Abstract
Aim
Beta tricalcium phosphate (beta-TCP) is an osteoconductive, resorbable material. Its clinical effectiveness has been proved in many indications. This study was clinical and radiographic study report obtained in patients undergoing anterior cervical discectomy and fusion ACDF in which PEEK cages were filled beta-TCP in an injectable form.
Material and methods
Between January 2010 and June 2011, 16 consecutive patients underwent ACDF using PEEK cages with beta-TCP. The cohort compromised 10 men and 6 women with a mean age of 45.2 years. The surgery was performed when the patient had myelopathy or radiculopathy with progressive neurological deficit, or failure of conservative treatment (a minimum of 3 months). The patients were evaluated by Odom criteria preoperatively and postoperative 3rd, 6th, 12th and 24th months. Preop and postop pain was evaluated with visual analogue scala (VAS). Disc height and fusion success rates were evaluated.
Results
Preoperative average VAS score was 7.9 (7–10) for neck pain and 8 (7–10) for arm pain. At the final follow-up, these scores became 1.5 and 1.4 for neck and arm pain, respectively. The average improvement rate was 81% for neck pain and 82.5% for arm pain. Postop ODOM's criteria main rate was 3.4. Bone fusion was achieved in 14 segments (70%) at 3rd month, 19 segments (95%) at 12th month follow-up assessment.
Conclusion
Clinical and radiological results revealed that B-TCP is a good alternative synthetic fusion material for cervical interbody fusion.
Level of evidence: Level IV, therapeutic study.
Keywords: Anterior cervical fusion, Interbody cage, Beta-tricalcium phosphate
Cervical spondylosis usually occurs in the discs, and may include disc herniation or osteophyte formation at endplates or uncovertebral joints. Anterior cervical discectomy and fusion (ACDF) has gained popularity since its introduction in the 1950s by Smith, Robinson and Cloward.1, 2, 3 Autograft is the most commonly used fusion material and has good or excellent results in the literature.4, 5 However, use of autologous graft entails second operation, generally on the iliac crest, and may be associated with important morbidity. Moreover, complications, such as long-term pain syndrome, femorocutaneous nerve damage, infection, or secondary fracture have been reported.6, 7 Interbody fusion devices were developed to avoid possible complications as result of donor bone harvesting. Criteria required for an ideal cage for cervical interbody fusion are the following: provide immediate stability, maintain spinal alignment and foraminal height, achieve greater or at least equal fusion success rate, and eliminate complications associated with use of autograft.2, 8, 9
Ideal graft materials should have 3 basic properties: osteogenicity, os-teoconductivity, and osteoinductivity. Beta-tricalcium phosphate (beta-TCP) has been widely used for bone regeneration due to its many positive physical properties.10, 11, 12 Beta-TCP is an osteoconductive, resorbable material, which has been used as a bone substitute for many years. Its clinical effectiveness has been proven in many applications.13, 14, 15, 16, 17, 18, 19
The purpose of the present study was to retrospectively analyze clinical and radiographical results of patients who underwent anterior cervical fusion for radicular-disc conflict due to soft disc herniation or spondylosis, This report evaluated patients who underwent ACDF in which polyetheretherketone (PEEK) cages were filled with commercially available granulated beta-TCP in injectable form (Suprabone Putty; BMT Calsis Sağlık Teknolojileri Sanayi Ticaret A.Ş., Ankara, Turkey).
Patients and methods
Between January 2010 and June 2011, 16 consecutive patients underwent ACDF using PEEK cages with beta-TCP in 20 disc segments at Department of Neurosurgery, Bakirkoy Research and Training Hospital for Neurology, Neurosurgery, and Psychiatry, Istanbul, Turkey. These 16 patients were followed prospectively. Cohort comprised 10 men and 6 women with mean age of 45.2 years. Clinical symptoms included cervical radiculopathy (10 patients) or myeloradiculopathy (6 patients) caused by nerve root or spinal cord compression. Surgery was performed when the patient had myelopathy or radiculopathy with progressive neurological deficit, or failure of conservative treatment was observed (minimum of 3 months).
