Abstract
Animal models of erythropoiesis have been, and will continue to be, important tools for understanding molecular mechanisms underlying the development of this cell lineage and the pathophysiology associated with various human erythropoietic diseases. In this regard, the mouse is probably the most valuable animal model available to investigators. The physiology and short gestational period of mice make them ideal for studying developmental processes and modeling human diseases. These attributes, coupled with cutting-edge genetic tools such as transgenesis, gene knockouts, conditional gene knockouts, and genome editing, provide a significant resource to the research community to test a plethora of hypotheses. This review summarizes the mouse models available for studying a wide variety of erythroid-related questions, as well as the properties inherent in each one.
Keywords: Mouse models, Transgenic mice, Knockout mice, Conditional knockout mice, Cre-loxP, Erythropoiesis, Globin gene switching, Hemoglobin
1. Introduction
Mouse models provide valuable resources to understand the molecular mechanisms underlying normal cellular processes of development, repair, and regeneration, as well as associated genetic perturbations resulting in disease. In addition, mouse models serve as experimental vehicles in which to test pharmaceutical and gene therapies. The common house mouse, Mus musculus, has been utilized in this capacity for over a century, beginning with inbred strains of mice as human disease models, leading to the mouse genome project, transgenics and genome editing capabilities of present day. Mice have a physiology similar to humans and their long history of use offers many advantages over other model systems. Early studies produced several thousand spontaneous and radiation-induced mutant strains, which are still available from a number of commercial vendors. These are useful because mutations in mouse genes homologous to their human counterparts often result in disease etiologies similar to humans. Later, inbred and congenic strains were created; these provide a uniform genetic background on which to perform studies free from the effect of modifier genes and polymorphisms. More recently, the advent of transgenic and gene targeting methodologies makes it possible to probe the biochemical and molecular pathways of human gene expression, offering the possibility that understanding these processes might lead to points of intervention in disease etiology. Variants of these two procedures made mice the animal of choice for modeling erythropoiesis and red blood cell diseases. Transgenesis allows introduction of human gene sequences or entire loci into the mouse genome or “knock-in” of human sequences to replace their mouse counterparts, while gene targeting to create gene “knockouts” or conditional knockouts, permits genetic studies of the endogenous globin loci, which often are arranged and regulated to a large degree like the human loci. Finally, the 21-day parturition period in mice compacts developmental studies into a useful time frame.
2. Globin Gene Switching and Erythropoiesis
Much of the technology utilized in modern mouse models was developed and/or applied to studies of the hematopoietic system, largely to work on globin gene switching and erythropoiesis. Novel transgenesis technology created opportunities for studying human gene expression in vivo within an animal model. Previous work was limited to analysis of transgenes in established cell lines. Murine transgenesis was particularly attractive, since endogenous globin synthesis had been well characterized during embryogenesis [1]. Developmental studies on hematopoiesis and globin gene switching could be carried out on staged embryos and fetuses during pregnancy beginning with the onset of erythropoiesis at day seven post-conception through post-partum in adult mice. All of the hematopoietic tissues present at a particular stage of development could be assessed, including yolk sac, liver, bone marrow, spleen, and peripheral blood.
3. Early Transgenic Models
Transgenic mice were first produced in the early 1980s by a number of investigators using simple gene or cDNA sequences linked to a promoter of choice. Most of the transgenes integrated in these mice suffered from position effect variegation (PEV) and as the technique developed, various cis-acting regulatory elements were included in transgene constructs in an attempt to overcome PEV, including enhancers, introns, insulators, and poly-adenylation signals. Only after locus control regions (LCRs) were discovered and incorporated into transgenes was PEV ameliorated [2].
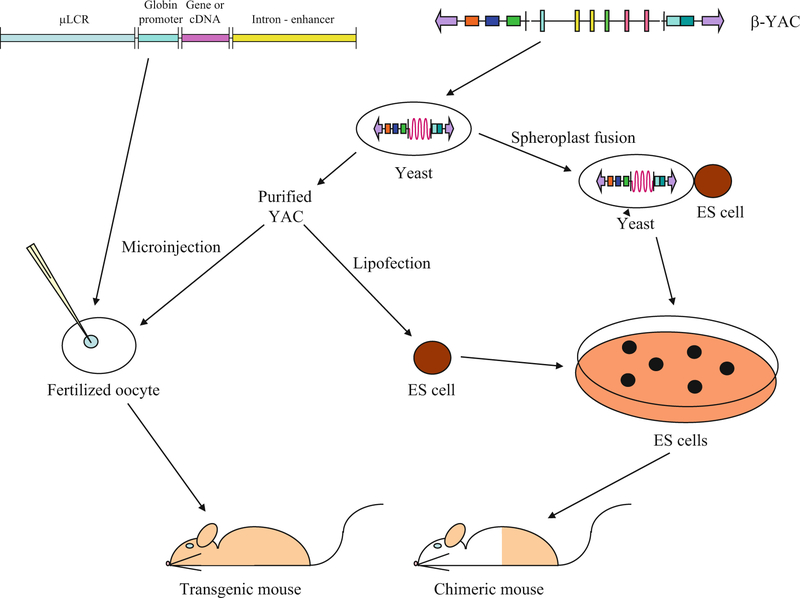
Initial experiments involved microinjection of simple trans-genes encompassing the human adult β-globin gene or the fetal γ-globin gene (Fig. 1, left side) [3, 4]. Expression was affected by position-of-integration of the transgenes within the murine genome and copy number-dependence was not observed, hallmarks of PEV. In spite of variable levels of expression associated with PEV, when the transgenes were expressed, correct regulation regarding developmental stage- and tissue-specificity was observed, demonstrating that cis-regulatory elements controlling spatial and temporal expression were gene-proximal. Discovery of the β-globin locus LCR in 1987 provided a major breakthrough in globin gene switching [2]. The LCR consists of a collection of DNA regions upstream of the β-like globin gene cluster that are hypersensitive to DNaseI. The LCR opens up the globin domain and makes it available for transcription and is required for high-level expression of all the genes within the cluster. When this element was linked to either a globin gene or a heterologous gene, PEV disappeared; that is, site-of-integration-independent, copy number-dependent expression of the linked transgene was obtained [2]. Generally, all of these transgenes used fragments that encompassed one or two human β-like globin genes coupled to derivative LCR sequences, such as mini- or micro-LCR cassettes (μLCR), individual, or multiple DNaseI-hypersensitive sites (HSs). As transgenic technology advanced, and more DNA could be successfully microinjected into oocytes in an intact state, multiple β-like genes could be linked to μLCRs or the intact LCR could be linked to single-globin genes in cosmid constructs [5].
Fig. 1.
Transgenesis with small recombinant or whole loci globin constructs. A prototypical small recombinant globin transgene is illustrated at the upper left. Most early transgenes consisted of LCR sequences (mini- or micro-LCRs, one or more 5′HSs, or the entire LCR), a globin promoter linked to its gene sequences, hybrid globin gene sequences, or a cDNA reporter (occasionally a non-globin promoter was linked to a globin gene or cDNA cassette), and a globin or heterologous intron and/or enhancer (or not). These purified transgenes were microinjected into the pronucleus of fertilized mouse oocytes to produce transgenic mice as shown schematically. Cosmid and bacterial artificial chromosomes (BACs) containing larger β-like globin locus inserts were similarly microinjected (not shown). Methods used to generate transgenic mice with yeast artificial chromosomes (YACs) are illustrated beginning with the human β-globin locus YAC (β-YAC) shown at the upper right. The β-YAC (155 or 215 kb) and other YACs ranging up to 650 kb in size may be purified from the yeast host and microinjected similar to small recombination DNA constructs or lipofected into embryonic stem (ES) cells, which are then utilized to produce chimeric mice and establish transgenic lines. Alternately, the YAC may be transferred to ES cells by yeast spheroplast-ES cell protoplast fusion. Lipofection or fusion must be employed when the YAC exceeds 650–800 kb in size
Analysis of α-globin transgenics identified the HS-40 upstream master regulatory region, and cis-regulatory elements controlling ζ-globin and α-globin gene regulation [6, 7]. Particularly notable were studies of the 3′untranslated region (UTR) and its role in α-globin mRNA stability (reviewed in [8]).
4. YAC and BAC Transgenesis
The 1990s saw the advent of whole human loci transgenesis using first yeast artificial chromosomes (YACs), then bacteriophage P1 artificial chromosomes (PACs), and later bacterial artificial chromosomes (BACs). YACs and BACs allow the manipulation of very large stretches of DNA, permitting the analysis of entire gene clusters such as the β-globin locus. Transgenesis with these vectors resulted in murine models that more accurately mirrored human gene expression [9–11]. The first human β-globin locus yeast artificial chromosome (β-YAC) transgenic mice were produced in 1993 [12, 13], ushering in the era of whole locus transgene studies (Fig. 1, right side). More recently, the use of β-globin locus bacterial artificial chromosomes (β-BACs), coupled with “recombineering,” has been employed [14]. Both types of transgenes allow the introduction of alterations within the context of the entire human β-locus using homologous recombination in either yeast or bacteria, respectively, without retention of exogenous DNA sequences. Thus, the effect of the mutation may be studied in an intact locus throughout development within the mouse. Initial studies demonstrated that the human globin gene expression pattern was, by and large, recapitulated during ontogeny in the mouse. Modified transgenes were utilized to produce hereditary persistence of fetal hemoglobin (HPFH) and sickle cell mouse models (reviewed in [15, 16]).
Many of these transgenics were mated with murine knockout mutant lines to produce the mouse models of sickle cell disease and thalassemia now utilized to study the pathophysiology of these diseases.
