Abstract
Activated B cells mature in germinal centers (GCs), but GC initiation during infection is poorly understood. Gaya et al. (2018) show that NKT cells, activated by CD169+ macrophages, produce an early wave of inter-leukin-4 (IL-4) that promotes GC formation during viral infection.
Following infection or immunization, activated B cells undergo affinity maturation and class switching to produce high-affinity antibodies whose specificity and function are tailored to the threat at hand. These processes take place within germinal centers (GCs), structured regions in lymphoid B cell follicles that facilitate interactions between activated B cells and accessory cells. Chief among the latter are follicular T helper (Tfh) cells, which promote B cell maturation through both ligand-receptor interactions and cytokine production. However, in certain infections, GCs can form prior to the appearance of a robust Tfh cell population, raising the question of how B cells initially seed the GC. In this issue, Gaya et al. (2018) show that NKT cells, innate-like T cells that recognize lipid antigens presented on the nonclassical MHC molecule CD1d, are critical for GC formation during viral infection. Their work provides insight not only into initiation of GCs and antibody responses, but also into a novel effector function for NKT cells in viral infection.
NKT cells are activated during infection not only with bacteria, many of which express lipid antigens that can be presented on CD1d (Brigl et al., 2003, 2011), but also with viruses, which lack known CD1d ligands (Tyznik et al., 2008). They are known to promote B cell responses in mice immunized with lipid-antigen conjugates (Galli et al., 2007; Leadbetter et al., 2008; Chang et al., 2011); however, a role for NKT cells in regulating B cell responses to viral infection has not been described. Gaya et al. (2018) now show that Cd1d−/− mice, which lack NKT cells, exhibit deficient GC formation and antibody production following infection with influenza virus, revealing a role for NKT cells in production of antiviral antibodies. In contrast to immunization with protein-lipid conjugates (Chang et al., 2011; Lead-better et al., 2008), they find that influenza infection does not elicit a Tfh cell-like phenotype in NKT cells, and GC formation and antibody production are not dependent on cognate interactions with B cells. Instead, the profound GC defects in NKT-deficient mice appeared to be entirely due to loss of IL-4 production by NKT cells early in infection, at a time when Tfh cells have not yet expanded or begun producing this cytokine (Figure 1). Il4−/− mice recapitulate the phenotype of Cd1d−/− mice, and in a key experiment, the authors use a fluorescent reporter of Il4 message to determine that NKT cells are the dominant population expressing Il4 transcript in lung-draining lymph nodes 3 days post-infection with influenza. In contrast, Tfh cells express little Il4 at this time but overtake NKT cells as the primary Il4 producers later in infection, peaking around day 9. It should be noted that secretion of IL-4 protein is post-transcriptionally regulated and that therefore the presence of Il4 transcript does not always correspond to secretion of IL-4 protein. For this reason, repetition of this experiment in KN2 mice, which report IL-4 protein (Mohrs et al., 2005), would be a valuable confirmation of the author’s model. Nevertheless, multiple additional experiments support the notion that NKT cells are essential early secretors of IL-4. Both Il4−/− mice and wild-type mice treated with anti-IL-4 recapitulated the phenotype of NKT-deficient Cd1d−/− mice, whereas treatment of CD1d-deficient mice with exogenous IL-4 completely rescued GC formation. Altogether, the authors show convincingly that NKT cells produce an early wave of IL-4 that is critical for GC development and antibody maturation during viral infection. A further inference of these experiments is that IL-4 production is the sole or primary contribution of NKT cells to GC formation during viral infection. Notably, although IL-4-producing NKT cells localized preferentially to follicular borders, the fact that exogenous IL-4 could substitute for NKT cells suggests that precise localization of early IL-4 production is not critical for proper GC formation, supporting previous work from Perona-Wright et al. (2010).
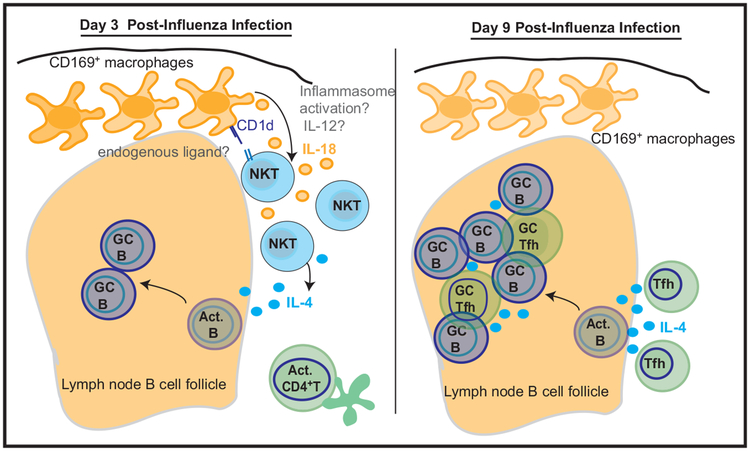
Figure 1. Early Production of IL-4 by NKT Cells Promotes Germinal Center Formation.
Soon after lung infection with influenza virus, CD169+ macrophages in the draining lymph nodes activate nearby NKT cells through both CD1d engagement and secretion of IL-18. These signals induce NKT cells to produce IL-4, which acts on B cells to promote germinal center (GC) formation and production of virus-specific antibodies (left). Later in infection, T follicular helper (Tfh) cells overtake NKT cells as the dominant IL-4 producers and, presumably, as principle orchestrators of B cell maturation (right). Outstanding questions include the nature of the CD1d ligand that contributes to activation of NKT cells; whether and how the inflammasome is activated to produce secreted IL-18; and whether other cytokines, such as IL-12, may be important in driving NKT cell activation. Abbreviation: Act., activated.