Discectomy and fusion with beta-TCP was performed using anterior approach in all patients included in this study. Transverse skin incision was made. Anterior cervical disc was approached using method described by Smith and Robinson (pre-sterno-cleidomastoid approach).3 Caspar distraction screw was used to distract disc space throughout the procedure. Cervical discectomy and removal of posterior hypertrophic osteophytes were performed thereafter. Upper and lower endplates were prepared by removing overlying cartilage and preserving hardest subchondral bone. Optimal size of PEEK cage was selected following completion of discectomy and end-plate preparation. Inner cavity of PEEK cage was filled with beta-TCP (SupraBone Putty; BMT Calsis Sağlık Teknolojileri Sanayi Ticaret A.Ş., Ankara, Turkey) (Fig. 1). PEEK cage with allograft was then implanted in disc space for fusion.
Fig. 1.
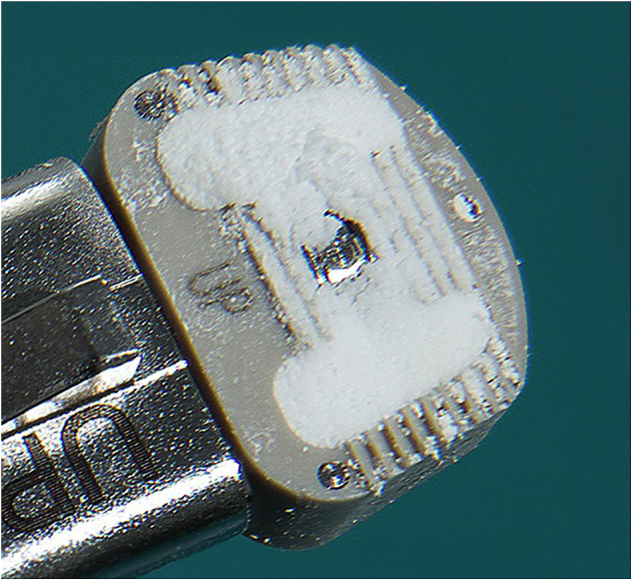
Polyetheretherketone cages containing granulated beta tricalcium phosphate.
Patients were allowed to stand the day after surgery and a soft collar was worn for 10 days. Radiographical follow-up was performed at postoperative 24 h (Fig. 2). Neck exercises were initiated 6 weeks after surgery and normal activity level was progressively resumed.
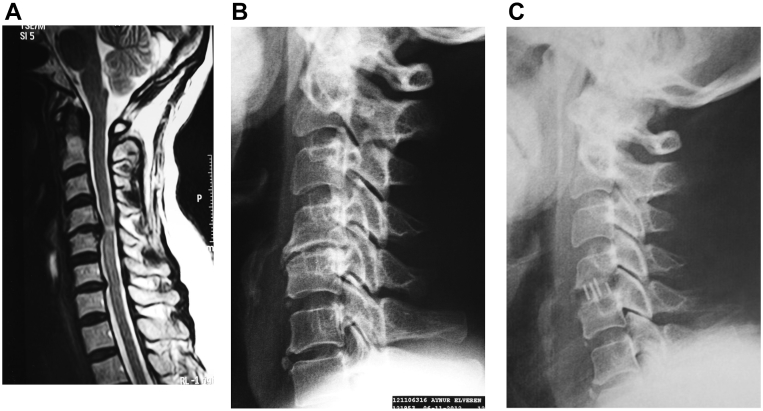
Fig. 2.
42-year-old female experienced neck pain with radiation to her right arm for 2 months. Conservative treatment failed. Severe spinal cord compression was observed in (A) magnetic resonance imaging. The patient underwent surgery for C4/5 disc herniation, and cages containing beta tricalcium phosphate graft were used. X-rays were obtained (B) pre-operatively, as well as (C) 6 months post surgery. Fusion was observed with trabecular bone bridging the involved vertebral bodies.