5. Knockout Models
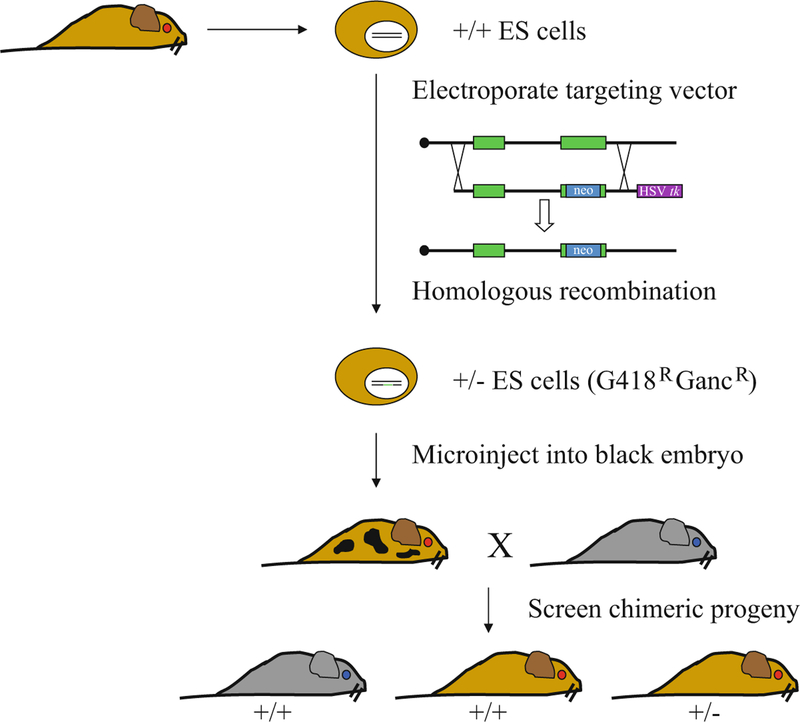
Coincidental and in parallel to transgenic studies, were projects analyzing the regulation of the endogenous murine β-like and α-like globin genes. The strategy in this case utilized gene targeting and chimeric mice to produce knockout mutations of genes or globin regulatory sequences (Fig. 2). In general, the deficiencies produced revealed similar mechanisms of action regarding globin gene switching in humans. One important caveat is that mice do not have a fetal globin analogous to the human γ-globin genes. A single switch from embryonic globin synthesis to definitive globin synthesis occurs, whereas two switches exist in humans, from primitive embryonic erythropoiesis to fetal definitive erythropoiesis and later from fetal definitive erythropoiesis to adult definitive erythropoiesis. However, the sites of hematopoiesis during development are conserved between mice and humans. Although γ-globin gene expression is confined to the fetal liver in humans, it is expressed mainly in the embryonic yolk sac in the mouse. Expression in the yolk sac can be attributed to the evolutionary homology of the human γ-globin and murine βh1-globin genes, both of which derive from an ancestral γ-globin gene. Mouse βh1-globin gene expression is limited to the embryonic yolk sac; the human γ-globin gene apparently “remembers” its evolutionary origin when in mice and is likewise expressed. However, unlike the murine βh1-globin gene, expression of the human γ-globin genes in the mouse continues during fetal liver definitive erythropoiesis and in a small population of adult definitive red cells [13]. Thus, studies on γ-globin gene expression can only be carried out in mice using the human transgene approach. Knockouts of adult α-globin and β-globin provided the mouse lines that were bred with human transgenic lines to produce mouse models of hemoglobinopathies, in which only human globin chains are incorporated into the hemoglobin molecule.
Fig. 2.
Generation of targeted mutations in endogenous murine globin loci. ES cells usually derived from 129 mice (brown coat color) are electroporated with a gene-targeting construct, a generalized version of which is shown. Following drug selection and screening for proper integration of the targeting vector, the ES cells are microinjected into blastocysts normally derived from C57Bl6 mice (black coat color). Chimeric mice are identified initially based on coat color, which is a varying mixture of brown and black. If the ES cells contributed to the germ line, the targeted mutation will be inherited by the next generation of mice as indicated at the bottom of the figure. Following homologous recombination in the target ES cells, the neo cassette confers resistance to the drug G418; loss of the HSVtk cassette following homologous recombination results in ganciclovir (GANC) resistance
6. Sickle Cell, Thalassemia, and Cooley’s Anemia Models
A number of mouse models for various hemoglobinopathies have been generated (Table 1). Using slightly different approaches for transgenesis and gene knockouts and knock-ins, alone or in combination, lines modeling sickle cell disease, β- or α-thalassemias, and Cooley’s anemia have been produced [17]. In many instances, the human phenotype for each of these diseases is remarkably conserved in the mouse (reviewed in [17, 18]). These mice offer the possibility for understanding the pathophysiology of these diseases in more detail, may serve as vehicles to test emerging pharmaceutical compounds and gene therapy approaches, will be useful for the isolation and proliferation of hematopoietic stem cells (HSCs), embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs), and for testing the efficacy of bone marrow transplantation and engraftment of gene-corrected stem cells.
Table 1.
Murine models of sickle cell disease, thalassemias and Cooley’s anemia
| Genotype | Phenotype | Severity of disease | |
|---|---|---|---|
| SCD mouse model | |||
| S Antilles | αH βS Antilles | Mild adult anemia Low irreversible sickling |
Mild |
| S+S Antilles | αH βS βS Antilles | Neonatal anemia Significant sickling |
Mild—moderate |
| SAD | αH βSAD | Neonatal anemia Low irreversible sickling Multi-organ pathology |
Moderate |
| Berkeley model (murine knockout) | αH βS γH δH | Significant adult anemia High irreversible sickling Chronic multi-organ damage |
Moderate—severe |
| Birmingham model (murine knockout) | αH βS γH | Severe anemia High irreversible sickling Multi-organ pathology |
Moderate—severe |
| San Francisco model (murine knockout) | αH βS γH δH (YAC) | Anemia Irreversible sickling |
Moderate—severe |
| Enhanced γ-globin expression models | αH βS (γHa/γHb/γHc) and αH βSAD γH | Decreased severity of disease with increasing HbF levels | Moderate—severe |
| Thalassemia mouse model | |||
| Carolina β0 (murine knockout) | Δb1/b2 | Heterozygous mice dramatically decreased hematocrit, hemoglobin, red blood cell counts, MCV, MCH, and MCHC, dramatically increased reticulocyte counts, serum bilirubin concentrations, and red cell distribution widths, display tissue and organ damage, spontaneous iron overload in spleen, liver, kidneys Homozygous mice: die in utero |
Severe |
| Birmingham β0 (murine knockout) | Δβmaj/βmin | Heterozygous mice: severely anemic, dramatically reduced hemoglobin levels, abnormal red cell morphology, splenomegaly, markedly increased reticulocyte counts Homozygous mice: die in utero |
Severe |
| βIVS-2-654 (murine knockout/human knock-in) | Δβmaj/βmin/HuβIVS-2–654 | Heterozygous mice: reduced mouse β globin chains and no human β globin Homozygous mice: do not survive postnatally |
Moderate |
| Cooley’s anemia (β thalassemia major) (murine knockout/human knock-in) | Δβmaj/βmin/Hu γβ0 Δα1/α2/Hu Δα2/α1 |
Heterozygous γβ0 knock-in mice: β thalassemia intermedia Homozygous mice: expire due to severe anemia upon completion of the HbF to adult switch after birth |
Severe |
| Cooley’s anemia (β thalassemia major) Preclinical model (murine knockout/human knock-in) | Δβmaj/βmin/Hu γHPFHδβ0 Δα1/α2/Hu Δα2/α1 |
Fully humanized mice survive postnatally by synthesizing predominantly HbF, some HbA2, completion of fetal to adult Hb switch after birth results in severe anemia marked by erythroid hyperplasia, ineffective erythropoiesis, hemolysis, and death | Severe |
| Berkeley α (murine knockout) | Δα1/α2 | Homozygous mice: become hydropic, die late in gestation, model for human hydrops fetalis | Severe |
αH, γH, δH, human α-, γ- and δ-globin genes; βS, human β-globin gene with HbS mutation; βS Antilles, human β-globin gene with HbS Antilles mutation; βSAD, human β-globin gene with HbS, HbS Antilles and HbD Punjab mutations; γHa/γHb/γHc: human γ-globin genes expressing three different levels of HbF. See text for references
Early models of sickle cell disease models (Table 1) include S Antilles and a modified version of this model [19, 20], S+S Antilles [21–23], and SAD [24, 25]. Although these models provided important information about sickle cell disease, they only mimicked the sickle cell trait exhibited in human βS heterozygous carriers, and did not present the severe hemolytic anemia that is a hallmark of homozygous sickle cell patients. The phenotypes associated with these mice were due to the inhibition of sickling by endogenous murine hemoglobin.
Newer SCD model mice (Table 1) express exclusively human globins and overcome the failure of earlier models to exhibit faithful sickle cell pathology. They include the Berkeley model, the Birmingham model, and the San Francisco model [33–36]. None of these models express murine α- and β-globin genes. Berkeley model mice express exclusively human α-, βS-, and γ-globin genes [26]. The Gγ-and Aγ-globin genes were included because γ-globin has anti-sickling properties that decreased the likelihood of fetal death during gestation as the switch from primitive embryonic erythropoiesis to fetal definitive erythropoiesis occurred. This model also showed some characteristics of β-thalassemia, including an increased susceptibility to oxidative damage due to free-radical production. The Birmingham mouse was the first model produced that displayed the sickle trait [27]. Subsequent improvements led to the creation of an improved SCD mouse model containing human Aγ-, βS-, and α1-globin genes driven by the human β-globin LCR [28]. Although coinjection of human globin constructs in the Berkeley and Birmingham models led to the integration of all the genes at a single chromosomal site, normal gene order and spatial organization required for appropriate expression of the members of the β-like globin gene family was disrupted. The San Francisco model partially remedied this by creating novel sickle cell mice harboring a βS-globin YAC containing the entire human β-globin gene cluster and the human α2-globin gene linked to a mini-LCR [29].