An intriguing question that arises from this and other studies is how viruses activate NKT cells through cognate interactions despite an apparent lack of pathogen-derived CD1d ligands. The role of CD1d in NKT activation varies with context: cognate CD1d-dependent interactions between NKT cells and B cells are required for antibody responses to lipid-antigen conjugates (Chang et al., 2011; Leadbetter et al., 2008) whereas NKT activation during bacterial infection is CD1d independent and was largely driven by IL-12 (Brigl et al., 2011). In the present work, Cre-Lox-mediated deletion of Cd1d is used to show that NKT activation by influenza requires cognate interactions not with B cells, but between NKT cells and CD169+ lymph node macrophages. Selective deletion of CD1d from CD169+ cells results in defective Il4 production and subsequently disrupted GC formation, despite an intact Tfh cell population. The nature of the ligands being presented on CD1d in this context are not clear, but they may include weakly recognized self-antigens that can be presented along with other activating signals during viral infection (Brigl et al., 2003). This finding highlights the importance of studying responses to infectious agents, since protein immunization alone is not sufficient to induce NKT activation in the absence of an exogenous glycolipid adjuvant (Leadbetter et al., 2008; Chang et al., 2011; Gaya et al., 2018).
Interestingly, full depletion of CD169+ cells disrupted Il4 production by NKT cells even more profoundly than CD169-restricted deletion of CD1d, indicating that CD169+ macrophages also contribute to NKT activation through CD1d-independent mechanisms. Investigating these, Gaya et al. (2018) found that mice lacking MyD88, a common signaling adaptor for both Toll-like receptors and several cytokine receptors, failed to upregulate Il4 upon viral infection. Further, mice lacking the receptor for IL-18, an NKT-activating cytokine that signals through MyD88, also exhibited defective Il4 production. It is curious that the authors did not examine the role of IL-12, since it is known to be critical for NKT activation in other contexts (Brigl et al., 2003, 2011) and its production by macrophages is likely to be MyD88 dependent (though the authors do show that IL-4 production is intact in the absence of TLR7, considered to be the primary TLR that recognizes influenza). Even so, the data are consistent with a model in which CD169+ macrophages activate NKT cells to produce IL-4 through both cognate interactions and production of IL-18.
IL-18 secretion requires inflammasome activation, which has been shown to occur in CD169+ lymph node macrophages during viral infection in vivo (Sagoo et al., 2016). However, inflammasome activation in this previous work leads to rapid death and disappearance of CD169+ cells, whereas in the current report, CD169+ cell populations remain intact at all time points examined. Thus, an outstanding question is whether, where, and how the inflammasome is activated to generate IL-18 in this model.
Finally, since NKT activation requirements and effector functions vary depending on context, it is important to consider how broadly these findings apply. Gaya et al. (2018) show that NKT cells are also important for GC formation during vaccinia infection, suggesting a general role in initiation of B cell responses to viruses. Furthermore, the authors present evidence that NKT cells perform a similar role in primates: analyzing lymph node transcriptional responses from rhesus macaques infected with Zika virus, they find that early strong expression of gene signatures for NKT cells, IL-4 and IL-18 signaling correlated with high titers of Zika-specific neutralizing antibodies later in infection. In contrast, no correlation was observed between early expression of Tfh cell genes and neutralizing antibody titers. These results suggest that early NKT activation is critical for high-affinity antibody production across multiple viral pathogens and host species.
In summary, this study builds on previous findings to fill a crucial gap in our understanding of the earliest steps in generation of antibodies, identifying NKT cells as central regulators of germinal center formation through non-cognate interactions during viral infection. By elucidating much of the mechanism of NKT cell activation in this context, Gaya et al. (2018) shed new light on how innate and innate-like cells cooperate to orchestrate adaptive responses. Through rapid production of IL-4 upon infection, NKT cells set the stage for optimal germinal center formation, “teeing up” B cells for efficient production of high-affinity antibody and ensuring that antiviral responses are up to par.
REFERENCES
- Brigl M, Bry L, Kent SC, Gumperz JE, and Brenner MB (2003). Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol 4, 1230–1237. [DOI] [PubMed] [Google Scholar]
- Brigl M, Tatituri RVV, Watts GFM, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu F-F, Besra GS, and Brenner MB (2011). Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med 208, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P-P, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, et al. (2011). Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol 13, 35–43. [DOI] [PubMed] [Google Scholar]
- Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, et al. (2007). Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl. Acad. Sci. USA 104, 3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaya M, Barral P, Burbage M, Aggarwal S, Montaner B, Warren Navia A, Aid M, Tsui C, Maldonado P, Nair U, et al. (2018). Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell 172, 517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, Besra GS, and Brenner MB (2008). NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl. Acad. Sci. USA 105, 8339–8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrs K, Wakil AE, Killeen N, Locksley RM, and Mohrs M (2005). A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona-Wright G, Mohrs K, and Mohrs M (2010). Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nat. Immunol 11, 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo P, Garcia Z, Breart B, Lemaître F, Michonneau D, Albert ML, Levy Y, and Bousso P (2016). In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat. Med 22, 64–71. [DOI] [PubMed] [Google Scholar]
- Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, and Kronenberg M (2008). Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J. Immunol 181, 4452–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]