Plain anteroposterior and lateral cervical spine radiographs were taken before and after surgery and at 3, 6, and 12 months postoperatively. One independent neurosurgeon assessed status of interbody height and final fusion results. Distance between midpoint of upper end plate and lower end plate was measured as interbody height. Radiographs obtained at 1-year follow-up were used to assess union. Union of cage was defined as no radiolucent line between cage and endplate, and no translation or angular change seen on lateral flexion and extension radiographs. Fine-cut computed tomography scan was done 3 months after surgery and at 3-month intervals until fusion was completed.
Neurological status in all patients, preoperatively and at discharge, 3, 6, and 12 months after surgery, was measured according to Odom's criteria.20 A 10-point visual analogue scale (VAS) with endpoint anchors of “no pain” (0)' and “worst possible pain”10 was also used to rate neck and arm pain before surgery and at each follow-up visit. Statistical analysis and comparisons of preoperative and postoperative results were performed using paired Student's t-tests. Level of significance was set at 0.05.
Ethics committee of Bakirkoy Research and Training Hospital for Neurology, Neurosurgery and Psychiartry approved the study on May 8, 2012 with approval number B.10.4.ISM.04.34.26.08.206.
Results
A total of 16 patients underwent anterior cervical discectomy and fusion with total of 20 cervical cages implanted. In this series, mean operation time was 62.1 min (range: 42–112 min), estimated blood loss was less than 50 mL each in all 16 patients, and mean length of hospital stay was 1.2 days (range: 1–2 days). All patients were followed-up for average 13 months (range 11–14 months). Patient demographic data are provided in Table 1. There were no early or late implant-related complications, and no additional surgeries were required for any reason.
Table 1.
Demographic, clinical, and radiological data of patients.
| Mean (low-high) | |
|---|---|
| Age | 44 ± 9.9 (31–59) |
| Duration of symptoms (months) | 8.9 ± 8 (3–36) |
| Preoperative neck pain (VAS) | 8.1 ± 0.9 (7–10) |
| Postperative neck pain (VAS) | 2 ± 1.7 (1–8) |
| Preoperative arm pain (VAS) | 8.2 ± 0.8 (7–10) |
| Postperative neck pain (VAS) | 1.5 ± 0.6 (1–3) |
| Postoperative Odom's criteria | 3.5 ± 0.5 (3–4) |
| Hospital stay (days) | 1. ± 0.5 (1–3) |
| Blood loss (mL) | 41.3 ± 24.7 (20–100) |
| Preoperative disc height (mm) | 6.3 ± 1.3 (4–8) |
| Postoperative early disc height (mm) | 7.8 ± 1.1 (7–10) |
| Postoperative late disc height (mm) | 8.2 ± 1 (7–10) |
Preoperative average VAS score was 7.9 (range: 7–10) for neck pain and 8 (range: 7–10) for arm pain. At final follow-up, these scores were 1.5 (range: 1–3) and 1.4 (range: 1–8) for neck and arm pain, respectively. Decreases were statistically significant for both neck and arm pain (p = 0.01 and p = 0.01, respectively). Average improvement rate was 81% for neck pain and 82.5% for arm pain. Postoperative Odom's criteria main rate was 3.4.
Bone fusion was achieved in 14 segments (70%) at 3rd month and 19 segments (95%) at 12th month follow-up assessment (Table 2).
Table 2.
Postoperative fusion rate.
| N | Fusion rate | P value | |
|---|---|---|---|
| Postop 3rd month | 20 | 70% | 0.002 |
| Postop 6th month | 20 | 85% | 0.013 |
| Postop 12th month | 20 | 95% | 0.030 |
Mean height of disc in preoperative period was 6.3 ± 1.3 mm (range: 4–8 mm). In early and late postoperative period, mean height of disc was 8.2 ± 1 mm (range: 7–10 mm) and 7.8 ± 1.1 mm (range: 7–10 mm), respectively. Postoperative height of disc was significantly greater at both early and late endpoint (p = 0.001 and p = 0.001) (Table 3).