One other group of SCD mouse models are the enhanced γ-globin expression models. Increasing the level of human γ-globin in transgenic mice to various levels in the SAD mouse improved life span, pathology, and hematological profile [30], thereby establishing the physiological range of HbF that would alleviate the SCD condition in mice. Another set of knockout mice expressing exclusively human globins and three levels of HbF (low, medium, and high) also showed a more faithful SCD pathology than the previous models [31]. In this model, murine knockout mice [26, 32] were mated with mice that expressed the co-integrated human mini-LCRα2 and mini-LCRβS used to create the S+S-Antilles model. Lethality of these mice was rescued by breeding with transgenic mice expressing three different human γ-globin constructs (γL, γM, γH) with increasing postnatal HbF levels. These mice exhibited more balanced α- and β-globin chain synthesis and thus present a closer approximation of human SCD.
Thalassemia model mice were generated with deletions of adult murine β-like globin genes, βmajor and βminor [32, 33] and both adult α-globin genes [34]. The disease phenotypes of these mice were rescued by the introduction of human globin transgenes. Mouse models with deletions in βmajor- and α-globin chains show varying severity of disease [33–35]. The βIVS-2–654 C→T mutation, which causes aberrant RNA splicing and leads to β0 thalassemia, has also been modeled in mice [36]. Two models of β thalassemia major or Cooley’s anemia were produced. Both are totally humanized; that is, they carry the human α2/α1 genes and a human γβ0 gene cassette [37] or γHPFHδβ0 gene cassette [38]. In the homozygous state, both the mouse strains expire shortly after birth due to severe anemia following the completion of the γ-globin to β0-globin switch. However, the later mouse model can be rescued from lethal anemia by regular blood transfusions.
7. Conditional Knockout Models
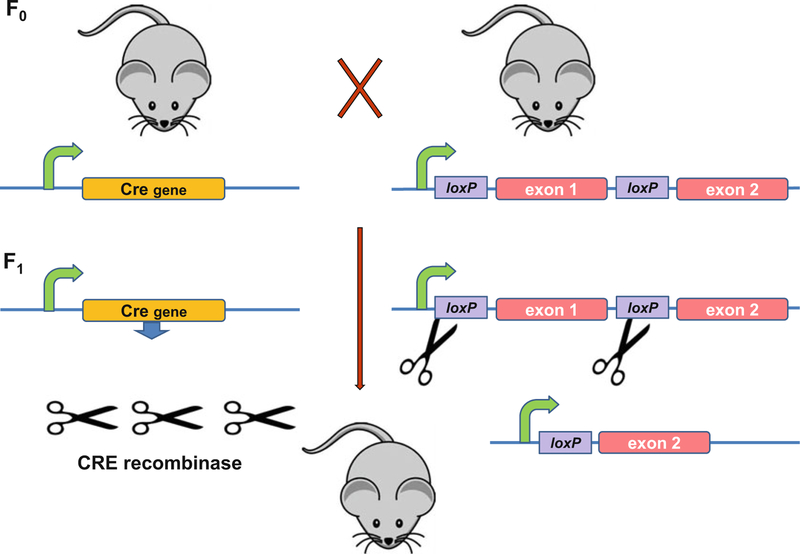
Manipulation of endogenous murine genes by gene targeting has been performed primarily in embryonic stem (ES) cells. Gene targeting uses homologous recombination to generate null alleles or gene “knockouts” or to substitute an altered transgene for the native chromosomal allele (“knock-ins”). The technique is a powerful tool for studying gene function in hematopoiesis. However, this classical method for producing null mutations results in the loss of gene expression in all tissues and at all developmental stages. The mutation may be lethal, or affect other tissues and developmental pathways not under analysis, consequently complicating interpretation of data. Thus, it is important that the inactivation of a particular gene occurs in a conditional manner, such as in a predefined tissue or at a certain stage of development. The gene-targeting approach to study gene function in mice has been refined with the advent of the bacteriophage P1 Cre-loxP site-specific recombination system. Using this technology, tissue- or developmental stage-specific gene knockouts may be produced. Genes are expressed normally in untargeted tissues and the effect of the null mutation is confined to the tissue of interest.
Cre is a 38 kDa protein that catalyzes reciprocal site-specific recombination between 34 bp loxP sequences on DNA (Fig. 3). If two loxPCre/loxP sites are in the same orientation in a genomic locus, expression of Cre results in the deletion of loxP-flanked DNA sequence in both the prokaryotic and eukaryotic cells. Generally, the two loxP sites are introduced into the genome such that they flank an essential portion of the target gene without affecting its function. Following Cre recombinase expression the gene is conditionally inactivated, depending upon the cell type-specific or inducible promoter that defines the spatial and temporal synthesis of Cre protein.
Fig. 3.
Conditional gene knockouts in mice using the Cre recombinase-loxP recombination system. A conditional knockout allele is produced by gene targeting as shown in Fig. 2. In this instance, the targeting construct contains two 34 bp loxP sites inserted in intergenic DNA sequence, usually in introns flanking an exon, multiple exons, or an exon plus the promoter of the targeted gene (right side of figure). The loxP sites do not interfere with gene expression. The resultant mice are bred with Cre expression mice (left side of figure), in which Cre recombinase gene expression is driven by a tissue-specific, developmental stage-specific, or inducible promoter, or a combination of these. Cre recombinase catalyzes excision of the loxP-flanked (floxed) DNA sequences, resulting in a targeted deletion and leaving behind a single loxP site. Intergenic sequences are shown in blue; promoters are indicated as curved green lines
A number of Cre drivers have been utilized to produce conditional knockouts in the hematopoietic/erythropoietic lineages. These are summarized in Table 2. The range of lineage specificity is wide; several are not strictly specific to erythroid, or more broadly, hematopoietic lineages. However even those with broad tissue/developmental range have been useful in the production of conditional knockouts, or activation of mutant versions of genes affecting erythropoiesis. Properties of these Cre transgenes, including name, tissue-specificity, developmental stage-specificity, and compound inducibility, are summarized in Table 2. The efficiency of recombination between loxP sites resulting in excision and production of null mutants varies between the available Cre drivers and in some instances efficiency is influenced by the floxed target. Many of the Cre driver mice are available commercially from vendors such as Jackson Laboratories. Wherever possible the reference describing the original synthesis of the Cre construct and generation of transgenic mice is included, so that an investigator desiring to obtain these mice will be able to contact the laboratory of the principal investigator where they were created.
Table 2.
Cre drivers
| Gene promoter controlling expression of Cre-recombinase | Tissues/cell types/stage-specificity | Reference |
|---|---|---|
| Erythropoietin receptor (EpoR) promoter (ErGFPcre) | Erythroid progenitors/erythroid-specific lineage. EpoR expression initiates at the erythroid burst-forming unit stage (BFU-E), and reaches maximal expression at the erythroid colony-forming unit (CFU-E) and/or proerythroblast stage, and decreases thereafter. | [42] |
| Vav promoter (VavCre) | Hematopoietic tissues and vascular endothelium. | [43] |
| Mx1 promoter (Mx-Cre or Mx1-Cre) | This promoter is silent unless activated via the administration of interferon a, interferon p, or a synthetic poly I:C double-stranded RNA. Expression is not tissue-specific or developmental stage-specific, and expression can be activated at any point during development. | [44] |
| GATA-1 promoter (GATA1-Cre) | GATA1 is a transcription factor necessary for erythroid-specific gene expression and is essential for both primitive and definitive erythropoiesis. Low level expression is achieved in CD34+ hematopoietic cells and high level expression is observed in eosinophils, mast cells, megakaryocytes, and erythroblasts. | [45] |
| Rosa26 promoter fused to the Cre ORF and the ligand- binding domain of the human estrogen receptor (Rosa 26CreER) | Rosa26 is highly and ubiquitously expressed in most cells and tissues of adult mice. CreER recombinase is inactive unless stimulated by the administration of the synthetic estrogen receptor ligand, 4-hydroxytamoxifen (OHT), allowing for temporal regulation of Cre activity. | [46] |
| Receptor tyrosine kinase Tek (Tie2) promoter/enhancer (Tie2Cre) | The Tie2 promoter/enhancer is expressed consistently in endothelial cells during embryogenesis and adulthood. The Tie2Cre system can delete floxed targets in vascular endothelial cells. | [47] |
| Mouse mammary tumor virus (MMTV) long terminal repeat (LTR) promoter (MMTV-LTR Cre) | MMTV-LTR promoter drives high levels of expression in virgin and lactating mammary gland, salivary gland, seminal vesicle, skin, erythrocytes, B cells, and T cells. | [48] |
| μ-LCR cassette-β-globin promoter-Cre construct (HBB-cre) | μ-LCR cassette-β-globin promoter stimulates high levels of Cre recombinase in a erythroid/megakaryocytic cell lineage-specific manner. | [49] |
| EllaCre | The adenovirus Ella promoter targets Cre recombinase expression to early mouse embryos. Cre expression is mosaic, and occurs in a wide variety of tissues including germ cells. | [50] |
| iCre | Mammalian codon-optimized Cre recombinase that improves Cre translation/function and reduces the high CpG content of the prokaryotic coding sequences, thus diminishing the chance of epigenetic silencing in mammalian systems. Included here for informational purposes as newer Cre drivers will utilize this variant with or without tamoxifen inducibility. | [51] |
Mice bearing floxed target genes important to erythropoiesis or some aspect of red blood cell biology have been culled from the literature and the list is summarized in Table 3. The name of each gene is listed, along with basic properties of the encoded protein, the major red cell property or process in which the gene product functions (and consequently that the null mutation affects), diseases modeled, and the earliest reference describing creation of the mice bearing the erythroid-specific null mutation (or activation of a mutated version of the gene in the erythroid compartment). Processes modeled by these Cre-lox mice include globin gene switching, hematopoietic stem cell function, erythropoiesis, and iron metabolism. Diseases modeled include paroxysmal nocturnal hemoglobinuria (PNH), polycythemia-like disease, erythrocytosis, chronic normocytic, normochromic anemia (preclinical model for the anemia of Epo deficiency), and human myelodysplastic syndromes (defects in cell cycle regulation and mitochondrial function).
