiecki E.M. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 11.Penning-van Beest F.J., Goettsch W.G., Erkens J.A. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28:236–242. doi: 10.1016/j.clinthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Cooper A., Drake J., Brankin E., PERSIST Investigators Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896–905. doi: 10.1111/j.1742-1241.2006.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansà M., Hernández C., Vidal M. Multidimensional analysis of treatment adherence in patients with multiple chronic conditions. A cross-sectional study in a tertiary hospital. Patient Educ Couns. 2010;81:161–168. doi: 10.1016/j.pec.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Shu A.D., Stedman M.R., Polinski J.M. Adherence to osteoporosis medications after patient and physician brief education: post hoc analysis of a randomized controlled trial. Am J Manag Care. 2009;15:417–424. [PMC free article] [PubMed] [Google Scholar]
- 15.Guilera M., Fuentes M., Grifols M., Ferrer J., Badia X. OPTIMA study investigators. Does an educational leaflet improve self-reported adherence to therapy in osteoporosis? The OPTIMA study. Osteoporos Int. 2006;17:664–671. doi: 10.1007/s00198-005-0031-8. [DOI] [PubMed] [Google Scholar]
- 16.Claxton A.J., Cramer J., Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 17.Richter A., Anton S.E., Koch P., Dennett S.L. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25:2307–2335. doi: 10.1016/s0149-2918(03)80222-9. [DOI] [PubMed] [Google Scholar]
- 18.Cotté F.E., Fautrel B., De Pouvourville G.A. Markov model simulation of the impact of treatment persistence in postmenopausal osteoporosis. Med Decis Making. 2009;29:125–139. doi: 10.1177/0272989X08318461. [DOI] [PubMed] [Google Scholar]
- 19.Tosteson A.N., Grove M.R., Hammond C.S. Early discontinuation of treatment for osteoporosis. Am J Meat. 2003;115:209–216. doi: 10.1016/s0002-9343(03)00362-0. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton B., McCoy K., Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14:259–262. doi: 10.1007/s00198-002-1370-3. [DOI] [PubMed] [Google Scholar]
- 21.Nahum R., Hirsch M., Kaplan B. Persistence and compliance with antiresorptive treatment for osteoporosis: the Israeli experience. Maturitas. 2009;63:S24. [Google Scholar]