Table 3.
Preoperative and postoperative clinical and radiological comparison of patients.
| Mean (low-high) | P value | ||
|---|---|---|---|
| Neck pain | Preoperative | 8.1 ± 0.9 (7–10) | 0.001 |
| Neck pain | Postperative | 2 ± 1.7 (1–8) | |
| Arm pain | Preoperative | 8.2 ± 0.8 (7–10) | 0.001 |
| Arm pain | Postperative | 1.5 ± 0.6 (1–3) | |
| Disc height (mm) | Preoperative | 6.3 ± 1.3 (4–8) | |
| Early disc height | Postoperative | 7.8 ± 1.1 (7–10) | 0.001 |
| Late disc height | Postoperative | 8.2 ± 1 (7–10) | 0.001 |
Discussion
Cervical spondylosis usually occurs in the discs, and may include disc herniation or osteophyte formation at endplates or uncovertebral joints. Today, first choice of treatment for cervical disc herniation and spondylotic radiculopathy or myelopathy is still ACDF.21 Achieving fusion using interbody cages has become increasingly popular. The major benefit of using prosthetic devices in ACDF is that complications arising from the use of autologous bone grafts are minimized. Moreover, these devices provide immediate stability, restore foraminal height and alignment, minimize operative time, and most importantly, eliminate donor site pain.14, 22
Autogenous bone is the optimal material for spinal fusion. However, such grafts are associated with increased surgical time, blood loss, and postoperative donor site pain.6, 7, 21 Therefore, allogen-derived fusion materials, such as tibia and femoral cortical bone grafts, bone morphogenetic protein (BMP), and demineralized bone matrix (DBM) are preferred materials for cervical interbody fusion surgery. Allografts obtained from cadavers have demonstrated some problems, such as bacterial contamination and viral transmission.23, 24, 25 All of these reasons support the importance of research and development of synthetic materials for use in spinal fusion.
Ceramics, such as hydroxyapatite and beta-TCP, have been used as bone graft material with good results.13, 15, 16, 18, 19, 26, 27, 28 These synthetic materials provide scaffold similar to that of autologous bone, are widely available, less expensive than BMP or DBM, and not associated with donor morbidity. Scaffolds made with beta-TCP provide osteoconduction for bone production as well as long-term stability and immediate osteogenic activity.17, 26
In order to reduce donor site complications and operation time, many different bone graft substitutes have been analyzed, but in the context of cervical fusion, none have proven to be superior to autologous bone.22 Beta-TCP substitutes have been used for reconstruction of metaphyseal defects in long bone fractures with good clinical success.14, 17, 18 To the best of our knowledge, only clinical study using interbody cages filled with beta-TCP was carried by Dai et al.15 According to Dai et al., anterior plate implanted to supplement interbody cages enhanced clinical outcomes and fusion rates. At 6 months, successful fusion rates were observed in all patients; no difference was seen between group with plate fixation and patient group without after 2 years.
In the present study, we used only PEEK interbody cages containing synthetic putty made of beta-TCP for cervical interbody fusion. Statistically significantly successful fusion rates and functional aspects were found at the end of our study. In recent years, cages augmented with such bone substitutes have been utilized to obtain solid interbody fusion in cases of cervical disease.9, 20 This procedure helps to avoids donor-site morbidity and at the same time reduces surgical time. This was confirmed in the present series, in which group had shorter surgical time, minor donor-site morbidity, and clinical results comparable to the BMP and DBM techniques.
The present study is not the first study to use PEEK cages filled with commercially available granulated beta-TCP in an injectable form in ACDF; however, only a handful of reports were available.15, 29, 30, 31, 32 Our results were consistent: results indicate that use of beta-TCP results in comparable fusion rates, and has the advantage of reduced morbidity associated with autogeneous grafts.
It was our finding that according to improvements in pain intensity, Neck Disability Index score, and clinical and radiological results, beta-TCP is a good alternative as synthetic fusion material for cervical interbody fusion.