Table 3.
Cre-loxP-mediated conditional knock-outs or knock-ins affecting erythropoiesis
| Floxed allele | Gene product description | Process under investigation | Phenotype/process affected | Reference |
|---|---|---|---|---|
| Zbtb7a | Transcription factor: Zinc finger and BTB domain containing 7A | Hematopoietic Stem Cell (HSC) maintenance | Embryonic lethal, anemia | [52] |
| Myc | Transcription factor: Myc proto-oncogene protein | Primitive Hematopoiesis | Embryonic lethal, blocks HSC differentiation, absence of primitive erythrocytes | [53] |
| SCL/Tal-1 | Transcription factor | Hematopoietic Stem Cells | Embryonic lethal, genesis of HSCs, differentiation into erythroid and megakaryocytic progenitors, anemia, thrombocytopenia | [54] |
| Asxl1 (also Asxl1: nlacZ/nGFP knock-in) | Transcription factor: Additional sex combs like transcriptional regulator 1 | Erythropoiesis | Embryonic lethal, myelodysplastic syndrome | [55] |
| Meis1 | Transcription factor: Homeobox protein Meis1 | Erythropoiesis | Primitive hematopoietic repopulating cells and megakaryocytic/erythroid progenitor expansion, abnormal HSC function | [56] |
| Klf1 | Transcription factor: Krüppel-like factor 1 | Hemoglobin Switching | Embryonic lethal, β-like globin gene expression, anemia | [57] |
| Klf2 | Transcription factor: Krüppel-like factor 2 | Hemoglobin Switching | Embryonic lethal, β-like globin gene expression, anemia | [57] |
| Bcl1 1a | Transcription factor: C2H2 type zinc-finger | Hemoglobin Switching | γ-globin (HbF) repression | [58] |
| Mi2/β | Chromodomain helicase DNA binding protein 4 | Hemoglobin Switching | γ-globin (HbF) repression | [59] |
| Atg7 | Interacting protein: Ubiquitin - activating enzyme E1-like protein | Erythropoiesis | Mitochondrial autophagy, splenomegaly, death via severe anemia | [60] |
| Med1 | Mediator complex subunit 1: required for SP1 activation as part of CRSP complex | Erythropoiesis | Block in erythroid development | [61] |
| Taf10 | Interacting protein: TATA-box binding protein associated factor 10 | Erythropoiesis | Embryonic lethal | [62] |
| Gfi 1b | Transcription factor: Growth factor independent 1 B transcription repressor | Erythropoiesis | Abnormal HSC function | [63] |
| Tsc 1/Rptor | Interacting protein: Tuberous sclerosis 1 interacting protein/regulatory associated protein of mTOR complex 1 | Erythropoiesis | Role of mTORC1 in erythropoiesis, increased neonatal mortality, macrocytic/microcytic anemia | [64] |
| Ldb1 | Interacting protein: LIM domain binding 1 | Erythropoiesis | Embryonic lethal, abnormal erythroid gene expression, defective primitive erythropoiesis, continuously required for definitive erythropoiesis and megakaryopoiesis | [65] |
| Pit1 | Interacting protein: Solute carrier family 20 (phosphate transporter) member 1 | Erythropoiesis | Erythroid maturation, anemia, myelodysplastic syndromes | [66] |
|
Sf3b1K700E knock-in |
Interacting protein: Splicing factor 3b subunit 1 | Erythropoiesis | Impaired erythropoiesis and aberrant splicing, myelodysplastic syndromes | [67] |
| Miz-1 | Transcription factor: Myc-interacting zinc finger protein 1 | Erythropoiesis | Embryonic lethal, required for embryonic and stress-induced erythropoiesis, perturbed erythroid differentiation and development, severe anemia | [68] |
| Brg1 | Transcription factor: Brahma-related gene 1 | Erythropoiesis, Vascular Development | Embryonic lethal, altered globin expression, anemia, erythropoietic/vascular abnormalities | [69] |
| RhoA | Hub protein: Ras homolog family member A | Erythropoiesis | Embryonic lethal, cytokinesis in erythroblasts, failed definitive erythropoiesis, anemia | [70] |
| Spry1 | Interacting protein: Sprouty RTK signaling antagonist 1 | Erythropoiesis | Perturbed erythroid development, reticulocytosis, heightened splenic erythropoiesis, regulator of erythropoiesis during anemia, transducer of EPOR signals, and candidate suppressor of Jak2 activity | [71] |
| Vegf | Growth factor: Vascular endothelial growth factor | Erythropoiesis | Erythropoietic lineage development, increased Gatal levels, increased erythroid differentiation | [72] |
| Stat5 | Transcription factor: Signal transducer and activator of transcription 5A | Erythropoiesis | Reduced transferrin receptor gene expression, microcytic, hypochromic anemia | [73] |
| cdc42 | Rho GTPase Cdc42: Cell division control protein 42 homolog | Erythropoiesis | Balance between myelopoiesis and erythropoiesis, altered gene expression, fatal myeloproliferative disorder, anemia, splenomegaly | [74] |
| Tfr2 | Membrane protein: Transferrin receptor 2 | Iron Metabolism | Liver iron overload, inadequate hepcidin levels, iron-deficient anemia | [75] |
| Mfrn1 | Mitochondrial iron transporter: Mitoferrin-1 | Iron Metabolism | Embryonic lethal, reduced erythroblast formation, severe anemia | [76] |
| Piga | Enzyme: Phosphatidylinositol glycan anchor biosynthesis class A | Erythropoiesis | Embryonic lethal, paroxysmal nocturnal hemoglobinuria (PNH) | [45] |
| Jak2V617F knock-in | Non-receptor tyrosine kinase: Janus kinase 2(JAK2) | Erythropoiesis | Polycythemia-like disease (all major features of human polycythemia vera) | [77] |
|
Phd1, Phd2, Phd3 |
Enzyme: Prolyl hydroxylase domain protein 1, 2, or 3 (3 PHD isoforms) | Erythropoiesis | Erythrocytosis | [78] |
| Epo | Growth factor: Erythropoietin (EPO) | Erythropoiesis | Embryonic lethal, chronic, normocytic, normochromic anemia, pre-clinical model for anemia of Epo deficiency | [79] |
| pRb | Tumor suppressor protein: Retinoblastoma protein | Erythropoiesis | Defects in cell cycle regulation and mitochondrial function, anemia, myelodysplastic syndromes | [80] |
| Trim33 | Transcriptional corepressor: Tripartite motif-containing 33, also known as transcriptional intermediary factor 1 gamma (TIF1-γ) | Erythropoiesis | Embryonic lethal | [81] |
| Bcl-x | Anti-apoptotic factor: Bcl-2-like protein 1 | Erythropoiesis | Required for the survival of erythroid cells at the end of maturation, which includes enucleated reticulocytes in circulation, hemolytic anemia, hyperplasia of immature erythroid cells, splenomegaly | [82] |
| Nix | Pro-apoptotic factor: BH3-only-like protein | Erythropoiesis | Reticulocytosis, thrombocytosis, splenomegaly, splenic and bone marrow erythroblastosis, reduced apoptosis during erythrocyte maturation | [83] |
| Tr2/Tr4 | Transcription factor: Testicular receptor 2 (TR2), Testicular receptor 4 (TR4) | Hemoglobin Switching | Embryonic lethal, γ-globin gene repression | [84] |
| Zbp-89 (Zfp148) | Transcription factor: Zinc finger protein 148 | Hematopoiesis | Stress erythropoiesis, erythroid lineage development, anemia, thrombocytopenia, myeloid and B lymphoid lineage anomalies, altered HSC gene expression | [85] |
| Trim28 | Transcriptional cofactor: Tripartite motif-containing protein 28, also known as transcriptional intermediary factor 1 beta (TIF1β) | Erythropoiesis | Cell- autonomous development of immature erythroblasts in bone marrow, essential for erythroblasts differentiation, anemia, increased apoptosis, reduced erythroid transcription factor expression, reduced heme biosynthesis | [86] |
| Ccbel | Lymphangiogenic factor: Collagen and calcium-binding epidermal growth factor domain-containing protein 1 | Primitive Erythropoiesis | Secreted lymphangiogenic CCBE1 is essential for fetal, but not postnatal erythropoiesis, loss of CCBE1 signaling impairs erythroblastic island formation and function, not required in hematopoietic cells, severe anemia, increased apoptosis | [87] |
| Sur | Inhibitor of apoptosis: Survivin | Erythropoiesis | Essential for steady-state hematopoiesis and survival of adult, high level expression critical for proper erythroid differentiation, bone marrow depletion, pancytopenia, erythrocyte abnormalities, hypocellular spleen, hematopoietic cell death | [88] |
| Runxl | Transcription factor: Runt-related transcription factor 1 | Hematopoiesis | Embryonic lethal, not required in adult hematopoietic compartment, abnormal HSCs, defective T- and B-cell maturation, inefficient platelet production, mild myeloproliferative phenotype | [89] |
| α4 | Transmembrane receptor: α4 integrin subunit | Hematopoietic Stem Cells | Progenitor cell influx into the peripheral blood, delayed erythroid and myeloid regeneration, hematopoietic abnormalities | [90] |
| Raf-1 | Enzyme: RAF proto-oncogene serine/threonine-protein kinase | Erythropoiesis | Embryonic lethal, anemia, accelerated erythroid differentiation | [91] |
| Jak2 | Non-receptor tyrosine kinase: Janus kinase 2 | Hematopoiesis | Embryonic lethal, impaired hematopoiesis, splenic atrophy, severely diminished erythropoiesis and thrombopoiesis, modestly affected granulopoiesis and monocytopoiesis | [92] |