This study was limited in that its non-inferiority design means that the sample size was relatively small. A superiority trial with a larger sample size might better compare the clinical and radiological outcomes of the beta TCP and BMP or DBM.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Cloward R.B. The anterior approach for ruptured cervical discs. J Neurosurg. 1958;15:602–614. doi: 10.3171/jns.1958.15.6.0602. [DOI] [PubMed] [Google Scholar]
- 2.Ryu S.I., Lim J.T., Kim S.-M., Paterno J., Willenberg R., Kim D.H. Comparison of the biomechanical stability of dense cancellous allograft with tricortical iliac autograft and fibular allograft for cervical interbody fusion. Eur Spine J. Sep; 2006;15(9):1339–1345. doi: 10.1007/s00586-005-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith G.W., Robinson R.A. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Jt Surg (Am) 1958;40:607–624. [PubMed] [Google Scholar]
- 4.Samartzis D., Shen F.H., Goldberg E.J., An H.S. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation. Spine. 2005;30:1756–1761. doi: 10.1097/01.brs.0000172148.86756.ce. [DOI] [PubMed] [Google Scholar]
- 5.Wright I.P., Eisenstein S.M. Anterior cervical discectomy and fusion without instrumentation. Spine. 2007;32:772–774. doi: 10.1097/01.brs.0000258846.86537.ad. [DOI] [PubMed] [Google Scholar]
- 6.Silber J.S., Anderson D.G., Daffner S.D. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Younger E.M., Chapman M.W. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Beaman F.D., Bancroft L.W., Peterson J.J., Kransdorf M.J. Bone graft materials and synthetic substitutes. Radiol Clin North Am. 2006;44:451–461. doi: 10.1016/j.rcl.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig S.C., Boden S.D. Osteoinductive bone graft substitutes for spinal fusion. Orthop Clin North Am. 1999;30:635–645. doi: 10.1016/s0030-5898(05)70116-4. [DOI] [PubMed] [Google Scholar]
- 10.Giannoudis P.V., Dinopoulos H., Tsiridis E. Bone substitutes: an update. Injury. 2005;36(suppl 3):S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Chau A.M., Xu L.L., Wong J.H., Mobbs R.J. Current status of bone graft options for anterior interbody fusion of the cervical and lumbar spine. Neurosurg Rev. 2014;37:23–37. doi: 10.1007/s10143-013-0483-9. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L., Ye F., Yang R. Osteoinduction of hydroxyapatite/beta-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010;6:1569–1574. doi: 10.1016/j.actbio.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Aunoble S., Clément D., Frayssinet P., Harmand M.F., Le Huec J.C. Biological performance of a new beta-TCP/PLLA composite material for applications in spine surgery: in vitro and in vivo studies. J Biomed Mater Res A. 2006;78(2):416–422. doi: 10.1002/jbm.a.30749. [DOI] [PubMed] [Google Scholar]
- 14.Burchardt H. The biology of bone graft repair. Clin Orthop Relat. Res. 1983;174:28–42. [PubMed] [Google Scholar]
- 15.Dai L., Jiang L. Anterior cervical fusion with interbody cage containing beta-tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2-year follow-up. Eur Spine J. 2008;17:698–705. doi: 10.1007/s00586-008-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai L.Y., Jiang L.S. Single-level instrumented posterolateral fusion of lumbar spine with b-tricalcium phosphate versus autograft: a prospective, randomized study with 3-year follow-up. Spine. 2008;May 20;33(12):1299–1304. doi: 10.1097/BRS.0b013e3181732a8e. [DOI] [PubMed] [Google Scholar]