| Abcb7 | Mitochondrial ATP-binding cassette sub-family B member 7 | Hematopoiesis | X-linked sideroblastic anemia with ataxia (XLSA/A) due to inhibition of heme biosynthesis, damaged mitochondria, pancytopenia, hypocellular bone marrow | [93] |
| Ufbp1 | Inhibitor of apoptosis in ER-stressed cells: Ufm1 binding protein 1 | Hematopoiesis | Embryonic lethal, impaired hematopoiesis, defective erythroid development, pancytopenia, suppressed GATA-1 and KLF1 expression | [94] |
| Ezh2 | Enzymatic component of Polycomb Repressive Complex 2: Enhancer of zeste homolog 2 | Hematopoietic Stem Cells | Embryonic lethal, essential for fetal liver, but not bone marrow erythropoiesis, defective HSC expansion, compromised lymphopoiesis, reduced H3K27me3 levels suggesting epigenetic status of HSCs is developmentally regulated, anemia | [95] |
| Rcor1 | Transcriptional corepressor: REST (repressor element-1 silencing transcription factor) corepressor 1 | Hematopoietic Stem Cells | Embryonic lethal, promotes erythropoiesis by repressing HSC and/or progenitor genes, as well as genes and signaling pathways that lead to myeloid cell fate, block in fetal erythropoiesis at the proerythroblast stage, anemia | [96] |
| Setd8 | Histone Methyltransferase: monomethylates histone H4 lysine 20 (H4K20mel) | Erythropoiesis | Embryonic lethal, defect in primitive erythropoiesis, abnormalities in cell cycle progression, regulates erythroid maturation, represses Gata2 expression, severe anemia | [97] |
| Smad4 | Signal transduction: Mothers against decapentaplegic homolog 4 |
Erythropoiesis | Not required for adult erythropoiesis, severe anemia due solely to blood loss, iron deficiency | [98] |
| Ulk1 | Enzyme: Serine/threonine-protein kinase | Erythropoiesis | Autophagy machinery that leads to the elimination of organelles in erythroid cells | [99] |
| Ck2β | Enzyme: serine-threonine kinase composed of two catalytic (α) and two regulatory (β) subunits | Hematopoiesis | Embryonic lethal, regulates definitive hematopoiesis of all hematopoietic cell lineages | [100] |
| Flvcr1a | Heme exporter membrane protein: Feline leukemia virus subgroup C receptor 1 | Erythropoiesis | Embryonic lethal, heme export control, severe macrocytic anemia, splenomegaly, iron accumulation in duodenum, liver and spleen, reduced BFU-E and CFU-E, defective erythroid differentiation | [101] |
| Rhau | RNA helicase: RNA helicase associated with AU-rich element | Hematopoiesis | Embryonic lethal, hemolytic anemia, differentiation defect at the proerythroblast stage | [102] |
| Add3 | Adducins: Skeletal protein involved in the assembly of spectrin-actin network | RBC membrane skeleton structure | Animals were viable and presented no obvious erythroid cell defects | [103] |
| cyclin ET74A T393A knock-in | Cell cycle regulator: Complexes with the CDK2 cyclin-dependent kinase subunit | Erythropoiesis | Links ubiquitin-proteasome pathway control of G1-to-S-phase progression to regulation of metabolism and gene expression in terminally differentiating bone marrow erythroid cells, abnormal erythropoiesis characterized by a large expansion of abnormally proliferating progenitors, impaired differentiation, dysplasia, and anemia, models early stage human refractory anemia/myelodysplastic syndrome | [104] |
| Rps14 | 40S ribosomal subunit protein S14 | Erythropoiesis | Erythroid differentiation defect dependent on p53 characterized by apoptosis during transition from polychromatic to orthochromatic erythroblasts, age-dependent progressive anemia, megakaryocyte dysplasia, block in erythroid differentiation, loss of HSC quiescence | [105] |
| Rps6 | 40s Ribosomal subunit protein S6 | Erythropoiesis | Hypoproliferative macrocytic anemia, granulocytopenia, thrombocytosis, lymphopenia (model for Diamond-Blackfan Anemia and myelodysplastic syndrome) | [106] |
| Ppp2ca | Enzyme: Serine/threonine phosphatase, protein phosphatase 2A | Erythropoiesis | Perturbed definitive erythropoiesis characterized by fetal liver atrophy, reduced Terll9+ cell number, atypical expression patterns of molecular markers, reduced colony formation, reduction in definitive globin gene expression | [107] |
| Dicer | Enzyme: Endoribonuclease | Hematopoietic Stem Cells | Down-regulation of erythroid genes, defective erythroid linage differentiation | [108] |
| Ml1 | Enzyme: Mixed-lineage leukemia | Hematopoietic Stem Cells | Embryonic lethal, reduced numbers of HSCs, abnormal HSC function | [109] |
| Ufm1 | Ubiquitin-like modification system: Ubiquitin-fold modifier 1 | Hematopoietic Stem Cells | Embryonic lethal, diminished hematopoietic development, increased HSC death, severe anemia, cytopenia, elevated endoplasmic reticulum stress, blocked autophagic degradation, increased ROS | [110] |
| Rac1, Rac2 | Rac GTPases: Racl and Rac2 regulates many aspects of intracellular actin dynamics | Erythropoiesis | Abnormal BFU-E and CFU-E morphology, decreased megakaryocyte-erythrocyte progenitors in bone marrow, increased splenic erythropoiesis | [111] |
| Adar1 | RNA-editing enzyme: Adenosine deaminase acting on RNA 1 | Hematopoietic Stem Cells | Embryonic lethal, abnormal HSC function, increased HSC apoptosis | [112] |
| Sod2 | Enzyme: Superoxide dismutase 2 | Hematopoietic Stem Cells | Increased ROS in erythroid progeny, diminished erythrocytes, decreased ferrochelatase activity, extramedullary hematopoiesis, systemic iron redistribution, abnormal gene expression (hematopoietic transcription factors, globins, and iron-response genes), changes in histone posttranslational modification | [113] |
A broad-coverage internet resource that may be used in conjunction with the mouse sources listed in this chapter is the International Mouse Strain Resource (IMSR, www.findmice.org), which offers users a catalog of worldwide mouse resources (live, cryopre-served, embryonic stem cells). A search engine is available on the home page that will perform a user-defined search of all or select resources. In addition, links are provided for direct access to the web sites of repositories, which describe holding resources of interest, descriptions of the holdings, and order forms. A link to the Mouse Genome Database (MGD, www.informatics.jax.org) is also provided for detailed information about the gene(s) involved, as well as the relationship of the mouse model(s) to human disease(s).
8. Reporter Mice
Mice with robust gene expression readouts in response to activation have utility in the screening or testing of potential compounds with therapeutic ramification in an in vivo setting. Reactivation of silenced γ-globin gene expression is of substantial clinical interest. A dual luciferase β-YAC transgenic mouse in which the Aγ-globin promoter was fused to firefly luciferase and the β-globin promoter was fused to Renilla luciferase was utilized to derive immortalized bone marrow cells that were subsequently used in a high-throughput screen (HTS) for γ-globin inducers [39]. Two other mouse models that serve a similar purpose is the so-called GG mouse, which carries a 183 kb human β-globin locus with eGFP fused to the Gγ-globin promoter [40] and a human β-globin locus Aγ-GFP (or GPA/GFP) β-DsRed PAC transgenic mouse [41].
9. Future Directions
Transgenesis via classic microinjection of oocytes or knock-in replacement of murine genes with their human counterparts in mouse ES cells remains cutting-edge and are important techniques for producing humanized mouse models. However, with the advent of genome editing via CRISPR/Cas9, TALENS, and zinc-finger nucleases, existing humanized mouse lines can be modified without the arduous process of generating an entire new mouse line. iPSCs or ESCs can be derived from existing mice, the cells subjected to genome editing, and new mice created from the engineered cells. The human transgenes themselves could be modified or endogenous mouse genes that play a role in transgene expression or gene product function could be modified. All of this would dramatically reduce the time required for mouse production, in many instances eliminating transgene introduction, gene targeting in ES cells, and many generations of breeding to produce mice with a desired combination of human transgenes and endogenous gene knockouts or mutants. Part of the power of this approach would be the ability to introduce subtle alterations affecting phenotype via a variety of hypomorphic mutations or changes that affect protein function including those affecting posttranslational modification, allostery, protein-protein interaction, and protein folding.
References
- 1.Trimborn T, Gribnau J, Grosveld F, Fraser P (1999) Mechanisms of developmental control of transcription in the murine α- and β-globin loci. Genes Dev 13(1):112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grosveld F, van Assendelft GB, Greaves DR, Kollias G (1987) Position-independent, high-level expression of the human β-globin gene in transgenic mice. Cell 51:975–985 [DOI] [PubMed] [Google Scholar]
- 3.Chada K, Magram J, Raphael K et al. (1985) Specific expression of a foreign β-globin gene in erythroid cells of transgenic mice. Nature 314:377–380 [DOI] [PubMed] [Google Scholar]
- 4.Kollias G, Wrighton N, Hurst J, Grosveld F (1986) Regulated expression of human Aγ-, β-, and hybrid γβ-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell 46:89–94 [DOI] [PubMed] [Google Scholar]
- 5.Enver T, Raich N, Ebens AJ et al. (1990) Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature 344:309–313 [DOI] [PubMed] [Google Scholar]
- 6.Higgs DR, Wood WG, Jarman AP et al. (1990) A major positive regulatory region located far upstream of the human α-globin gene locus. Genes Dev 4:1588–1601 [DOI] [PubMed] [Google Scholar]
- 7.Sharpe JA, Wells DJ, Whitelaw E et al. (1993) Analysis of the human α-globin gene cluster in transgenic mice. Proc Natl Acad Sci U S A 90:11262–11266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waggoner SA, Liebhaber SA (2003) Regulation of α-globin mRNA stability. Exp Biol Med 228:387–395 [DOI] [PubMed] [Google Scholar]
- 9.Kaufman RM, Pham CT, Ley TJ (1999) Transgenic analysis of a 100-kb human β-globin cluster-containing DNA fragment propagated as a bacterial artificial chromosome. Blood 94:3178–3184 [PubMed] [Google Scholar]
- 10.Peterson KR (2003) Transgenic mice carrying yeast artificial chromosomes. Expert Rev Mol Med 2003:1–25 [DOI] [PubMed] [Google Scholar]
- 11.Katsantoni EZ, de Krom M, Kong-a-San J et al. (2004) An embryonic-specific repressor element located 3′ to the Aγ-globin gene influences transcription of the human β-globin locus in transgenic mice. Exp Hematol 32:224–233 [DOI] [PubMed] [Google Scholar]
- 12.Gaensler KM, Kitamura M, Kan YW (1993) Germ-line transmission and developmental regulation of a 150-kb yeast artificial chromo-some containing the human β-globin locus in transgenic mice. Proc Natl Acad Sci U S A 90:11381–11385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson KR, Clegg CH, Huxley C et al. (1993) Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human β-globin locus display proper developmental control of human globin genes. Proc Natl Acad Sci U S A 90:7593–7597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayanan K, Williamson R, Zhang Y, Stewart AF, Ioannou PA (1999) Efficient and precise engineering of a 200 kb β-globin human/bacterial artificial chromosome in E. coli DH10B using an inducible homologous recombination system. Gene Ther 6:442–447 [DOI] [PubMed] [Google Scholar]
- 15.Harju S, McQueen KJ, Peterson KR (2002) Chromatin structure and control of β-like globin gene switching. Exp Biol Med 227:683–700 [DOI] [PubMed] [Google Scholar]
- 16.de Laat W, Grosveld F (2003) Spatial organization of gene expression: the active chromatin hub. Chromosome Res 11:447–459 [DOI] [PubMed] [Google Scholar]
- 17.Paw BH, Choe S-K, Costa FC, Sundar SV, Peterson KR (2006) Vertebrate models for sickle cell disease research In: Pace BS (ed) Renaissance of sickle cell disease research in the genomic era. World Scientific and Imperial College Press, London, pp 237–257 [Google Scholar]
- 18.Nagel RL, Fabry ME (2001) The panoply of animal models for sickle cell anaemia. Br J Haematol 112:19–25 [DOI] [PubMed] [Google Scholar]
- 19.Rubin EM, Witkowska HE, Spangler E et al. (1991) Hypoxia-induced in vivo sickling of transgenic mouse red cells. J Clin Invest 87:639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popp RA, Popp DM, Shinpock SG et al. (1997) A transgenic mouse model of hemoglobin S Antilles disease. Blood 89:4204–4212 [PubMed] [Google Scholar]
- 21.Fabry ME, Costantini F, Pachnis A et al. (1992) High expression of human βS- and α-globins in transgenic mice: erythrocyte abnormalities, organ damage, and the effect of hypoxia. Proc Natl Acad Sci U S A 89:12155–12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabry ME, Nagel RL, Pachnis A, Suzuka SM, Costantini F (1992) High expression of human βS- and α-globins in transgenic mice: hemoglobin composition and hematological consequences. Proc Natl Acad Sci U S A 89:12150–12154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabry ME, Sengupta A, Suzuka SM et al. (1995) A second generation transgenic mouse model expressing both hemoglobin S (HbS) and HbS-Antilles results in increased phenotypic severity. Blood 86:2419–2428 [PubMed] [Google Scholar]
- 24.Trudel M, Saadane N, Garel MC et al. (1991) Towards a transgenic mouse model of sickle cell disease: hemoglobin SAD. EMBO J 10:3157–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trudel M, De Paepe ME, Chretien N et al. (1994) Sickle cell disease of transgenic SAD mice. Blood 84:3189–3197 [PubMed] [Google Scholar]
- 26.Paszty C, Brion CM, Manci E et al. (1997) Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science 278:876–878 [DOI] [PubMed] [Google Scholar]
- 27.Ryan TM, Townes TM, Reilly MP et al. (1990) Human sickle hemoglobin in transgenic mice. Science 247:566–568 [DOI] [PubMed] [Google Scholar]
- 28.Ryan TM, Ciavatta DJ, Townes TM (1997) Knockout-transgenic mouse model of sickle cell disease. Science 278:873–876 [DOI] [PubMed] [Google Scholar]
- 29.Chang JC, Lu R, Lin C et al. (1998) Transgenic knockout mice exclusively expressing human hemoglobin S after transfer of a 240-kb βS-globin yeast artificial chromosome: a mouse model of sickle cell anemia. Proc Natl Acad Sci U S A 95:14886–14890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blouin MJ, Beauchemin H, Wright A et al. (2000) Genetic correction of sickle cell disease: insights using transgenic mouse models. Nat Med 6:177–182 [DOI] [PubMed] [Google Scholar]
- 31.Fabry ME, Suzuka SM, Weinberg RS et al. (2001) Second generation knockout sickle mice: the effect of HbF. Blood 97:410–418 [DOI] [PubMed] [Google Scholar]
- 32.Yang B, Kirby S, Lewis J et al. (1995) A mouse model for β0-thalassemia. Proc Natl Acad Sci U S A 92:11608–11612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciavatta DJ, Ryan TM, Farmer SC, Townes TM (1995) Mouse model of human beta zero thalassemia: targeted deletion of the mouse βmaj- and βmin-globin genes in embryonic stem cells. Proc Natl Acad Sci U S A 92:9259–9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paszty C, Mohandas N, Stevens ME et al. (1995) Lethal α-thalassaemia created by gene targeting in mice and its genetic rescue. Nat Genet 11:33–39 [DOI] [PubMed] [Google Scholar]
- 35.Skow LC, Burkhart BA, Johnson FM et al. (1983) A mouse model for β-thalassemia.Cell 34:1043–1052 [DOI] [PubMed] [Google Scholar]
- 36.Lewis J, Yang B, Kim R et al. (1998) A common human β globin splicing mutation modeled in mice. Blood 91:2152–2156 [PubMed] [Google Scholar]
- 37.Huo Y, McConnell SC, Liu S-R et al. (2009) Humanized model of Cooley’s anemia. J Biol Chem 284:4889–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huo Y, McConnell SC, Ryan TM (2009) Preclinical transfusion-dependent humanized mouse model of β thalassemia major. Blood 113:4763–4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson KR, Costa FC, Fedosyuk F, Neades RY, Chazelle AM, Zelenchuk L, Fonteles AH, Dalal P, Roy A, Chaguturu R, Li B, Pace BS (2014) A cell-based high-throughput screen for novel inducers of fetal hemoglobin for treatment of hemoglobinopathies. PLoS One 9:e107006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McColl B, Kao BR, Lourthai P et al. (2014) An in vivo model for analysis of developmental erythropoiesis and globin gene regulation. FASEB J 28:2306–2317 [DOI] [PubMed] [Google Scholar]
- 41.Papadopoulos P, Gutierrez L, van der Linden R et al. (2012) A dual reporter mouse model of the human b-globin locus: applications and limitations. PLoS One 7(12):e51272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinrich AC, Pelanda R, Klingmuller U (2004) A mouse model for visualization and conditional mutations in the erythroid line-age. Blood 104(3):659–666. doi: 10.1182/blood-2003-05-1442 [DOI] [PubMed] [Google Scholar]
- 43.Georgiades P, Ogilvy S, Duval H, Licence DR, Charnock-Jones DS, Smith SK, Print CG (2002) VavCre transgenic mice: a tool for mutagenesis in hematopoietic and endothelial lineages. Genesis 34(4):251–256. doi: 10.1002/gene.10161 [DOI] [PubMed] [Google Scholar]
- 44.Yao YC, Zi XY, Li JX, Xiong J, Xie QD, Wang XM, Hu YP (2001) Generation of Mx-cre transgenic mice. Yi Chuan Xue Bao 28 (4):313–316 [PubMed] [Google Scholar]
- 45.Jasinski M, Keller P, Fujiwara Y, Orkin SH, Bessler M (2001) GATA1-Cre mediates Piga gene inactivation in the erythroid/megakaryocytic lineage and leads to circulating red cells with a partial deficiency in glycosyl phosphatidylinositol-linked proteins (paroxysmal nocturnal hemoglobinuria type II cells). Blood 98(7):2248–2255. doi: 10.1182/blood.V98.7.2248 [DOI] [PubMed] [Google Scholar]
- 46.Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21(1):70–71. doi: 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- 47.Kisanuki YY (2001) Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 230(2):230–242. doi: 10.1006/dbio.2000.0106 [DOI] [PubMed] [Google Scholar]