- 17.Hernigou P., Roussignol X., Flouzat-Lachaniette C.H., Filippini P., Guissou I., Poignard A. Opening wedge tibial osteotomy for large varus deformity with Ceraver resorbable beta tricalcium phosphate wedges. Int Orthop. 2010;34(2):191–199. doi: 10.1007/s00264-009-0875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirata M., Murata H., Takeshita H., Sakabe T., Tsuji Y., Kubo T. Use of purified beta- tricalcium phosphate for filling defects after of benign bone tumors. Int Orthop. 2006;30(6):510–513. doi: 10.1007/s00264-006-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogose A., Hotta T., Hatano H. Histological examination of beta-tricalcium phosphate graft in human femur. J Biomed Mater Res. 2002;63(5):601–604. doi: 10.1002/jbm.10380. [DOI] [PubMed] [Google Scholar]
- 20.Odom G.L., Finney W., Woodhall B. Cervical disk lesions. J AmMed Assoc. 1958;166(1):23–28. doi: 10.1001/jama.1958.02990010025006. [DOI] [PubMed] [Google Scholar]
- 21.Bono C.M., Lee C.K. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years : influence of technique on fusion rate and clinical outcome. Spine (Phila Pa 1976) 2004;29:455–463. doi: 10.1097/01.brs.0000090825.94611.28. discussion Z5. [DOI] [PubMed] [Google Scholar]
- 22.Chau A.M., Mobbs R.J. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J. 2009;18(4):449–464. doi: 10.1007/s00586-008-0878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop R.C., Moore K.A., Hadley M.N. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85:206–210. doi: 10.3171/jns.1996.85.2.0206. [DOI] [PubMed] [Google Scholar]
- 24.Delloye C., Cornu O., Druez V., Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Jt Surg Br. 2007;89:574–579. doi: 10.1302/0301-620X.89B5.19039. [DOI] [PubMed] [Google Scholar]
- 25.Floyd T., Ohnmeiss D. A meta-analysis of autograft versus allograft. Eur Spine J. 2000;9:398–403. doi: 10.1007/s005860000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandoff J.F., Silber J.S., Vaccaro A.R. Contemporary alternatives to synthetic bone grafts for spine surgery. Am J Orthop (Belle Mead NJ) 2008;37:410–414. [PubMed] [Google Scholar]
- 27.Hing K.A., Wilson L.F., Buckland T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007;7:475–490. doi: 10.1016/j.spinee.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Moro-Barrero L., Acebal-Cortina G., Suárez-Suárez M., Pérez-Redondo J., Murcia-Mazón A., López-Muñiz A. Radiographic analysis of fusion mass using fresh autologous bone marrow with ceramic composites as an alternative to autologous bone graft. J Spinal Disord Tech. 2007;20:409–415. doi: 10.1097/bsd.0b013e318030ca1e. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Zhang F., Lv F. Osteoinductive activity of ErhBMP-2 after anterior cervical diskectomy and fusion with a B-TCP interbody cage in a goat model. Orthop (United States) Feb 2014;37(2):pe123–pe131. doi: 10.3928/01477447-20140124-13. [DOI] [PubMed] [Google Scholar]
- 30.Brenke C., Kindling S., Scharf J. Short term experience with a new absorbable composite cage ((sup) tricalcium phosphate polylactic acid) in patients after stand alone anterior cervical discectomy and fusion. Spine (Phila Pa 1976) (United States) May 15 2013;38(11):pE635–pE640. doi: 10.1097/BRS.0b013e31828d65bb. [DOI] [PubMed] [Google Scholar]
- 31.Zagra A., Zagra L., Scaramuzzo L. Anterior cervical fusion for radicular-disc conflict performed by three different procedures: clinical and radiographic analysis at long-term follow-up. Eur Spine J (Germany) Nov 2013;22(suppl 6):pS905–pS909. doi: 10.1007/s00586-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi Jemin, Lee Gun Woo, Nam Woo Dong. A prospective randomized clinical trial comparing bone union rate following anterior cervical discectomy and fusion using a polyetheretherketone cage: hydroxyapatite/B-tricalcium phosphate mixture versus hydroxyapatite/demineralized bone matrix mixture. Asian Spine J. 2015;9(1):30–38. doi: 10.4184/asj.2015.9.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]