- 48.Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L (1997) Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res 25(21):4323–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson KR, Fedosyuk H, Zelenchuk L, Nakamoto B, Yannaki E, Stamatoyannopoulos G, Ciciotte S, Peters LL, Scott LM, Papayannopoulou T (2004) Transgenic Cre expression mice for generation of erythroidspecific gene alterations. Genesis 39(1):1–9. doi: 10.1002/gene.20020 [DOI] [PubMed] [Google Scholar]
- 50.Holzenberger M, Lenzner C, Leneuve P, Zaoui R, Hamard G, Vaulont S, Le Bouc Y (2000) Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a target gene. Nucleic Acids Res 28 (21):e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R (2002) Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis 32(1):19–26 [DOI] [PubMed] [Google Scholar]
- 52.Maeda T, Ito K, Merghoub T, Poliseno L, Hobbs RM, Wang G, Dong L, Maeda M, Dore LC, Zelent A, Luzzatto L, Teruya-Feldstein J, Weiss MJ, Pandolfi PP (2009) LRF is an essential downstream target of GATA1 in erythroid development and regulates BIM-dependent apoptosis. Dev Cell 17 (4):527–540. doi: 10.1016/j.devcel.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, Martin GR, Bishop JM (2001) c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature 414(6865):768–773. doi: 10.1038/414768a [DOI] [PubMed] [Google Scholar]
- 54.Mikkola HKA, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH (2003) Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature 421(6922):547–551. doi: 10.1038/nature01345 [DOI] [PubMed] [Google Scholar]
- 55.Abdel-Wahab O, Gao J, Adli M, Dey A, Trimarchi T, Chung YR, Kuscu C, Hricik T, Ndiaye-Lobry D, LaFave LM, Koche R, Shih AH, Guryanova OA, Kim E, Li S, Pandey S, Shin JY, Telis L, Liu J, Bhatt PK, Monette S, Zhao X, Mason CE, Park CY, Bernstein BE, Aifantis I, Levine RL (2013) Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. J Exp Med 210 (12):2641–2659. doi: 10.1084/jem.20131141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Unnisa Z, Clark JP, Roychoudhury J, Thomas E, Tessarollo L, Copeland NG, Jenkins NA, Grimes HL, Kumar AR (2012) Meis1 preserves hematopoietic stem cells in mice by limiting oxidative stress. Blood 120 (25):4973–4981. doi: 10.1182/blood-2012-06-435800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alhashem YN, Vinjamur DS, Basu M, Klingmüller U, Gaensler KML, Lloyd JA (2011) Transcription factors KLF1 and KLF2 positively regulate embryonic and fetal β-globin genes through direct promoter binding*. J Biol Chem 286(28):24819–24827. doi: 10.1074/jbc.M111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sankaran VG, Xu J, Ragoczy T, Ippolito GC, Walkley CR, Maika SD, Fujiwara Y, Ito M, Groudine M, Bender MA, Tucker PW, Orkin SH (2009) Developmental and species-divergent globin switching are driven by BCL11A. Nature 460(7259):1093–1097. doi: 10.1038/nature08243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa FC, Fedosyuk H, Chazelle AM, Neades RY, Peterson KR (2012) Mi2β is required for γ-globin gene silencing: temporal assembly of a GATA-1-FOG-1-Mi2 repressor complex in β-YAC transgenic mice. PLoS Genet 8(12): e1003155. doi: 10.1371/journal.pgen.1003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mortensen M, Ferguson DJP, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK (2010) Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A 107(2):832–837. doi: 10.1073/pnas.0913170107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stumpf M, Yue X, Schmitz S, Luche H, Reddy JK, Borggrefe T (2010) Specific erythroid lineage defect in mice conditionally deficient for Mediator subunit Med1. Proc Natl Acad Sci U S A 107(50):21541–21546. doi: 10.1073/pnas.1005794107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohan IW, Scheer E, Wendling O, Metzger D, Tora L (2003) TAF10 (TAFII30) Is Necessary for TFIID Stability and Early Embryo-genesis in Mice. Mol Cell Biol 23 (12):4307–4318. doi: 10.1128/mcb.23.12.4307-4318.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khandanpour C, Sharif-Askari E, Vassen L, Gaudreau M-C, Zhu J, Paul WE, Okayama T, Kosan C, Möröy T (2010) Evidence that Growth factor independence 1b regulates dormancy and peripheral blood mobilization of hematopoietic stem cells. Blood 116 (24):5149–5161. doi: 10.1182/blood-2010-04-280305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knight ZA, Schmidt SF, Birsoy K, Tan K, Friedman JM (2014) A critical role for mTORC1 in erythropoiesis and anemia. eLife 3:e01913. doi: 10.7554/eLife.01913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L, Lee JY, Gross J, Song SH, Dean A, Love PE (2010) A requirement for Lim domain binding protein 1 in erythropoiesis. J Exp Med 207(12):2543–2550. doi: 10.1084/jem.20100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L, Sánchez-Bonilla M, Crouthamel M, Giachelli C, Keel S (2013) Mice lacking the sodium-dependent phosphate import protein, PiT1 (SLC20A1), have a severe defect in terminal erythroid differentiation and early B cell development. Exp Hematol 41 (5):432–443. e437. doi: 10.1016/j.exphem.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Obeng EA et al. (2016) Physiologic expression of Sf3b1K700E causes impaired erythropoiesis, aberrant splicing, and sensitivity to therapeutic spliceosome modulation. Cancer Cell 30:404. doi: 10.1016/j.ccell.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kosan C, Rashkovan M, Ross J, Schaffer AM, Saba I, Lemsaddek W, Trudel M, Möröy T (2014) The transcription factor Miz-1 is required for embryonic and stress-induced erythropoiesis but dispensable for adult erythropoiesis. Am J Blood Res 4(1):7–19 [PMC free article] [PubMed] [Google Scholar]
- 69.Griffin CT, Brennan J, Magnuson T (2008) The chromatin-remodeling enzyme BRG1 plays an essential role in primitive erythropoiesis and vascular development. Development 135(3):493–500. doi: 10.1242/dev.010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Konstantinidis DG, Giger KM, Risinger M, Pushkaran S, Zhou P, Dexheimer P, Yerneni S, Andreassen P, Klingmüller U, Palis J, Zheng Y, Kalfa TA (2015) Cytokinesis failure in RhoA-deficient mouse erythroblasts involves actomyosin and midbody dysregulation and triggers p53 activation. Blood 126 (12):1473–1482. doi: 10.1182/blood-2014-12-616169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sathyanarayana P, Dev A, Pradeep A, Ufkin M, Licht JD, Wojchowski DM (2012) Spry1 as a novel regulator of erythropoiesis, EPO/EPOR target, and suppressor of JAK2. Blood 119(23):5522–5531. doi: 10.1182/blood-2011-11-392571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drogat B, Kalucka J, Gutiérrez L, Hammad H, Goossens S, Ghahremani MF, Bartunkova S, Haigh K, Deswarte K, Nyabi O, Naessens M, Ferrara N, Klingmüller U, Lambrecht BN, Nagy A, Philipsen S, Haigh JJ (2010) Vegf regulates embryonic erythroid development through Gata1 modulation. Blood 116 (12):2141–2151. doi: 10.1182/blood-2010-01-264143 [DOI] [PubMed] [Google Scholar]
- 73.Zhu B-M, McLaughlin SK, Na R, Liu J, Cui Y, Martin C, Kimura A, Robinson GW, Andrews NC, Hennighausen L (2008) Hematopoietic-specific Stat5-null mice display microcytic hypochromic anemia associated with reduced transferrin receptor gene expression. Blood 112(5):2071–2080. doi: 10.1182/blood-2007-12-127480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang L, Wang L, Kalfa TA, Cancelas JA, Shang X, Pushkaran S, Mo J, Williams DA, Zheng Y (2007) Cdc42 critically regulates the balance between myelopoiesis and erythropoiesis. Blood 110(12):3853–3861. doi: 10.1182/blood-2007-03-079582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roetto A, Cunto FD, Pellegrino RM, Hirsch E, Azzolino O, Bondi A, Defilippi I, Carturan S, Miniscalco B, Riondato F, Cilloni D, Silengo L, Altruda F, Camaschella C, Saglio G (2010) Comparison of 3 Tfr2-deficient murine models suggests distinct functions for Tfr2-α and Tfr2-β isoforms in different tissues. Blood 115(16):3382–3389. doi: 10.1182/blood-2009-09-240960 [DOI] [PubMed] [Google Scholar]
- 76.Troadec MB, Warner D, Wallace J, Thomas K, Spangrude GJ, Phillips J, Khalimonchuk O, Paw BH, Ward DM, Kaplan J (2011) Targeted deletion of the mouse Mitoferrin1 gene: from anemia to protoporphyria. Blood 117(20):5494–5502. doi: 10.1182/blood-2010-11-319483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG (2010) Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 115(17):3589–3597. doi: 10.1182/blood-2009-04-215848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH (2008) Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood 111(6):3229–3235. doi: 10.1182/blood-2007-09-114561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeigler BM, Vajdos J, Qin W, Loverro L, Niss K (2010) A mouse model for an erythropoietin-deficiency anemia. Dis Model Mech 3(11–12):763–772. doi: 10.1242/dmm.004788 [DOI] [PubMed] [Google Scholar]
- 80.Sankaran VG, Orkin SH, Walkley CR (2008) Rb intrinsically promotes erythropoiesis by coupling cell cycle exit with mitochondrial biogenesis. Genes Dev 22(4):463–475. doi: 10.1101/gad.1627208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J, Kaartinen V (2008) Generation of mice with a conditional allele for Trim33. Genesis 46(6):329–333. doi: 10.1002/dvg.20401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagner KU, Claudio E, Rucker EB 3rd, Riedlinger G, Broussard C, Schwartzberg PL, Siebenlist U, Hennighausen L (2000) Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development 127 (22):4949–4958 [DOI] [PubMed] [Google Scholar]
- 83.Diwan A, Koesters AG, Odley AM, Pushkaran S, Baines CP, Spike BT, Daria D, Jegga AG, Geiger H, Aronow BJ, Molkentin JD, Macleod KF, Kalfa TA, Dorn GW (2007) Unrestrained erythroblast development in Nix−/− mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proc Natl Acad Sci U S A 104(16):6794–6799. doi: 10.1073/pnas.0610666104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cui S, Tanabe O, Sierant M, Shi L, Campbell A, Lim KC, Engel JD (2015) Compound loss of function of nuclear receptors Tr2 and Tr4 leads to induction of murine embryonic β-type globin genes. Blood 125 (9):1477–1487. doi: 10.1182/blood-2014-10-605022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, Romain RD, Park D, Scadden DT, Merchant JL, Arnaout MA (2014) Stress hematopoiesis is regulated by the Krüppel-like transcription factor ZBP-89. Stem Cells 32 (3):791–801. doi: 10.1002/stem.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hosoya T, Clifford M, Losson R, Tanabe O, Engel JD (2013) TRIM28 is essential for erythroblast differentiation in the mouse. Blood 122(23):3798–3807. doi: 10.1182/blood-2013-04-496166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou Z, Enis DR, Bui H, Khandros E, Kumar V, Jakus Z, Thom C, Yang Y, Dhillon V, Chen M, Lu M, Weiss MJ, Kahn ML (2013) The secreted lymphangiogenic factor CCBE1 is essential for fetal liver erythropoiesis. Blood 121(16):3228–3236. doi: 10.1182/blood-2012-10-462689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leung CG, Xu Y, Mularski B, Liu H, Gurbuxani S, Crispino JD (2007) Requirements for survivin in terminal differentiation of erythroid cells and maintenance of hematopoietic stem and progenitor cells. J Exp Med 204 (7):1603–1611. doi: 10.1084/jem.20062395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Growney JD, Shigematsu H, Li Z, Lee BH, Adelsperger J, Rowan R, Curley DP, Kutok JL, Akashi K, Williams IR, Speck NA, Gilli-land DG (2005) Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood 106 (2):494–504. doi: 10.1182/blood-2004-08-3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scott LM, Priestley GV, Papayannopoulou T (2003) Deletion of α4 Integrins from Adult Hematopoietic Cells Reveals Roles in Homeostasis, Regeneration, and Homing. Mol Cell Biol 23(24):9349–9360. doi: 10.1128/mcb.23.24.9349-9360.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kolbus A, Pilat S, Husak Z, Deiner EM, Stengl G, Beug H, Baccarini M (2002) Raf-1 antagonizes erythroid differentiation by restraining caspase activation. J Exp Med 196(10):1347–1353. doi: 10.1084/jem.20020562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park SO, Wamsley HL, Bae K, Hu Z, Li X, Choe S, Slayton WB, Oh SP, Wagner KU, Sayeski PP (2013) Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One 8(3): e59675. doi: 10.1371/journal.pone.0059675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pondarre C, Campagna DR, Antiochos B, Sikorski L, Mulhern H, Fleming MD (2007) Abcb7, the gene responsible for X-linked side-roblastic anemia with ataxia, is essential for hematopoiesis. Blood 109(8):3567–3569. doi: 10.1182/blood-2006-04-015768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai Y, Pi W, Sivaprakasam S, Zhu X, Zhang M, Chen J, Makala L, Lu C, Wu J, Teng Y, Pace B, Tuan D, Singh N, Li H (2015) UFBP1, a key component of the Ufm1 conjugation system, is essential for ufmylation-mediated regulation of erythroid development. PLoS Genet 11(11):e1005643. doi: 10.1371/journal.pgen.1005643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mochizuki-Kashio M, Mishima Y, Miyagi S, Negishi M, Saraya A, Konuma T, Shinga J, Koseki H, Iwama A (2011) Dependency on the polycomb gene Ezh2 distinguishes fetal from adult hematopoietic stem cells. Blood 118(25):6553–6561. doi: 10.1182/blood-2011-03-340554 [DOI] [PubMed] [Google Scholar]
- 96.Yao H, Goldman DC, Nechiporuk T, Kawane S, McWeeney SK, Tyner JW, Fan G, Kerenyi MA, Orkin SH, Fleming WH, Mandel G (2014) Corepressor Rcor1 is essential for murine erythropoiesis. Blood 123 (20):3175–3184. doi: 10.1182/blood-2013-11-538678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malik J, Getman M, Lillis J, Gallagher PG, Steiner LA (2015) The histone methyltransferase Setd8 is essential for mammalian erythropoiesis. Blood 126:3577 [Google Scholar]
- 98.Pan D, Schomber T, Kalberer CP, Terracciano LM, Hafen K, Krenger W, Hao-Shen H, Deng C, Skoda RC (2007) Normal erythropoiesis but severe polyposis and bleeding anemia in Smad4-deficient mice. Blood 110 (8):3049–3055. doi: 10.1182/blood-2007-02-074393 [DOI] [PubMed] [Google Scholar]
- 99.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, Selak MA, Ney PA, Thompson CB (2008) Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112(4):1493–1502. doi: 10.1182/blood-2008-02-137398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tubi LQ, Nunes SC, Casellato A, Mandato E,Zaffino F, Brancalion A, Filhol-Cochet O, Boldyreff B, Manni S, Semenzato G, Piazza F (2014) Csnk2β knockout during hematopoiesis results in lethality at mid/late gestation mostly due to impaired fetal erythropoiesis. Blood 124:4329 [Google Scholar]
- 101.Mercurio S, Petrillo S, Chiabrando D, Bassi ZI, Gays D, Camporeale A, Vacaru A, Minis-calco B, Valperga G, Silengo L, Altruda F, Baron MH, Santoro MM, Tolosano E (2015) The heme exporter Flvcr1 regulates expansion and differentiation of committed erythroid progenitors by controlling intracellular heme accumulation. Haematologica 100 (6):720–729. doi: 10.3324/haematol.2014.114488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai JC, Ponti S, Pan D, Kohler H, Skoda RC,Matthias P, Nagamine Y (2012) The DEAH-box helicase RHAU is an essential gene and critical for mouse hematopoiesis. Blood 119 (18):4291–4300. doi: 10.1182/blood-2011-08-362954 [DOI] [PubMed] [Google Scholar]
- 103.Sahr KE, Lambert AJ, Ciciotte SL, Mohandas N, Peters LL (2009) Targeted deletion of the γ-adducin gene (Add3) in mice reveals differences in α-adducin interactions in erythroid and nonerythroid cells. Am J Hematol 84 (6):354–361. doi: 10.1002/ajh.21427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minella AC, Loeb KR, Knecht A, Welcker M, Varnum-Finney BJ, Bernstein ID, Roberts JM, Clurman BE (2008) Cyclin E phosphorylation regulates cell proliferation in hematopoietic and epithelial lineages in vivo. Genes Dev 22(12):1677–1689. doi: 10.1101/gad.1650208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schneider RK, Schenone M, Ferreira MV, Kramann R, Joyce CE, Hartigan C, Beier F, Brümmendorf TH, Germing U, Platzbecker U, Büsche G, Knüchel R, Chen MC, Waters CS, Chen E, Chu LP, Novina CD, Lindsley RC, Carr SA, Ebert BL (2016) Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by S100A8 and S100A9. Nat Med 22:288–297. doi: 10.1038/nm.4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keel S, Phelps S, Sabo K, O’Leary MN, Kirn-Safran CB, Abkowitz JL (2012) Establishing Rps6 hemizygous mice as a model for studying how ribosomal protein haploinsufficiency impairs erythropoiesis. Exp Hematol 40 (4):290–294. doi: 10.1016/j.exphem.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen W, Gu P, Jiang X, Ruan HB, Li C, Gao X (2011) Protein Phosphatase 2A Catalytic Subunit α (PP2Acα) Maintains Survival of Committed Erythroid Cells in Fetal Liver Erythropoiesis through the STAT5 Pathway. Am J Pathol 178(5):2333–2343. doi: 10.1016/j.ajpath.2011.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Buza-Vidas N, Cismasiu VB, Moore S, Mead AJ, Woll PS, Lutteropp M, Melchiori L, Luc S, Bouriez-Jones T, Atkinson D, O’Carroll D, Jacobsen SEW, Nerlov C (2012) Dicer is selectively important for the earliest stages of erythroid development. Blood 120 (12):2412–2416. doi: 10.1182/blood-2011-10-383653 [DOI] [PubMed] [Google Scholar]
- 109.McMahon KA, Hiew SY, Hadjur S, Veiga-Fernandes H, Menzel U, Price AJ, Kioussis D, Williams O, Brady HJ (2007) Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell 1 (3):338–345. doi: 10.1016/j.stem.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 110.Zhang M, Zhu X, Zhang Y, Cai Y, Chen J, Sivaprakasam S, Gurav A, Pi W, Makala L, Wu J, Pace B, Tuan-Lo D, Ganapathy V, Singh N, Li H (2015) RCAD|[sol]|Ufl1, a Ufm1 E3 ligase, is essential for hematopoietic stem cell function and murine hematopoiesis. Cell Death Differ 22(12):1922–1934. doi: 10.1038/cdd.2015.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kalfa TA, Pushkaran S, Zhang X, Johnson JF,Pan D, Daria D, Geiger H, Cancelas JA, Williams DA, Zheng Y (2010) Rac1 and Rac2 GTPases are necessary for early erythropoietic expansion in the bone marrow but not in the spleen. Haematologica 95(1):27–35. doi: 10.3324/haematol.2009.006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.XuFeng R, Boyer MJ, Shen H, Li Y, Yu H, Gao Y, Yang Q, Wang Q, Cheng T (2009) ADAR1 is required for hematopoietic progenitor cell survival via RNA editing. Proc Natl Acad Sci U S A 106(42):17763–17768. doi: 10.1073/pnas.0903324106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Case AJ et al. (2013) Manganese superoxide dismutase depletion in murine hematopoietic stem cells perturbs iron homeostasis, globin switching, and epigenetic control in erythrocyte precursorcells. Free Radic Biol Med 56:17–27. doi: 10.1016/j.freeradbiomed.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]